Selective Separation of Rare Earth Ions from Mine Wastewater Using Synthetic Hematite Nanoparticles from Natural Pyrite
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Reagents
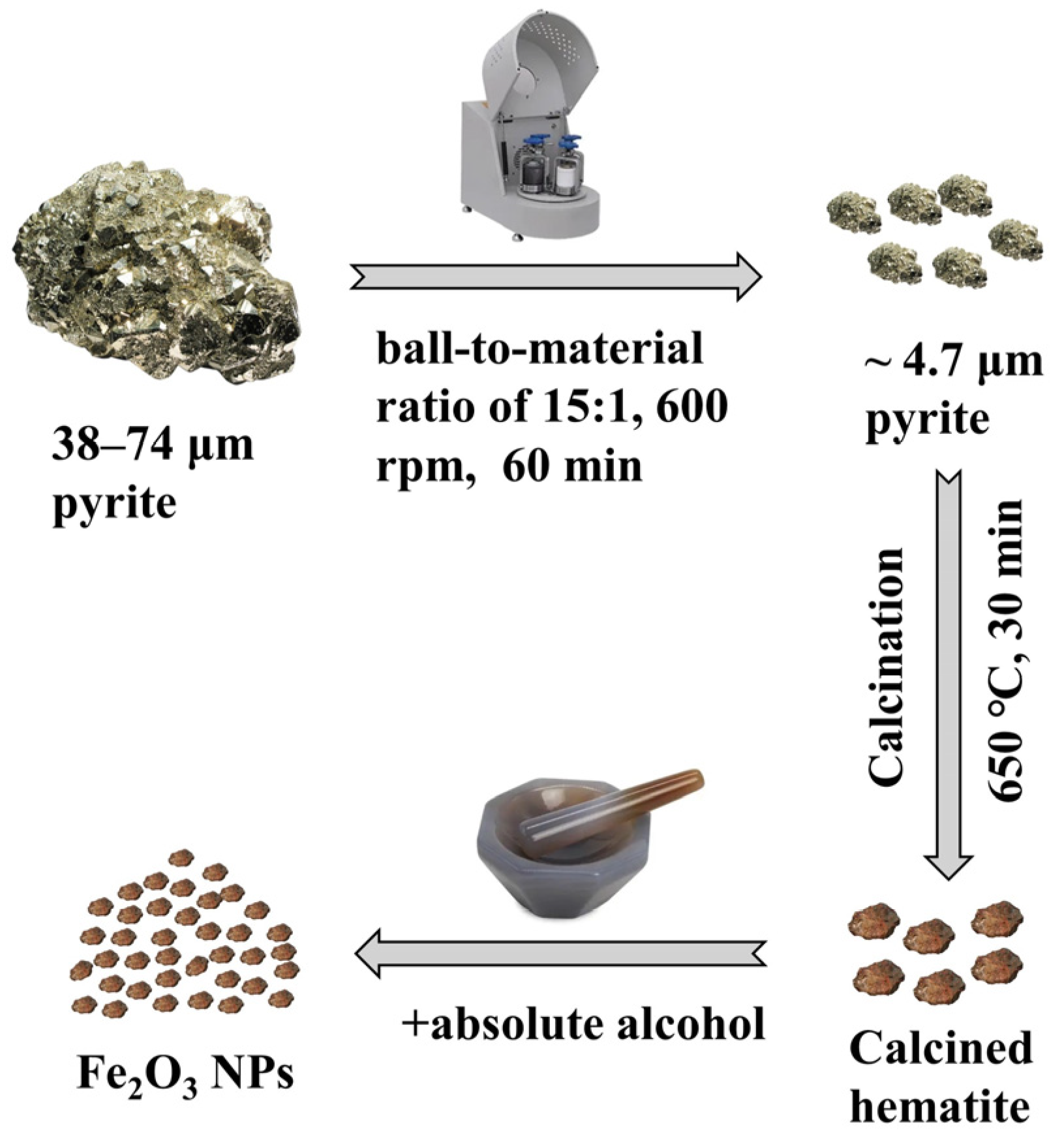
2.2. Preparation of Fe2O3 NPs
2.3. Adsorption and Desorption Experiments
2.4. Characterizations
3. Results and Discussion
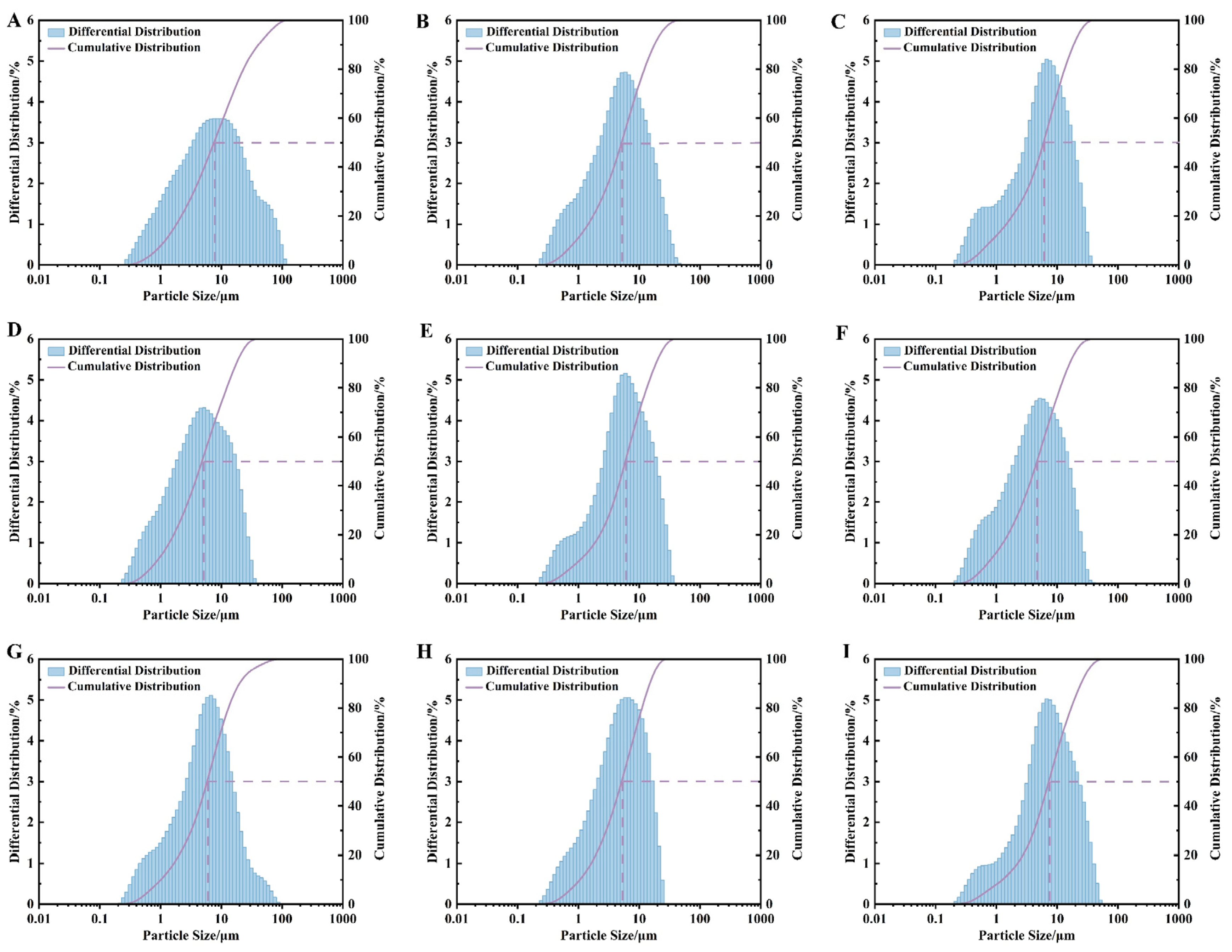
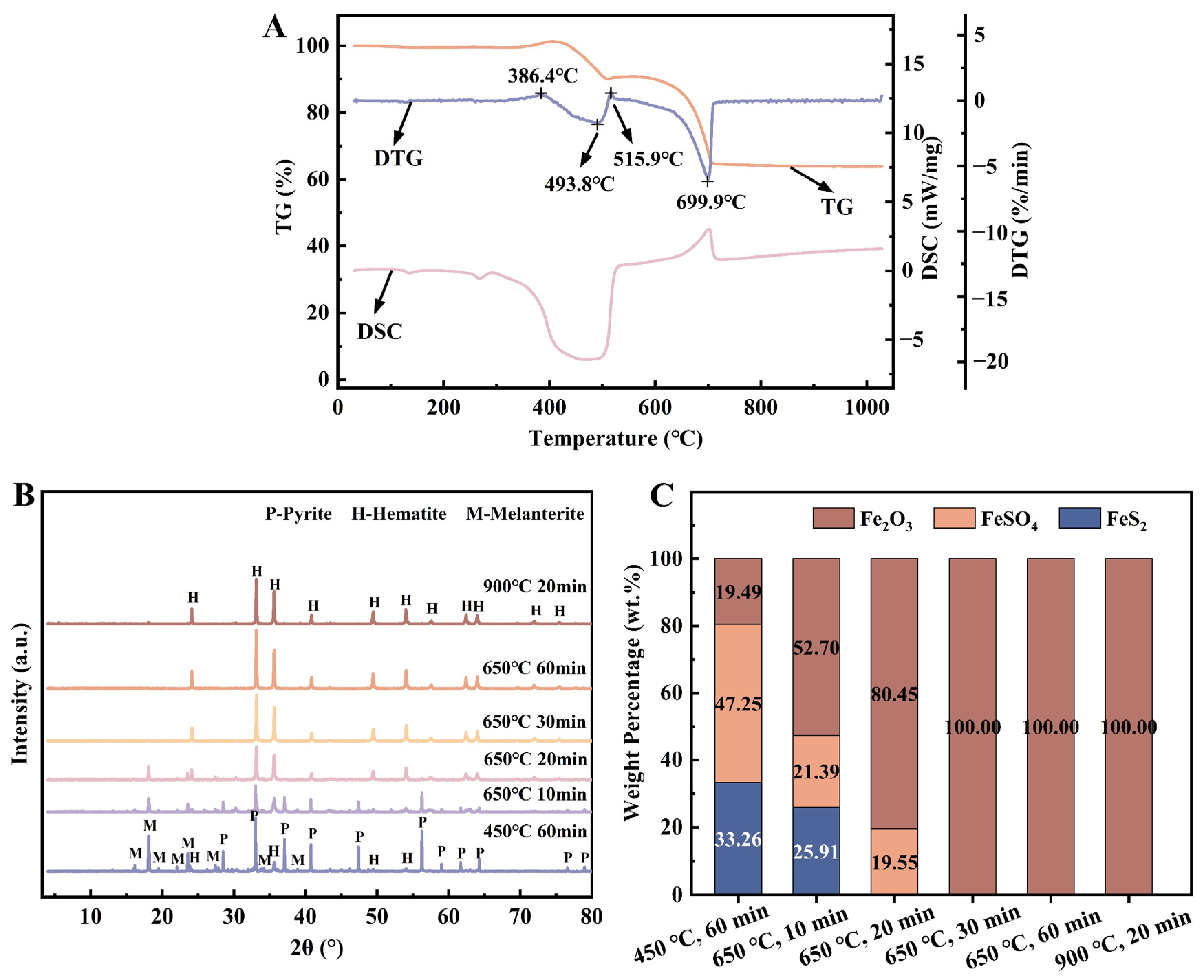
3.1. Preparation and Characterization of Fe2O3 NPs
3.2. Factors Affecting the Adsorption Efficiency of RE3+
3.2.1. Time
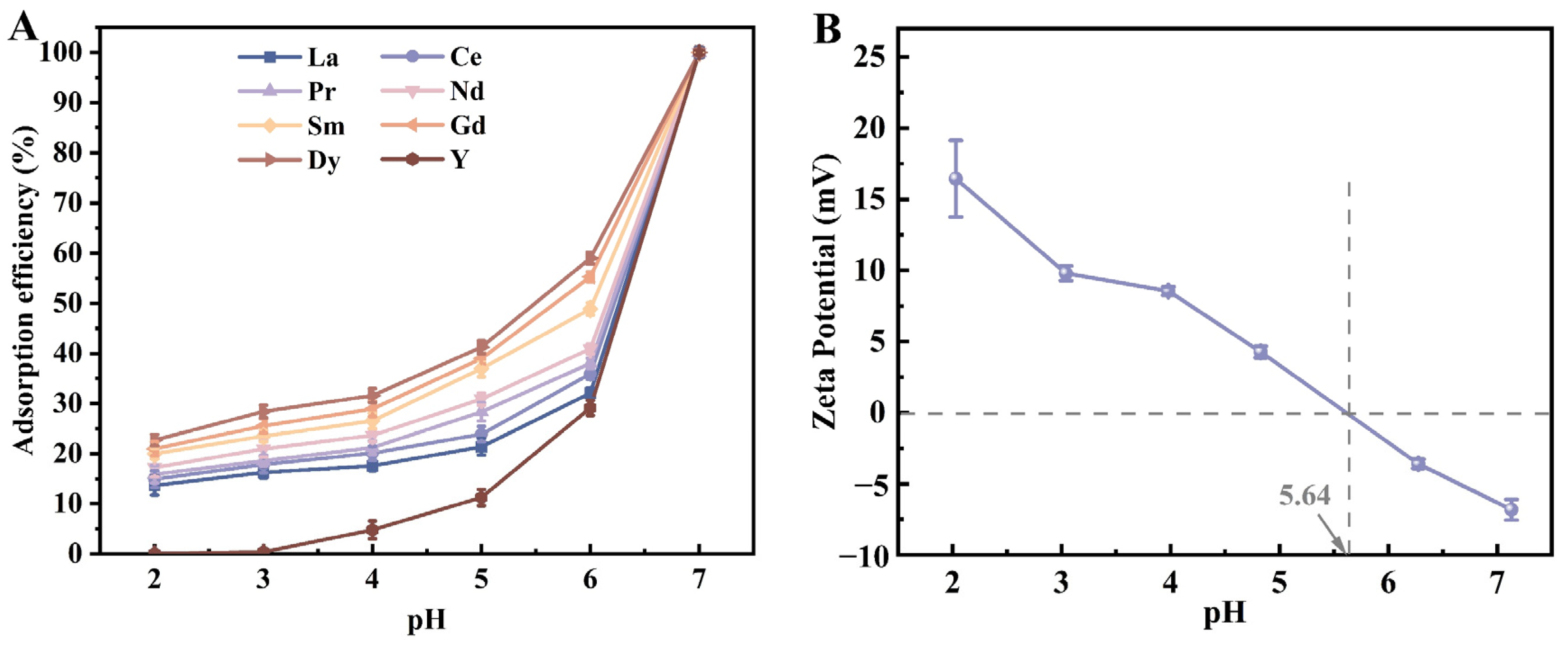
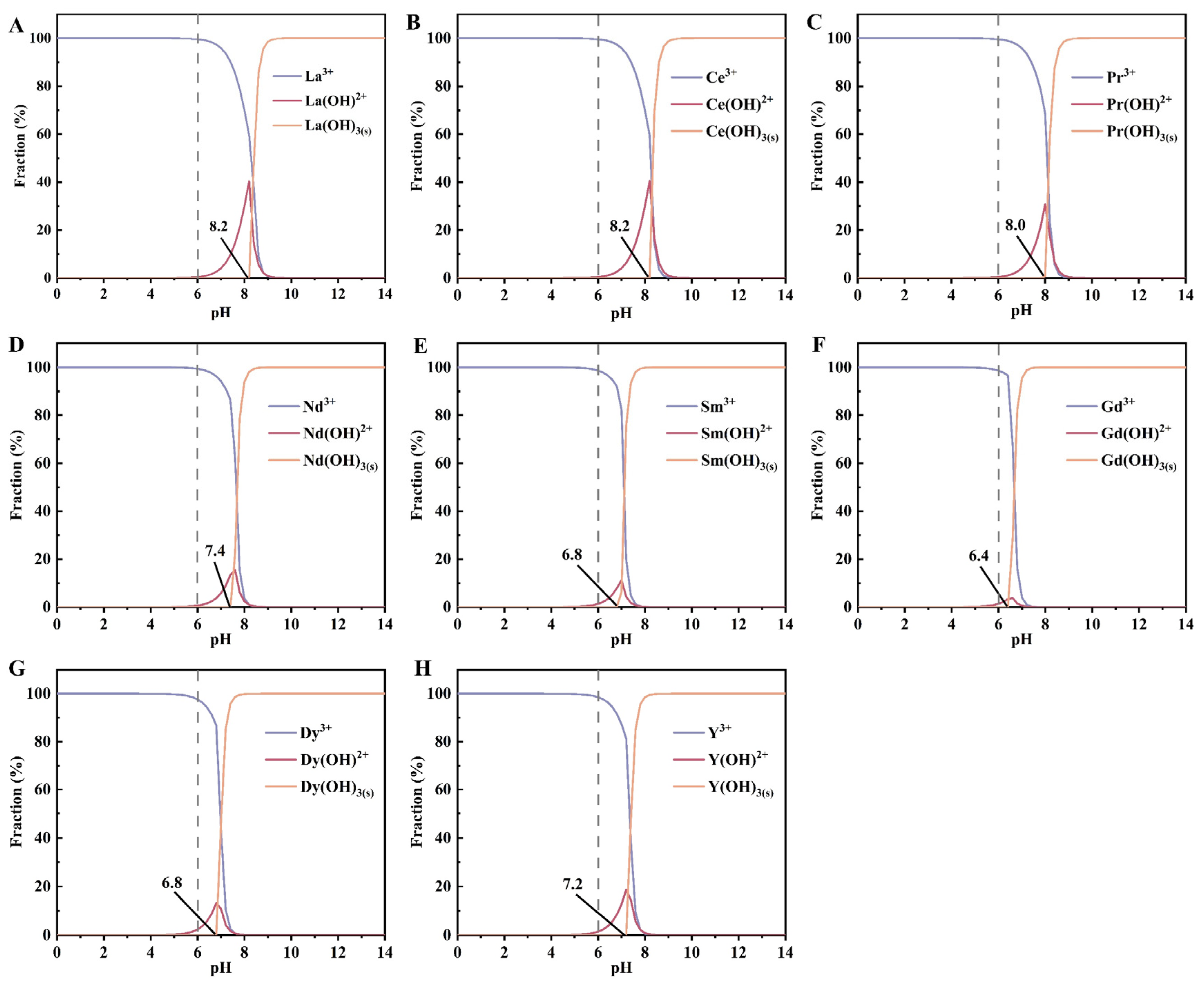
3.2.2. pH
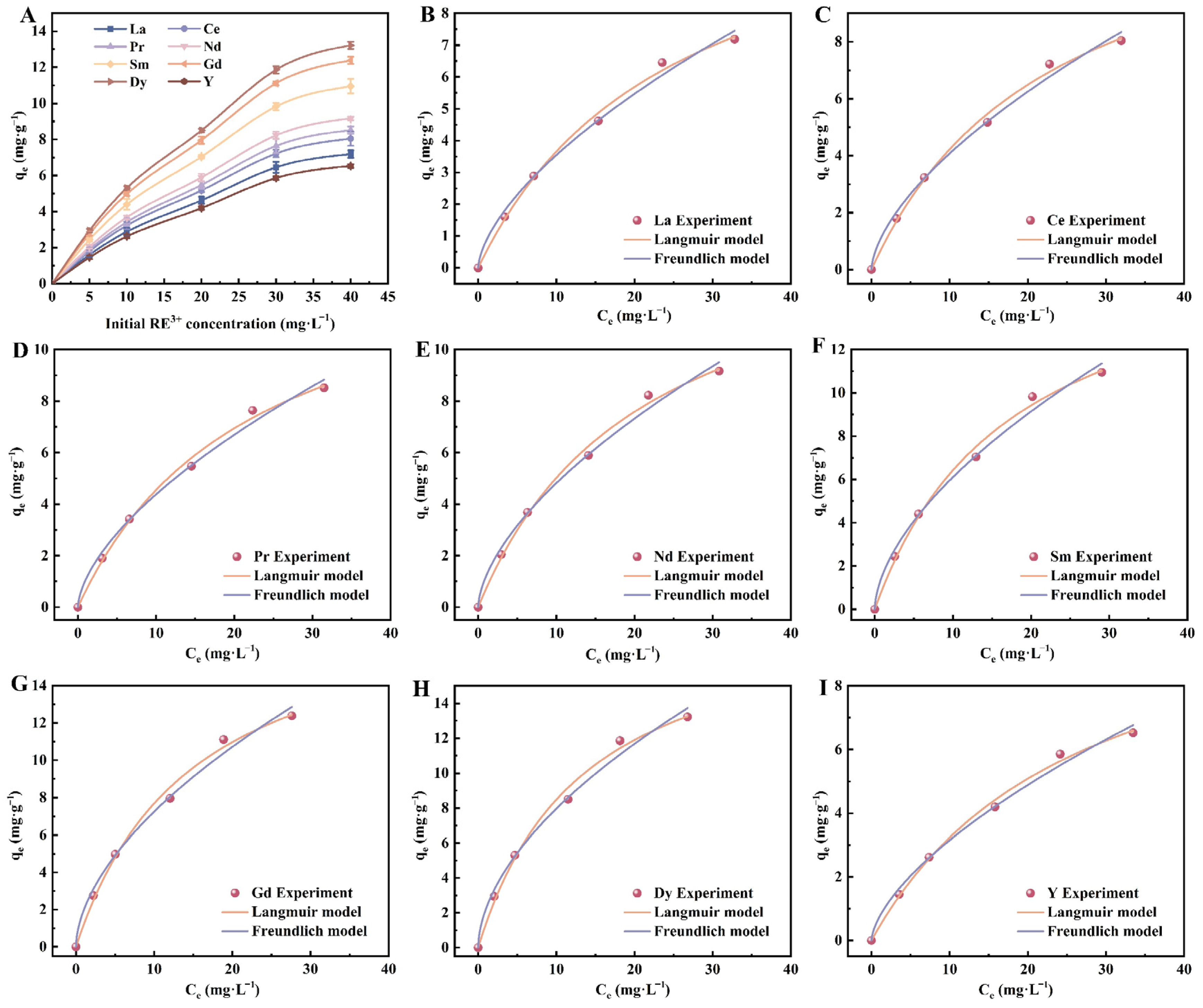
3.2.3. Initial RE3+ Concentration
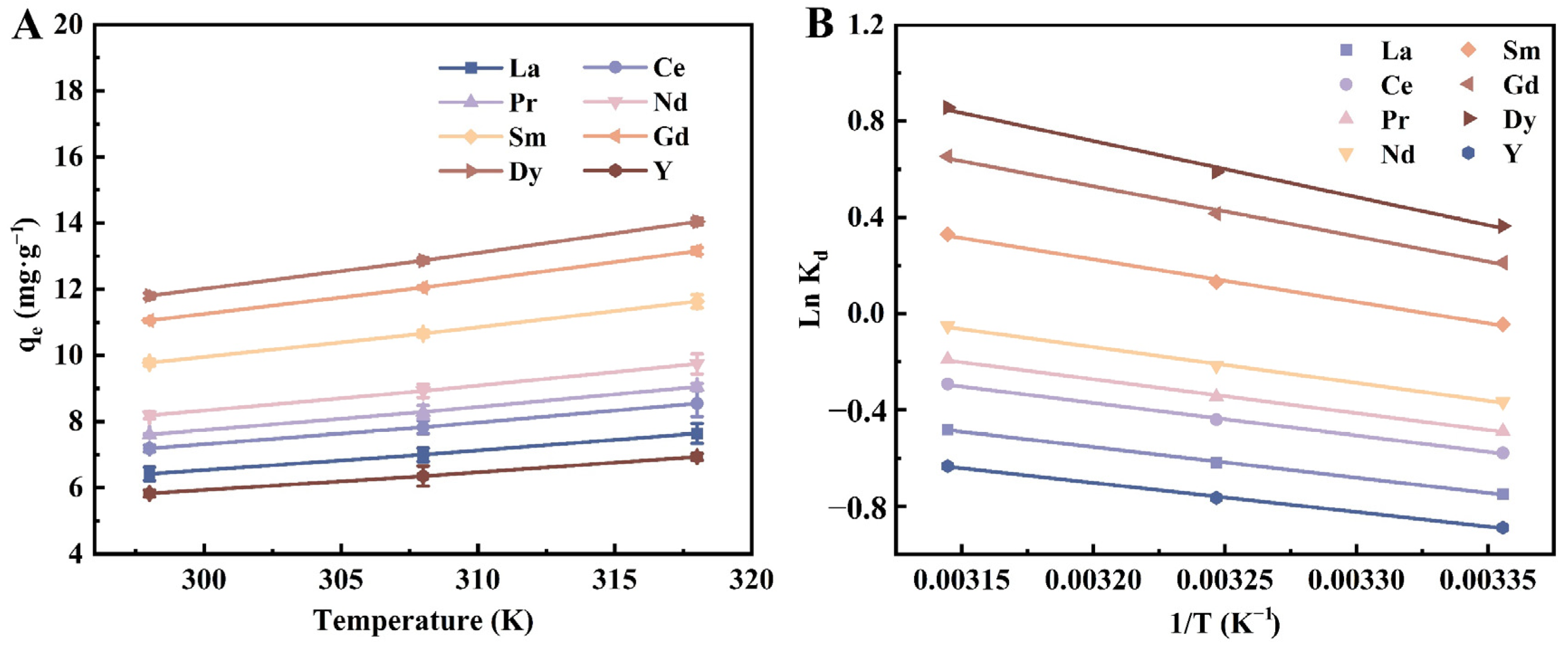
3.2.4. Temperature
3.3. Selective Separation
3.4. Reusability
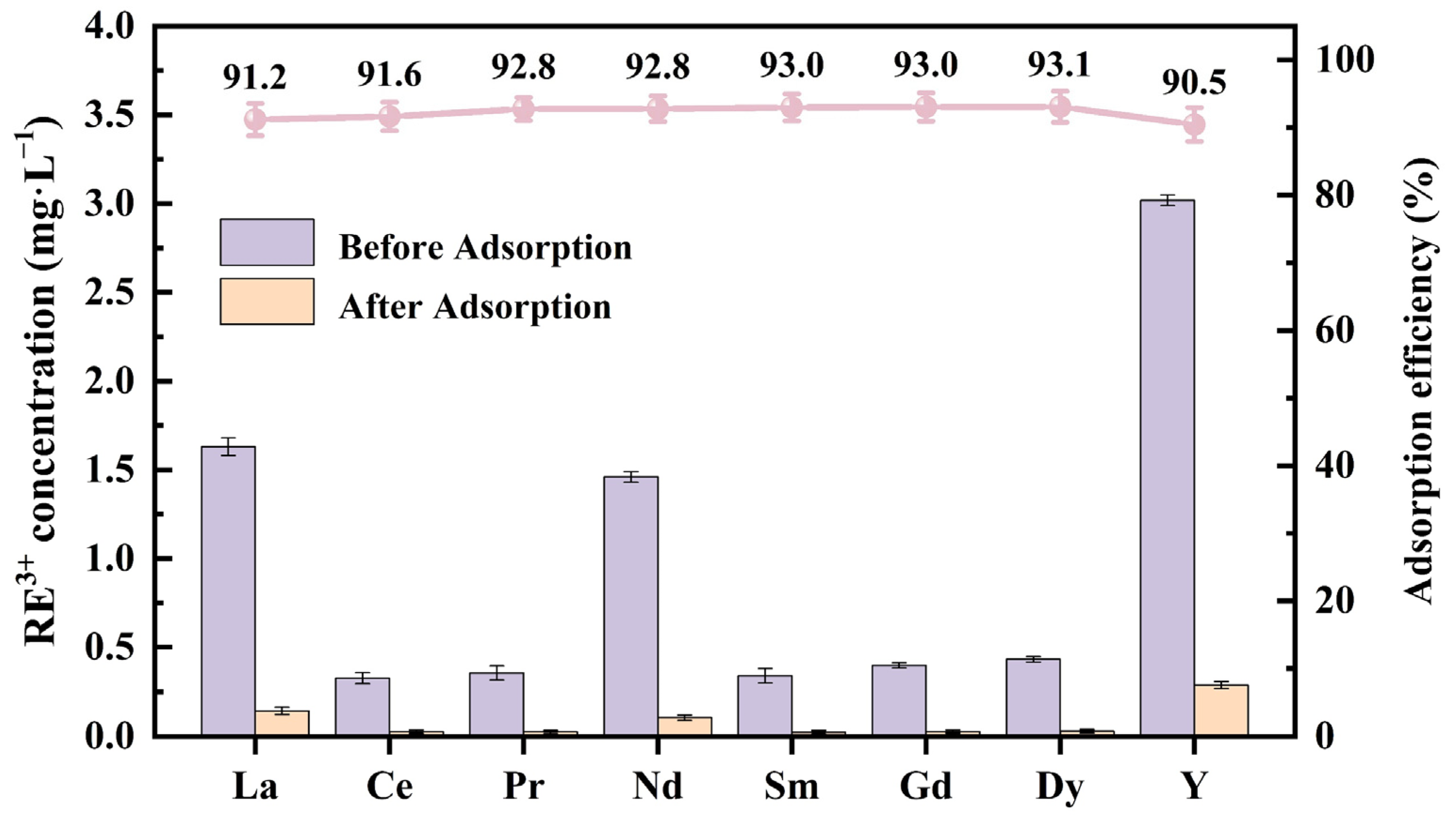
3.5. Application of Fe2O3 NPs to Actual Mine Wastewater
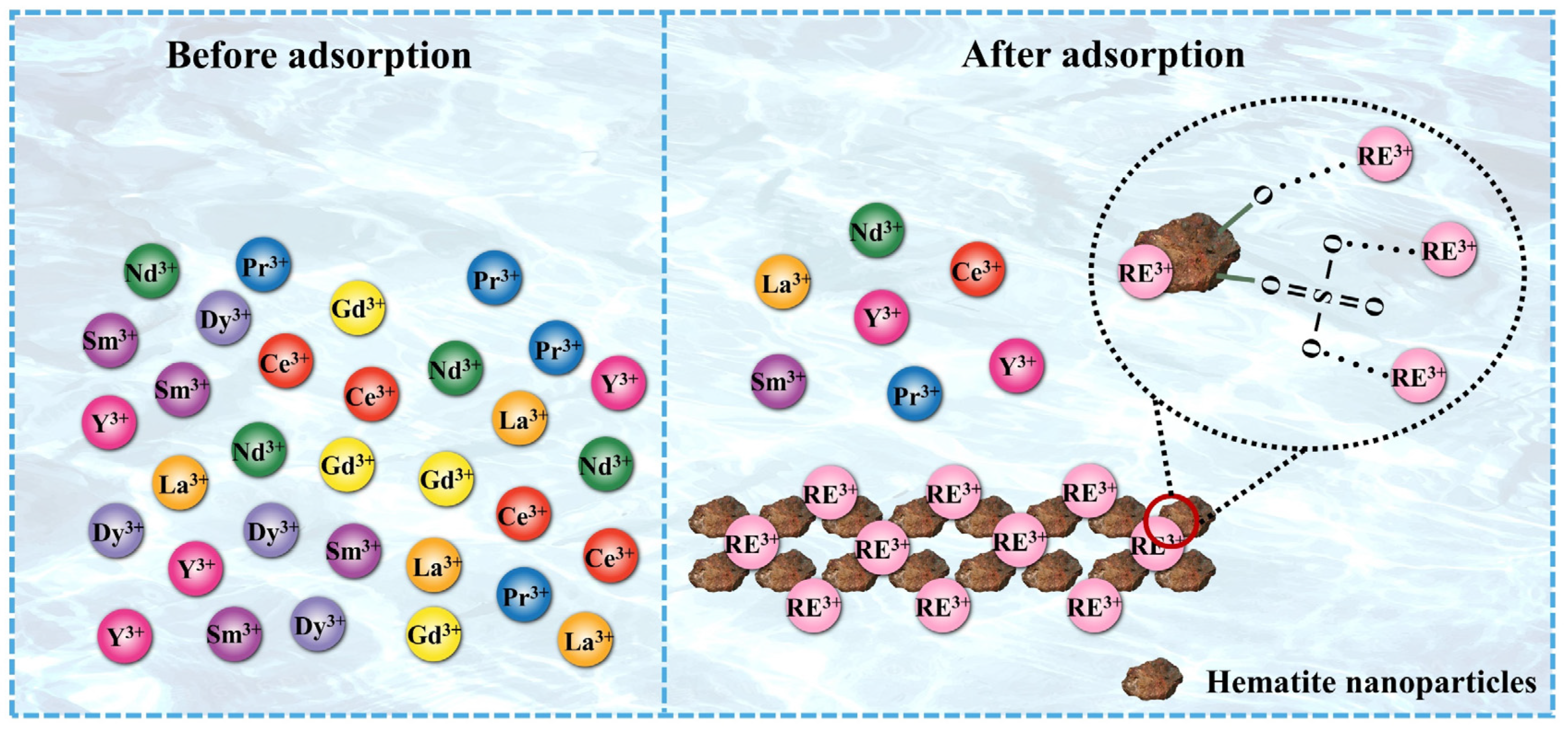
3.6. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Weng, X.; Chen, Z. Recovery of rare earth elements from mine wastewater using biosynthesized reduced graphene oxide. J. Colloid Interface Sci. 2023, 638, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, Q.; Gao, J.; Guo, Y.; Cheng, F. The selective adsorption of rare earth elements by modified coal fly ash based SBA-15. Chin. J. Chem. Eng. 2022, 47, 155–164. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.S.; Huot, H.; Guo, M.N.; Zhu, S.C.; Zheng, H.X.; Morel, J.L.; Tang, Y.T.; Qiu, R.L. Biogeochemical cycles of nutrients, rare earth elements (REEs) and Al in soil-plant system in ion-adsorption REE mine tailings remediated with amendment and ramie (Boehmeria nivea L.). Sci. Total Environ. 2022, 809, 152075. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tan, X.; Yu, Y.; Li, Y.; Wang, P.; Liang, Y.; Yan, Y. Effectively auto-regulated adsorption and recovery of rare earth elements via an engineered E. coli. J. Hazard. Mater. 2022, 424 Pt C, 127642. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Q.; Li, Z.; Liu, C.; Liu, X.; Liu, Y.; Dong, G.; Lan, T.; Wei, Y. Fabrication of efficient alginate composite beads embedded with N-doped carbon dots and their application for enhanced rare earth elements adsorption from aqueous solutions. J. Colloid Interface Sci. 2020, 562, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar, S.; Srivastava, V.; Hammouda, S.B.; Sillanpaa, M. Fabrication of novel metal ion imprinted xanthan gum-layered double hydroxide nanocomposite for adsorption of rare earth elements. Carbohydr. Polym. 