Source and Migration Pathways of Heavy Metals in Soils from an Iron Mine in Baotou City, China
Abstract
1. Introduction
2. Materials and Methods
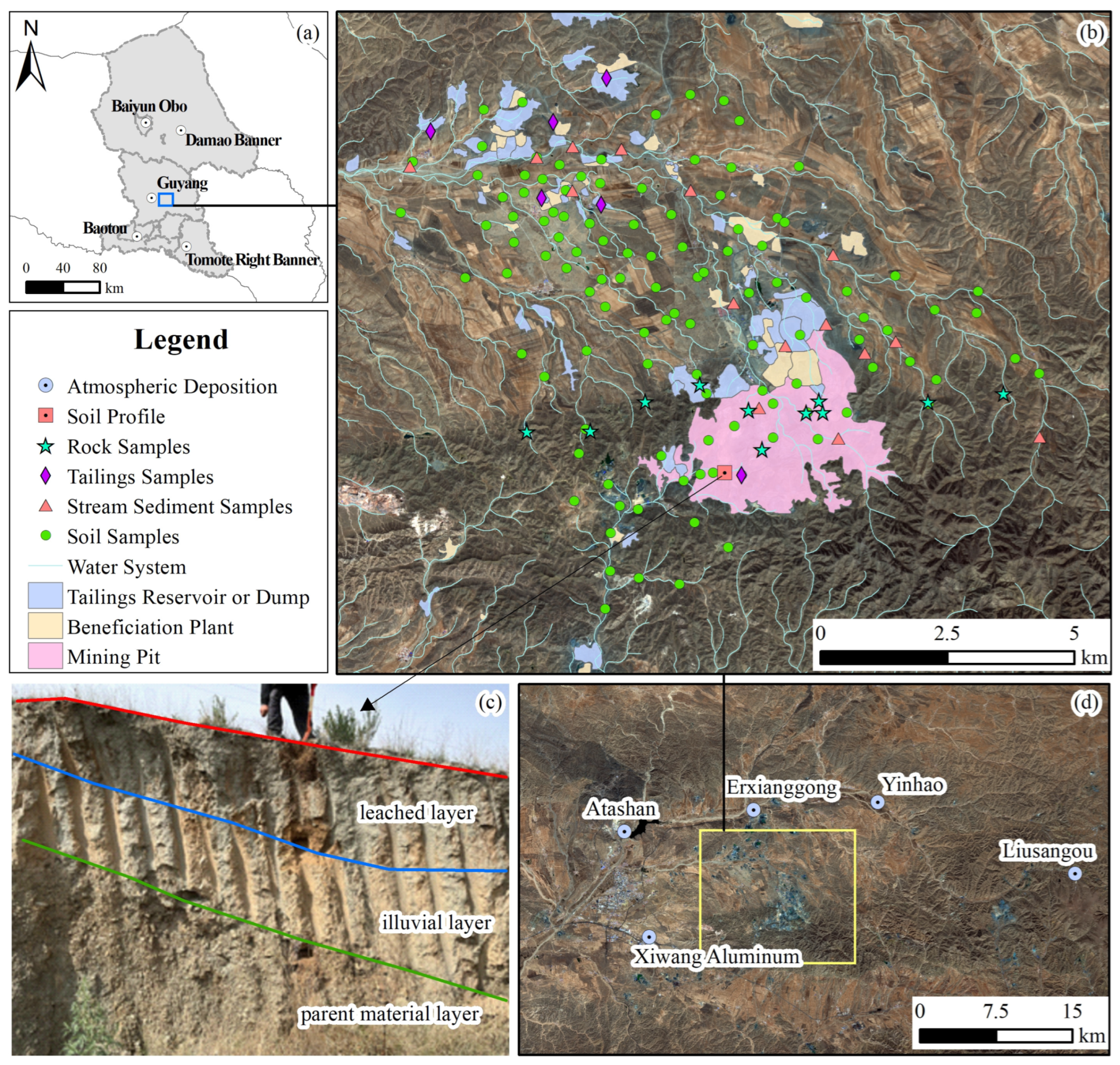
2.1. Overview of Study Area
2.2. Sample Collection and Processing
2.2.1. Soil Sample
2.2.2. Tailings and Rock Sample
2.2.3. Soil Profile Sample
2.2.4. Stream Sediment and Atmospheric Deposition Sample
2.3. Analytical Methods
2.4. Statistical Method
2.5. Enrichment Factor
2.6. Geoaccumulation Index
2.7. Chemical Index of Alteration
3. Results
3.1. Characterization of Heavy Metal and Major Element
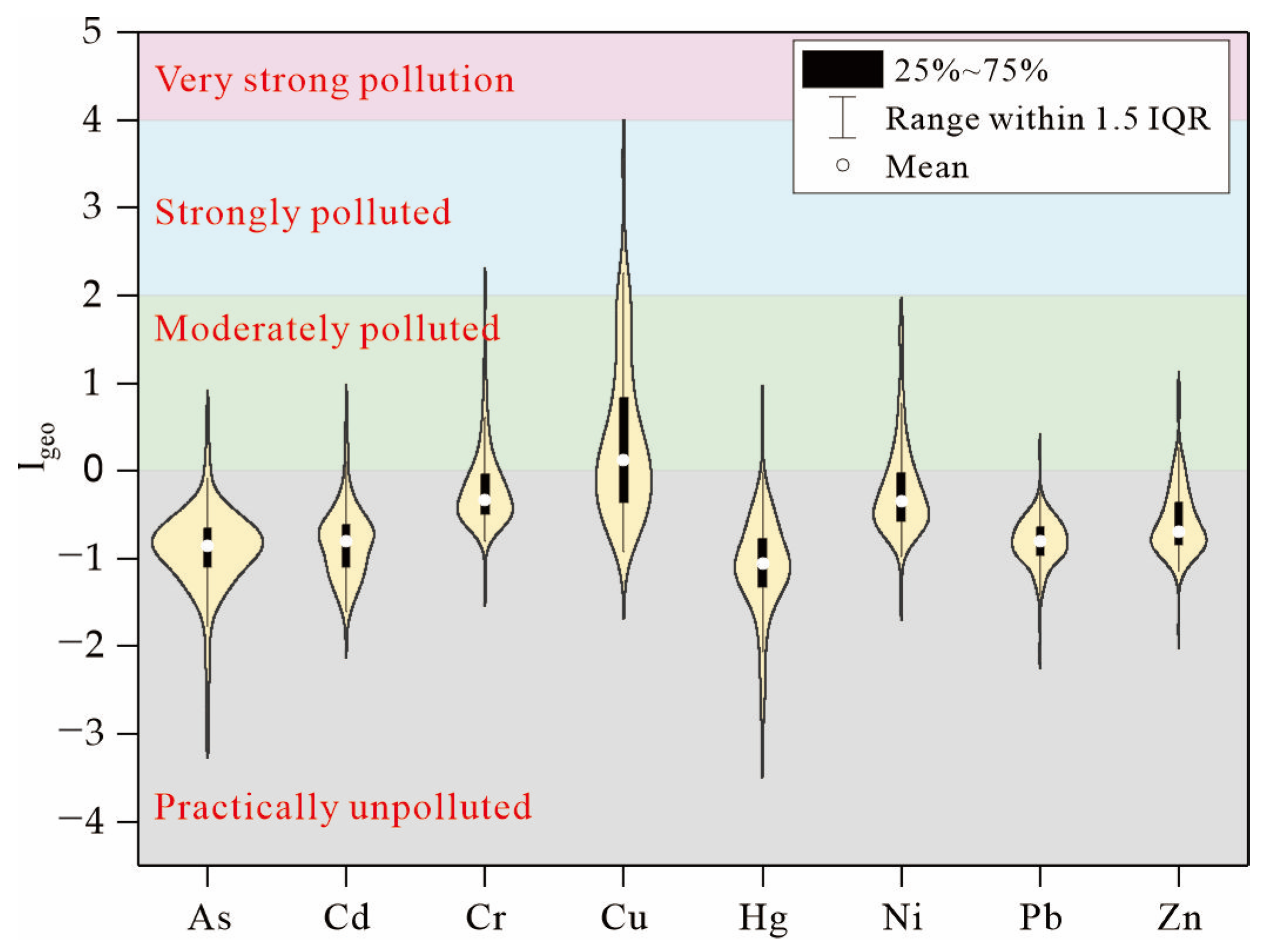
3.2. Heavy Metals Pollution Assessment
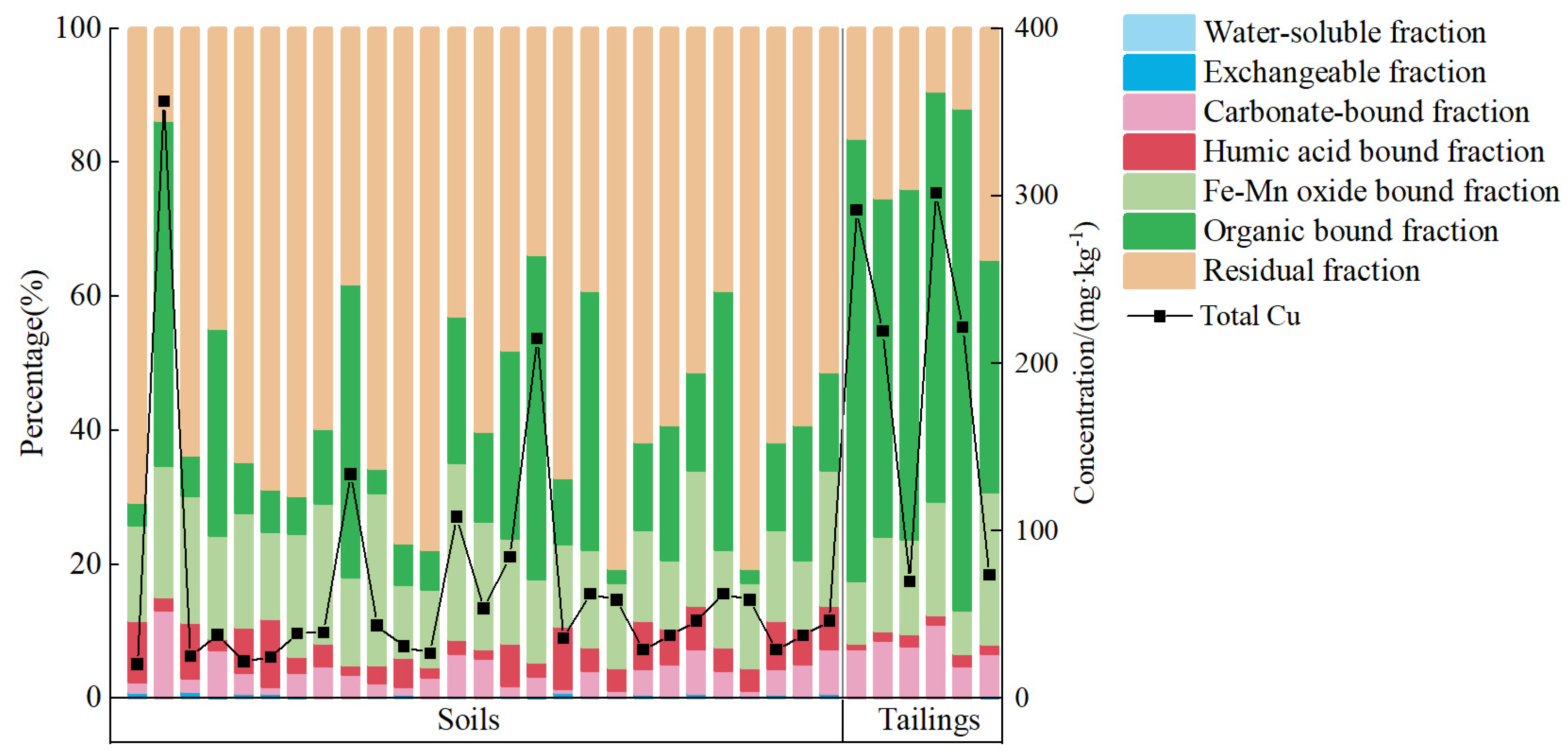
3.3. Geochemical Speciation of Cu
3.4. Mineral Composition
4. Discussion
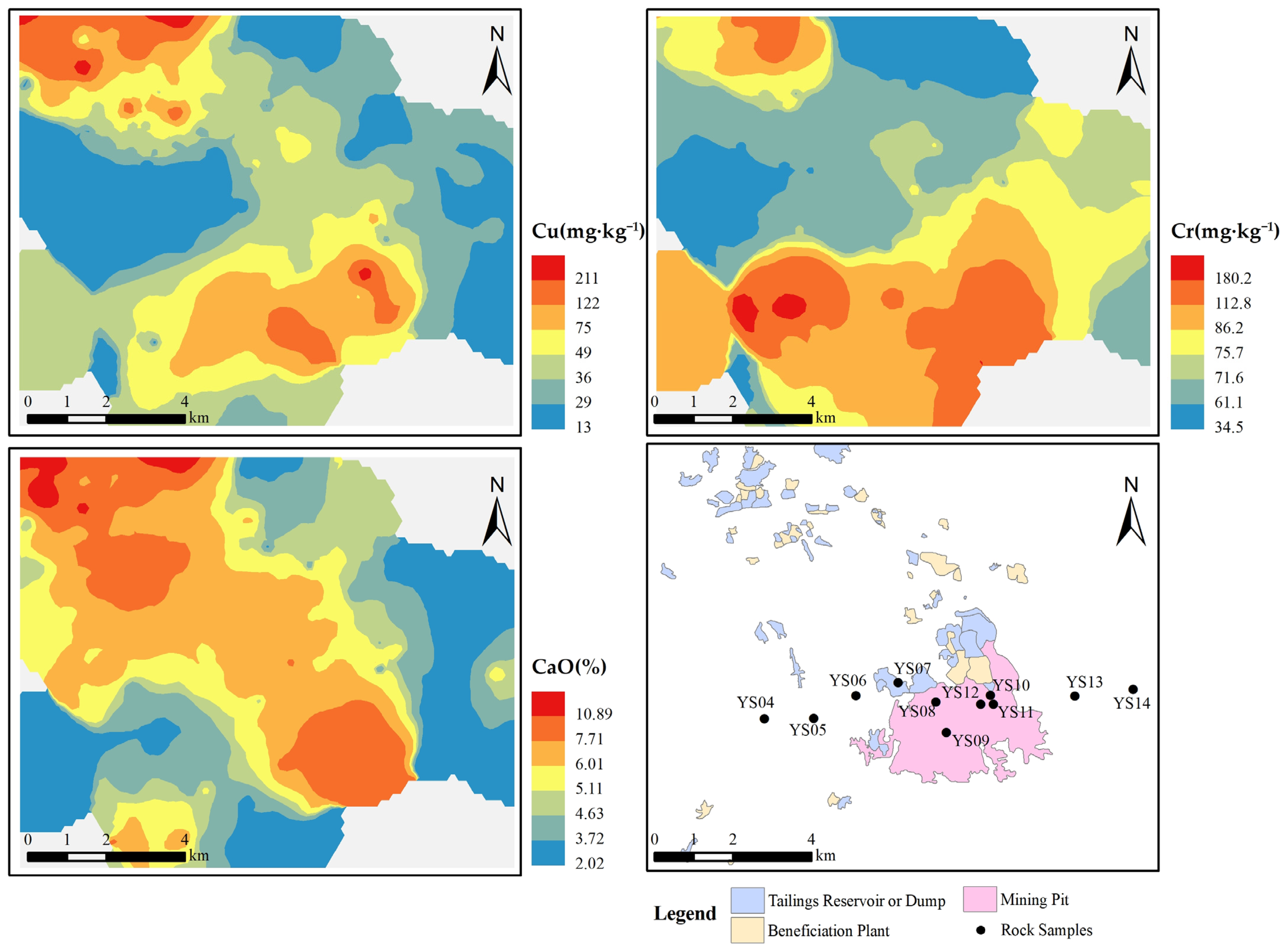
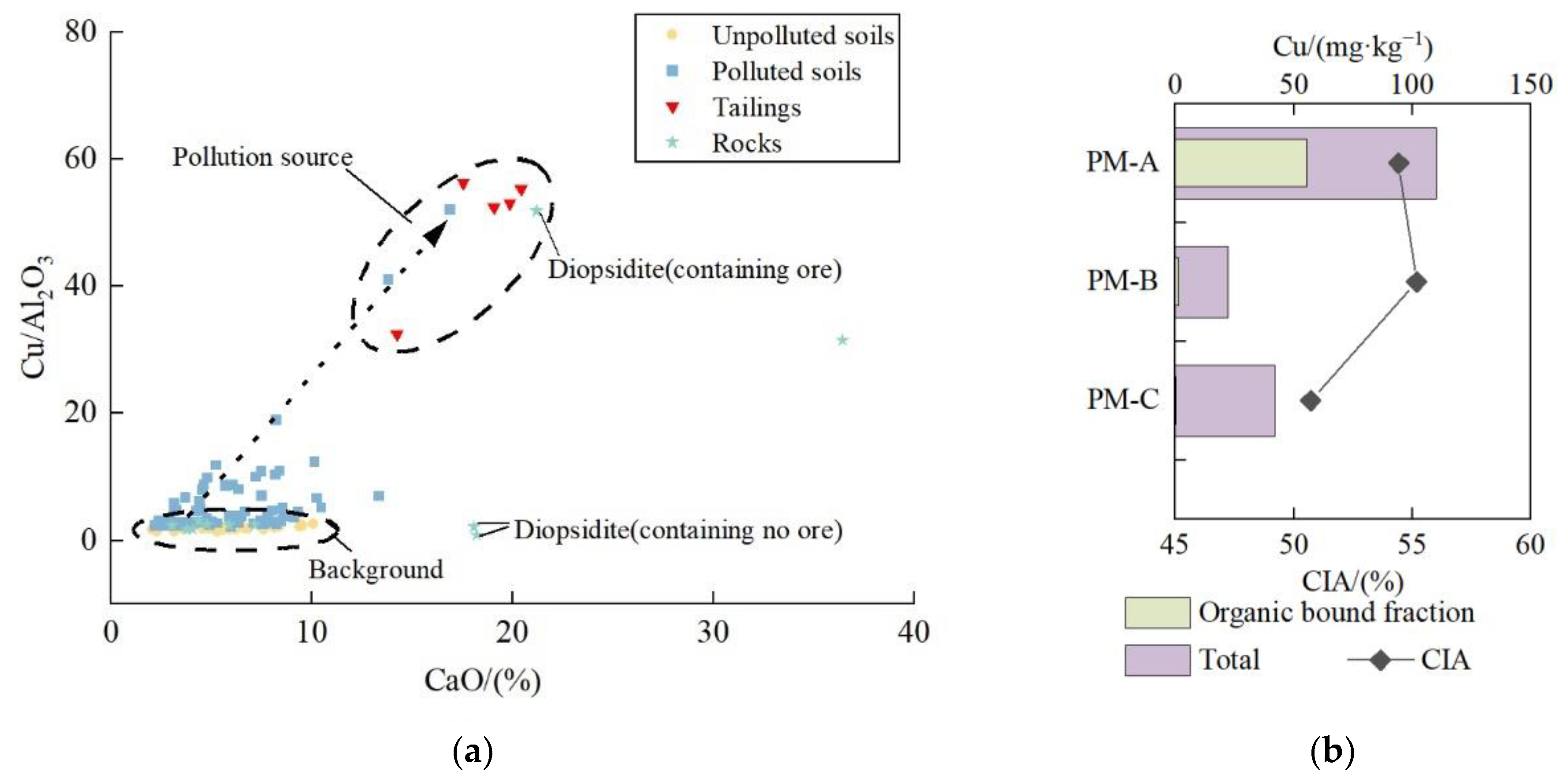
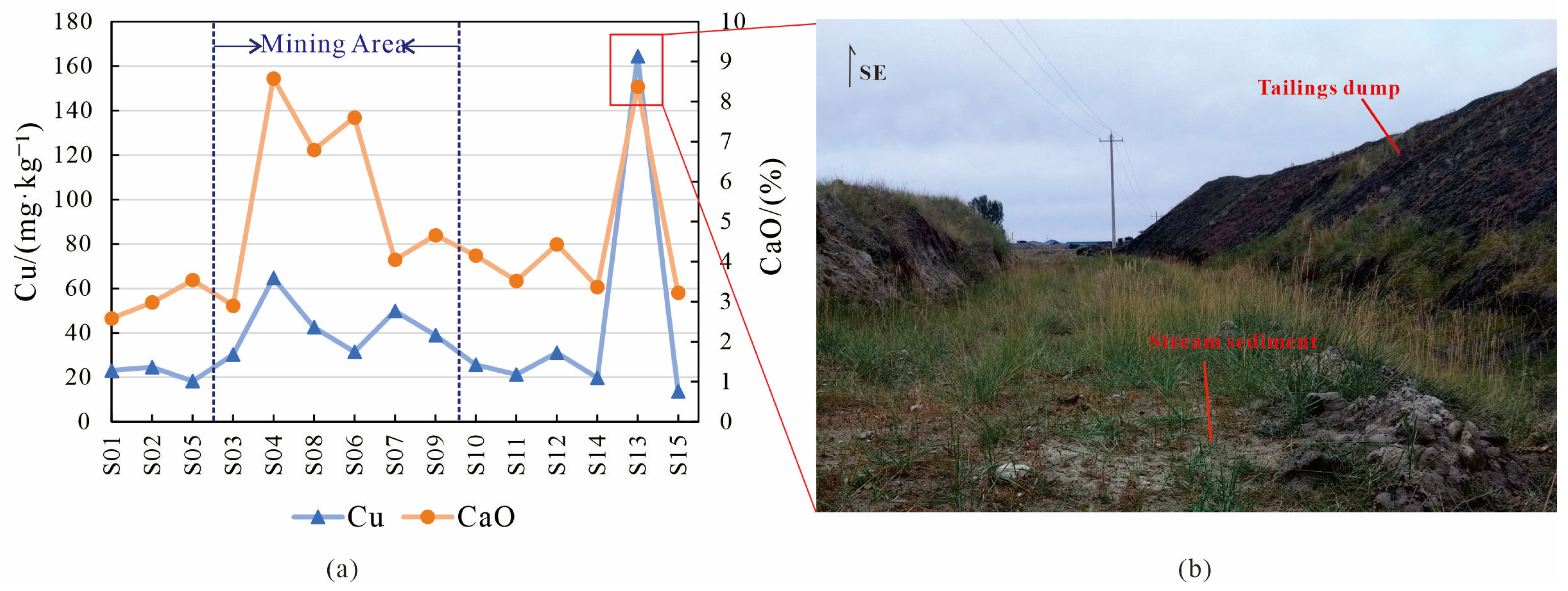
4.1. Source Identification
4.2. Migration Pathways
5. Conclusions
- (1)
- The average concentrations of Cu in the soils, tailings, and stream sediments were higher than the background value. A total of 61.9% of soils are polluted, and the unpolluted to moderately polluted state was the main pollution level.
- (2)
- The tailings were mainly composed of diopside, biotite, and amphibole, while soils were mainly composed of quartz, albite, and biotite. In tailings, the organic bound fraction was always the dominant form, while in soils, the residual fraction was the dominant form. The differences in mineral composition and chemical speciation between tailings and soils can be used to identify the source of Cu.
- (3)
- The high value of Cu/Al2O3 and concentration of CaO in polluted soils, tailings, and ore-bearing rock indicated that tailings may be the source of Cu pollution. The variation in CIA value, mineral composition, and the form of Cu in the soil profile can also serve as evidence to support the possibility that Cu pollution in the topsoil originated from tailings.
- (4)
- The migration pathways of Cu might be mainly hydraulic transport and wind transport.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, C.; Zhang, Y.; Zhang, J.; Chen, Y.; Duan, C.; Qi, J.; Cheng, Z.; Pan, Z. Comprehensive evaluation of the eco-geological environment in the concentrated mining area of mineral resources. Sustainability 2022, 14, 6808. [Google Scholar] [CrossRef]
- Strzałkowski, P.; Litwa, P. Environmental protection problems in the areas of former mines with emphasis on sinkholes: Selected examples. Int. J. Environ. Sci. Technol. 2020, 18, 771–780. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Ordoñez-Alcivar, R.; Arguello-Guadalupe, C.; Carrera-Silva, K.; D’Orio, G.; Straface, S. History, Socioeconomic Problems and Environmental Impacts of Gold Mining in the Andean Region of Ecuador. Int. J. Environ. Res. Public Health 2022, 19, 1190. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, J.; Qi, Q.; Liu, W.; Li, X. Study on chain relationship and risk assessment model of coal mine geological disasters. Arab. J. Geosci. 2022, 15, 900. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, D.; Muzammal, M. Mine Geological Disaster Risk Assessment and Management Based on Multisensor Information Fusion. Mob. Inf. Syst. 2022, 2022, 1757026. [Google Scholar] [CrossRef]
