Recovery of Apatite from Magnetic Concentration Tailings by Flotation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Sample
2.2. Flotation Tests, Zeta Potential, and Contact Angle Measurements with Pure Apatite
2.3. Flotation Tests of Tailings Containing Apatite
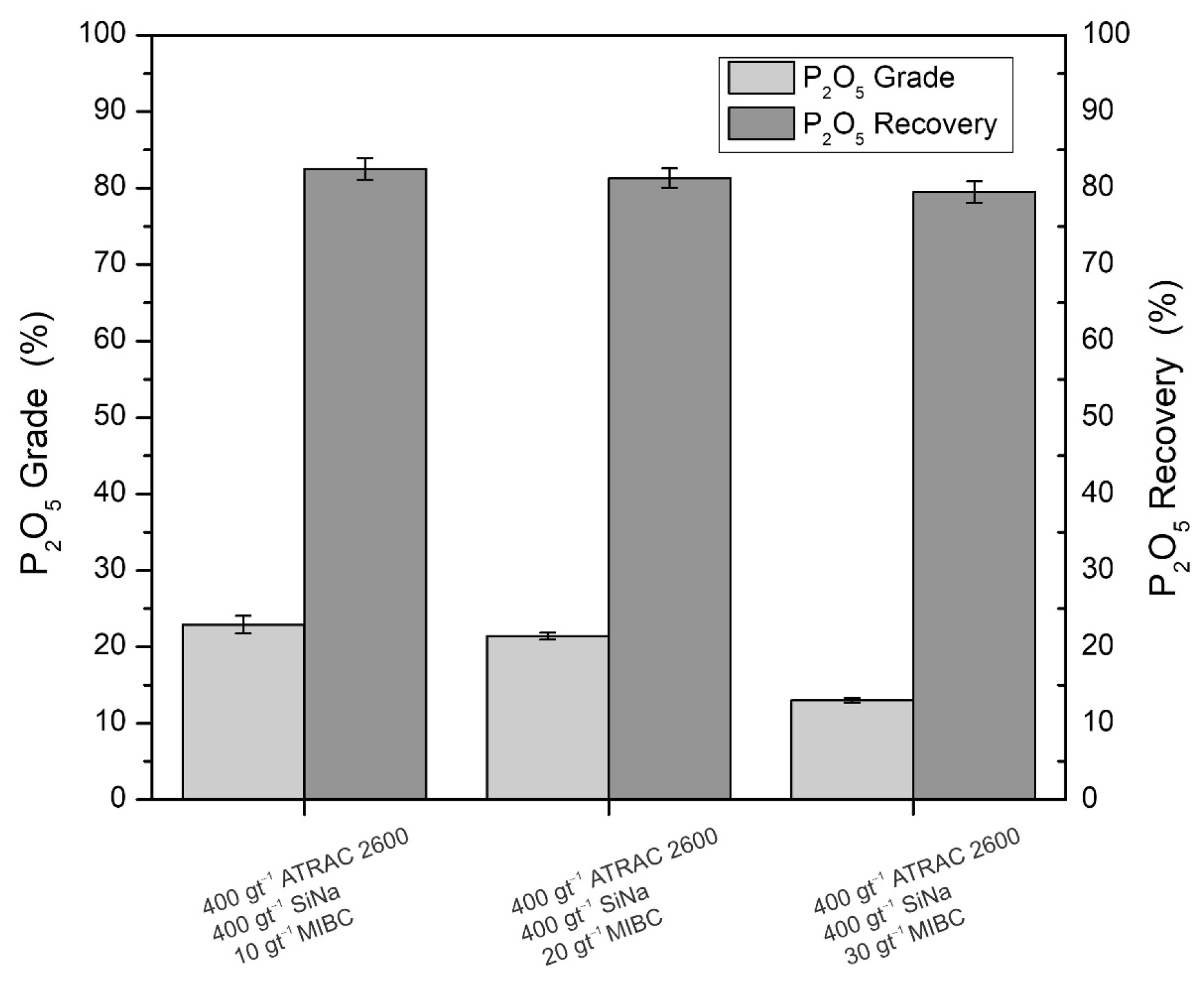
2.4. Kinetic Flotation Tests and Open Cycle Tests
3. Results
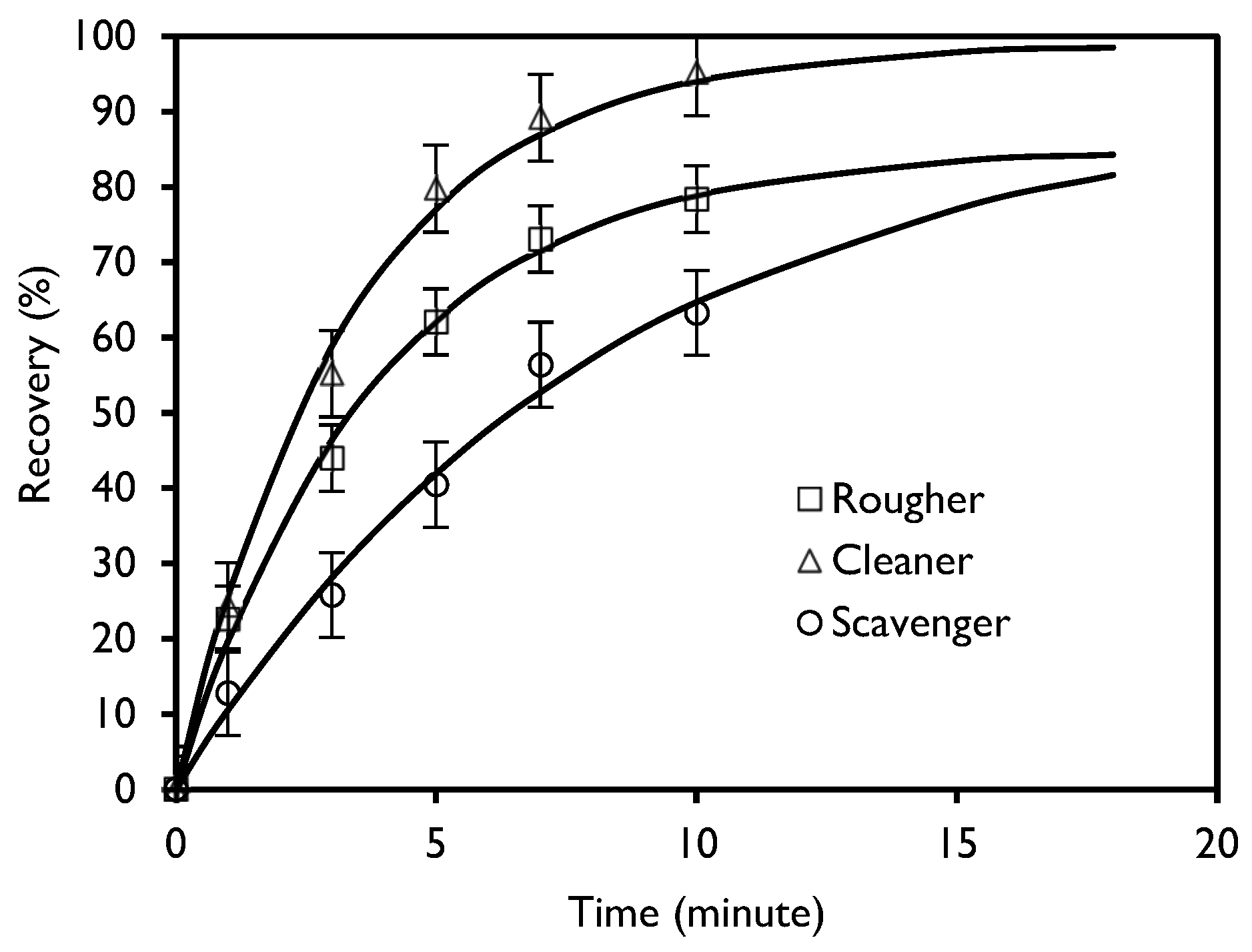
3.1. Flotation Kinetics
3.2. Determination of the Split Factor
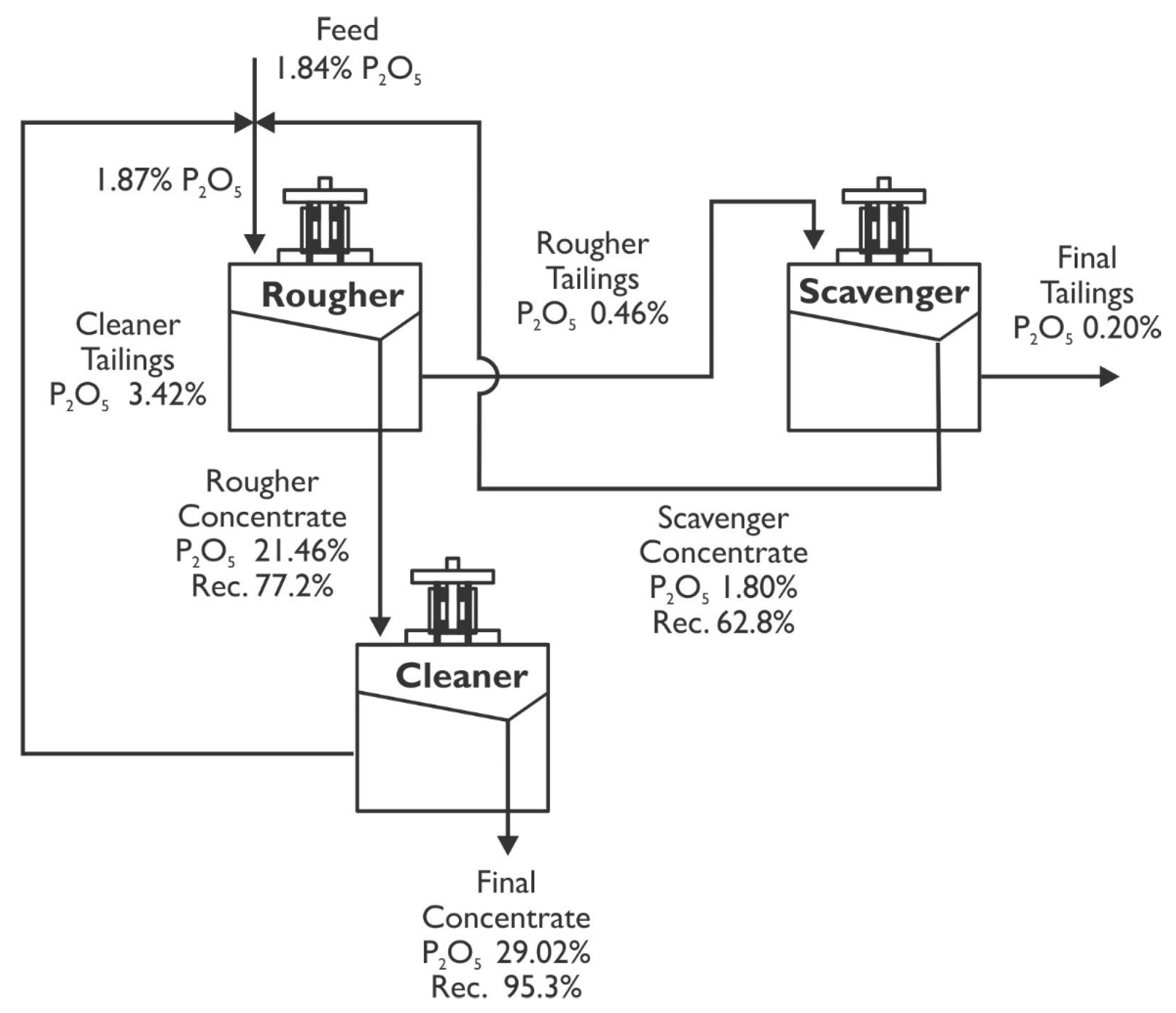
3.3. Simulation of Flotation Circuits
4. Conclusions
- The utilization of the Atrac-2600 collector significantly enhanced the contact angle and hydrophobicity of the apatite. However, no adsorption of this reagent was observed on the surfaces of the gangue minerals;