2018, 194, 274–284. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Sun, M.; Liang, X.; He, H.; Zhu, J.; Takahashi, Y. Environmental risk assessment of the potential “Chemical Time Bomb” of ion-adsorption type rare earth elements in urban areas. Sci. Total Environ. 2022, 822, 153305. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-R.; Liu, W.-S.; Tang, Y.-T.; Wang, S.-Z.; Cao, Y.-J.; Chen, Z.-W.; Xie, C.-D.; Liu, C.; Guo, M.-N.; Qiu, R.-L. Effects of in situ leaching on the origin and migration of rare earth elements in aqueous systems of South China: Insights based on REE patterns, and Ce and Eu anomalies. J. Hazard. Mater. 2022, 435, 128959. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Z.; Xu, L.; Hu, F. Efficient and rapid adsorption of rare earth elements from water by magnetic Fe3O4/MnO2 decorated reduced graphene oxide. J. Mol. Liq. 2020, 313, 113510. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Meng, Y.; Wang, X.; Yin, D.; Sillanpää, M. An EDTA-β-cyclodextrin material for the adsorption of rare earth elements and its application in preconcentration of rare earth elements in seawater. J. Colloid Interface Sci. 2016, 465, 215–224. [Google Scholar] [CrossRef]
- Xu, W.; Yu, C.; Liu, L.; Zhai, Y.; Xu, R.; Hou, H. An O- modified coordination polymer for rapid and selective adsorption of rare earth elements from aqueous solution. Colloids Surf. A 2020, 607, 125464. [Google Scholar] [CrossRef]
- Li, J.; Gong, A.; Li, F.; Qiu, L.; Zhang, W.; Gao, G.; Liu, Y.; Li, J. Synthesis and characterization of magnetic mesoporous Fe3O4@mSiO2-DODGA nanoparticles for adsorption of 16 rare earth elements. RSC Adv. 2018, 8, 39149–39161. [Google Scholar] [CrossRef] [PubMed]
- Gomes Rodrigues, D.; Monge, S.; Pellet-Rostaing, S.; Dacheux, N.; Bouyer, D.; Faur, C. A new carbamoylmethylphosphonic acid-based polymer for the selective sorption of rare earth elements. Chem. Eng. J. 2019, 371, 857–867. [Google Scholar] [CrossRef]
- Gomes Rodrigues, D.; Monge, S.; Pellet-Rostaing, S.; Dacheux, N.; Bouyer, D.; Faur, C. Sorption properties of carbamoylmethylphosphonated-based polymer combining both sorption and thermosensitive properties: New valuable hydrosoluble materials for rare earth elements sorption. Chem. Eng. J. 2019, 355, 871–880. [Google Scholar] [CrossRef]
