Ultrasound-Assisted Selective Leaching of Arsenic from Copper Smelting Flue Dust
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
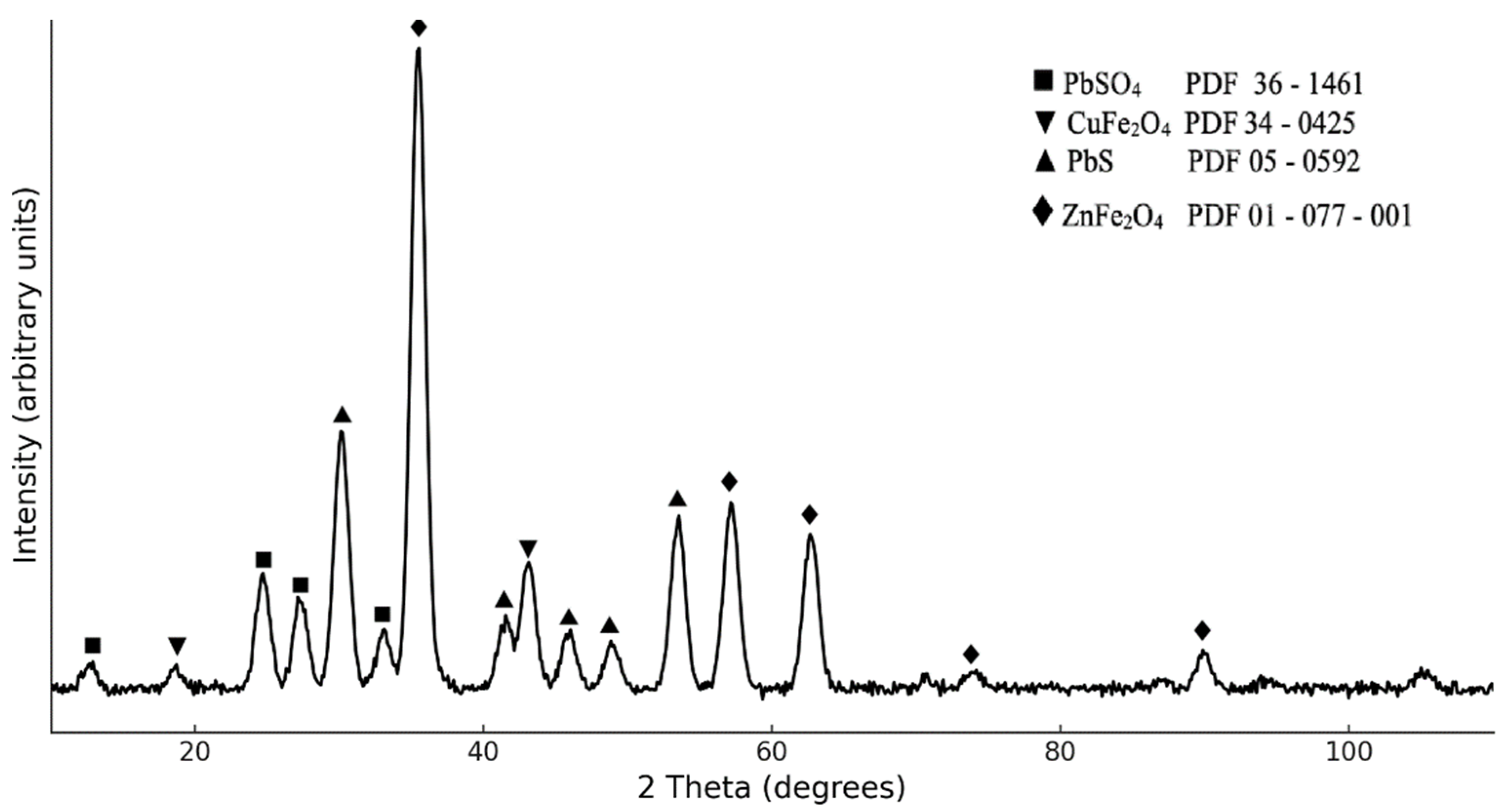
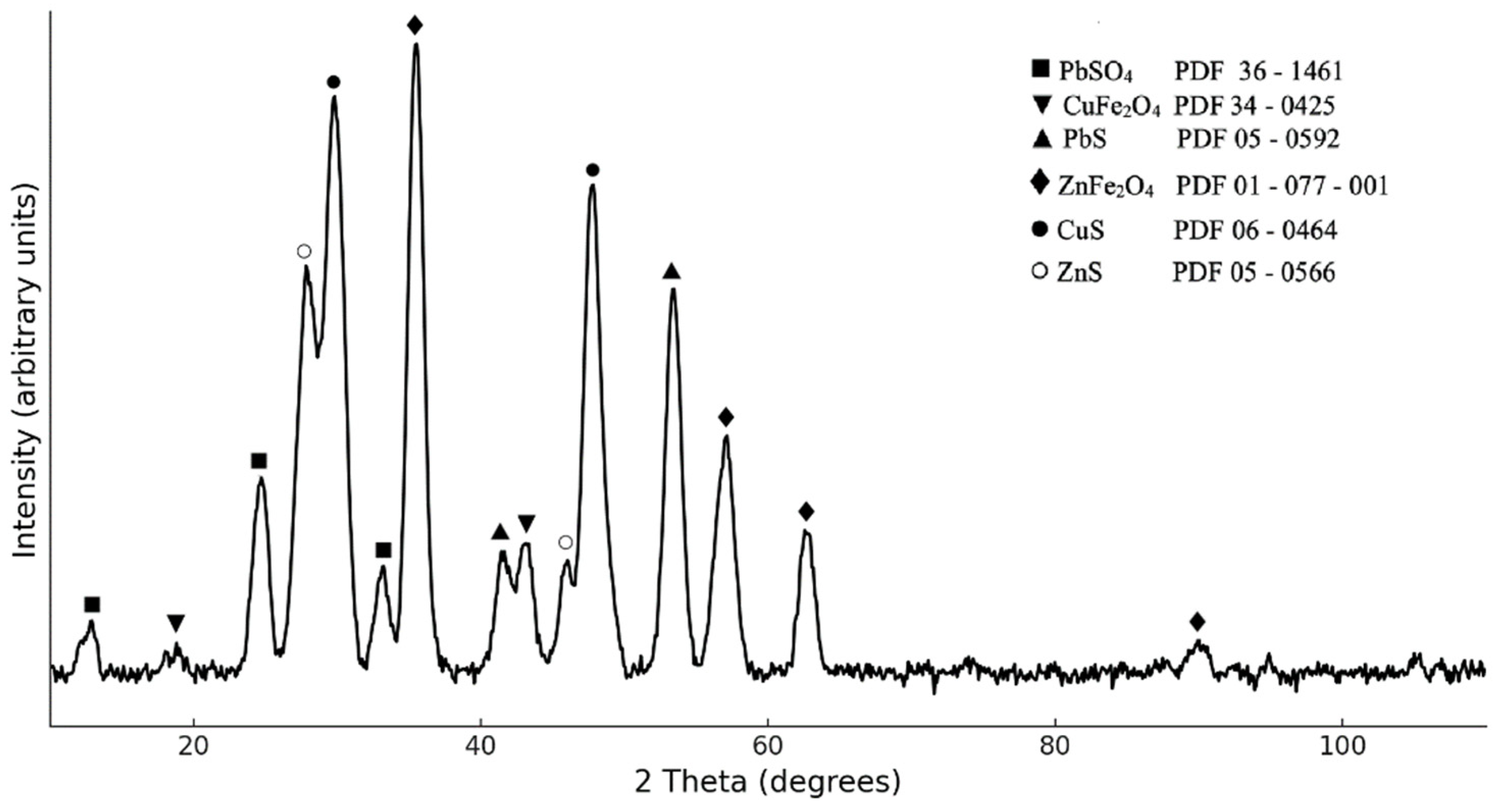
3.1. Characterization of CFD Sample
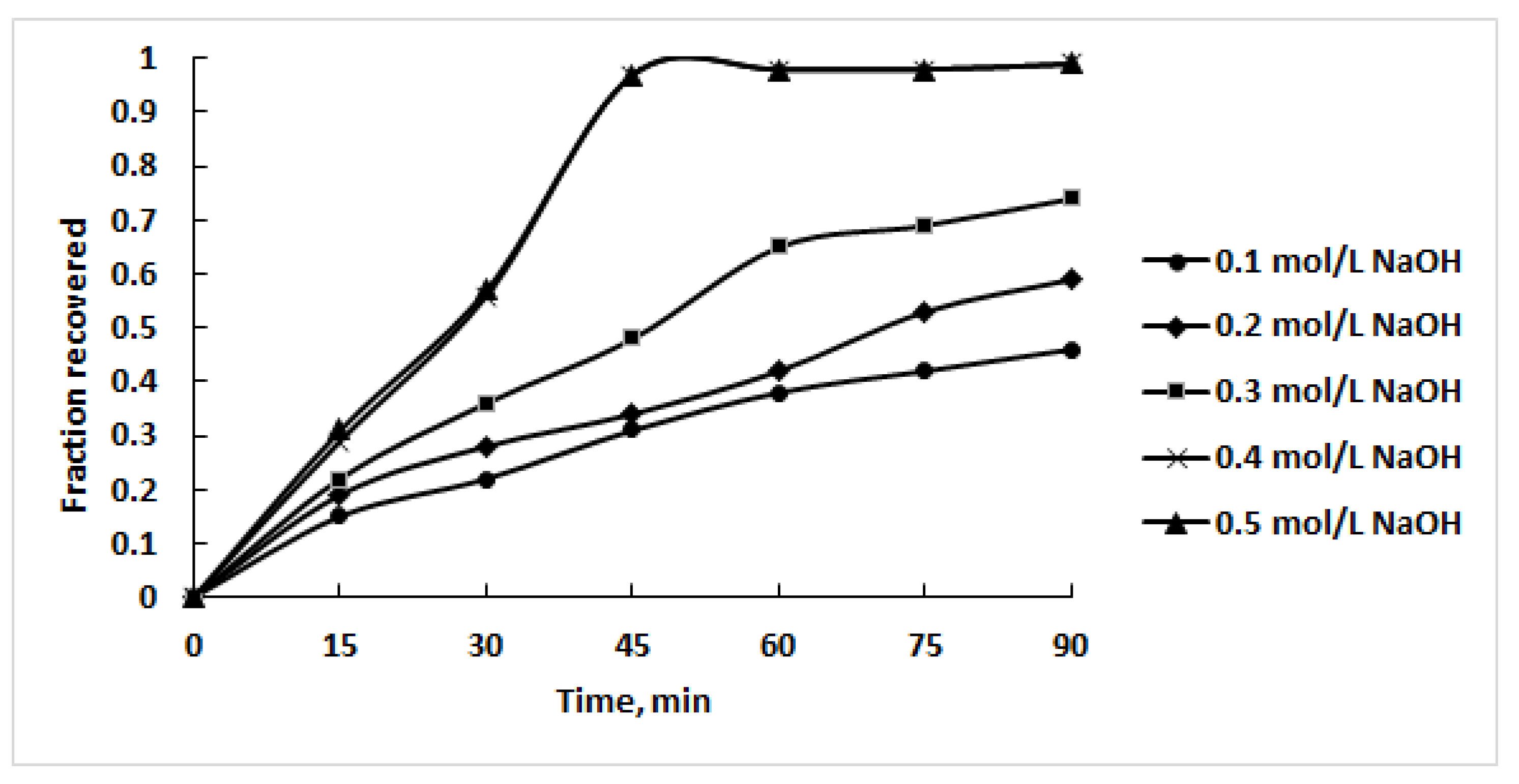
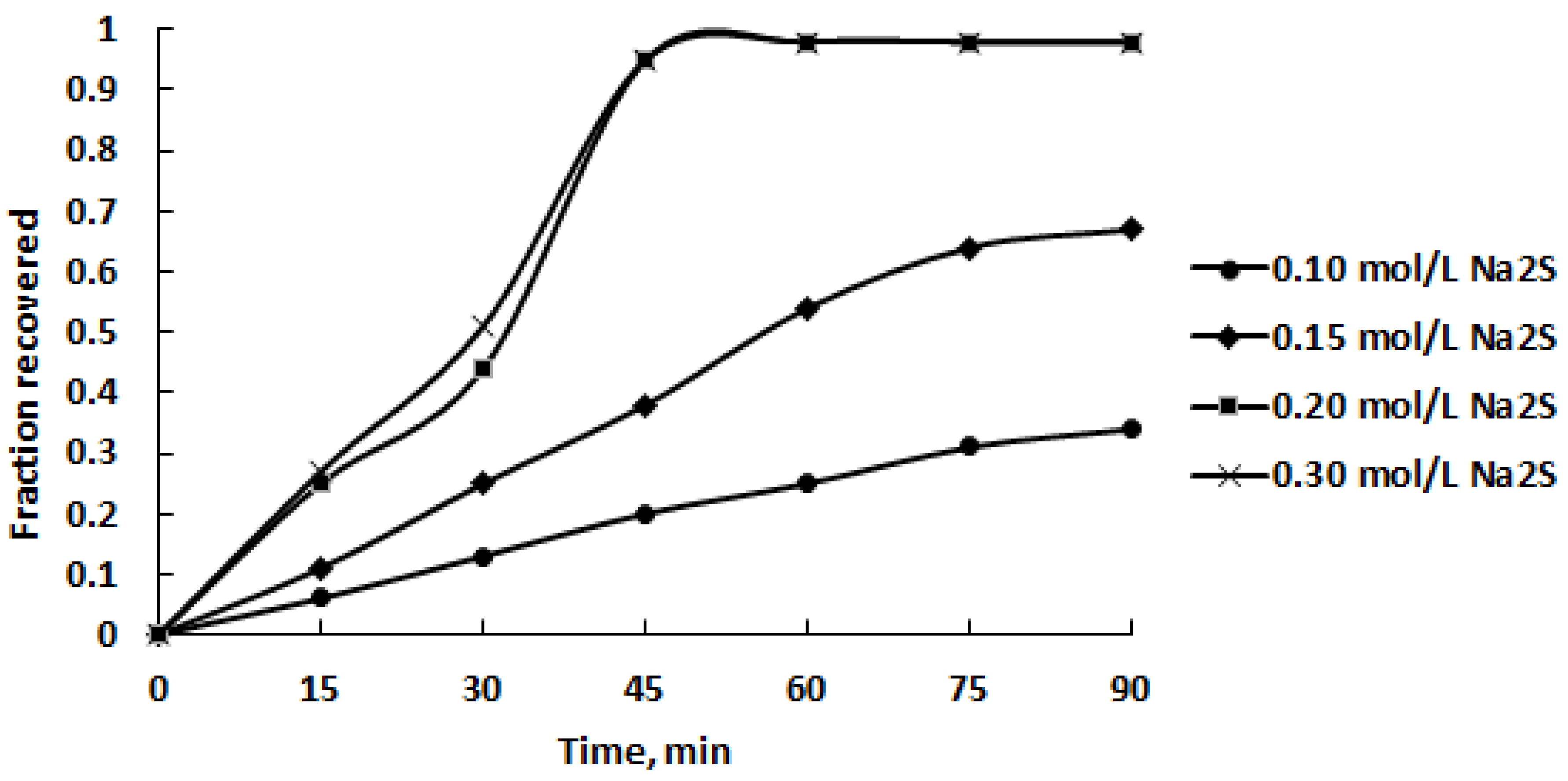
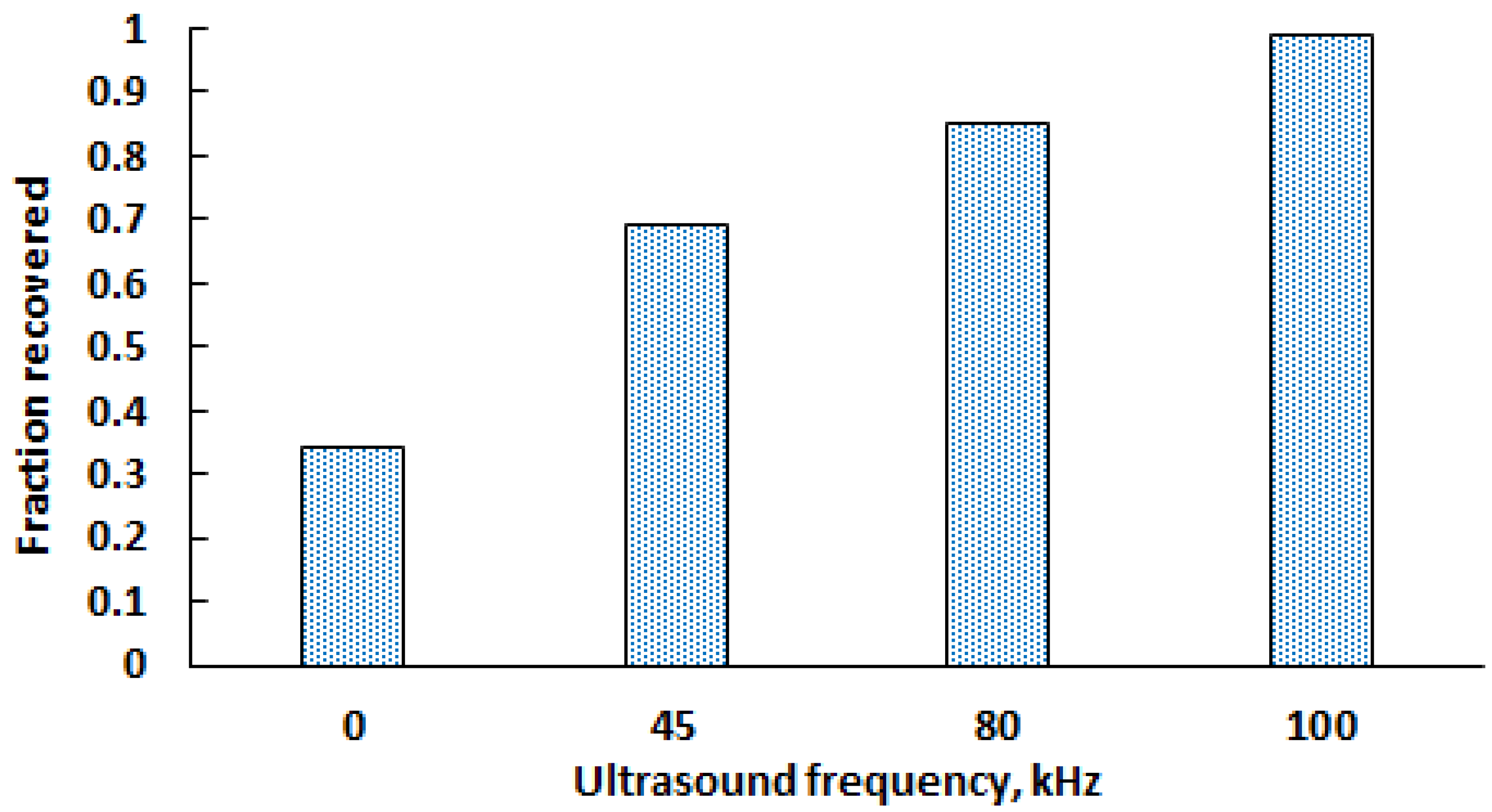
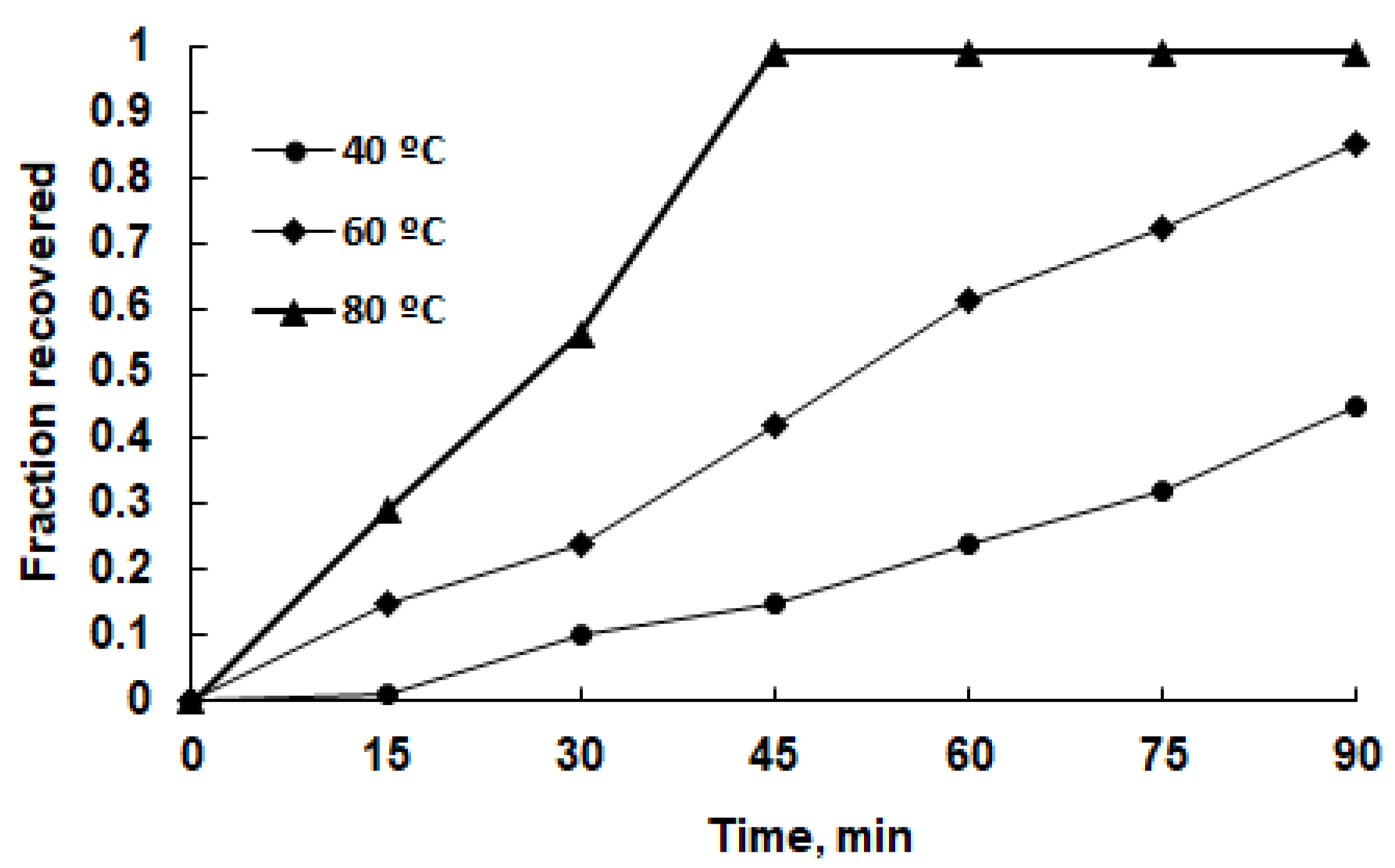
3.2. Leaching Experiments
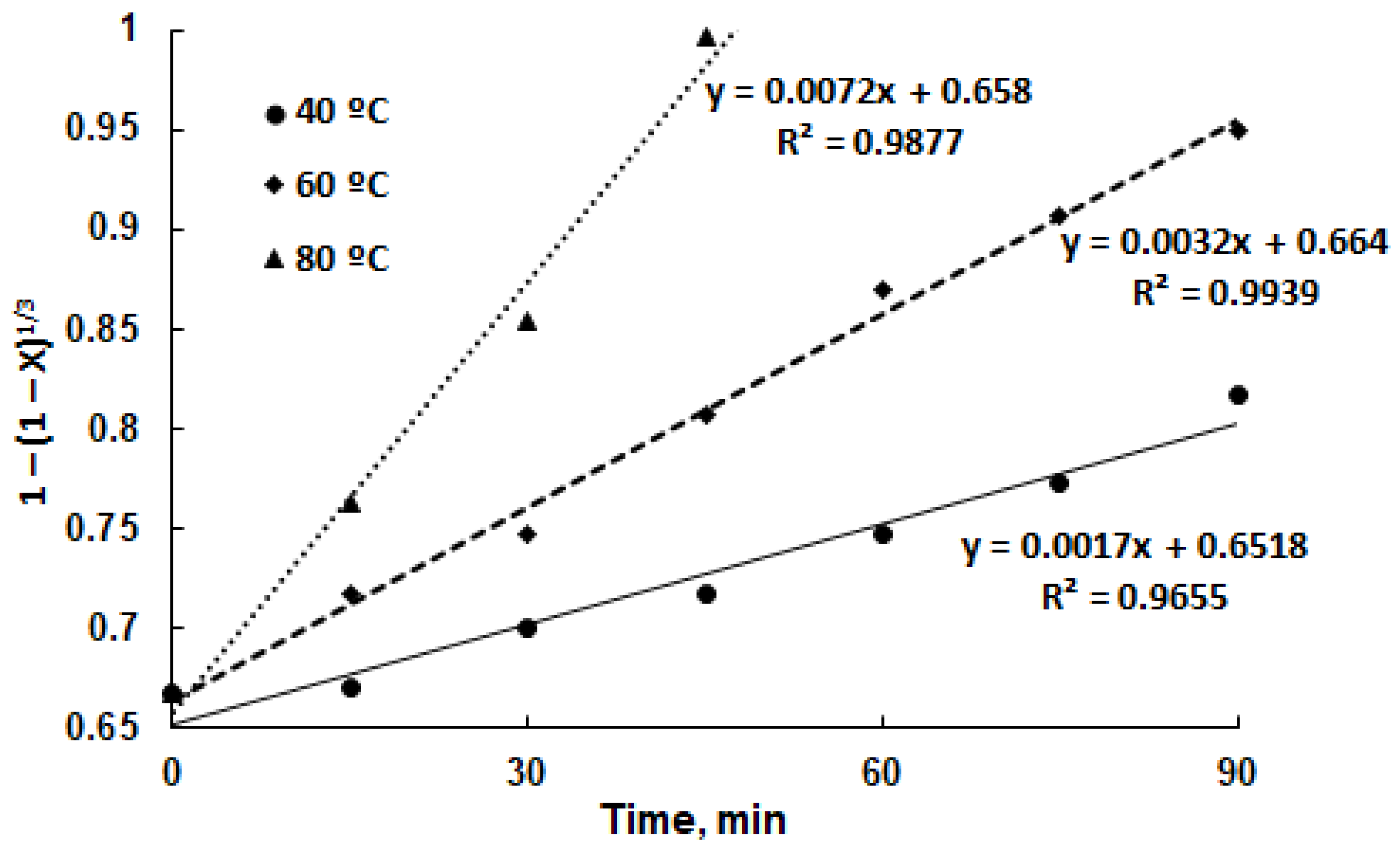
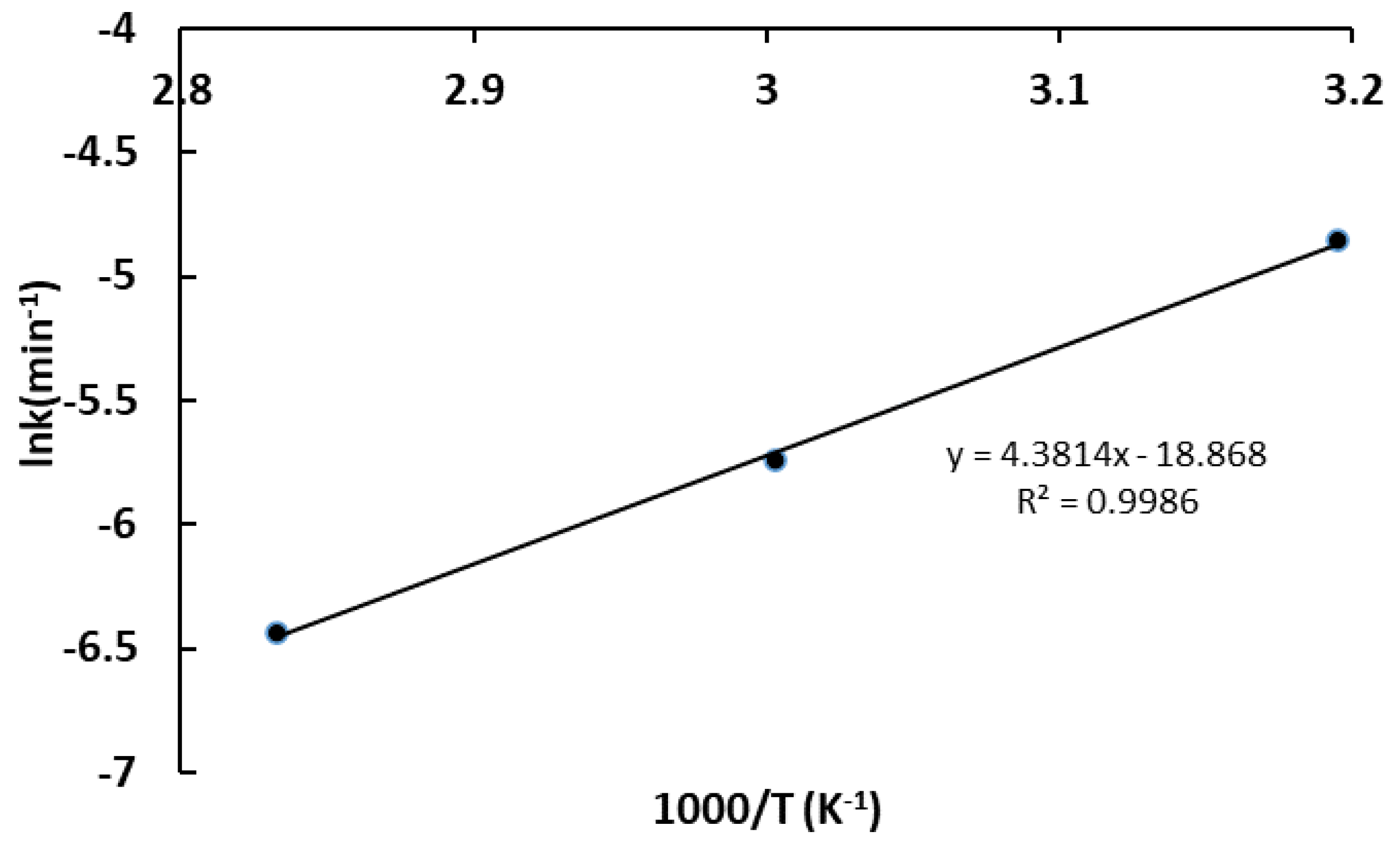
3.3. Leaching Kinetics Analysis
3.4. Leaching Residue Analysis
3.5. Possible Processes during Ultrasound-Assisted Selective Leaching of Arsenic from CFD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, L.; Lan, J.; Du, Y.; Zhang, T.C.; Du, D. Microwave-enhanced selective leaching of arsenic from copper smelting flue dusts. J. Hazard. Mater. 2020, 386, 121964. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ma, Y.; Gao, W.; Yang, J.; Yang, Y.; Li, Q.; Jiang, T. A review of the comprehensive recovery of valuable elements from copper smelting open-circuit dust and arsenic treatment. JOM 2020, 72, 3860–3875. [Google Scholar] [CrossRef]
- Feng, S.; Che, J.; Zhang, W.; Zuo, Y.; Wang, C.; Ma, B.; Chen, Y. A sustainable approach for recovering copper and zinc from copper smelting flue dust: Paving the path for waste reduction. Sep. Purif. Technol. 2024, 342, 127037. [Google Scholar] [CrossRef]
- Gonzalez-Montero, P.; Iglesias-Gonzalez, N.; Romero, R.; Mazuelos, A.; Carranza, F. Recovery of zinc and copper from copper smelter flue dust. Optimisation of sulphuric acid leaching. Environ. Technol. 2018, 41, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Cruells, M.; Roca, A.; Bergó, R. Treatment of copper flash smelter flue dusts for copper and zinc extraction and arsenic stabilization. Hydrometallurgy 2010, 105, 148–154. [Google Scholar] [CrossRef]