- The flotation circuit must comprise the rougher–scavenger–cleaner stages to produce concentrates with P2O5 grades and recoveries of 29.02% and 89.7%, respectively. The iron tailings must undergo a 10 min conditioning process with 400 gt−1 of Atrac-2600 collector, 400 gt−1 sodium silicate dispersant, pH adjustment to 10, and flotation times for each stage set at 10, 7.6, and 6.8 min, respectively;
- The results presented in this study demonstrate that apatite can be recovered from the tailings of the magnetic concentration process of the Minera del Pacífico Company and can be a relevant source of phosphate, showing that it is feasible to transform an environmental liability into an economic resource within the mining sector, thus facilitating virtuous, inclusive, and sustainable mining.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jasinski, S.M. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2020.
- COCHILCO—Yearbook: Copper and Other Mineral Statistics 2003–2022. Available online: https://www.cochilco.cl/Lists/Anuario/Attachments/27/ANUARIO_ESTADISTICO_COCHILCO%20A%C3%91O%202022.pdf (accessed on 15 March 2023).
- Esposito, M.; Tse, T.; Soufani, K. Is the circular economy a new fast-expanding market? Thunderbird Int. Bus. Rev. 2017, 59, 9–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Zang, L.; Li, Z.; Qin, J. Discussion on the model of mining circular economy. Energy Procedia 2012, 16, 438–443. [Google Scholar] [CrossRef]
- Solomons, I. Mine Tailings Recovery Can Be Value Generator. Says Council for Geoscience. Mining Weekly, 9 June 2017. Available online: http://www.miningweekly.com/article/mine-tailings-recovery-can-be-value-generator-reduces-enviro-impact-2017-06-09 (accessed on 10 March 2023).
