Influence of Mineralogy and Mineralogy Approach to Optimize Processing: A Case Study of Tin–Copper Polymetallic Ore
Abstract
:1. Introduction
2. Samples and Methods
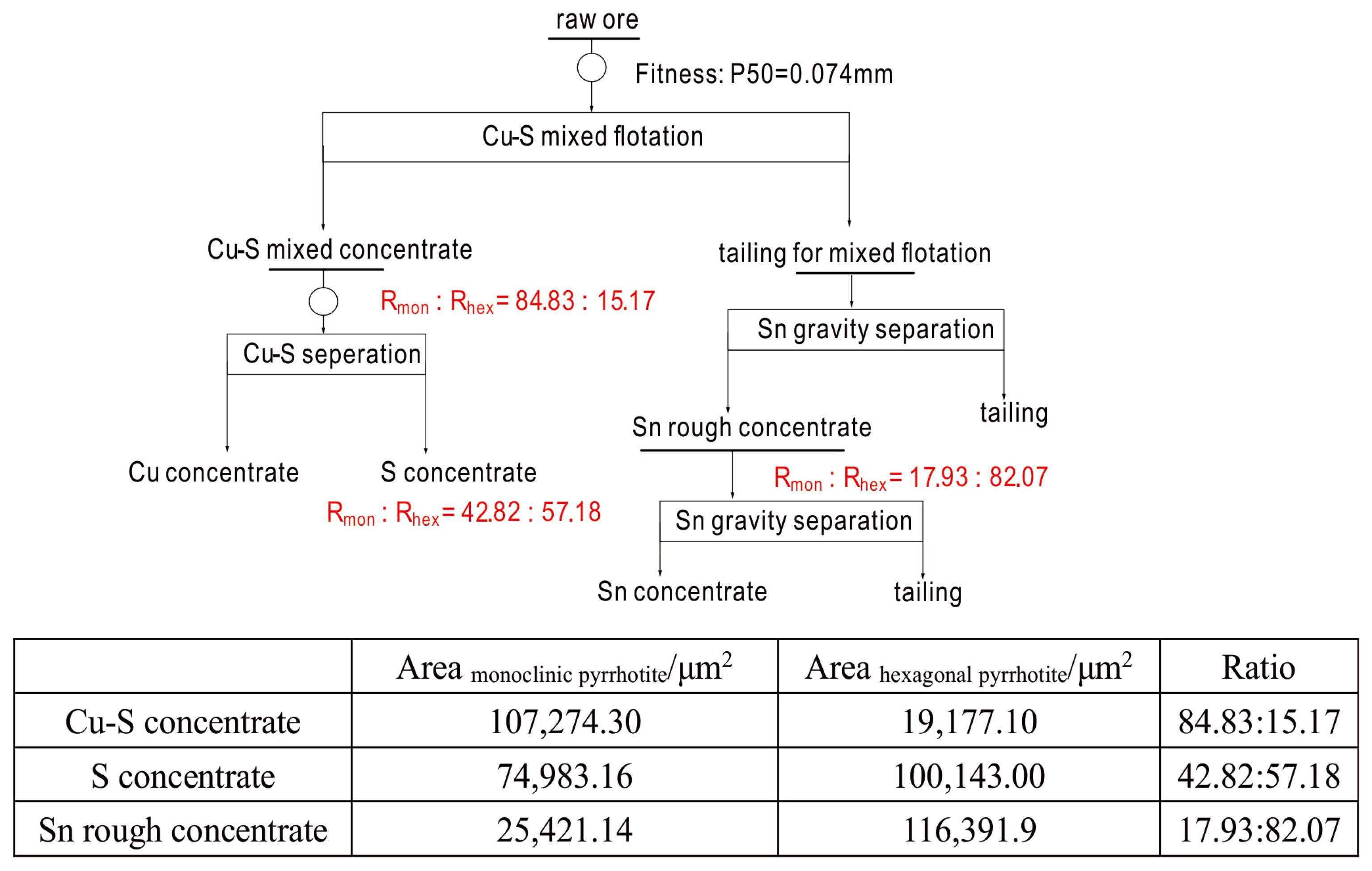
2.1. Process Flowsheet, Problems, and Sample Selection
- Cu-S mixed concentrate was selected to understand the problem of an insufficient copper concentrate grade. During the copper–sulfur separation process, a process mineralogy study based on Cu-S mixed concentrate can provide mineralogical data, like the Cu mineral size and liberation degree, to help determine the reasons for a low Cu grade and a method to change it.
