The Occurrence and Distribution of Nitrogen in Coal of Different Ranks and Densities
Abstract
1. Introduction
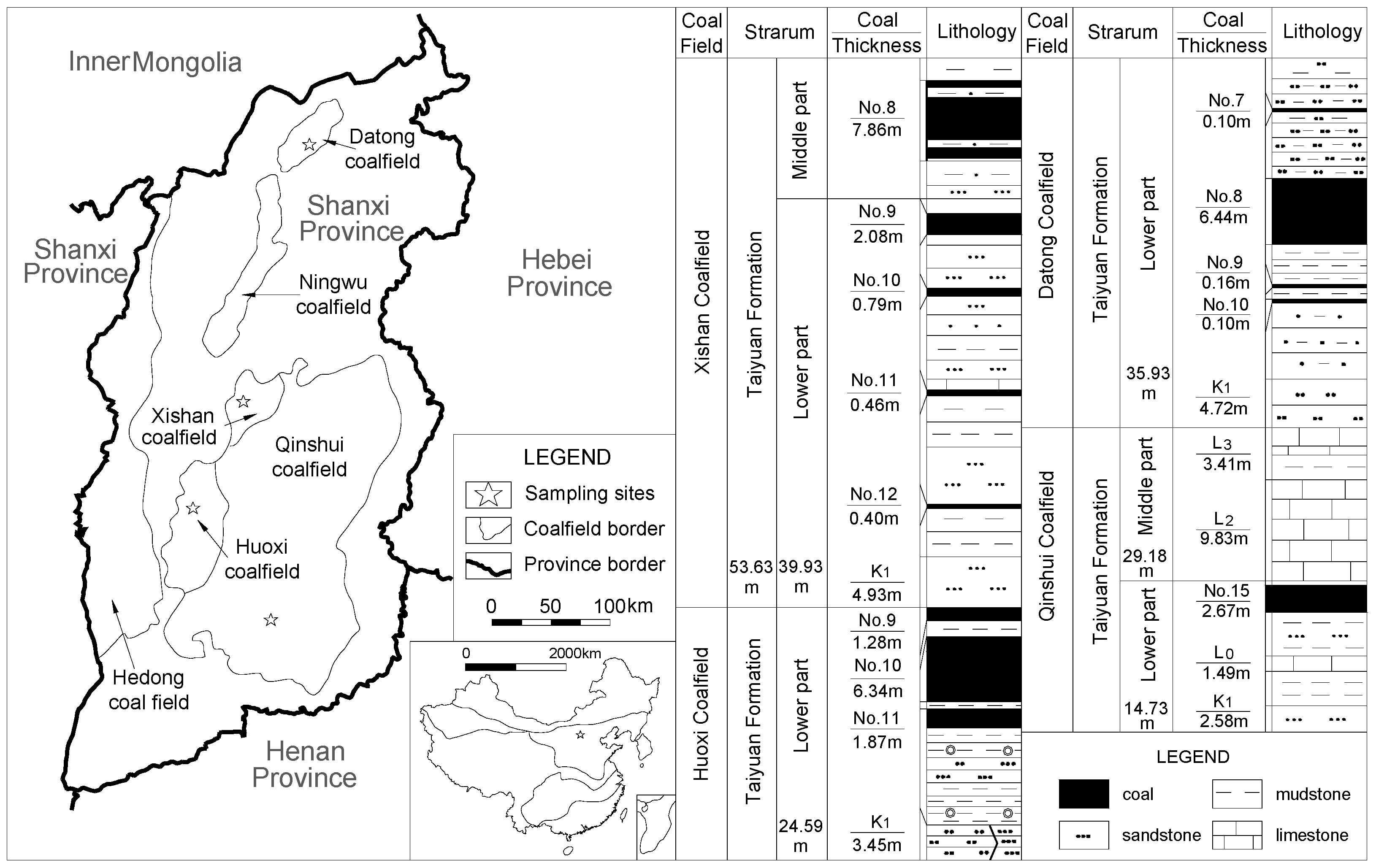
2. Geological Setting
3. Materials and Methods
4. Results and Discussion
4.1. Coal Petrology and Coal Quality
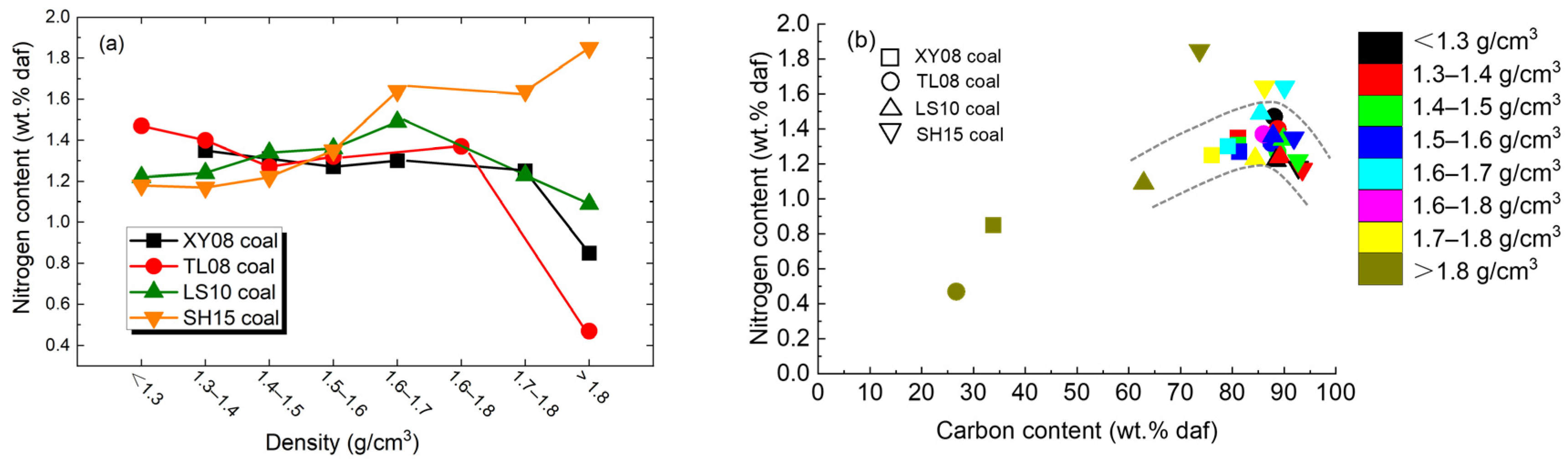