- Barros, K.S.; Vielmo, V.S.; Moreno, B.G.; Riveros, G.; Cifuentes, G.; Bernardes, A.M. Chemical composition data of the main stages of copper production from sulfide minerals in Chile: A review to assist circular economy studies. Minerals 2022, 12, 250. [Google Scholar] [CrossRef]
- Dosmukhamedov, N.; Zholdasbay, E.; Argyn, A. Extraction of Pb, Cu, Zn and As from Fine Dust of Copper Smelting Industry via Leaching with Sulfuric Acid. Sustainability 2023, 15, 15881. [Google Scholar] [CrossRef]
- Zheng, G.H.; Kozinsk, J.A. Solid waste remediation in the metallurgical industry: Application and environmental impact. Environ. Prog. 1996, 15, 283–292. [Google Scholar] [CrossRef]
- Jarošíková, A.; Ettler, V.; Mihaljevič, M.; Penížek, V.; Matoušek, T.; Culka, A.; Drahota, P. Transformation of arsenic-rich copper smelter flue dust in contrasting soils: A 2-year field experiment. Environ. Pollut. 2018, 237, 83–92. [Google Scholar] [CrossRef]
- Balladares, E.; Kelm, U.; Helle, S.; Parra, R.; Araneda, E. Chemical-mineralogical characterization of copper smelting flue dust. Dyna 2014, 81, 11–18. [Google Scholar] [CrossRef]
- Zhang, W.; Che, J.; Xia, L.; Wen, P.; Chen, J.; Ma, B.; Wang, C. Efficient removal and recovery of arsenic from copper smelting flue dust by a roasting method: Process optimization, phase transformation and mechanism investigation. J. Hazard. Mater. 2021, 412, 125232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C. Formation mechanism of arsenic-containing dust in the flue gas cleaning process of flash copper pyrometallurgy: A quantitative identification of arsenic speciation. Chem. Eng. J. 2021, 423, 130193. [Google Scholar] [CrossRef]