- S concentrate was selected to solve the problem of As in S concentrate. Through process mineralogical research of S concentrate, types of As minerals and the relationship between sulfur minerals and arsenic minerals in products can be clarified. These data can answer whether there is a possibility of reducing arsenic content in sulfur concentrate.
- Sn rough concentrate processed after the first gravity separation process was selected to understand why a large quantity of pyrrhotite and pyrite enters gravity separation, resulting in a high S grade in Sn concentrate. Through mineralogy research, the size distribution of pyrrhotite and pyrite and their relationship with cassiterite can be obtained.
2.2. Method
2.2.1. Chemical Analysis
2.2.2. SEM-Based Automated Mineralogy and SEM Analysis
2.2.3. EMPA Analysis of Mineral
2.2.4. XRD
2.2.5. Microscope Analysis
3. Results
3.1. Cu-S Mixed Concentrate
3.1.1. Chemical Composition
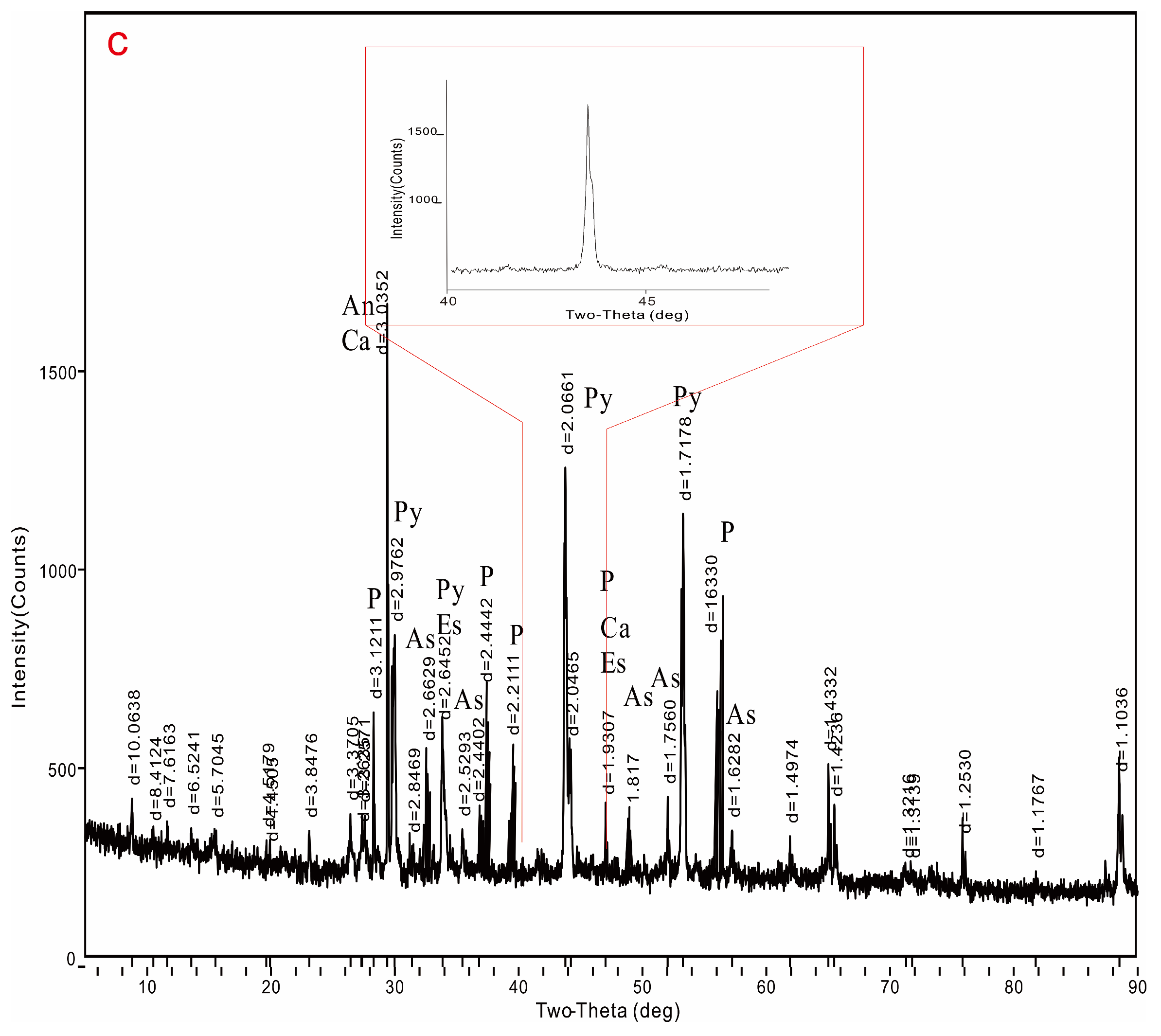
3.1.2. XRD Result
3.1.3. Mineral Composition
3.1.4. Mineral Size Distribution of Main Sulfide Minerals
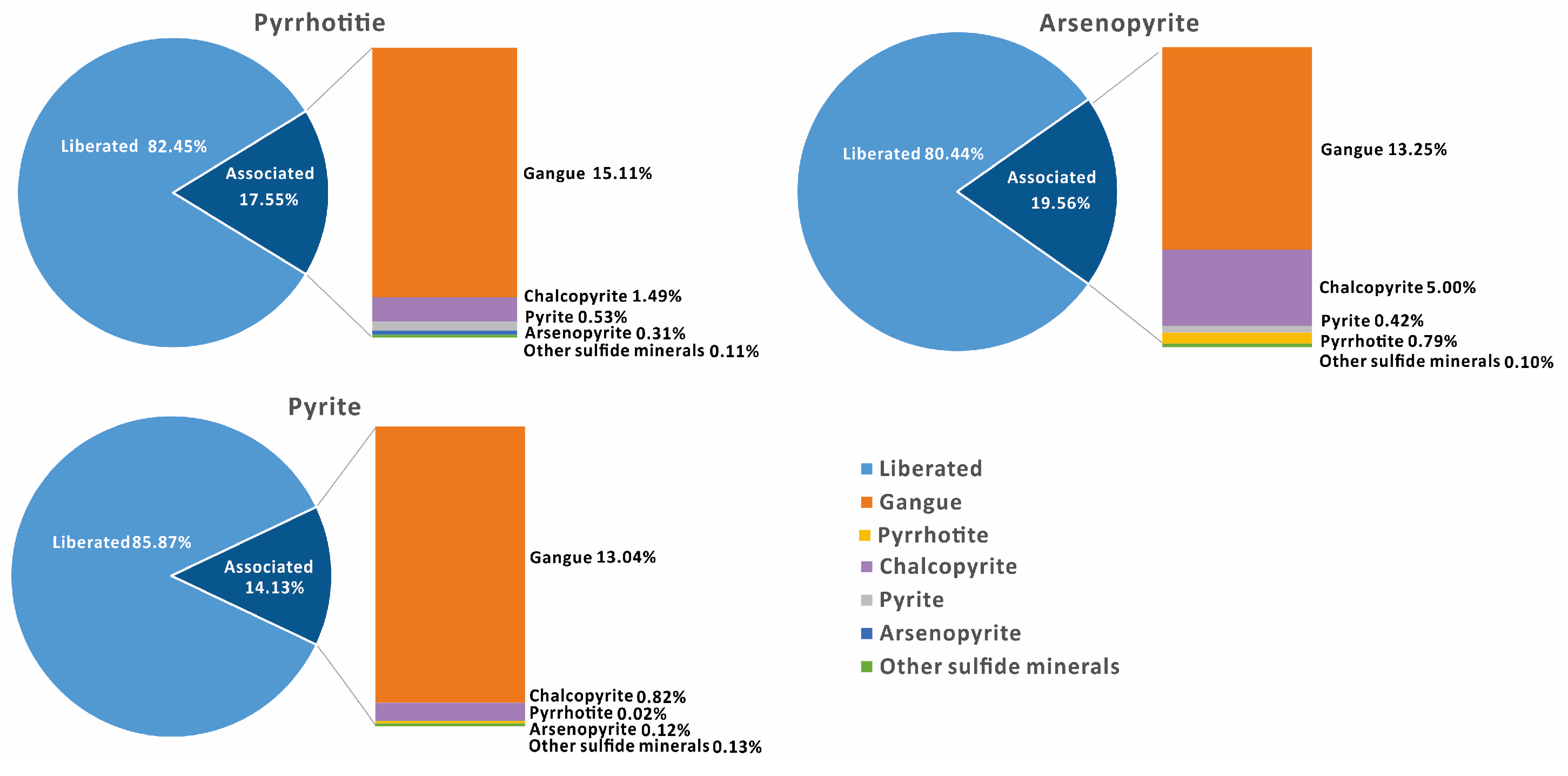
3.1.5. Liberation of Main Sulfide Minerals
3.1.6. In Situ Chemical Composition of Pyrrhotite
3.2. S concentrate
3.2.1. Chemical Composition
3.2.2. XRD Result
3.2.3. Mineral Composition