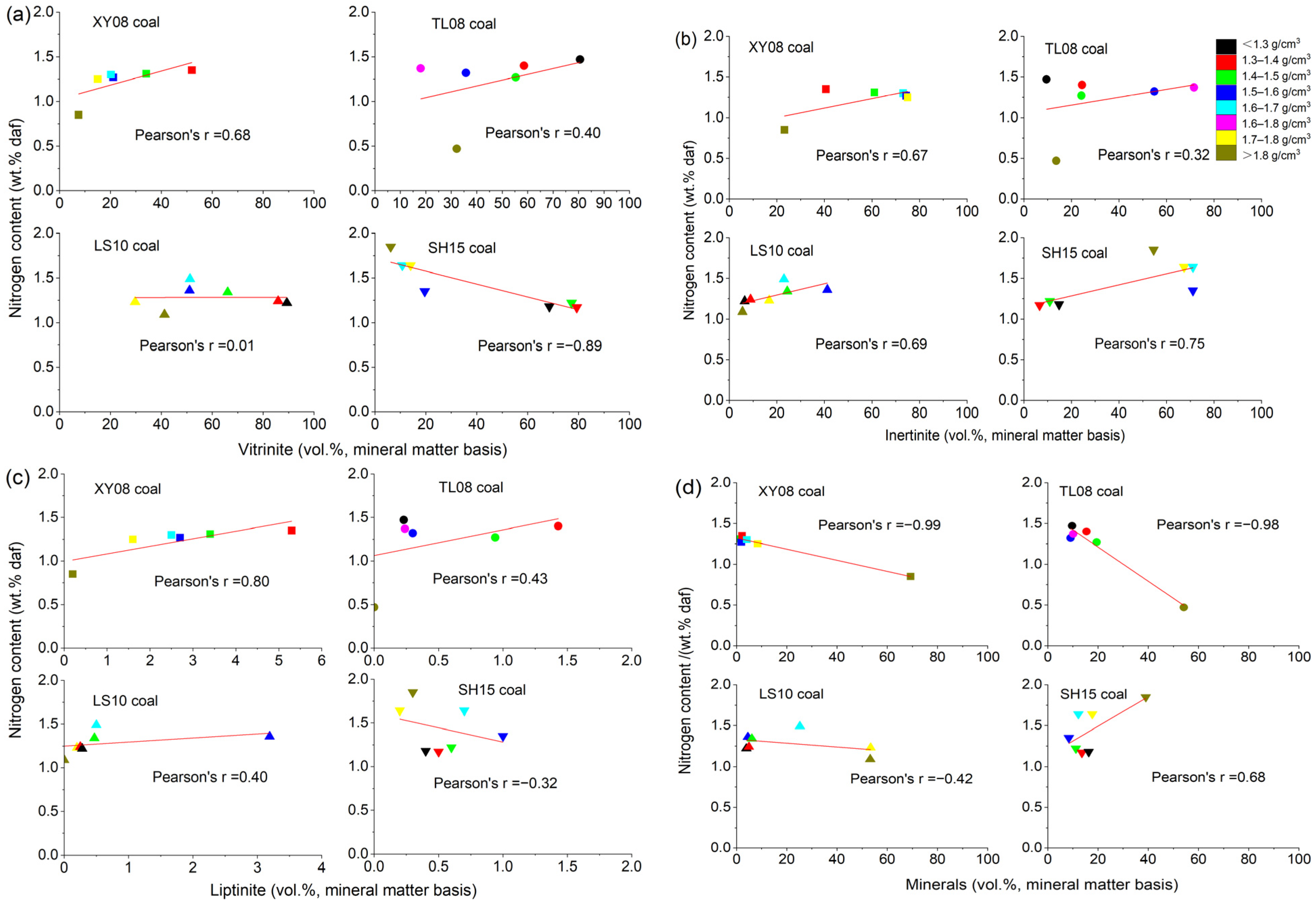
4.2. Nitrogen Content
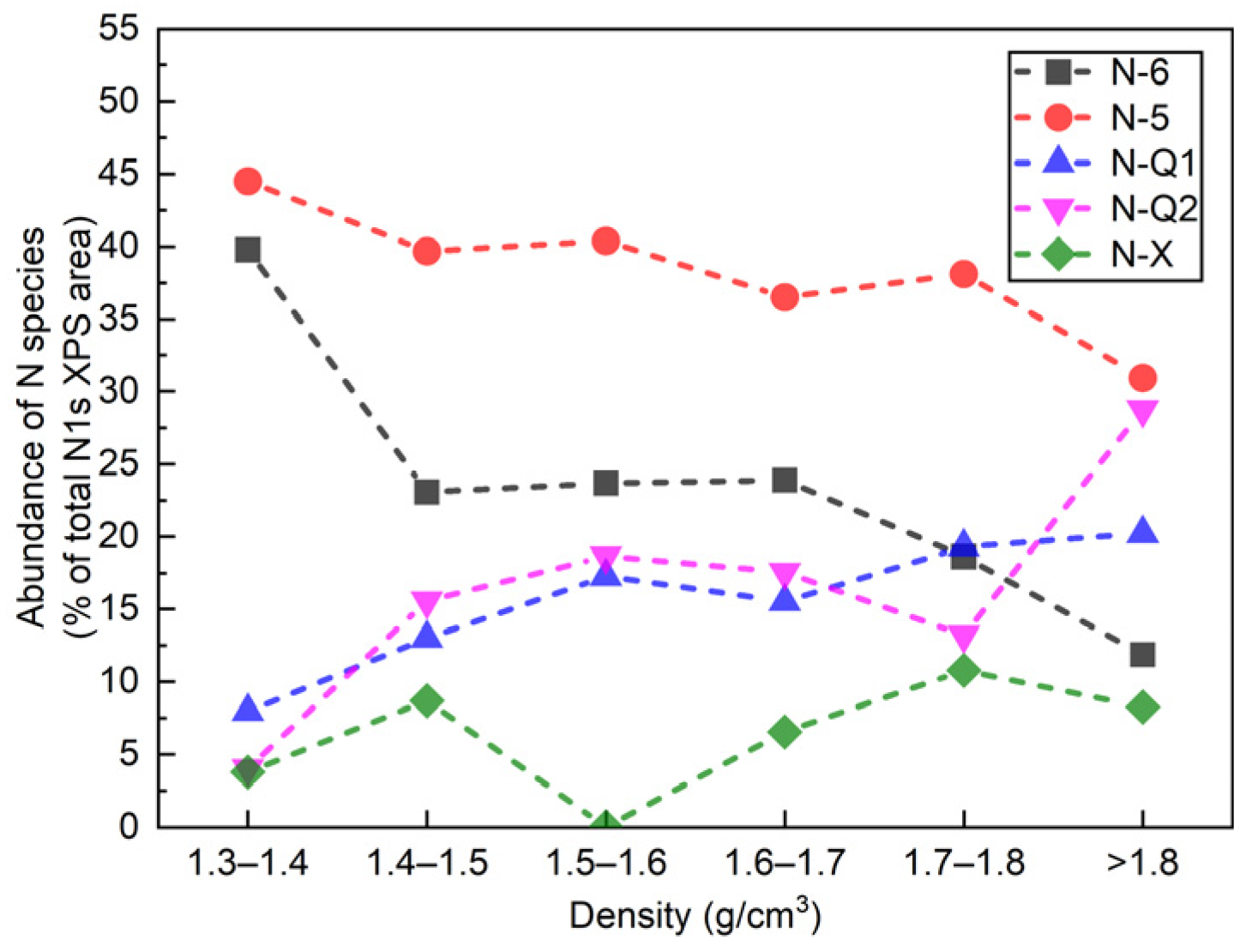
4.3. Nitrogen Occurrence State of DDF Samples
4.3.1. Sub-Peaks N-X: Oxidized Nitrogen
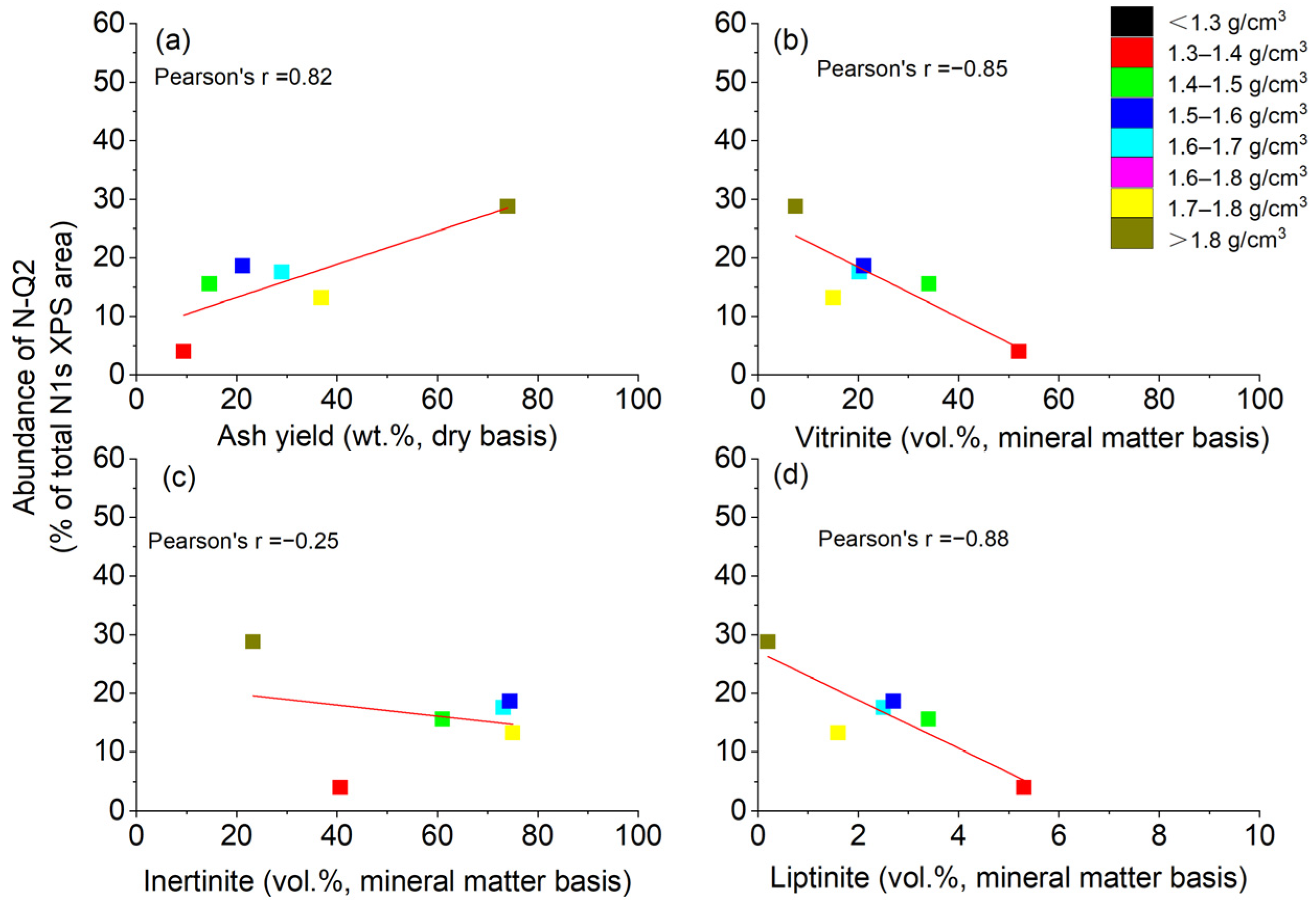
4.3.2. Sub-Peak N-Q2: Contributing to Inorganic Nitrogen or Organic Nitrogen
4.3.3. Sub-Peaks N-6, N-5, and N-Q1: Contributing to the Certain Organic Nitrogen
5. Conclusions
- (1)
- The nitrogen content was about 0.47%–1.85%, and the maximum nitrogen content was positively correlated with the rank of the coal, but the difference was not obvious. The Ndaf% reached a maximum value near 85 wt.% of carbon content.