- Chen, G.E.; Sun, D.; Xu, Z.L. Rare Earth Ion from Aqueous Solution Removed by Polymer Enhanced Ultrafiltration Process. Adv. Mater. Res. 2011, 233–235, 959–964. [Google Scholar] [CrossRef]
- Javadian, H.; Ruiz, M.; Saleh, T.A.; Sastre, A.M. Ca-alginate/carboxymethyl chitosan/Ni0.2Zn0.2Fe2.6O4 magnetic bionanocomposite: Synthesis, characterization and application for single adsorption of Nd+3, Tb+3, and Dy+3 rare earth elements from aqueous media. J. Mol. Liq. 2020, 306, 112760. [Google Scholar] [CrossRef]
- Ashour, R.M.; El-sayed, R.; Abdel-Magied, A.F.; Abdel-khalek, A.A.; Ali, M.M.; Forsberg, K.; Uheida, A.; Muhammed, M.; Dutta, J. Selective separation of rare earth ions from aqueous solution using functionalized magnetite nanoparticles: Kinetic and thermodynamic studies. Chem. Eng. J. 2017, 327, 286–296. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, C.; Liu, R.; Xie, Z.; Liu, Z.; Cen, S.; Tao, C.; Guo, S. Leaching kinetics of manganese from pyrolusite using pyrite as a reductant under microwave heating. Sep. Purif. Technol. 2021, 277, 119472. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, B.; Lyu, H.; Li, D. Development of a novel pyrite/biochar composite (BM-FeS2@BC) by ball milling for aqueous Cr(VI) removal and its mechanisms. J. Hazard. Mater. 2021, 413, 125415. [Google Scholar] [CrossRef]
- Karagiorgakis, A.L.; Schindler, M.; Spiers, G.A. Retention of rare earth elements in authigenic phases following biogeochemical dissolution of ore from Elliot Lake, Ontario. Hydrometallurgy 2018, 177, 9–20. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Guo, J.; Song, Y.; Hou, Y.; Lu, C.; Han, Y.; Shen, X.; Liu, B. Effect of calcinated pyrite on simultaneous ammonia, nitrate and phosphorus removal in the BAF system and the Fe2+ regulatory mechanisms: Electron transfer and biofilm properties. Environ. Res. 2021, 194, 110708. [Google Scholar] [CrossRef] [PubMed]
- Trang, V.T.; Tam, L.T.; Van Quy, N.; Phan, V.N.; Van Tuan, H.; Huy, T.Q.; Dinh, N.X.; Le, A.-T. Enhanced adsorption efficiency of inorganic chromium (VI) ions by using carbon-encapsulated hematite nanocubes. J. Sci. Adv. Mater. Devices 2020, 5, 392–399. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.; Xiong, J.; Hou, J.; Wang, X.; Tan, W. Al-substitution-induced defect sites enhance adsorption of Pb2+ on hematite. Environ. Sci. Nano 2019, 6, 1323–1331. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, F.; Chen, X.; Xia, W.; Zou, Y.; Zhou, X.; Liu, H.; Song, Y.; He, J.; Ma, S. Effective Cu(II) ions adsorption from aqueous solutions using low grade oolitic hematite tailing with phosphorus: Response surface methodology. Desalination Water Treat. 2022, 265, 57–70. [Google Scholar] [CrossRef]
- Estes, S.L.; Powell, B.A. Enthalpy of Uranium Adsorption onto Hematite. Environ. Sci. Technol. 2020, 54, 15004–15012. [Google Scholar] [CrossRef] [PubMed]
- Denys, A.; Janots, E.; Auzende, A.-L.; Lanson, M.; Findling, N.; Trcera, N. Evaluation of selectivity of sequential extraction procedure applied to REE speciation in laterite. Chem. Geol. 2021, 559, 119954. [Google Scholar] [CrossRef]
- Viana, T.; Henriques, B.; Ferreira, N.; Pinto, R.J.B.; Monteiro, F.L.S.; Pereira, E. Insight into the mechanisms involved in the removal of toxic, rare earth, and platinum elements from complex mixtures by Ulva sp. Chem. Eng. J. 2023, 453, 139630. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, B.; Liao, R.; Hong, M.; Yu, S.; Liu, S.; Wang, J.; Qiu, G. Combined effect and mechanism of visible light and Ag+ on chalcopyrite bioleaching. Miner. Eng. 2022, 175, 107283. [Google Scholar] [CrossRef]
- Yan, Q.; Yang, Y.; Chen, W.; Weng, X.; Owens, G.; Chen, Z. Recovery and removal of rare earth elements from mine wastewater using synthesized bio-nanoparticles derived from Bacillus cereus. Chem. Eng. J. 2023, 459, 141585. [Google Scholar] [CrossRef]
- Pechishcheva, N.V.; Estemirova, S.K.; Kim, A.V.; Zaitceva, P.V.; Sterkhov, E.V.; Shchapova, Y.V.; Zhidkov, I.S.; Skrylnik, M.Y. Adsorption of hexavalent chromium on mechanically activated graphite. Diam. Relat. Mater. 2022, 127, 109152. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Lamsayah, M.; Butylina, S.; Geng, S.; Esmaeili, M.; Giove, A.; Massima Mouele, E.S.; Virolainen, S.; El Barkany, S.; Ouammou, A.; et al. Sustainable composite material based on glutenin biopolymeric-clay for efficient separation of rare earth elements. Chem. Eng. J. 2022, 440, 135959. [Google Scholar] [CrossRef]
- Ren, J.; Zheng, L.; Su, Y.; Meng, P.; Zhou, Q.; Zeng, H.; Zhang, T.; Yu, H. Competitive adsorption of Cd(II), Pb(II) and Cu(II) ions from acid mine drainage with zero-valent iron/phosphoric titanium dioxide: XPS qualitative analyses and DFT quantitative calculations. Chem. Eng. J. 2022, 445, 136778. [Google Scholar] [CrossRef]