- Pecina, V.; Juřička, D.; Hedbávný, J.; Klimánek, M.; Kynický, J.; Brtnický, M.; Komendová, R. The impacts of mining on soil pollution with metal(loid)s in resource-rich Mongolia. Sci. Rep. 2023, 13, 2763. [Google Scholar] [CrossRef]
- Buch, A.C.; Sims, D.B.; de Ramos, L.M.; Marques, E.D.; Ritcher, S.; Abdullah, M.M.S.; Silva-Filho, E.V. Assessment of environmental pollution and human health risks of mine tailings in soil: After dam failure of the Córrego do Feijão Mine (in Brumadinho, Brazil). Environ. Geochem. Health 2024, 46, 72. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Banerjee, S.; Ghosh, S.; Majumder, S.; Mandal, J.; Roy, P.K.; Bhattacharyya, P. Appraisal of pollution and health risks associated with coal mine contaminated soil using multimodal statistical and Fuzzy-TOPSIS approaches. Front. Environ. Sci. Eng. 2024, 18, 60. [Google Scholar] [CrossRef]
- Rouhani, A.; Skousen, J.; Tack, F.M.G. An Overview of Soil Pollution and Remediation Strategies in Coal Mining Regions. Minerals 2023, 13, 1064. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, T.; Liu, X.; Ma, X.; Yang, Z.; Hou, Q.; Xia, X.; Li, F. Research progress in current status of soil heavy metal pollution and analysis technology. Geol. China 2021, 48, 460–476. [Google Scholar] [CrossRef]
- Hamada, N.H.S.; Ali, R.A.S.; El-Tabakh, M.A.M.; Bream, A.S. Environmental monitoring: Tobara fish as bioindicators of heavy metal pollution in a coastal ecosystem. Reg. Stud. Mar. Sci. 2024, 69, 103335. [Google Scholar] [CrossRef]
- Saidon, N.B.; Szabó, R.; Budai, P.; Lehel, J. Trophic transfer and biomagnification potential of environmental contaminants (heavy metals) in aquatic ecosystems. Environ. Pollut. 2024, 340, 122815. [Google Scholar] [CrossRef]
- Tumanyan, A.F.; Seliverstova, A.P.; Zaitseva, N.A. Effect of Heavy Metals on Ecosystems. Chem. Technol. Fuels Oils 2020, 56, 390–394. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, D.; Wu, Y.; Zhang, L.; Chen, R.; Li, R.; Gu, W.; Zhang, L.; Liu, C.; Sun, Q. Heavy metal risk of disposable food containers on human health. Ecotoxicol. Environ. Saf. 2023, 255, 114797. [Google Scholar] [CrossRef]
- Mahmoud, N.; Al-Shahwani, D.; Al-Thani, H.; Isaifan, R.J. Risk Assessment of the Impact of Heavy Metals in Urban Traffic Dust on Human Health. Atmosphere 2023, 14, 1049. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Ma, Y.; Guo, C.; Jia, J. An Assessment Framework for Human Health Risk from Heavy Metals in Coal Chemical Industry Soils in Northwest China. Sustainability 2023, 15, 14768. [Google Scholar] [CrossRef]
- Panqing, Y.; Abliz, A.; Xiaoli, S.; Aisaiduli, H. Human health-risk assessment of heavy metal–contaminated soil based on Monte Carlo simulation. Sci. Rep. 2023, 13, 7033. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Ji, W.; Wei, N.; Liao, Q.; Huang, D.; Meng, X.; Song, Y. Influencing Factors of Elevated Levels of Potentially Toxic Elements in Agricultural Soils from Typical Karst Regions of China. Agronomy 2023, 13, 2230. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Tao, C.; Ji, W.; Huang, S.; Zhou, M.; Meng, X. Bioavailability of Cd in Agricultural Soils Evaluated by DGT Measurements and the DIFS Model in Relation to Uptake by Rice and Tea Plants. Agronomy 2023, 13, 2378. [Google Scholar] [CrossRef]