- (2)
- In the low-rank coal, the nitrogen content was mainly related to vitrinite and inertinite, while in the middle–high-rank coal, the nitrogen content was mainly related to inertinite and minerals. The higher rank coal had higher nitrogen content when the density was >1.6 g/cm3, while it had lower nitrogen content when the density was <1.5 g/cm3.
- (3)
- Pyrrolic (N-5) and pyridinic (N-6) were the main forms of nitrogen in the low-rank coal. The contents of N-6 and N-5 decreased with increases in the coal density, but the contents of quaternary N-Q1 and quaternary N-Q2 increased. In addition, the vitrinite and liptinite may have contained more N-6 and less N-Q1, while the inertinite may have contained less N-6 and more N-Q1. N-Q2 mainly came from fixed ammonia nitrogen in minerals. The nitrogen tended to form a more stable structure in the high-density-level samples.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, B.; Goldblatt, C. The Nitrogen Budget of Earth. Earth Sci. Rev. 2015, 148, 150–173. [Google Scholar] [CrossRef]
- Golubyatnikov, L.L.; Mokhov, I.I.; Eliseev, A.V. Nitrogen Cycle in the Earth Climatic System and Its Modeling. Izv. Atmos. Ocean. Phys. 2013, 49, 229–243. [Google Scholar] [CrossRef]
- Krevelen, D.W. Coal: Typology, Chemistry, Physics, Constitution; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1993; pp. 145–155. [Google Scholar]
- Meyers, R.A. Coal Structure; Academic Press: New York, NY, USA, 1982; pp. 85–90. [Google Scholar]
- Buckley, A.N.; Kelly, M.D.; Nelson, P.F.; Riley, K.W. Inorganic Nitrogen in Australian Semi-Anthracites; Implications for Determining Organic Nitrogen Functionality in Bituminous Coals by X-Ray Photoelectron Spectroscopy. Fuel Process. Technol. 1995, 43, 47–60. [Google Scholar] [CrossRef]
- Valentim, B.; Guedes, A.; Boavida, D. Nitrogen Functionality in “Oil Window” Rank Range Vitrinite Rich Coals and Chars. Org. Geochem. 2011, 42, 502–509. [Google Scholar] [CrossRef]
- Müller, P.J. CN Ratios in Pacific Deep-Sea Sediments: Effect of Inorganic Ammonium and Organic Nitrogen Compounds Sorbed by Clays. Geochim. Cosmochim. Acta 1977, 41, 765–776. [Google Scholar] [CrossRef]
- Wu, D.S.; Zheng, B.S.; Tang, X.Y.; Wang, M.S.; Hu, J.; Li, S.H.; Wang, B.B.; Finkelman, R.B. Contents and Distribution of Nitrogen in Chinese Coals. Earth Environ. 2006, 34, 1–6, (In Chinese with English Abstract). [Google Scholar]
- Chen, Y.F.; Jiang, Y.; Chen, W.M.; Qin, C.X. Study on Distribution of Nitrogen Content in Chinese Coal. Clean Coal Technol. 2008, 14, 71–74, (In Chinese with English Abstract). [Google Scholar]
- Xiao, H.Y.; Liu, C.Q. The Elemental and Isotopic Composition of Sulfur and Nitrogen in Chinese Coals. Org. Geochem. 2011, 42, 84–93. [Google Scholar] [CrossRef]
- Shamooni, A.; Debiagi, P.; Wang, B.; Luu, T.D.; Stein, O.T.; Kronenburg, A.; Bagheri, G.; Stagni, A.; Frassoldati, A.; Faravelli, T.; et al. Carrier-Phase DNS of Detailed NOx Formation in Early-Stage Pulverized Coal Combustion with Fuel-Bound Nitrogen. Fuel 2021, 291, 119998. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhou, Y.G.; Bai, N.M.; Han, J.C. Experimental Investigation of the Characteristics of NOx Emissions with Multiple Deep Air-Staged Combustion of Lean Coal. Fuel 2020, 280, 118416. [Google Scholar] [CrossRef]
- Li, Z.X.; Miao, Z.Q. Effects of Moisture and Its Input Form on Coal Combustion Process and NOx Transformation Characteristics in Lignite Boiler. Fuel 2020, 266, 116970. [Google Scholar] [CrossRef]
- Valentim, B.; Guedes, A.; Rodrigues, S.; Flores, D. Case Study of Igneous Intrusion Effects on Coal Nitrogen Functionalities. Int. J. Coal Geol. 2011, 86, 291–294. [Google Scholar] [CrossRef]
- Valentim, B.; Algarra, M.; Guedes, A.; Ruppert, L.F.; Hower, J.C. Notes on the Origin of Copromacrinite Based on Nitrogen Functionalities and δ13C and δ15N Determined on Samples from the Peach Orchard Coal Bed, Southern Magoffin County, Kentucky. Int. J. Coal Geol. 2016, 160–161, 63–72. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Gorbaty, M.L.; Kwiatek, P.J.; Fletcher, T.H.; Watt, M.; Solum, M.S.; Pugmire, R.J. Nitrogen Transformations in Coal during Pyrolysis. Energy Fuels 1998, 12, 159–173. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A.; Matzner, S.; Boehm, H.-P. The Development of Nitrogen Functionality in Model Chars during Gasification in CO2 and O2. Carbon 1999, 37, 1143–1150. [Google Scholar] [CrossRef]