- Smith, Y.R.; Bhattacharyya, D.; Willhard, T.; Misra, M. Adsorption of aqueous rare earth elements using carbon black derived. Chem. Eng. J. 2016, 296, 102–111. [Google Scholar] [CrossRef]
- Ambroz, A.; Ban, I.; Luxbacher, T. Assessment of the Capability of Magnetic Nanoparticles to Recover Neodymium Ions from Aqueous Solution. Acta Chim. Slov. 2022, 69, 826–836. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhu, J.; Chen, Y.; Chen, F.; Zhu, J.; Liu, M.; Zhang, K.; Gan, M. Pyrite-activated persulfate for simultaneous 2,4-DCP oxidation and Cr(VI) reduction. Chem. Eng. J. 2021, 406, 126758. [Google Scholar] [CrossRef]
- Labus, M. Pyrite thermal decomposition in source rocks. Fuel 2021, 287, 119529. [Google Scholar] [CrossRef]
- Abeshu, G.W.; Li, H.-Y.; Zhu, Z.; Tan, Z.; Leung, L.R. Median bed-material sediment particle size across rivers in the contiguous US. Earth Syst. Sci. Data 2022, 14, 929–942. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Xiao, S.; Liu, J. Comparative study of interaction between pyrite and cysteine by thermogravimetric and electrochemical techniques. Hydrometallurgy 2010, 101, 88–92. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Huang, M.; Zhang, S.; Frost, R.L. Application of TG-FTIR to study SO2 evolved during the thermal decomposition of coal-derived pyrite. Thermochim. Acta 2013, 555, 1–6. [Google Scholar] [CrossRef]
- Yan, X.; Shao, J.; Wen, Q.; Shen, J. Stabilization of soil arsenic by natural limonite after mechanical activation and the associated mechanisms. Sci. Total Environ. 2020, 708, 135118. [Google Scholar] [CrossRef]
- Yu, S.H.; Yao, Q.Z.; Zhou, G.T.; Fu, S.Q. Preparation of hollow core/shell microspheres of hematite and its adsorption ability for samarium. ACS Appl. Mater. Interfaces 2014, 6, 10556–10565. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr(VI) and Pb(II) ions by MOF derived Co-Al layered double hydroxide @hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Hughes, I.D.; Dane, M.; Ernst, A.; Hergert, W.; Luders, M.; Poulter, J.; Staunton, J.B.; Svane, A.; Szotek, Z.; Temmerman, W.M. Lanthanide contraction and magnetism in the heavy rare earth elements. Nature 2007, 446, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, J.; Al Gharabli, S.; Szymczyk, A.; Terzyk, A.P.; Boncel, S.; Knozowska, K.; Li, G.; Kujawski, W. On membrane-based approaches for rare earths separation and extraction—Recent developments. Coord. Chem. Rev. 2023, 493, 215340. [Google Scholar] [CrossRef]