- Zhang, W.; Che, J.; Wen, P.; Xia, L.; Ma, B.; Chen, J.; Wang, C. Co-treatment of copper smelting flue dust and arsenic sulfide residue by a pyrometallurgical approach for simultaneous removal and recovery of arsenic. J. Hazard. Mater. 2021, 416, 126149. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.K.; Dash, B.; Sahu, S.; Bhattacharya, I.N.; Subbaiah, T. Extraction of copper by leaching of electrostatic precipitator dust and two step removal of arsenic from the leach liquor. Korean J. Chem. Eng. 2012, 29, 1638–1642. [Google Scholar] [CrossRef]
- Che, J.; Zhang, W.; Deen, K.M.; Wang, C. Eco-friendly treatment of copper smelting flue dust for recovering multiple heavy metals with economic and environmental benefits. J. Hazard. Mater. 2024, 465, 133039. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Long, D.; Zhong, H.; Wang, S.; Liu, L. Comprehensive recovery of arsenic and antimony from arsenic-rich copper smelter dust. J. Hazard. Mater. 2021, 413, 125365. [Google Scholar] [CrossRef]
- Ke, J.J.; Qiu, R.Y.; Chen, C.Y. Recovery of metal values from copper smelter flue dust. Hydrometallurgy 1984, 12, 217–224. [Google Scholar] [CrossRef]
- Yao, W.M.; Min, X.B.; Li, Q.Z.; Li, K.Z.; Wang, Y.Y.; Wang, Q.W.; Liu, H.; Qu, S.L.; Dong, Z.Q.; Qu, C.; et al. Formation of arsenic− copper-containing particles and their sulfation decomposition mechanism in copper smelting flue gas. Trans. Nonferrous Met. Soc. China 2021, 31, 2153–2164. [Google Scholar] [CrossRef]