- Phiri, Z.; Everson, R.C.; Neomagus, H.W.J.P.; Wood, B.J. The Effect of Acid Demineralising Bituminous Coals and De-Ashing the Respective Chars on Nitrogen Functional Forms. J. Anal. Appl. Pyrol. 2017, 125, 127–135. [Google Scholar] [CrossRef]
- Boudou, J.P.; Schimmelmann, A.; Ader, M.; Mastalerz, M.; Sebilo, M.; Gengembre, L. Organic Nitrogen Chemistry during Low-Grade Metamorphism. Geochim. Cosmochim. Acta 2008, 72, 1199–1221. [Google Scholar] [CrossRef]
- Mitra-Kirtley, S.; Mullins, O.C.; Branthaver, J.F.; Cramer, S.P. Nitrogen Chemistry of Kerogens and Bitumens from X-Ray Absorption near-Edge Structure Spectroscopy. Energy Fuels 1993, 7, 1128–1134. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Gorbaty, M.L.; Kwiatek, P.J. Quantification of Nitrogen Forms in Argonne Premium Coals. Energy Fuels 1994, 8, 896–906. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Freund, H.; Gorbaty, M.L.; Kwiatek, P.J. Thermal Chemistry of Nitrogen in Kerogen and Low-Rank Coal. Energy Fuels 1999, 13, 529–538. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Afeworki, M.; Gorbaty, M.L.; Kwiatek, P.J.; Sansone, M.; Walters, C.C.; Cohen, A.D. Thermal Transformations of Nitrogen and Sulfur Forms in Peat Related to Coalification. Energy Fuels 2006, 20, 635–652. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhang, J.; Sheng, C.D.; Chen, J.; Liu, Y.X.; Zhao, L.; Xie, F. X-Ray Photoelectron Spectroscopy (XPS) Investigation of Nitrogen Functionalities during Coal Char Combustion in O2/CO2 and O2/Ar Atmospheres. Energy Fuels 2011, 25, 240–245. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, Q.; Lin, Z.M.; Whiddon, R.; Qiu, K.; Kuang, M.; Cen, K.F. Transformation of Nitrogen and Sulphur Impurities during Hydrothermal Upgrading of Low Quality Coals. Fuel 2016, 164, 254–261. [Google Scholar] [CrossRef]
- Geng, W.H.; Kumabe, Y.; Nakajima, T.; Takanashi, H.; Ohki, A. Analysis of Hydrothermally-Treated and Weathered Coals by X-Ray Photoelectron Spectroscopy (XPS). Fuel 2009, 88, 644–649. [Google Scholar] [CrossRef]
- Kawashima, H.; Koyano, K.; Takanohashi, T. Changes in Nitrogen Functionality Due to Solvent Extraction of Coal during HyperCoal Production. Fuel Process. Technol. 2013, 106, 275–280. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, B.; Tian, C.; Sheng, Q.; Li, Z.; Zhang, N.; Wang, J.; Yang, X. Polyaspartic Acid, an Eco-Friendly Regulator for the Improvement of Coking Coal Flotation: Applications and Mechanism. Int. J. Coal Prep. Util. 2024, 1–20. [Google Scholar] [CrossRef]
- Gui, X.H.; Liu, J.T.; Cao, Y.J.; Miao, Z.Y.; Li, S.L.; Xing, Y.W.; Wang, D.P. Coal Preparation Technology: Status and Development in China. Energy Environ. 2015, 26, 997–1013. [Google Scholar] [CrossRef]
- Li, Z.; Yu, W.; Yang, C.; Zhou, A.N. Actuality and Expectation on Upgrading Utilization of Low-Rank Coal. Min. Process. Equip. 2013, 41, 1–6, (In Chinese with English Abstract). [Google Scholar]
- Chen, Q.; Chen, S. Research Status of Coal Desulfurization by Microbial Pretreatment and Flotation Separation. Clean Coal Technol. 2015, 21, 118–120, (In Chinese with English Abstract). [Google Scholar]
- Song, D.Y.; Zhang, X.K.; Zhang, J.Y.; Zheng, C.G. Migration Characteristics of Hazardous Trace Elements in Coal in the Process of Flotation. J. China Coal Soc. 2010, 35, 1170–1176, (In Chinese with English Abstract). [Google Scholar]
- Khorasanipour, M.; Karimabadi, F.R.; Espahbodi, M.; Ebrahimnejad, M.R. The Effect of Flotation Desulfurization on the Trace Element Geochemistry of Sarcheshmeh Mine Tailings, SE of Iran: Recycling and the Environmental Opportunities. Environ. Earth Sci. 2021, 80, 420. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, K.; Wei, Y. Characteristics of Tectonic Evolution of Carboniferous-Permian Coal Measures in the North-Central Qinshui Basin. Coal Geol. Explor. 2020, 48, 85–91, (In Chinese with English Abstract). [Google Scholar]
- GB/T 478–2008; Method for Float and Sink Analysis of Coal. Standards Press of China: Beijing, China, 2008. (In Chinese)
- ISO 17246: 2010; Coal-Proximate Analysis. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 17247: 2013; Coal Ultimate Analysis. International Organisation for Standardisation: Geneva, Switzerland, 2013.