- Molina, L.; Gaete, J.; Alfaro, I.; Ide, V.; Valenzuela, F.; Parada, J.; Basualto, C. Synthesis and characterization of magnetite nanoparticles functionalized with organophosphorus compounds and its application as an adsorbent for La (III), Nd (III) and Pr (III) ions from aqueous solutions. J. Mol. Liq. 2019, 275, 178–191. [Google Scholar] [CrossRef]
- Javadian, H.; Ruiz, M.; Sastre, A.M. Response surface methodology based on central composite design for simultaneous adsorption of rare earth elements using nanoporous calcium alginate/carboxymethyl chitosan microbiocomposite powder containing Ni0.2Zn0.2Fe2.6O4 magnetic nanoparticles: Batch and column studies. Int. J. Biol. Macromol. 2020, 154, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Peng, Y.; Ma, J.; Shi, Z.; Jia, Q. Construction of hypercrosslinked polymers with styrene-based copolymer precursor for adsorption of rare earth elements. Sep. Purif. Technol. 2022, 285, 120378. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Gan, M. Controllable biosynthesis of nanoscale schwertmannite and the application in heavy metal effective removal. Appl. Surf. Sci. 2020, 529, 147012. [Google Scholar] [CrossRef]
- Zhou, F.; Xiao, Y.; Guo, M.; Wang, S.; Qiu, R.; Morel, J.L.; Simonnot, M.O.; Zhang, W.X.; Zhang, W.; Tang, Y.T. Insights into the Selective Transformation of Ceria Sulfation and Iron/Manganese Mineralization for Enhancing the Selective Recovery of Rare Earth Elements. Environ. Sci. Technol. 2023, 57, 3357–3368. [Google Scholar] [CrossRef]
- Gunawardhana, B.P.N.; Gunathilake, C.A.; Dayananda, K.E.D.Y.T.; Dissanayake, D.M.S.N.; Mantilaka, M.M.M.G.P.G.; Kalpage, C.S.; Rathnayake, R.M.L.D.; Rajapakse, R.M.G.; Manchanda, A.S.; Etampawala, T.N.B.; et al. Synthesis of Hematite Nanodiscs from Natural Laterites and Investigating Their Adsorption Capability of Removing Ni2+ and Cd2+ Ions from Aqueous Solutions. J. Compos. Sci. 2020, 4, 57. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Zeng, G.; Huang, B.; Dong, H.; Huang, J.; Yang, Z.; Wei, J.; Hu, L.; Zhang, Q. Phase transformation of crystalline iron oxides and their adsorption abilities for Pb and Cd. Chem. Eng. J. 2016, 284, 247–259. [Google Scholar] [CrossRef]




| Ions | Concentration (mg·L−1) |