- Kumar, M.; Seth, A.; Singh, A.K.; Rajput, M.S.; Sikandar, M. Remediation strategies for heavy metals contaminated ecosystem: A review. Environ. Sustain. Indic. 2021, 12, 100155. [Google Scholar] [CrossRef]
- Zaka, S.; Aqeel, M.; Mahmood, A.; Noman, A.; Rizvi, Z.F.; Sarfraz, W.; Nazir, A.; Arshad, K.; Khalid, N. Integrative Evaluation of the Ecological Hazards by Microplastics and Heavy Metals in Wetland Ecosystem. Bull. Environ. Contam. Toxicol. 2023, 110, 81. [Google Scholar] [CrossRef]
- Hou, C.; Feng, C.; Liu, S. Distribution, Risk Evaluation, and Source Analysis of the Heavy Metals in the Sediment Deposition of the Lower Shichuanhe River; Acta Geochimica: Shaanxi, China, 2023; Volume 42, pp. 832–844. [Google Scholar]
- Xie, S.; Lan, T.; Xing, A.; Chen, C.; Meng, C.; Wang, S.; Xu, M.; Hong, M. Spatial distribution and ecological risk of heavy metals and their source apportionment in soils from a typical mining area, Inner Mongolia, China. J. Arid Land 2023, 5, 1196–1215. [Google Scholar] [CrossRef]
- Mostafa, M.T.; El-Nady, H.; Gomaa, R.M.; Abdelgawad, H.F.; Abdelhafiz, M.A.; Salman, S.A.E.; Khalifa, I.H. Urban geochemistry of heavy metals in road dust from Cairo megacity, Egypt: Enrichment, sources, contamination, and health risks. Environ. Earth Sci. 2023, 83, 37. [Google Scholar] [CrossRef]
- Tang, X.-F.; Wu, Y.; Han, L.-B.; Lan, Z.; Rong, X.-P. Characteristics of heavy metal migration in farmland. Environ. Earth Sci. 2022, 81, 338. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Zeng, J.; Yuan, S.; Luo, X.; Wu, C.; Xue, S. Migration and distribution characteristics of soil heavy metal (loid)s at a lead smelting site. J. Environ. Sci. 2024, 135, 600–609. [Google Scholar] [CrossRef]
- Qin, M.; Jin, Y.; Peng, T.; Zhao, B.; Hou, D. Heavy metal pollution in Mongolian-Manchurian grassland soil and effect of long-range dust transport by wind. Environ. Int. 2023, 177, 108019. [Google Scholar] [CrossRef]
- Bux, R.K.; Batool, M.; Shah, S.M.; Solangi, A.R.; Shaikh, A.A.; Haider, S.I.; Shah, Z.-u.-H. Mapping the Spatial distribution of Soil heavy metals pollution by Principal Component Analysis and Cluster Analyses. Water Air Soil Pollut. 2023, 234, 330. [Google Scholar] [CrossRef]
- Liu, J.; Kang, H.; Tao, W.; Li, H.; He, D.; Ma, L.; Tang, H.; Wu, S.; Yang, K.; Li, X. A spatial distribution—Principal component analysis (SD-PCA) model to assess pollution of heavy metals in soil. Sci. Total Environ. 2023, 859, 160112. [Google Scholar] [CrossRef]
- Aidoo, E.N.; Appiah, S.K.; Awashie, G.E.; Boateng, A.; Darko, G. Geographically weighted principal component analysis for characterising the spatial heterogeneity and connectivity of soil heavy metals in Kumasi, Ghana. Heliyon 2021, 7, e08039. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Liu, J.; Xing, Y.; Liu, Q.; Liu, J.; Liu, F. Assessment of Heavy Metal Pollution and Ecological Risk in Soils of the Southwestern Part of the Xiongan New Area. Earth Sci. Front. 2021, 28, 238–249. [Google Scholar]