3.2.4. Mineral Size Distribution of Main Sulfide Minerals
3.2.5. Liberation of Main Sulfide Minerals
3.2.6. In Situ Chemical Composition of Pyrrhotite
3.3. Sn Rough Concentrate
3.3.1. Chemical Composition
3.3.2. XRD Results
3.3.3. Mineral Composition
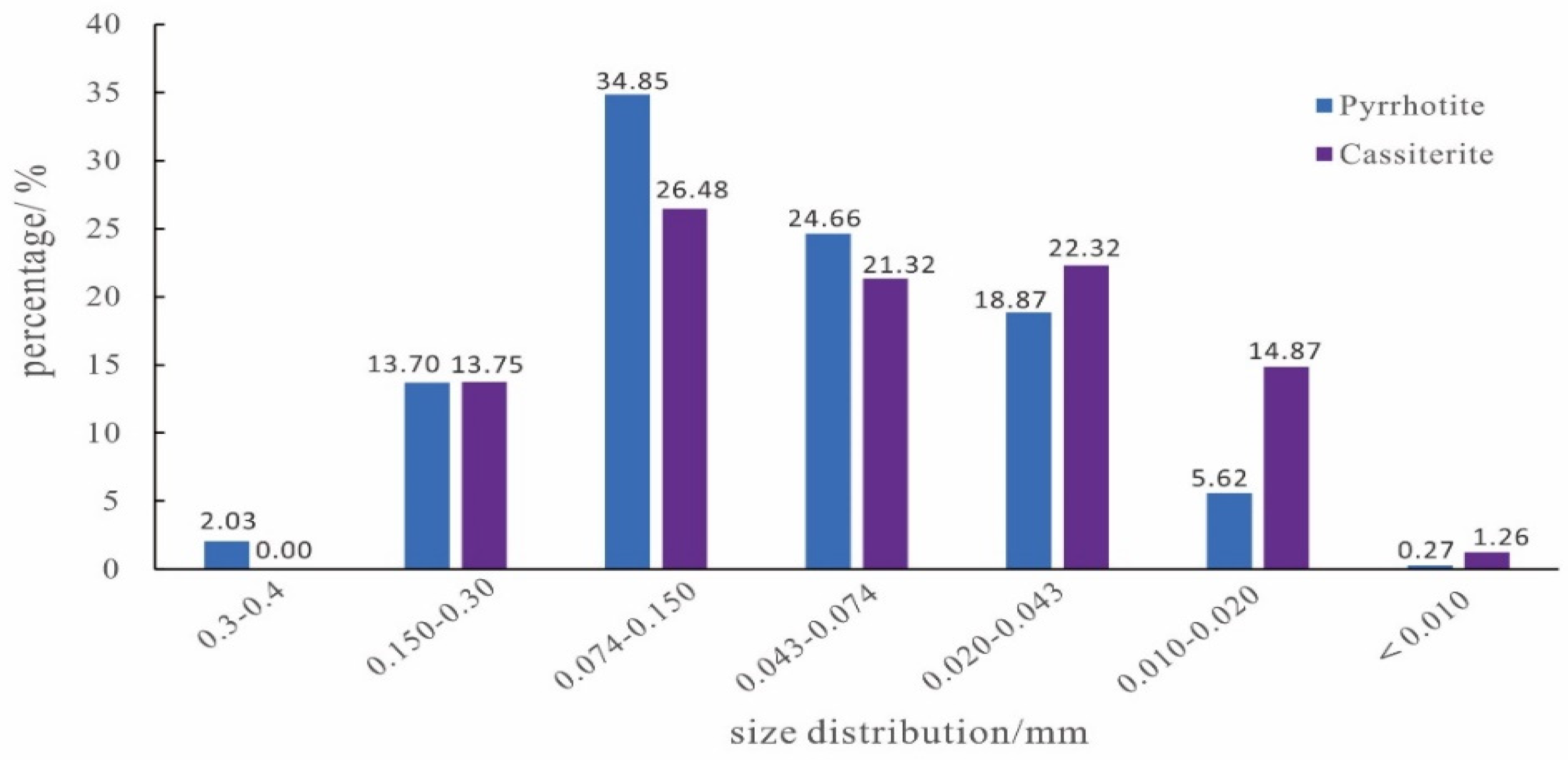
3.3.4. Mineral Size Distribution of Pyrrhotite and Cassiterite
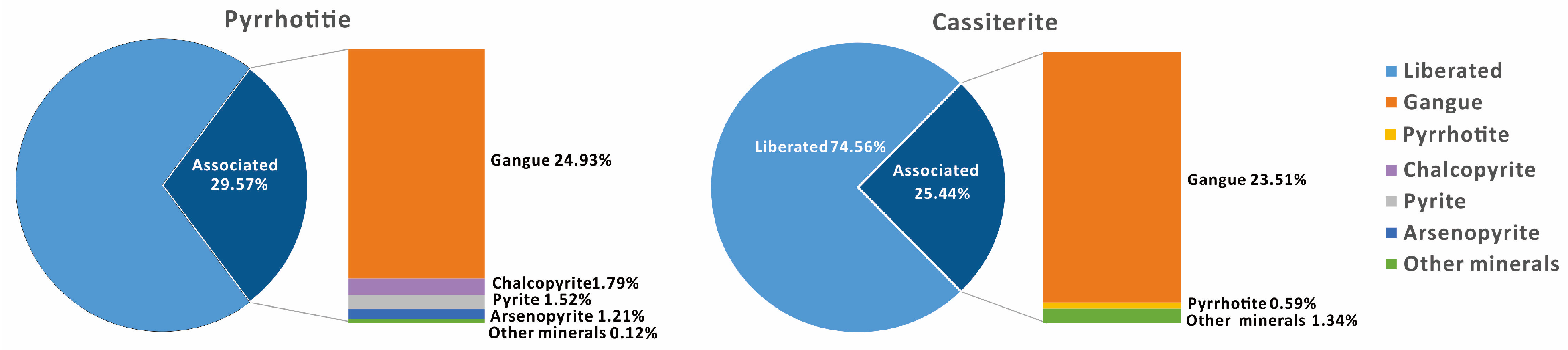
3.3.5. Liberation of Pyrrhotite and Cassiterite
3.3.6. In Situ Chemical Composition of Pyrrhotite
4. Discussion
4.1. Process Mineralogy Factors Affecting the Process Indicators
4.1.1. Liberation Degree and Size Distribution of Target Minerals