- Castillo, C.; Ihle, C.F.; Jeldres, R.I. Chemometric Optimisation of a Copper Sulphide Tailings Flocculation Process in the Presence of Clays. Minerals 2019, 9, 582. [Google Scholar] [CrossRef]
- Vivallo, W.; Henríquez, F.; Espinoza, S. Los depósitos de hierro del tipo magnetita-apatita: Geoquímica de las rocas volcanicas asociadas y potencialidad de la mena de hierro como fuente de mineralización de oro. Rev. Geol. Chile 1995, 22, 159–175. [Google Scholar] [CrossRef]
- Chen, H.; Cooke, D.R.; Baker, M.J. Mesozoic iron oxide copper-gold mineralization in the central Andes and the Gondwana Supercontinent breakup. Econ. Geol. 2013, 108, 37–44. [Google Scholar] [CrossRef]
- Brito, B.; Jerez, O.; Gutierrez, L. Incorporation of Rheological Characterization in Grinding and Tailings Slurries to Optimize the CMP Magnetic Separation Plant. Minerals 2021, 11, 386. [Google Scholar] [CrossRef]
- Xia, W.C.; Ma, G.X.; Bu, X.N.; Peng, Y.L. Effect of particle shape on bubble-particle attachment angle and flotation behavior of glass beads and fragments. Powder Technol. 2018, 338, 168–172. [Google Scholar] [CrossRef]
- Santos, E.P.; Dutra, A.J.B.; Oliveira, J.F. The effect of jojoba oil on the surface properties of calcite and apatite aiming at their selective flotation. Int. J. Miner. Process. 2015, 143, 34–38. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Lafhaj, Z.; Fornasiero, D. The role of a fatty alcohol in improving calcium minerals flotation with oleate. Colloids Surf. A 2019, 560, 410–417. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Jong, K.; Han, Y.; Ryom, S. Flotation mechanism of oleic acid amide on apatite. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 127–131. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Araujo, A.C.; Peres, A.E.C. Reagents in igneous phosphate ores flotation. Miner. Eng. 2005, 18, 199–204. [Google Scholar] [CrossRef]
- Costa, D.S.; Alves, A.S.; Budke, R.; Mendes, R.M.M.; Peres, A.E.C. Flotabilidade de apatita usando óleos vegetais da Amazônia como coletores. In Proceedings of the XXIV Encontró Nacional de Tratamento de Minérios e Metalurgia Extrativa, Salvador, Brazil, 16–19 October 2011; pp. 237–244. [Google Scholar]
- De Oliveira, P.; Mansur, H.; Mansur, A.; Da Silva, G.; Peres, A. Apatite flotation using pataua palm tree oil as collector. J. Mater. Res. Technol. 2019, 28, 4612–4619. [Google Scholar] [CrossRef]
- Gorochovceva, N.; Klingberg, A.; Lannefors, J. Development of anionic collectors for direct flotation of apatite from complex siliceous ores with a focus on sustainability. In Proceedings of the International Mineral Processing Congress. Proceedings: XXVII International Mineral Processing, Santiago, Chile, 20–24 October 2014; pp. 68–78. [Google Scholar]
- Shengchao, Y.; Wei, L. Rare earth elements in the iron-oxide apatite (IOA) deposit: Insights from apatite. Int. Geol. Rev. 2022, 64, 1–18. [Google Scholar] [CrossRef]
- Taylor, R.D.; Shah, A.K.; Walsh, G.J.; Taylor, C.D. Geochemistry and geophysics of iron oxide-apatite deposits and associated tailings piles with implications for potential rare earth element resources from historic minerals and mine tailings in the eastern Adirondack New York, USA. Econ. Geol. 2019, 114, 1569–1598. [Google Scholar] [CrossRef]
- Birinci, M. Enrichment of Apatite-Bearing Iron Ore by Magnetic Separation and Flotation. Eur. J. Tech. (EJT) 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Pålsson, B.I.; Fredriksson, A. Apatite for extraction. II. Flotation of apatite and rare earth elements from old tailings dumps. In Proceedings of the XXVI International Mineral Processing Congress—IMPC 2012, New Delhi, India, 24–28 September 2012; ISBN 81-901714-3-7. [Google Scholar]
- Oliveira, M.S.; Santana, R.C.; Ataíde, C.H.; Barrozo, M.A. Recovery of apatite from flotation tailings. Sep. Purif. Technol. 2011, 79, 79–84. [Google Scholar] [CrossRef]
- Valderrama, L.; Oliva, J.; Gómez, O.; Zazzali, B. Recuperación de apatita de relaves producidos en la concentración magnética de hierro. Rev. Holos 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Atrac-2600. Available online: https://www.nouryon.com/product/atrac-2600-anionic-surfactant-blend/ (accessed on 1 March 2023).