- Liu, P.; Wu, Q.; Hu, W.; Tian, K.; Huang, B.; Zhao, Y. Effects of atmospheric deposition on heavy metals accumulation in agricultural soils: Evidence from field monitoring and Pb isotope analysis. Environ. Pollut. 2023, 330, 121740. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Yang, B.; Yang, S.; Ye, J.; Li, J.; Ren, L.; Liu, Z.; Bi, X.; Liu, J. Application of Pb Isotopes and REY Patterns in Tracing Heavy Metals in Farmland Soils from the Upper-Middle Area of Yangtze River. Int. J. Environ. Res. Public Health 2023, 20, 966. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Liu, H.; Dinis, F.; Zhang, Q.; Jing, P.; Liu, F.; Ju, X. Contamination Evaluation and Source Analysis of Heavy Metals in Karst Soil Using UNMIX Model and Pb-Cd Isotopes. Int. J. Environ. Res. Public Health 2022, 19, 12478. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Shen, Z.; Wang, Y.; Mei, N.; Li, C.; Liu, Y.; Ma, W.; Zhang, C.; Wang, D. Tracing and quantifying the source of heavy metals in agricultural soils in a coal gangue stacking area: Insights from isotope fingerprints and receptor models. Sci. Total Environ. 2023, 863, 160882. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, Y.; Jiang, X.; Duan, H.; Ruan, X.; Li, Z.; Li, Y. Combination of UNMIX, PMF model and Pb-Zn-Cu isotopic compositions for quantitative source apportionment of heavy metals in suburban agricultural soils. Ecotoxicol. Environ. Saf. 2022, 234, 113369. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, X.; Han, J.; Zhu, J.-M.; Li, S.; Liang, L.; Wu, P.; Wu, Q.; Qiu, G. Heavy metal(loid)s in agricultural soils in the world’s largest barium-mining area: Pollution characteristics, source apportionment, and health risks using PMF model and Cd isotopes. Process Saf. Environ. Prot. 2022, 166, 669–681. [Google Scholar] [CrossRef]
- Han, Y.; Hu, Y.; Qang, Q.; Shi, M.; Zhou, L. Progress of copper isotope and its application in environmental pollution tracing. Bull. Geol. Sci. Technol. 2023, 42, 378–387. [Google Scholar]
- Wang, L.; Jin, Y.; Weiss, D.J.; Schleicher, N.J.; Wilcke, W.; Wu, L.; Guo, Q.; Chen, J.; O’Connor, D.; Hou, D. Possible application of stable isotope compositions for the identification of metal sources in soil. J. Hazard. Mater. 2021, 407, 124812. [Google Scholar] [CrossRef]
- Zhang, M.; Song, H.; Shang, J.; Liu, X.; Qi, S.; Li, H. Spectroscopic methods for isotope analysis of heavy metal atoms: A review. Spectrochim. Acta Part B At. Spectrosc. 2023, 207, 106740. [Google Scholar] [CrossRef]
- Madadi, R.; Mejjad, N.; De-la-Torre, G.E. Geochemical speciation, ecological risk, and source identification of heavy metal(loid)s in sediments and waters from Musa Estuary, Persian Gulf. Mar. Pollut. Bull. 2023, 190, 114836. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Chen, X.; Liu, H.; Li, X. Source apportionment of heavy metal(loid)s in sediments of a typical karst mountain drinking-water reservoir and the associated risk assessment based on chemical speciations. Environ. Geochem. Health 2023, 45, 7585–7601. [Google Scholar] [CrossRef]