- Filippou, D.; Demopoulos, G.P. Arsenic immobilization by controlled scorodite precipitation. JOM 1997, 49, 52–55. [Google Scholar] [CrossRef]
- Kashiwakura, S.; Ohno, H.; Matsubae-Yokoyama, K.; Kumagai, Y.; Kubo, H.; Nagasaka, T. Removal of arsenic in coal fly ash by acid washing process using dilute H2SO4 solvent. J. Hazard. Mater. 2010, 181, 419–425. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, T.; Li, G.; Chen, B.; Shi, X. Recovery of bismuth and arsenic from copper smelter flue dusts after copper and zinc extraction. Miner. Eng. 2012, 39, 23–28. [Google Scholar] [CrossRef]
- Karimov, K.A.; Naboichenko, S.S. Sulfuric acid leaching of high-arsenic dust from copper smelting. Metallurgist 2016, 60, 456–459. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, X.; Wang, Y.; Han, H.; Sun, W.; Yue, T.; Sun, J. Alkali circulating leaching of arsenic from copper smelter dust based on arsenic-alkali efficient separation. J. Environ. Manag. 2021, 287, 112348. [Google Scholar] [CrossRef] [PubMed]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Selective leaching of arsenic from enargite in NaHS–NaOH media. Hydrometallurgy 2010, 101, 64–68. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandström, Å. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Guo, L.; Hu, Z.; Du, Y.; Zhang, T.C.; Du, D. Mechanochemical activation on selective leaching of arsenic from copper smelting flue dusts. J. Hazard. Mater. 2021, 414, 125436. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Li, Q.; Zhao, Z.; Liu, Z.; Zeng, L.; Li, L. Removal of arsenic from arsenate complex contained in secondary zinc oxide. Hydrometallurgy 2011, 109, 237–244. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Majzlan, J.; Königsberger, E. Thermodynamic properties for arsenic minerals and aqueous species. Rev. Mineral. Geochem. 2014, 79, 217–255. [Google Scholar] [CrossRef]
- Bu, X.; Danstan, J.K.; Hassanzadeh, A.; Behrad Vakylabad, A.; Chelgani, S.C. Metal extraction from ores and waste materials by ultrasound-assisted leaching-an overview. Miner. Process. Extr. Metall. Rev. 2024, 45, 28–45. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.P. A review of the application of ultrasound in bioleaching and insights from sonication in (bio) chemical processes. Resources 2017, 7, 3. [Google Scholar] [CrossRef]
- Bao, S.; Chen, B.; Zhang, Y.; Ren, L.; Xin, C.; Ding, W.; Zhang, W. A comprehensive review on the ultrasound-enhanced leaching recovery of valuable metals: Applications, mechanisms and prospects. Ultrason. Sonochem. 2023, 98, 106525. [Google Scholar] [CrossRef] [PubMed]
- Luque-Garcıa, J.L.; De Castro, M.L. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Väisänen, A.; Suontamo, R. Comparison of ultrasound-assisted extraction, microwave-assisted acid leaching and reflux for the determination of arsenic, cadmium and copper in contaminated soil samples by electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 2002, 17, 739–742. [Google Scholar] [CrossRef]
- Li, X.; Zhu, R.; Hu, S.; Lei, Y.; Zhang, H.; Yao, J.; Xu, C.; Li, S. Leaching of arsenic from flue gas desulphurisation gypsum using ultrasound-enhanced sulfuric acid. J. Clean. Prod. 2022, 381, 135163. [Google Scholar] [CrossRef]