- Hanumantha Rao, K.; Dwari, R.K.; Lu, S.; Vilinska, A.; Somasundaran, P. Flotation of phosphate gangue from magnetite fines—Non-ionic surfactant as Atrac collector modifier. In Proceedings of the XXV International mineral processing Congress Proceedings, Brisbane, QLD, Australia, 6–10 September 2010; pp. 1933–1943. [Google Scholar]
- Amankonah, J.; Somasundaran, P. Effects of dissolved mineral species on the electrokinetic behavior of calcite and apatite. Colloids Surf. 1985, 15, 335–353. [Google Scholar] [CrossRef]
- Vučinić, D.R.; Radulović, D.S.; Deušić, S.D. Electrokinetic properties of hydroxyapatite under flotation conditions. J. Colloid Interface Sci. 2010, 343, 239–245. [Google Scholar] [CrossRef]
- Barros, L.A.F.; Ferreira, E.E.; Peres, A.E.C. Floatability of apatites and gangue minerals of an igneous phosphate ore. Miner. Eng. 2008, 21, 994–999. [Google Scholar] [CrossRef]
- Albino, K.I.P.; Gorochovceva, N.; Klingberg, A.; Lima, O.A. New synthetic collector for the direct flotation of apatite from complex ore. In Proceedings of the XXVI Encontro Nacional de Tratamento de Minérios e Metalurgia Extrativa, Poços de Caldas, MG, Brazil, 18–22 October 2015. [Google Scholar]