- Chen, B.; He, R.; Cai, P.; Huang, G.; Wang, F. Geochemical Speciation, Risk Assessment, and Sources Identification of Heavy Metals in Mangrove Surface Sediments from the Nanliu River Estuary of the Beibu Gulf, China. Sustainability 2022, 14, 9112. [Google Scholar] [CrossRef]
- Cheng, L.; Ning, X. The assessment of social progress index in the eco-city construction for Baotou. J. Arid. Land Resour. Environ. 2014, 28, 12–16. [Google Scholar] [CrossRef]
- Bai, Y.; Li, Y.; Fang, L. The current situation and prevention proposals of the mine geological environment in Baotou city. China Min. Mag. 2020, 29, 114–116. [Google Scholar]
- Guo, W.; Zhao, R.; Zhang, J.; Bao, Y.; Wang, H.; Yang, M.; Sun, X.; Jin, K. Distribution characteristic and assessment of soil heavy metal pollution in the iron mining of Baotou in Inner Mongolia. Environ. Sci. 2011, 32, 3099–3105. [Google Scholar]
- Wang, C.; Li, Y.; Zhou, W.-h.; Mao, L.; Lu, Z.; Hu, H.; Du, X.; Bian, P.; Gao, Q. Determination and health hazard assessment of multi-element in soils from Guyang County, Baotou City. Rock Miner. Anal. 2022, 41, 476–487. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y.; Deng, Y.; Wang, G. Progress and Prospect of Mine Ecological Restoration in China. J. Resour. Ecol. 2023, 4, 681–682. [Google Scholar]
- Hao, G.; Wang, H.; Niu, G.; Kang, J.; Hao, S. Metamorphic geotectonic research and scientific significance in China. Geol. Surv. Res. 2020, 43, 89–96. [Google Scholar]
- Liu, F.; Su, S.; Yu, X.; Liang, F.; Hu, Y.; Niu, X. Characteristics and petrogenesis of zoned amphiboles in Wengeqi mafic-ultramafic complex, Inner Mongolia. Earth Sci. Front. 2013, 20, 206–222. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, L.; Gao, L. Chemical characteristics and source apportionment of atmospheric precipitation in Baotou urban area. Environ. Chem. 2024, 43, 1–14. [Google Scholar]
- Wang, J.; Zuo, L.; Li, Z.; Mu, H.; Zhou, P.; Yang, J.; Zhao, Y.; Qin, K. A detection method of trace metal elements in black soil based on hyperspectral technology: Geological implications. J. Geomech. 2021, 27, 418–429. [Google Scholar]
- Wu, C.; Sun, B.; Tian, M.; Cheng, X.; Liu, D.; Zhou, Y. Enrichment Characteristics and Ecological Risk Assessment of Heavy Metals in a Farmland System with High Geochemical Background in the Black Shale Region of Zhejiang, China. Minerals 2024, 14, 375. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Dong, L. XRD Bulk Mineral Distribution Patterns of Surface Sediments in the Western Arctic Ocean and Bering Sea. Acta Sedimentol. Sin. 2023, 41, 1054–1066. [Google Scholar]
- Zhang, G.; Chen, S.; Long, R.; Ma, B.; Chang, Y.; Mao, C. Distribution of Heavy Metals in Surface Sediments of a Tropical Mangrove Wetlands in Hainan, China, and Their Biological Effectiveness. Minerals 2023, 13, 1476. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Su, M.; Li, S.; Wang, Z.; Zhao, S.; Zhang, Q. Characteristics of reference and background values of soils in Hetao area. Rock Miner. Anal. 2007, 26, 287–292. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Nasir, M.J.; Wahab, A.; Ayaz, T.; Khan, S.; Khan, A.Z.; Lei, M. Assessment of heavy metal pollution using contamination factor, pollution load index, and geoaccumulation index in Kalpani River sediments, Pakistan. Arab. J. Geosci. 2023, 16, 143. [Google Scholar] [CrossRef]