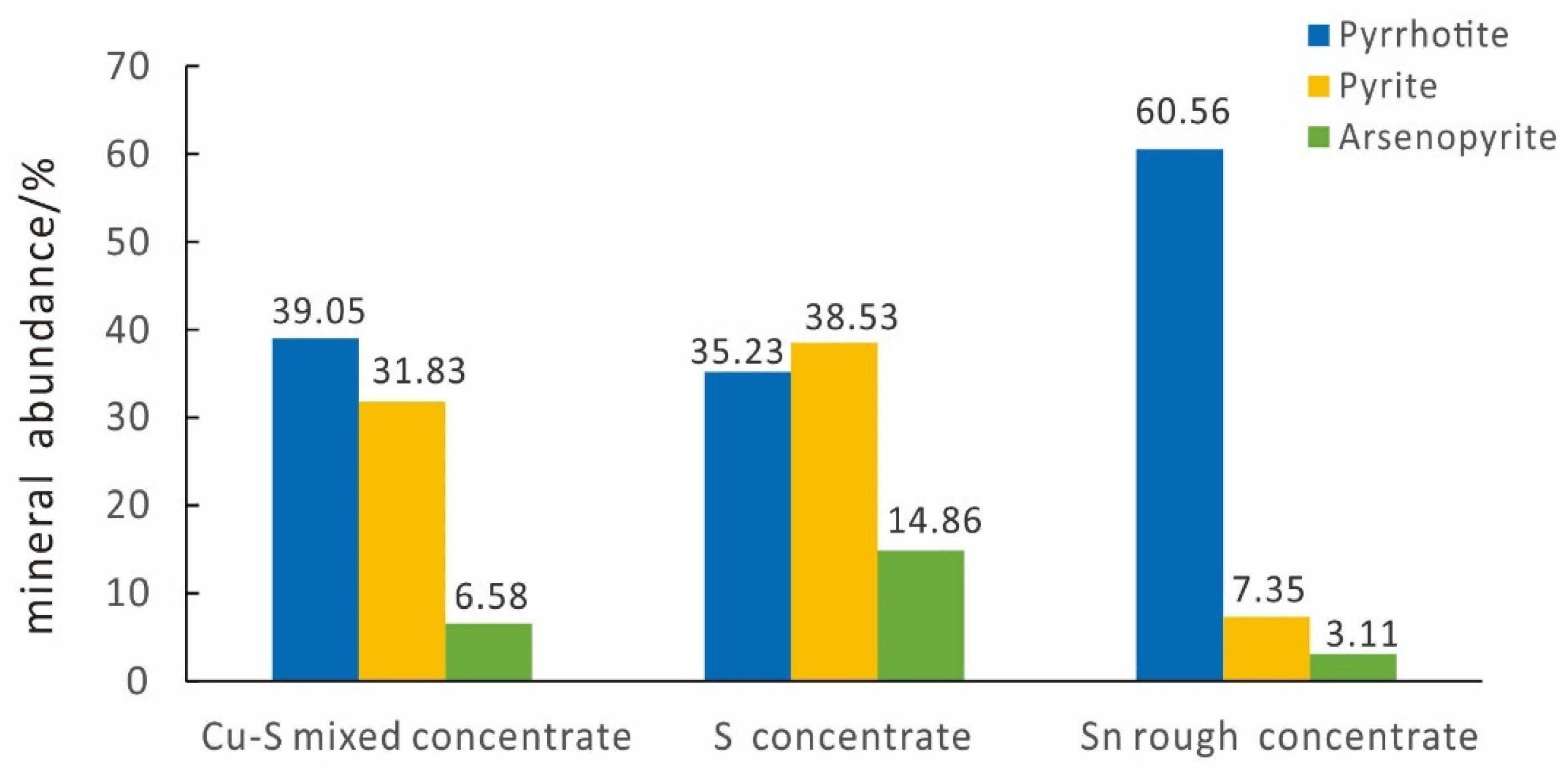
4.1.2. Main Minerals and Their Abundance
4.2. Classification of Pyrrhotite
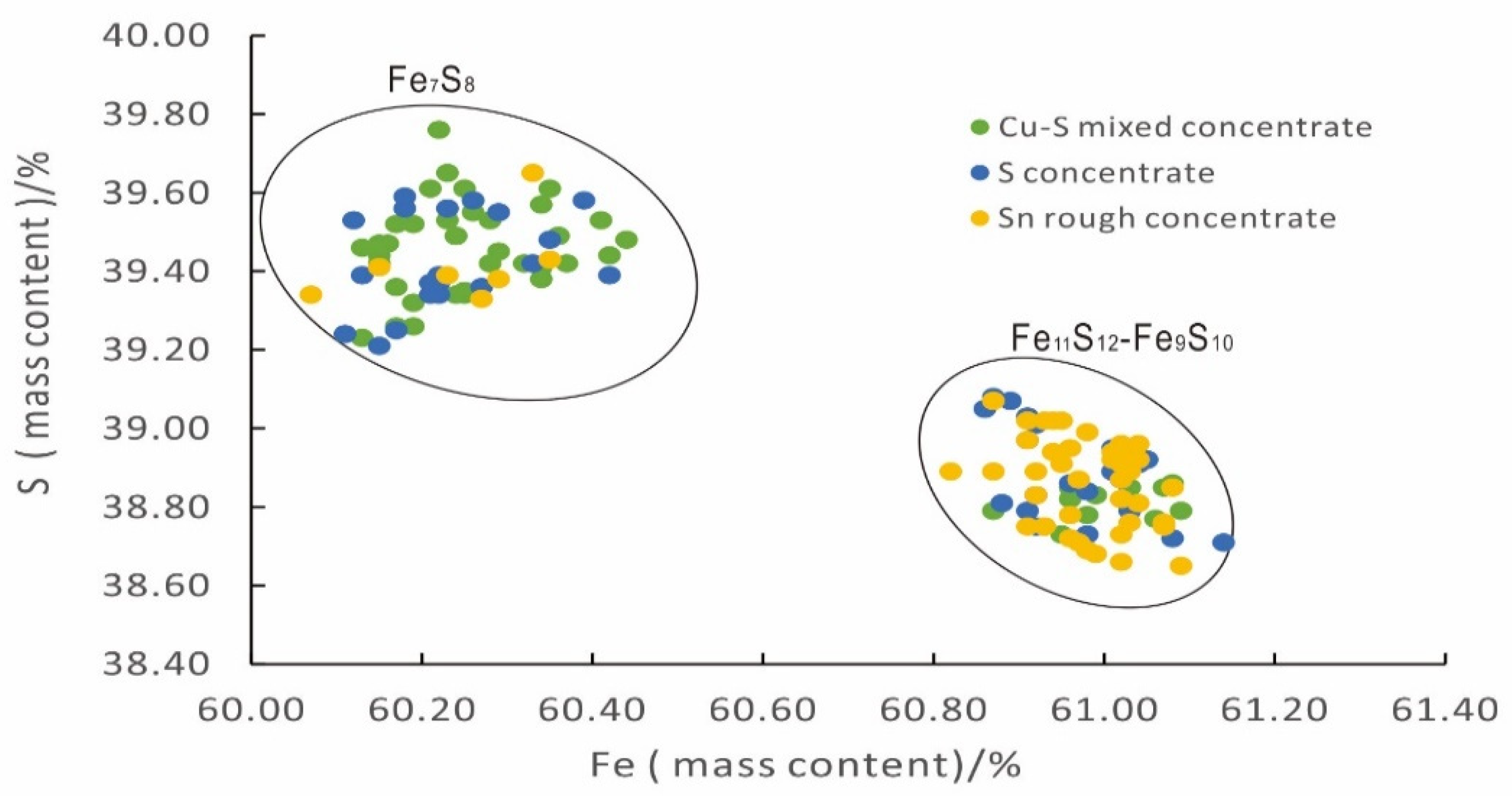
4.2.1. Fe and S Content of Pyrrhotite in Different Samples
4.2.2. XRD Peak Ratios of Pyrrhotite in Different Samples
4.3. Ratio Calculation for Different Superstructures of Pyrrhotite in Different Samples
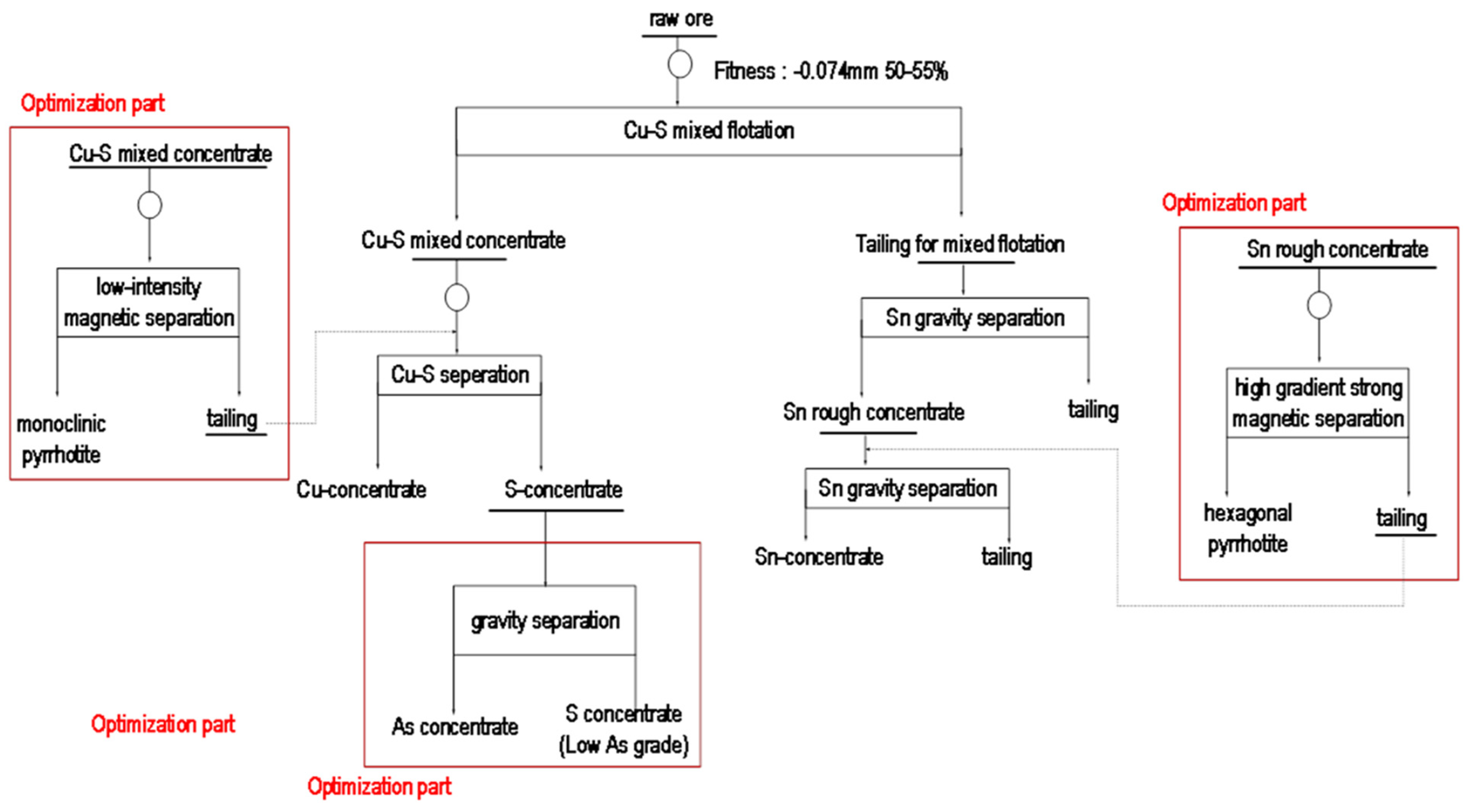
4.4. Optimization Test Results for Mineralogical Evidence Suggestion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdollahia, M.; Bahramib, A.; Mirmohammadic, S.M.; Kazemid, F.; Daneshe, A.; Ghorbanif, Y. A process mineralogy approach to optimize