- Xie, J.; Li, X.; Mao, S.; Li, L.; Ke, B.; Zhang, Q. Effects of structure of fatty acid collectors on the adsorption of fluorapatite (001) surface: A first-principles calculations. Appl. Surf. Sci. 2018, 444, 699–709. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Y.; Hou, J. Behaviour and mechanism of collophane and dolomite separation using alkyl hydroxamic acid as a flotation collector. Physicochem. Probl. Min. Process. 2016, 52, 155–169. [Google Scholar] [CrossRef]
- García Zúñiga, H. La recuperación por flotación es una función es exponencial del tiempo. Bol. Min. Soc. Nac. Min. 1935, XLVIII, 83–86. [Google Scholar]
- Silva, J.; Baltar, C.; Gonzaga, R.; Peres, A.; Leite, J. Identification of sodium silicate species used as flotation depressants. Min. Metall. Explor. 2012, 29, 207. [Google Scholar] [CrossRef]
- Qi, G.W.; Klauber, C.; Warren, L.J. Mechanism of action of sodium silicate in the flotation of apatite from hematite. Int. J. Miner. Process. 1993, 39, 251–273. [Google Scholar] [CrossRef]
- Brown, A.M. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput. Methods Programs Biomed. 2001, 65, 191–200. [Google Scholar] [CrossRef]