- Huang, X.; Fu, Q.; Wang, J. Distribution and Pollution Assessment by Index of Geoaccumulation about the Heavy Metals in Sediments of Dexing Copper Mine Rainwater Network. IOP Conf. Ser. Earth Environ. Sci. 2020, 455, 012186. [Google Scholar] [CrossRef]
- Négrel, P.; Ladenberger, A.; Reimann, C.; Demetriades, A.; Birke, M.; Sadeghi, M.; Albanese, S.; Andersson, M.; Baritz, R.; Batista, M.J.; et al. GEMAS: Chemical weathering of silicate parent materials revealed by agricultural soil of Europe. Chem. Geol. 2023, 639, 121732. [Google Scholar] [CrossRef]
- Kalinin, P.I.; Kudrevatykh, I.Y.; Malyshev, V.V.; Pilguy, L.S.; Buhonov, A.V.; Mitenko, G.V.; Alekseev, A.O. Chemical weathering in semi-arid soils of the Russian plain. Catena 2021, 206, 105554. [Google Scholar] [CrossRef]
- GB15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Li, F.; Li, S.; Yang, Y.; Cheng, L. Advances in the study of weathering products of primary silicate minerals, exemplified by mica and feldspar. Acta Petrol. Mineral. 2006, 25, 440–448. [Google Scholar]
- Niu, S.; Fang, Q.; Yu, J.; Wu, J. Heavy Metals Present in the Soils from Extremely Large Opencast Iron Mine Pit. Bull. Environ. Contam. Toxicol. 2021, 107, 984. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhang, X.; Wang, Y.; Li, C.; TANG, B. Heavy Metal Pollution in Soils Surrounding an Iron Tailings in Upstream Areas of Hanjiang River, Shaanxi Province. J. Agro-Environ. Sci. 2015, 9, 1707–1714. [Google Scholar]
- Sun, H.; Wei, X.; Jia, F.; He, Z.; Sun, X. Geochemical baseline and ecological risk accumulation effect of soil heavy metals in the small-scale drainage catchment of V-Ti-magnetite in the Yixun River basin, Chengde. Acta Geol. Sin. 2021, 95, 588–604. [Google Scholar]
- Hasan, O.; Miko, S.; Mesić, S.; Peh, Z. Chemical Weathering Rates of Soils Developed on Eocene Marls and Sandstones in a Mediterranean Catchment (Istria, Croatia). Land 2023, 12, 913. [Google Scholar] [CrossRef]
- Tongggiroh, A.; Imran, A.M.; Haerany, S. Environmental Geochemistry of Heavy Metals and Plagioclase Background Enrichment Factor in Coastal Sediments at Lumpue—Parepare, South Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 370, 012002. [Google Scholar] [CrossRef]
- Oke, S. Intensity of Chemical Weathering Over Three Meta-igneous Rocks: Importance for Trace Metals Enrichment in Soil Profiles. In Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions, 2nd ed.; EMCEI 2019. Environmental Science and Engineering; Springer: Cham, Germany, 2021; pp. 1287–1291. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, X.; Tang, L.; Ji, H.; Jin, Y. Studies on the distribution and chemical speciation of heavy metals in a iron mine soil of the upstream area of Miyun Reservoir, Beijing. China Environ. Sci. 2012, 32, 1632–1639. [Google Scholar]
- DZ/T 0295-2016; Specification of Land Quality Geochemical Evaluation. China Geological Survey: Beijing, China, 2016.