- Pumure, I.; Renton, J.J.; Smart, R.B. Accelerated aqueous leaching of selenium and arsenic from coal associated rock samples with selenium speciation using ultrasound extraction. Environ. Geol. 2009, 56, 985–991. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, G.; Wang, S.; Liu, H.; Fu, L.; Xiang, D.; Zhu, M. Ultrasonic-enhanced selective arsenic removal and sulfide conversion from iron slag. J. Clean. Prod. 2024, 450, 141885. [Google Scholar] [CrossRef]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, J.; Li, Y.; Wang, R.; Xu, Z. Deep separation of arsenic and alkali from alkaline arsenic containing solution using hydrothermal lime precipitation method. J. Environ. Chem. Eng. 2024, 12, 112294. [Google Scholar] [CrossRef]
- Gbor, P.K.; Jia, C.Q. Critical evaluation of coupling particle size distribution with the shrinking core model. Chem. Eng. Sci. 2004, 59, 1979–1987. [Google Scholar] [CrossRef]
- Nadirov, R.; Karamyrzayev, G. Selective ozone-assisted acid leaching of copper from copper smelter slag by using isopropanol as a solvent. Minerals 2022, 12, 1047. [Google Scholar] [CrossRef]
- Nadirov, R.; Karamyrzayev, G. Enhancing Synthetic Zinc Ferrite Hydrochloric Acid Leaching by Using Isopropanol as a Solvent. Min. Metall. Explor. 2022, 39, 1743–1751. [Google Scholar] [CrossRef]
- Safari, V.; Arzpeyma, G.; Rashchi, F.; Mostoufi, N. A shrinking particle—Shrinking core model for leaching of a zinc ore containing silica. Int. J. Miner. Process. 2009, 93, 79–83. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Taskinen, P.; Peng, N.; Peng, B.; Jokilaakso, A.; Liu, H.; Liang, Y.; Zhao, Z.; Wang, Z. Treatment of high-arsenic copper smelting flue dust with high copper sulfate: Arsenic separation by low temperature roasting. Miner. Eng. 2021, 164, 106796. [Google Scholar] [CrossRef]