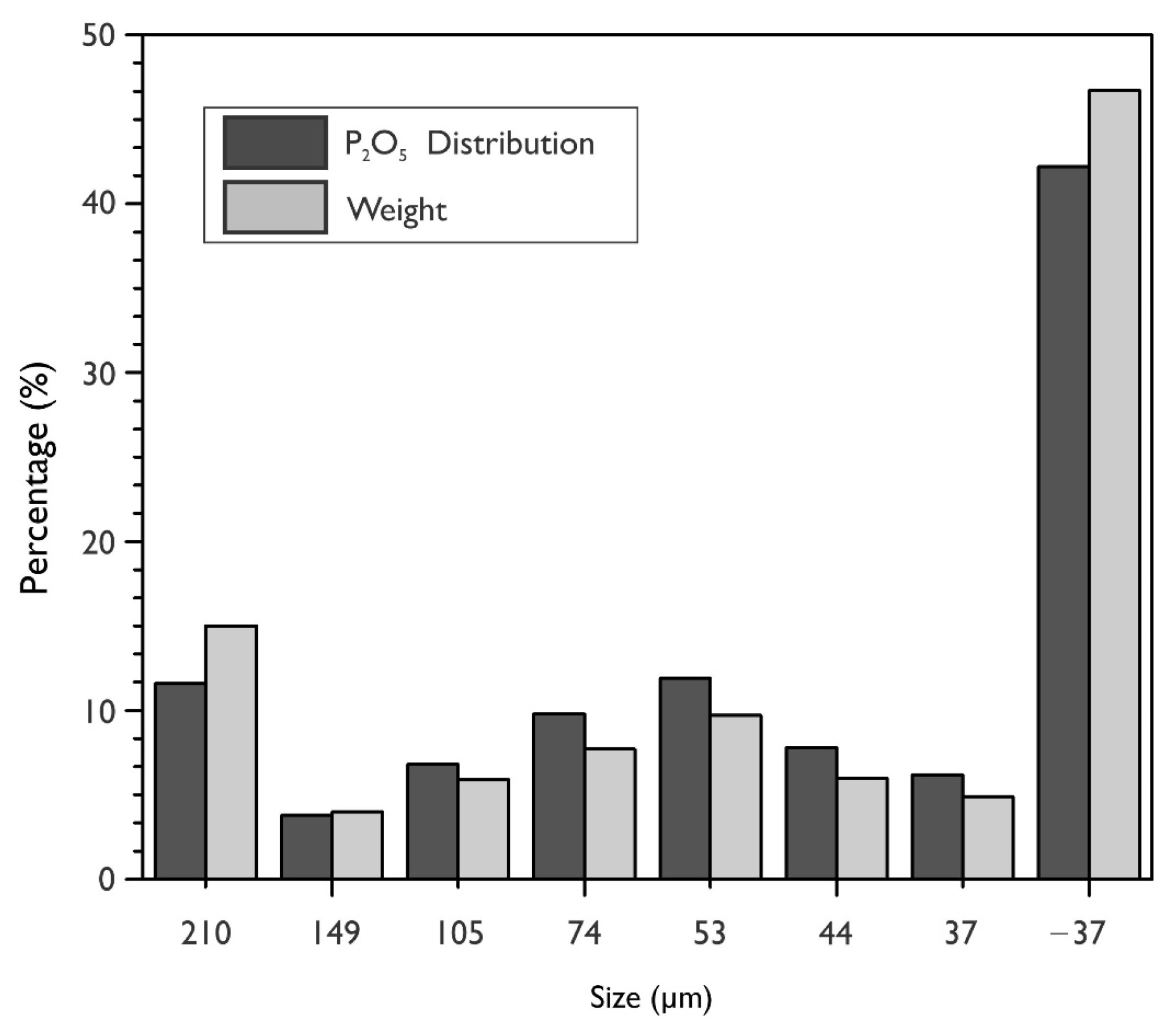
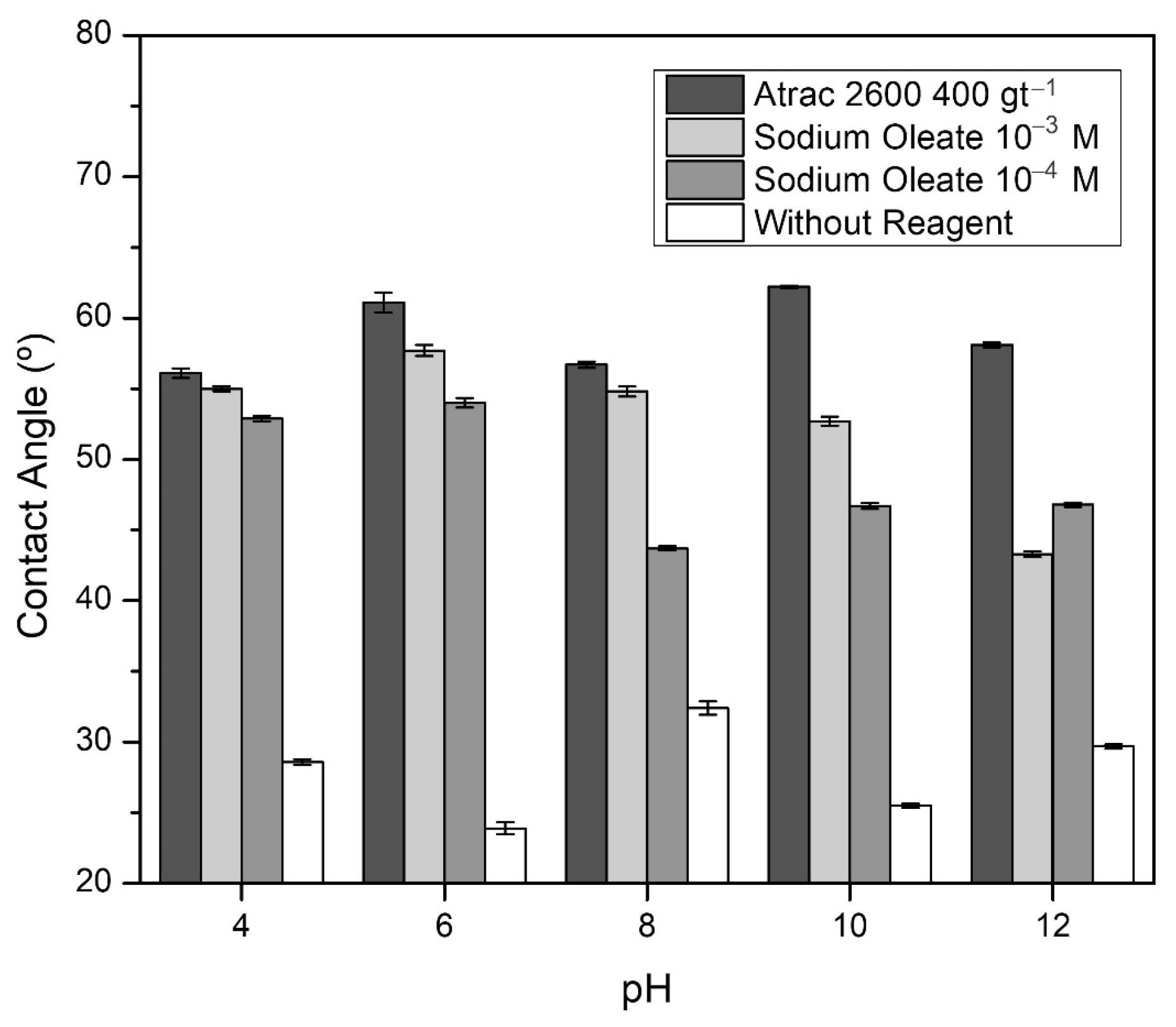
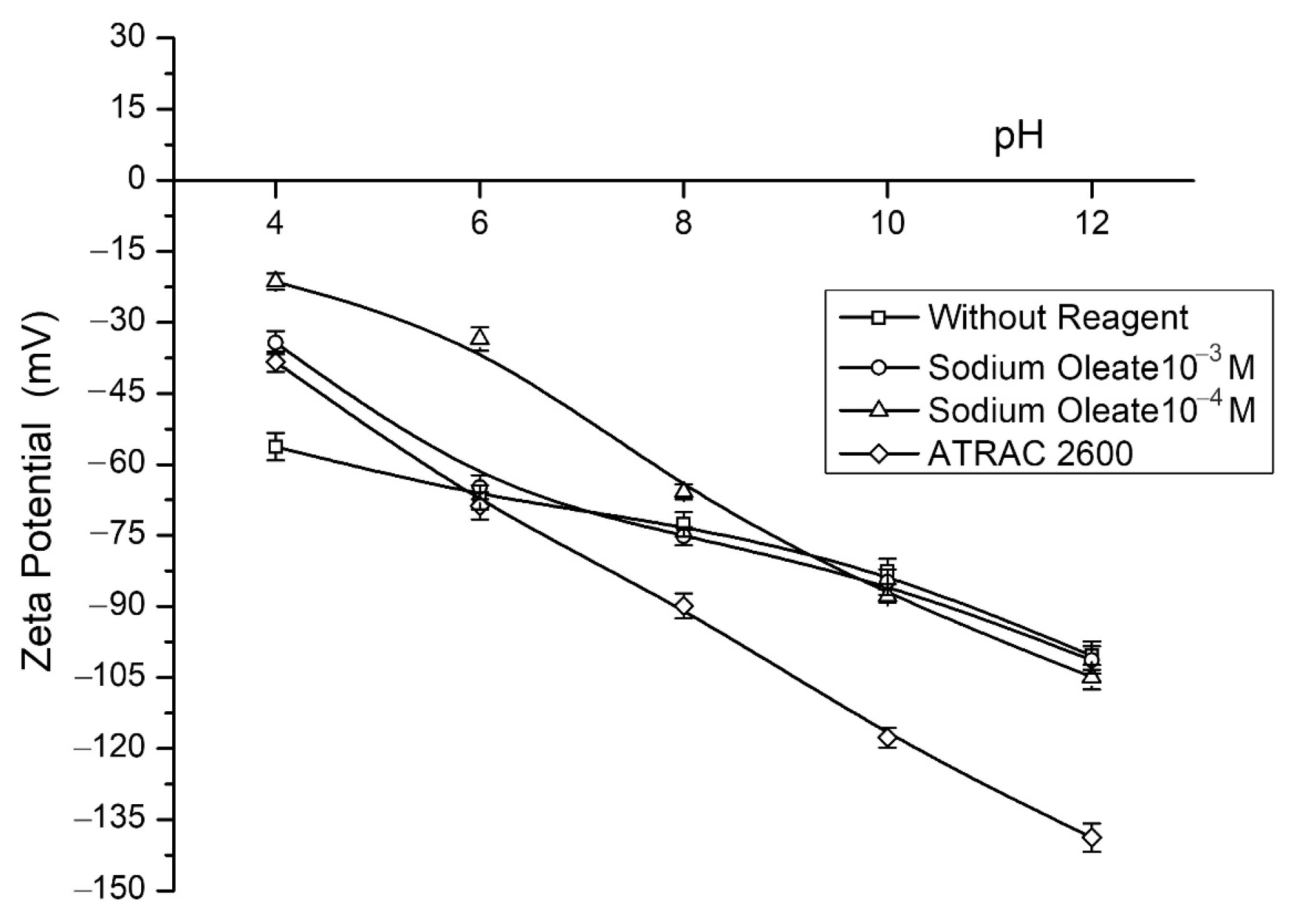
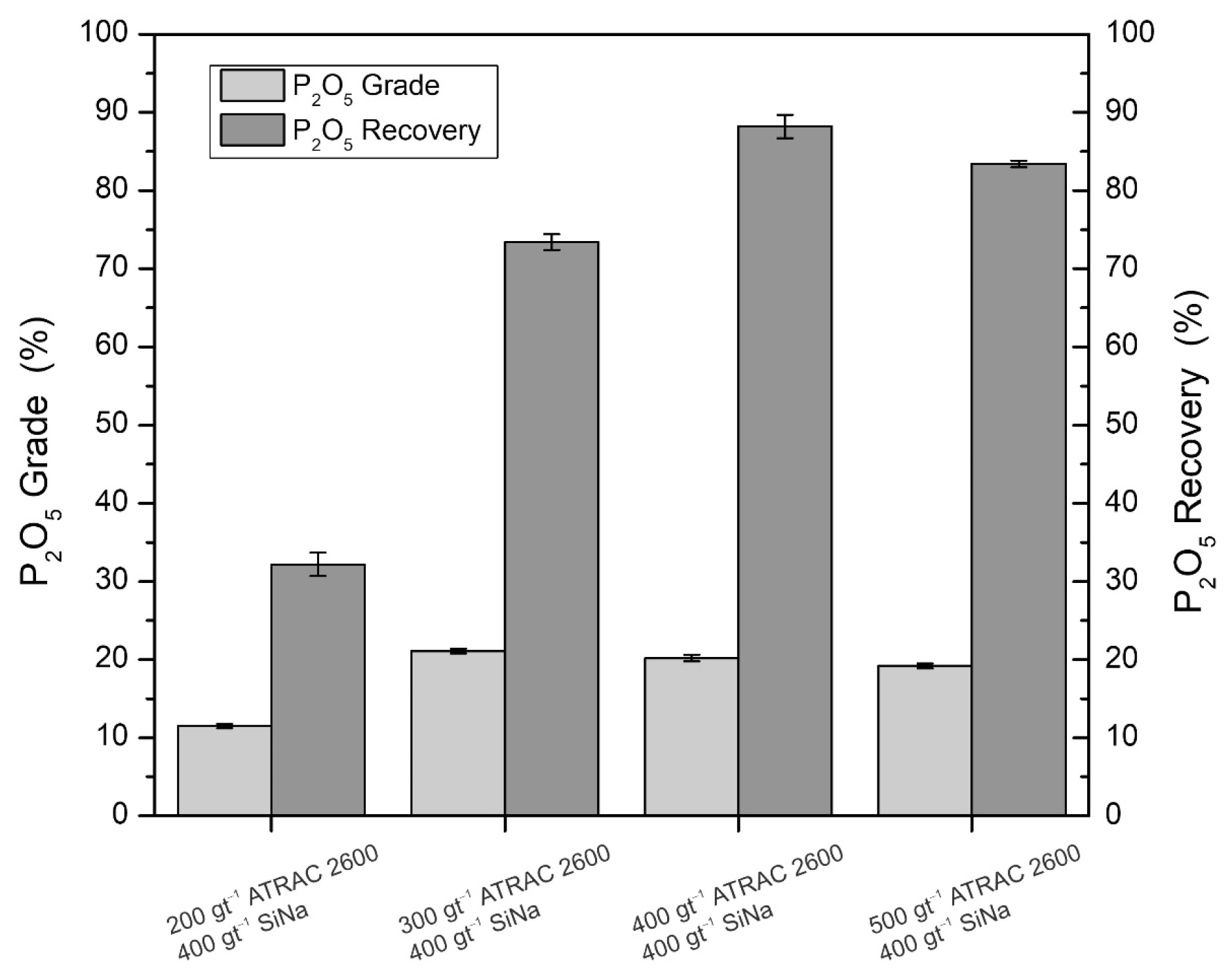
| Species | P2O5 | SiO2 | FeT | Al2O3 | CaO | MgO | Na2O |
|---|---|---|---|---|---|---|---|
| Composition, % | 1.8 | 46.4 | 25.9 | 6.8 | 6.1 | 4.9 | 2.7 |
| Mineral | Percentage | Mineral | Percentage |
|---|---|---|---|
| FeOx/Hydroxide | 30.7 | Pyroxene | 2.3 |
| Chalcopyrite | 0.1 | Epidote | 3.0 |
| Pyrite | 2.6 | Chlorite | 6.8 |
| Quartz | 3.1 | Micas | 2.3 |
| Feldspars | 18.5 | Apatite | 3.4 |
| Amphibole | 19.9 | Others | 6.8 |
| Element | CaO | P2O5 | Cl | SiO2 | Fe2O3 | MgO | Al2O3 |
|---|---|---|---|---|---|---|---|
| (%) | 37.3 | 37.1 | 3.8 | 2.3 | 1.84 | 0.59 | 0.16 |
| Stage | R∞, % | K, (min−1) | C.I. | S.E. | R2 |
|---|---|---|---|---|---|
| Rougher | 85.1 | 0.26 | 4,41 | 2.07 | 0.992 |
| Cleaner | 99.0 | 0.30 | 5.76 | 2.70 | 0.991 |
| Scavenger | 91.8 | 0.12 | 5.65 | 2.65 | 0.984 |
| Stage | Apatite Recovery, % | Weight Recovery, % |
|---|---|---|
| Rougher | 6.72 | 77.2 |
| Cleaner | 70.47 | 95.3 |