- Jones, M.C.; Peteet, D.M.; Sambrotto, R. Late-Glacial and Holocene δ15N and δ13C Variation from a Kenai Peninsula, Alaska Peatland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 293, 132–143. [Google Scholar] [CrossRef]
- Broder, T.; Blodau, C.; Biester, H.; Knorr, K.H. Peat Decomposition Records in Three Pristine Ombrotrophic Bogs in Southern Patagonia. Biogeosciences 2012, 9, 1479–1491. [Google Scholar] [CrossRef]
- Andersson, R.A.; Meyers, P.; Hornibrook, E.; Kuhry, P.; Mörth, C.M. Elemental and Isotopic Carbon and Nitrogen Records of Organic Matter Accumulation in a Holocene Permafrost Peat Sequence in the East European Russian Arctic. J. Quat. Sci. 2012, 27, 545–552. [Google Scholar] [CrossRef]
- Boudou, J.P.; Mariotti, A.; Oudin, J.L. Unexpected Enrichment of Nitrogen during the Diagenetic Evolution of Sedimentary Organic Matter. Fuel 1984, 63, 1508–1510. [Google Scholar] [CrossRef]
- Burchill, P.; Welch, L.S. Variation of Nitrogen Content and Functionality with Rank for Some UK Bituminous Coals. Fuel 1989, 68, 100–104. [Google Scholar] [CrossRef]
- Hindmarsh, C.J.; Wang, W.X.; Thomas, K.M.; Crelling, J.C. The Release of Nitrogen during the Combustion of Macerals, Microlithotypes and Their Chars. Fuel 1994, 73, 1229–1234. [Google Scholar] [CrossRef]
- Xie, K.C.; Zhang, Y.F.; Li, C.Z.; Ling, D.Q. Pyrolysis Characteristics of Macerals Separated from a Single Coal and Their Artificial Mixture. Fuel 1991, 70, 474–479. [Google Scholar] [CrossRef]
- Chang, H.Z.; Wang, C.G.; Zeng, F.; Li, J.; Li, W.Y.; Xie, K.C. XPS Comparative Analysis of Coal Macerals with Different Reducibility. J. Fuel Chem. Technol. 2006, 34, 389–394, (In Chinese with English Abstract). [Google Scholar]
- Gong, B.; Buckley, A.N.; Lamb, R.N.; Nelson, P.F. XPS Determination of the Forms of Nitrogen in Coal Pyrolysis Chars. Surf. Interface Anal. 1999, 28, 126–130. [Google Scholar] [CrossRef]
- Zhu, H.W.; Cheng, M.K.; Xu, J.Y.; Chen, S.; Niu, F.; Yu, D.X. Nitrogen Migration and Transformation from Ammonia to Char during Ammonia-Coal/Char Co-Pyrolysis. Int. J. Hydrogen Energy 2024, 49, 137–148. [Google Scholar] [CrossRef]
- Li, Q.G.; An, L.; Wu, P.; Wang, S.; Gu, S.; Yuan, Y.; Fu, Y. The Introduction of Nitrogen from Coal into the Surface Watershed Nitrogen Cycle Due to Coal Mining Activity. Sci. Total Environ. 2023, 900, 165822. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Li, C.Z. Formation of NOx and SOx Precursors during the Pyrolysis of Coal and Biomass. Part I. Effects of Reactor Configuration on the Determined Yields of HCN and NH3 during Pyrolysis. Fuel 2000, 79, 1883–1889. [Google Scholar] [CrossRef]
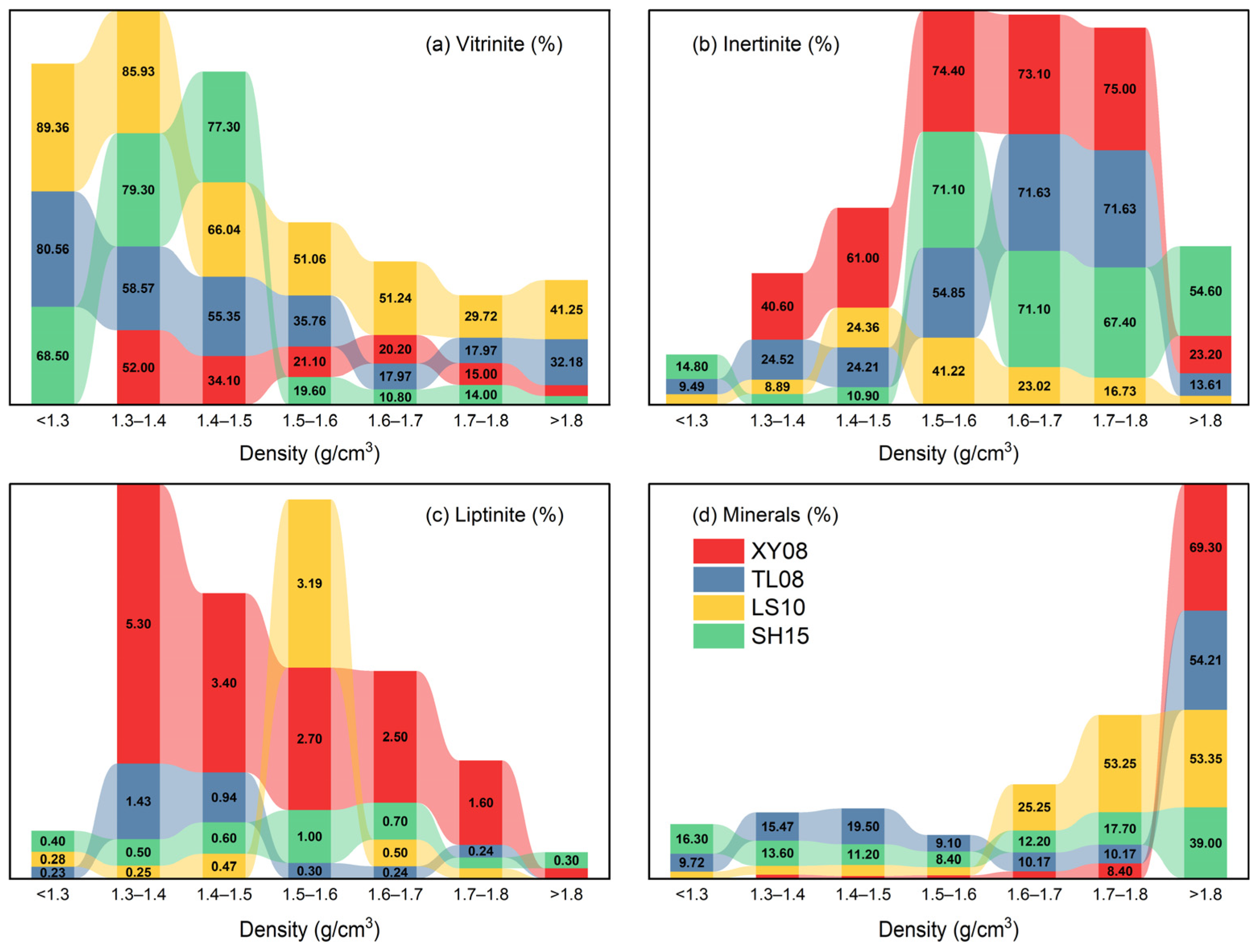