- Jarošíková, A.; Ettler, V.; Mihaljevič, M.; Drahota, P.; Culka, A.; Racek, M. Characterization and pH-dependent environmental stability of arsenic trioxide-containing copper smelter flue dust. J. Environ. Manag. 2018, 209, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Zhang, Z.; Zhang, G. Removal of zinc from basic oxygen steelmaking filter cake by selective leaching with butyric acid. J. Clean. Prod. 2019, 209, 1–9. [Google Scholar] [CrossRef]
- Şahin, M.; Erdem, M. Cleaning of high lead-bearing zinc leaching residue by recovery of lead with alkaline leaching. Hydrometallurgy 2015, 153, 170–178. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.Y.; Tian, Q.H.; Qin, H. Selective removal of arsenic from high arsenic dust in the NaOH-S system and leaching behavior of lead, antimony, zinc and tin. Hydrometallurgy 2021, 202, 105607. [Google Scholar] [CrossRef]
- Dutra, A.J.B.; Paiva, P.R.P.; Tavares, L.M. Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 2006, 19, 478–485. [Google Scholar] [CrossRef]
- Youcai, Z.; Stanforth, R. Extraction of zinc from zinc ferrites by fusion with caustic soda. Miner. Eng. 2000, 13, 1417–1421. [Google Scholar] [CrossRef]
- Terzano, R.; Spagnuolo, M.; Vekemans, B.; De Nolf, W.; Janssens, K.; Falkenberg, G.; Fiore, S.; Ruggiero, P. Assessing the origin and fate of Cr, Ni, Cu, Zn, Pb, and V in industrial polluted soil by combined microspectroscopic techniques and bulk extraction methods. Environ. Sci. Technol. 2007, 41, 6762–6769. [Google Scholar] [CrossRef]