|---|---|
| Y | 3.019 |
| La | 1.630 |
| Nd | 1.461 |
| Dy | 0.432 |
| Gd | 0.399 |
| Pr | 0.356 |
| Sm | 0.341 |
| Ce | 0.327 |
| Ca | 34.884 |
| K | 11.151 |
| Na | 7.502 |
| Mg | 5.982 |
| Al | 3.060 |
| Mn | 2.025 |
| Test | Ball-to-Material Ratio (A) | Rotation Speed, Rpm (B) | Grinding Time, h (C) | D50 of Pyrite, μm |
|---|---|---|---|---|
| 1 | 10:1 | 400 | 1 | 7.563 |
| 2 | 10:1 | 500 | 2 | 5.132 |
| 3 | 10:1 | 600 | 3 | 5.956 |
| 4 | 15:1 | 400 | 2 | 4.803 |
| 5 | 15:1 | 500 | 3 | 5.912 |
| 6 | 15:1 | 600 | 1 | 4.669 |
| 7 | 20:1 | 400 | 3 | 5.841 |
| 8 | 20:1 | 500 | 1 | 5.137 |
| 9 | 20:1 | 600 | 2 | 7.282 |
| I | 18.651 | 18.207 | 17.369 | T = 52.295 |
| II | 15.384 | 16.181 | 17.217 | |
| III | 18.260 | 17.907 | 17.709 | |
| K1 | 6.217 | 6.069 | 5.790 | |
| K2 | 5.128 | 5.394 | 5.739 | |
| K3 | 6.087 | 5.969 | 5.903 | |
| R | 1.089 | 0.675 | 0.164 |
| Ions | qe,exp/ (mg·g−1) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|
| qe,cal/ (mg·g−1) | k1/ min−1 | R2 | qe,cal/ (mg·g−1) | k2/ g·mg−1·min−1 | R2 | ||
| La3+ | 0.99 | 0.95 | 0.48 | 0.984 | 1.00 | 0.85 | 0.999 |
| Ce3+ | 1.19 | 1.15 | 0.70 | 0.988 | 1.19 | 1.23 | 0.996 |
| Pr3+ | 1.43 | 1.36 | 0.88 | 0.983 | 1.39 | 1.47 | 0.992 |
| Nd3+ | 1.53 | 1.48 | 0.87 | 0.988 | 1.51 | 1.36 | 0.997 |
| Sm3+ | 1.83 | 1.78 | 0.75 | 0.991 | 1.83 | 0.91 | 0.998 |
| Gd3+ | 1.93 | 1.89 | 0.72 | 0.997 | 1.94 | 0.84 | 0.998 |
| Dy3+ | 2.07 | 2.01 | 0.68 | 0.994 | 2.08 | 0.71 | 0.998 |
| Y3+ | 0.58 | 0.53 | 0.42 | 0.947 | 0.56 | 1.12 | 0.986 |
| REEs | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|
| qm/(mg·g−1) | kL/(L·mg−1) | R2 | KF/(mg·g−1) | 1/n | R2 | |
| La | 12.804 | 0.040 | 0.992 | 0.840 | 0.625 | 0.991 |
| Ce | 14.023 | 0.043 | 0.993 | 0.986 | 0.617 | 0.991 |
| Pr | 14.665 | 0.045 | 0.991 | 1.072 | 0.612 | 0.990 |
| Nd | 15.520 | 0.048 | 0.992 | 1.198 | 0.604 | 0.991 |
| Sm | 17.659 | 0.057 | 0.990 | 1.595 | 0.583 | 0.988 |
| Gd | 19.160 | 0.067 | 0.991 | 1.980 | 0.564 | 0.990 |
| Dy | 19.940 | 0.074 | 0.990 | 2.237 | 0.552 | 0.986 |
| Y | 11.816 | 0.038 | 0.994 | 0.736 | 0.632 | 0.991 |
| Adsorbents | Ions | Adsorption Capacity (mg·g−1) | Ref. |
|---|---|---|---|
| Hollow core/shell hematite microspheres | Sm | 14.48 | [41] |
| Functionalized Fe3O4 NPs | La, Nd, Gd, Y | 32.5, 41.0, 52.0, 35.8 | [17] |
| Calcium alginate/carboxymethyl chitosan/Ni0.2Zn0.2Fe2.6O4 | Nd, Tb, Dy | 23.15, 24.41, 25.24 | [47] |