| Sample | Ro,max (%) | Density (g/cm3) | Mineral Matter Basis (vol.%) | Mineral-Free Basis (vol.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Liptinite | Mineral | Vitrinite | Inertinite | Liptinite | ||||
| XY08 | XY081 | 0.82 | 1.3–1.4 | 52.00 | 40.60 | 5.30 | 2.20 | 53.12 | 41.47 | 5.41 |
| XY082 | 1.4–1.5 | 34.10 | 61.00 | 3.40 | 1.50 | 34.62 | 61.93 | 3.45 | ||
| XY083 | 1.5–1.6 | 21.10 | 74.40 | 2.70 | 1.90 | 21.49 | 75.76 | 2.75 | ||
| XY084 | 1.6–1.7 | 20.20 | 73.10 | 2.50 | 4.10 | 21.09 | 76.30 | 2.61 | ||
| XY085 | 1.7–1.8 | 15.00 | 75.00 | 1.60 | 8.40 | 16.38 | 81.88 | 1.75 | ||
| XY086 | >1.8 | 7.50 | 23.20 | 0.20 | 69.30 | 24.27 | 75.08 | 0.65 | ||
| TL08 | TL080 | 1.10 | <1.3 | 80.56 | 9.49 | 0.23 | 9.72 | 89.23 | 10.51 | 0.26 |
| TL081 | 1.3–1.4 | 58.57 | 24.52 | 1.43 | 15.47 | 69.30 | 29.01 | 1.69 | ||
| TL082 | 1.4–1.5 | 55.35 | 24.21 | 0.94 | 19.50 | 68.75 | 30.07 | 1.17 | ||
| TL083 | 1.5–1.6 | 35.76 | 54.85 | 0.30 | 9.10 | 39.33 | 60.33 | 0.33 | ||
| TL0845 | 1.6–1.8 & | 17.97 | 71.63 | 0.24 | 10.17 | 20.00 | 79.73 | 0.26 | ||
| TL086 | >1.8 | 32.18 | 13.61 | 0.00 | 54.21 | 70.28 | 29.72 | 0.00 | ||
| LS10 | LS100 | 1.32 | <1.3 | 89.36 | 6.44 | 0.28 | 3.92 | 93.00 | 6.71 | 0.29 |
| LS101 | 1.3–1.4 | 85.93 | 8.89 | 0.25 | 4.94 | 90.39 | 9.35 | 0.26 | ||
| LS102 | 1.4–1.5 | 66.04 | 24.36 | 0.47 | 6.14 | 72.67 | 26.81 | 0.52 | ||
| LS103 | 1.5–1.6 | 51.06 | 41.22 | 3.19 | 4.53 | 53.48 | 43.18 | 3.34 | ||
| LS104 | 1.6–1.7 | 51.24 | 23.02 | 0.50 | 25.25 | 68.54 | 30.79 | 0.66 | ||
| LS105 | 1.7–1.8 | 29.72 | 16.73 | 0.20 | 53.25 | 63.71 | 35.87 | 0.42 | ||
| LS106 | >1.8 | 41.25 | 5.50 | 0.00 | 53.35 | 88.24 | 11.76 | 0.00 | ||
| SH15 | SH150 | 1.77 | <1.3 | 68.50 | 14.80 | 0.40 | 16.30 | 81.84 | 17.68 | 0.48 |
| SH151 | 1.3–1.4 | 79.30 | 6.60 | 0.50 | 13.60 | 91.78 | 7.64 | 0.58 | ||
| SH152 | 1.4–1.5 | 77.30 | 10.90 | 0.60 | 11.20 | 87.05 | 12.27 | 0.68 | ||
| SH153 | 1.5–1.6 | 19.60 | 71.10 | 1.00 | 8.40 | 21.37 | 77.54 | 1.09 | ||
| SH154 | 1.6–1.7 | 10.80 | 71.10 | 0.70 | 12.20 | 13.08 | 86.08 | 0.85 | ||
| SH155 | 1.7–1.8 | 14.00 | 67.40 | 0.20 | 17.70 | 17.16 | 82.60 | 0.25 | ||
| SH156 | >1.8 | 6.20 | 54.60 | 0.30 | 39.00 | 10.15 | 89.36 | 0.49 | ||
| Sample | Density (g/cm3) | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Vdaf | FCd | Ad | Cdaf | Hdaf | N*daf | Odaf | St,daf | ||
| XY081 | 1.3–1.4 | 1.32 | 36.05 | 57.97 | 9.36 | 81.10 | 5.16 | 1.35 | 11.07 | 1.32 |
| XY082 | 1.4–1.5 | 1.59 | 32.46 | 57.53 | 14.53 | 81.17 | 4.67 | 1.31 | 11.91 | 0.92 |
| XY083 | 1.5–1.6 | 1.54 | 30.28 | 55.00 | 21.12 | 81.35 | 4.44 | 1.27 | 12.21 | 0.75 |
| XY084 | 1.6–1.7 | 1.71 | 30.47 | 49.38 | 28.98 | 79.10 | 4.62 | 1.30 | 14.25 | 0.75 |
| XY085 | 1.7–1.8 | 1.43 | 33.40 | 42.08 | 36.82 | 76.02 | 4.81 | 1.25 | 16.95 | 0.97 |