- Li, W. Synthesis and Solubility of Arsenic Tri-Sulfide and Sodium Arsenic Oxy-Sulfide Complexes in Alkaline Sulfide Solutions. Ph.D. Dissertation, University of British Columbia, Vancouver, BC, Canada, 2013. [Google Scholar]
- Wilkin, R.T.; Ford, R.G. Arsenic solid-phase partitioning in reducing sediments of a contaminated wetland. Chem. Geol. 2006, 228, 156–174. [Google Scholar] [CrossRef]





| Element (wt.%) | |||||
|---|---|---|---|---|---|
| Cu | S | Pb | Fe | Zn | As |
| 3.76 | 14.11 | 24.27 | 1.94 | 6.17 | 12.35 |
| Equation | Temperature, °C (K) | ||
|---|---|---|---|
| 40 (313) | 60 (333) | 80 (353) | |
| (4) | R2 = 0.7971 | R2 = 0.9268 | R2 = 0.9055 |
| (5) | R2 = 0.9655 | R2 = 0.9939 | R2 = 0.9877 |
| (6) | R2 = 0.7318 | R2 = 0.7267 | R2 = 0.6777 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenzhaliyev, B.; Ketegenov, T.; Mussapyrova, L.; Nadirov, R. Ultrasound-Assisted Selective Leaching of Arsenic from Copper Smelting Flue Dust. Minerals 2024, 14, 532. https://doi.org/10.3390/min14060532
Kenzhaliyev B, Ketegenov T, Mussapyrova L, Nadirov R. Ultrasound-Assisted Selective Leaching of Arsenic from Copper Smelting Flue Dust. Minerals. 2024; 14(6):532. https://doi.org/10.3390/min14060532
Chicago/Turabian StyleKenzhaliyev, Bagdaulet, Tlek Ketegenov, Lyazzat Mussapyrova, and Rashid Nadirov. 2024. "Ultrasound-Assisted Selective Leaching of Arsenic from Copper Smelting Flue Dust" Minerals 14, no. 6: 532. https://doi.org/10.3390/min14060532
APA StyleKenzhaliyev, B., Ketegenov, T., Mussapyrova, L., & Nadirov, R. (2024). Ultrasound-Assisted Selective Leaching of Arsenic from Copper Smelting Flue Dust. Minerals, 14(6), 532. https://doi.org/10.3390/min14060532