| Magnetite nanoparticles functionalized with organophosphorus compounds | La, Pr, Nd | 8.3, 8.7, 8.9 | [46] |
| Fe2O3 NPs (this work) | La, Ce, Pr, Nd, Sm, Gd, Dy, Y | 12.8, 14.0, 14.7, 15.5, 17.7, 19.2, 19.9, 11.8 |
| RE3+ | (kJ·mol−1) | (kJ·mol−1) | (J·mol−1·K−1) | R2 | ||
|---|---|---|---|---|---|---|
| 298K | 308K | 318K | ||||
| La | 1.857 | 1.588 | 1.274 | 10.551 | 29.149 | 0.997 |
| Ce | 1.435 | 1.130 | 0.774 | 11.278 | 33.002 | 0.997 |
| Pr | 1.212 | 0.886 | 0.506 | 11.723 | 35.243 | 0.997 |
| Nd | 0.910 | 0.555 | 0.138 | 12.400 | 38.525 | 0.996 |
| Sm | 0.111 | −0.336 | −0.871 | 14.735 | 49.027 | 0.994 |
| Gd | −0.525 | −1.065 | −1.728 | 17.378 | 60.012 | 0.992 |
| Dy | −0.902 | −1.508 | −2.267 | 19.413 | 68.091 | 0.990 |
| Y | 2.202 | 1.959 | 1.675 | 10.050 | 26.313 | 0.997 |
| SF | 298K | 308K | 318K | SF | 298K | 308K | 318K |
|---|---|---|---|---|---|---|---|
| Gd3+/La3+ | 2.62 | 2.82 | 3.11 | Dy3+/La3+ | 3.05 | 3.35 | 3.82 |
| Gd3+/Ce3+ | 2.21 | 2.36 | 2.58 | Dy 3+/Ce3+ | 2.57 | 2.80 | 3.16 |
| Gd3+/Pr3+ | 2.02 | 2.14 | 2.33 | Dy 3+/Pr3+ | 2.35 | 2.55 | 2.85 |
| Gd3+/Nd3+ | 1.78 | 1.88 | 2.03 | Dy 3+/Nd3+ | 2.08 | 2.24 | 2.48 |
| Gd3+/Sm3+ | 1.29 | 1.33 | 1.38 | Dy3+/Sm3+ | 1.51 | 1.58 | 1.70 |
| Gd3+/Dy3+ | 0.86 | 0.84 | 0.82 | Dy 3+/Gd3+ | 1.16 | 1.19 | 1.23 |
| Gd3+/Y3+ | 3.01 | 3.26 | 3.62 | Dy 3+/Y3+ | 3.50 | 3.87 | 4.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Wang, J.; Yang, B.; Liu, Y.; Qiu, G. Selective Separation of Rare Earth Ions from Mine Wastewater Using Synthetic Hematite Nanoparticles from Natural Pyrite. Minerals 2024, 14, 464. https://doi.org/10.3390/min14050464
Zhao C, Wang J, Yang B, Liu Y, Qiu G. Selective Separation of Rare Earth Ions from Mine Wastewater Using Synthetic Hematite Nanoparticles from Natural Pyrite. Minerals. 2024; 14(5):464. https://doi.org/10.3390/min14050464
Chicago/Turabian StyleZhao, Chunxiao, Jun Wang, Baojun Yang, Yang Liu, and Guanzhou Qiu. 2024. "Selective Separation of Rare Earth Ions from Mine Wastewater Using Synthetic Hematite Nanoparticles from Natural Pyrite" Minerals 14, no. 5: 464. https://doi.org/10.3390/min14050464
APA StyleZhao, C., Wang, J., Yang, B., Liu, Y., & Qiu, G. (2024). Selective Separation of Rare Earth Ions from Mine Wastewater Using Synthetic Hematite Nanoparticles from Natural Pyrite. Minerals, 14(5), 464. https://doi.org/10.3390/min14050464