| XY086 | >1.8 | 0.52 | 16.09 | 9.92 | 73.98 | 33.86 | 7.11 | 0.85 | 35.17 | 23.02 |
| TL080 | <1.3 | 0.43 | 25.90 | 72.94 | 1.57 | 88.07 | 4.88 | 1.47 | 3.63 | 1.96 |
| TL081 | 1.3–1.4 | 0.55 | 21.85 | 74.50 | 4.68 | 88.76 | 4.6 | 1.40 | 3.69 | 1.56 |
| TL082 | 1.4–1.5 | 0.49 | 20.39 | 68.75 | 13.65 | 88.73 | 4.42 | 1.27 | 4.26 | 1.33 |
| TL083 | 1.5–1.6 | 0.53 | 19.36 | 61.72 | 23.46 | 87.54 | 4.29 | 1.32 | 5.37 | 1.50 |
| TL0845 | 1.6–1.8 | 0.63 | 18.70 | 54.18 | 33.36 | 86.03 | 4.05 | 1.37 | 6.90 | 1.67 |
| TL086 | >1.8 | 0.54 | 22.51 | 51.57 | 33.45 | 26.66 | 2.42 | 0.47 | 54.79 | 15.69 |
| LS100 | <1.3 | 0.60 | 18.08 | 80.09 | 2.23 | 88.45 | 4.49 | 1.22 | 2.75 | 3.10 |
| LS101 | 1.3–1.4 | 0.48 | 17.06 | 78.15 | 5.78 | 89.23 | 4.46 | 1.24 | 1.89 | 3.18 |
| LS102 | 1.4–1.5 | 0.60 | 16.86 | 71.36 | 14.18 | 89.86 | 4.47 | 1.34 | 1.04 | 3.30 |
| LS103 | 1.5–1.6 | 0.64 | 18.26 | 62.89 | 23.06 | 87.87 | 4.24 | 1.36 | 2.09 | 4.43 |
| LS104 | 1.6–1.7 | 0.59 | 21.46 | 54.36 | 30.78 | 85.48 | 4.10 | 1.49 | 4.67 | 4.28 |
| LS105 | 1.7–1.8 | 0.54 | 22.56 | 48.96 | 36.77 | 84.41 | 4.03 | 1.23 | 1.61 | 8.71 |
| LS106 | >1.8 | 0.40 | 15.56 | 30.36 | 54.08 | 62.87 | 3.92 | 1.09 | 0.17 | 31.97 |
| SH150 | <1.3 | 1.29 | 6.59 | 86.10 | 7.82 | 92.75 | 3.04 | 1.18 | 2.60 | 0.41 |
| SH151 | 1.3–1.4 | 1.22 | 5.90 | 87.85 | 6.64 | 93.52 | 3.12 | 1.17 | 1.83 | 0.36 |
| SH152 | 1.4–1.5 | 1.37 | 6.14 | 85.91 | 8.48 | 92.65 | 2.97 | 1.22 | 2.81 | 0.36 |
| SH153 | 1.5–1.6 | 1.25 | 7.97 | 76.39 | 16.99 | 91.89 | 3.18 | 1.35 | 3.19 | 0.36 |
| SH154 | 1.6–1.7 | 0.85 | 10.81 | 64.56 | 27.61 | 90.08 | 3.67 | 1.64 | 4.23 | 0.36 |
| SH155 | 1.7–1.8 | 0.97 | 17.69 | 52.85 | 35.79 | 86.22 | 3.40 | 1.64 | 8.41 | 0.34 |
| SH156 | >1.8 | 3.21 | 40.16 | 28.10 | 53.04 | 73.64 | 3.19 | 1.85 | 21.06 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhang, Q.; Zhao, F.; Liu, X.; Zhang, S. The Occurrence and Distribution of Nitrogen in Coal of Different Ranks and Densities. Minerals 2024, 14, 549. https://doi.org/10.3390/min14060549
Liu D, Zhang Q, Zhao F, Liu X, Zhang S. The Occurrence and Distribution of Nitrogen in Coal of Different Ranks and Densities. Minerals. 2024; 14(6):549. https://doi.org/10.3390/min14060549
Chicago/Turabian StyleLiu, Dongna, Qi Zhang, Fenghua Zhao, Xile Liu, and Shangqing Zhang. 2024. "The Occurrence and Distribution of Nitrogen in Coal of Different Ranks and Densities" Minerals 14, no. 6: 549. https://doi.org/10.3390/min14060549
APA StyleLiu, D., Zhang, Q., Zhao, F., Liu, X., & Zhang, S. (2024). The Occurrence and Distribution of Nitrogen in Coal of Different Ranks and Densities. Minerals, 14(6), 549. https://doi.org/10.3390/min14060549