- Zhou, Z.H.; Liu, T.; Dudagula; Ren, L.; He, Z.; Lu, Z.; He, J. Characteristics of Atmospheric Pollutants and Influencing Factors in Baotou. Res. Environ. Sci. 2017, 30, 202–213. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, K.; Jiang, N.; Zou, X.; Wu, B.; He, W. Dust amoun treaching typical cities from dust emissions due to soil wind erosion in Beijing-Tianjins and storm source regions and its source analysis. Chin. Sci. Bull. 2023, 68, 801–816. [Google Scholar] [CrossRef]

| Element | Detection Limit | Element | Detection Limit |
|---|---|---|---|
| As | 0.5 | SiO2 | 0.1 * |
| Cd | 0.03 | Al2O3 | 0.05 * |
| Cr | 2.5 | Fe2O3 | 0.05 * |
| Cu | 1 | K2O | 0.05 * |
| Hg | 0.0005 | Na2O | 0.05 * |
| Ni | 2 | CaO | 0.05 * |
| Pb | 2 | MgO | 0.05 * |
| Zn | 4 |
| Range of Concentration | Accuracy | Precision |
| (Calculation method: ) | (Calculation method: ) | |
| Within 3 times detection limit | ≤0.12 | ≤0.17 |
| Exceeding 3 times detection limit | ≤0.10 | ≤0.15 |
| 1%~5% | ≤0.07 | ≤0.10 |
| >5% | ≤0.05 | ≤0.08 |
| Step | Extractant | Operating Procedure | |
|---|---|---|---|
| F1 | Water-soluble fraction | distilled water | Take 25 mL, shake, oscillate for 30 min, centrifuge for 20 min, and filter. |
| F2 | Exchangeable fraction | 1 mol·L−1 magnesium chloride solution | Take 25 mL, shake, oscillate for 30 min, centrifuge for 20 min, and filter. |
| F3 | Carbonate bonding fraction | 1 mol·L−1 sodium acetate solution | Take 25 mL, shake, oscillate for 1 h, centrifuge for 20 min, and filter. |
| F4 | Humic acid bonding fraction | 0.1 mol·L−1 sodium pyrophosphate solution | Take 50 mL, shake, oscillate for 40 min, wait 2 h, centrifuge for 20 min, and filter. |
| F5 | Fe-Mn oxidation fraction | 0.25 mol·L−1 mixture of hydroxyamine hydrochloride and hydrochloric acid | Take 50 mL, shake, oscillate for 1 h, centrifuge for 20 min, and filter. |
| F6 | Organic bonding fraction | 30% hydrogen peroxide, (1 + 1) nitric acid solution, 3.2 mol·L−1 ammonium acetate–nitric acid mixture | Take 3 mL HNO3 and 5 mL H2O2, shake, then bathe in a constant-temperature water bath for 1.5 h, add 3 mL H2O2, and bathe in the water bath for 70 min. Finally, add 2.5 mL of the ammonium acetate–nitric acid solution, dilute to 25 mL and leave for 10 h, centrifuge for 20 min, and filter. |
| F7 | Residual fraction | - | Step F6 leftover residue is air-dried, finely powdered, and weighed. |
| Igeo-Class | Geoaccumulation Index | Pollution Intensity |
|---|---|---|
| 6 | >5 | Very strong pollution |
| 5 | >4–5 | Strong to very strong |
| 4 | >3–4 | Strongly polluted |
| 3 | >2–3 | Moderately to strongly |
| 2 | >1–2 | Moderately polluted |
| 1 | >0–1 | Unpolluted to moderate |
| 0 | <0 | Practically unpolluted |
| As | Cd | Cr | Cu | Hg | Ni | Pb | Zn | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | SiO2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soils | Min | 1.8 | 0.05 | 34.5 | 15 | 0.004 | 14 | 7 | 24 | 7.27 | 2.02 | 2.30 | 1.69 | 1.30 | 0.63 | 45.8 |
| Max | 15.3 | 0.30 | 352.0 | 378 | 0.058 | 114 | 33 | 153 | 14.98 | 16.88 | 12.16 | 3.08 | 11.64 | 3.15 | 70.7 | |
| Mean | 7.7 | 0.10 | 78.8 | 49 | 0.019 | 34 | 16 | 59 | 11.30 | 5.99 | 5.10 | 2.16 | 2.89 | 2.19 | 61.2 | |
| SD | 2.3 | 0.04 | 37.4 | 53 | 0.007 | 17 | 3 | 20 | 1.11 | 2.58 | 1.56 | 0.23 | 1.56 | 0.37 | 3.9 | |
| CV(%) | 29.3 | 33.8 | 47.5 | 107.9 | 38.5 | 49.2 | 20.0 | 34.6 | 9.9 | 43.1 | 30.6 | 10.7 | 54.1 | 17.1 | 6.3 | |
| Tailings | Min | 1.4 | 0.14 | 52.7 | 265 | 0.005 | 43 | 3 | 90 | 5.63 | 14.24 | 8.37 | 0.77 | 8.06 | 0.65 | 43.3 |
| Max | 2.8 | 0.20 | 345.0 | 341 | 0.010 | 120 | 9 | 209 | 9.03 | 20.43 | 15.36 | 1.79 | 11.37 | 1.36 | 50.8 | |
| Mean | 2.1 | 0.17 | 120.0 | 312 | 0.006 | 64 | 5 | 133 | 7.02 | 17.65 | 10.51 | 1.18 | 10.10 | 0.90 | 47.2 | |
| SD | 0.4 | 0.03 | 111.0 | 28 | 0.002 | 29 | 2 | 54 | 1.51 | 2.64 | 2.75 | 0.39 | 1.17 | 0.26 | 2.6 | |
| CV(%) | 22.4 | 16.4 | 92.8 | 9.1 | 29.4 | 44.9 | 39.0 | 40.1 | 21.5 | 15.0 | 26.1 | 32.8 | 11.5 | 28.5 | 5.5 | |