| Scavenger | 15.89 | 62.8 |
| Stage | Grade of P2O5, % | Recovery, % | ||
|---|---|---|---|---|
| Feed | Concentrate | Tailing | ||
| Rougher | 1.87 | 21.46 | 0.46 | 77.2 |
| Cleaner | 21.46 | 29.02 | 3.42 | 95.3 |
| Scavenger | 0.46 | 1.80 | 0.20 | 62.8 |
| Global flotation circuit | 1.84 | 29.02 | 0.20 | 89.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valderrama, L.; Gómez, O.; Pavez, O.; Santander, M. Recovery of Apatite from Magnetic Concentration Tailings by Flotation. Minerals 2024, 14, 441. https://doi.org/10.3390/min14050441
Valderrama L, Gómez O, Pavez O, Santander M. Recovery of Apatite from Magnetic Concentration Tailings by Flotation. Minerals. 2024; 14(5):441. https://doi.org/10.3390/min14050441
Chicago/Turabian StyleValderrama, Luis, Osvaldo Gómez, Osvaldo Pavez, and Mario Santander. 2024. "Recovery of Apatite from Magnetic Concentration Tailings by Flotation" Minerals 14, no. 5: 441. https://doi.org/10.3390/min14050441
APA StyleValderrama, L., Gómez, O., Pavez, O., & Santander, M. (2024). Recovery of Apatite from Magnetic Concentration Tailings by Flotation. Minerals, 14(5), 441. https://doi.org/10.3390/min14050441