| Stream sediments | Min | 0.9 | 0.04 | 58.4 | 13 | 2.292 | 17 | 10 | 35 | 9.75 | 2.59 | 2.79 | 2.35 | 1.27 | 1.69 | 50.6 |
| Max | 3.8 | 0.10 | 133.2 | 169 | 5.750 | 49 | 16 | 92 | 14.91 | 8.58 | 8.50 | 3.99 | 7.61 | 4.75 | 62.0 | |
| Mean | 2.0 | 0.06 | 86.6 | 39 | 0.004 | 30 | 12 | 56 | 13.08 | 4.72 | 4.90 | 3.22 | 2.98 | 3.54 | 57.7 | |
| SD | 1.0 | 0.02 | 24.1 | 38 | 1.162 | 10 | 2 | 15 | 1.53 | 2.06 | 1.60 | 0.48 | 2.00 | 1.14 | 3.2 | |
| CV(%) | 47.1 | 29.8 | 27.8 | 98.4 | 32.8 | 32.5 | 16.4 | 26.6 | 11.7 | 43.7 | 32.7 | 14.9 | 67.2 | 32.3 | 5.5 | |
| Background values 1 | 9.7 | 0.12 | 56.4 | 19 | 0.025 | 25 | 19 | 56 | 11.03 | 5.89 | 3.62 | 2.26 | 1.91 | 1.92 | 65.13 | |
| Risk screening values 2 | 20 | 0.6 | 250 | 100 | 3.4 | 190 | 170 | 300 | - | - | - | - | - | - | - | |
| Sample | Rock Identification | As | Ni | Cu | Pb | Cr | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YS04 | diorite | 1.9 | 14 | 36 | 10 | 33 | 15.33 | 3.06 | 2.20 | 1.39 | 4.95 | 1.44 | 68.00 |
| YS05 | diorite | 1.1 | 65 | 45 | 7 | 221 | 14.74 | 4.36 | 2.47 | 1.56 | 4.02 | 3.98 | 60.88 |
| YS06 | leptynite | 4.4 | 51 | 24 | 19 | 111 | 14.24 | 3.79 | 4.90 | 3.54 | 3.02 | 3.13 | 59.40 |
| YS07 | marble | 6.5 | 2 | 24 | 5 | 10 | 0.77 | 36.46 | 0.37 | 0.26 | 0.08 | 14.86 | 16.76 |
| YS08 | diopside rock | 0.9 | 53 | 5 | 3 | 111 | 5.75 | 18.24 | 4.65 | 2.23 | 0.74 | 13.49 | 46.53 |
| YS09 | diopside rock | 0.9 | 68 | 11 | 3 | 81 | 5.15 | 18.09 | 13.20 | 1.14 | 0.74 | 9.06 | 36.99 |
| YS10 | monzonite | 0.3 | 530 | 25 | 9 | 977 | 10.54 | 7.17 | 2.89 | 2.71 | 2.30 | 12.65 | 49.73 |
| YS11 | diorite | 0.4 | 26 | 45 | 20 | 75 | 17.90 | 4.77 | 2.96 | 3.32 | 4.66 | 2.90 | 56.67 |
| YS12 | diopside rock (ore-bearing) | 1.2 | 45 | 124 | 4 | 76 | 2.39 | 21.22 | 12.25 | 0.38 | 0.36 | 11.18 | 38.14 |
| YS13 | monzonite | 0.7 | 25 | 38 | 14 | 92 | 15.56 | 5.87 | 3.74 | 3.03 | 3.70 | 4.34 | 55.36 |
| YS14 | diorite | 0.4 | 66 | 28 | 18 | 187 | 16.02 | 4.06 | 2.62 | 4.21 | 3.06 | 4.29 | 59.03 |
| Location | Geoaccumulation Index | Reference | ||
|---|---|---|---|---|
| Element | Range | Average | ||
| Ma’anshan | Cu | −2.67~1.39 | 0.71 | [64] |
| Zn | −0.38~1.30 | 0.38 | ||
| Hanzhong | Cd | 0.92~4.47 | 2.79 | [65] |
| Hg | 1.23~3.94 | 2.67 | ||
| As | −1.28~4.65 | 1.26 | ||
| Zn | 0.65~3.51 | 0.65 | ||
| Ni | 0.06~2.76 | 0.06 | ||
| Chengde | Cu | Approximately 0 to 2.3 | 1.22 | [66] |
| Amphibole | Albite | Chlorite | Quartz | Biotite | Orthoclase | Diopside | |
|---|---|---|---|---|---|---|---|
| PM-A | 12.23 | 34.54 | 8.34 | 12.27 | 14.48 | 14.56 | 3.59 |
| PM-B | 3.46 | 33.65 | 5.85 | 27.61 | 4.56 | 24.87 | 0 |
| PM-C | 9.96 | 53.63 | 0.91 | 7.44 | 11.56 | 16.5 | 0 |
| As | Cr | Cu | Pb | Cd | Hg | |
|---|---|---|---|---|---|---|
| mg·(m2·a)−1 | μg·(m2·a)−1 | |||||
| Atashan | 3.33 | 15.91 | 11.69 | 18.42 | 238.10 | 10.01 |
| Liusangou | 1.27 | 8.50 | 8.30 | 3.88 | 58.45 | 7.73 |
| Yinhao | 2.21 | 15.52 | 14.16 | 5.89 | 64.04 | 8.28 |
| Xiwang Aluminum | 4.76 | 30.89 | 19.41 | 38.13 | 173.68 | 17.86 |
| Erxianggong | 1.90 | 13.12 | 18.67 | 5.08 | 61.69 | 9.36 |
| Average value of China | 2.45 | 15.08 | 13.09 | 22.99 | 482.17 | 36.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Xu, D.; Li, Y.; Zhou, W.; Bian, P.; Zhang, S. Source and Migration Pathways of Heavy Metals in Soils from an Iron Mine in Baotou City, China. Minerals 2024, 14, 506. https://doi.org/10.3390/min14050506
Wang C, Xu D, Li Y, Zhou W, Bian P, Zhang S. Source and Migration Pathways of Heavy Metals in Soils from an Iron Mine in Baotou City, China. Minerals. 2024; 14(5):506. https://doi.org/10.3390/min14050506
Chicago/Turabian StyleWang, Changyu, Danhong Xu, Yongli Li, Wenhui Zhou, Peng Bian, and Siyuan Zhang. 2024. "Source and Migration Pathways of Heavy Metals in Soils from an Iron Mine in Baotou City, China" Minerals 14, no. 5: 506. https://doi.org/10.3390/min14050506
APA StyleWang, C., Xu, D., Li, Y., Zhou, W., Bian, P., & Zhang, S. (2024). Source and Migration Pathways of Heavy Metals in Soils from an Iron Mine in Baotou City, China. Minerals, 14(5), 506. https://doi.org/10.3390/min14050506