molybdenite flotation in copper-molybdenum processing plants. Miner. Eng. 2020, 157, 106557. [Google Scholar] [CrossRef]
- Wightman, E.; Evans, C. Representing and interpreting the liberation spectrum in a processing context. Miner. Eng. 2014, 61, 121–125. [Google Scholar] [CrossRef]
- Can, N.M.; Celik, I.B.; Bicak, O.; Altun, O. Mass balance and quantitative mineralogy studies for circuit modification. Miner. Process. Extr. Metall. Rev. 2013, 34, 348–365. [Google Scholar] [CrossRef]
- Ghorbani, Y.; Fitzpatrick, R.; Kinchington, M.; Rollinson, G.; Hegarty, P. A process mineralogy approach to gravity concentration of Tantalum bearing minerals. Minerals 2017, 7, 194. [Google Scholar] [CrossRef]
- Rincon, J.; Gaydardzhiev, S.; Stamenov, L. Investigation on the flotation recovery of copper sulfosalts through an integrated mineralogical approach. Miner. Eng. 2019, 130, 36–47. [Google Scholar] [CrossRef]
- Tungpalan, K.; Wightman, E.; Manlapig, E. Relating mineralogical and textural characteristics to flotation behaviour. Miner. Eng. 2015, 82, 136–140. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Tian, Z.; Wu, C.; Qin, W. Cassiterite beneficiation in China: A mini-review. J. Cent. South Univ. 2023, 30, 1–19. [Google Scholar] [CrossRef]
- Bulatovic, S.; De Silvio, E. Process development for impurity removal from a tin gravity concentrate. Miner. Eng. 2000, 13, 871–879. [Google Scholar] [CrossRef]
- Angadi, S.I.; Sreenivas, T.; Jeon, H.S.; Baek, S.H.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Miner. Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
- Tomance, R.; Blagojev, M. Quantifying mineral liberation—A conventional and new automatic sophisticated techniques approach. J. Pol. Miner. Eng. Soc. 2020, 2, 211–216. [Google Scholar] [CrossRef]
- Kelebek, S.; Tukel, C.J. Separation of nickeliferous hexagonal pyrrhotite from pentlandite in Ni-Cu sulphide ores: Recovery by size performance. Miner. Eng. 2018, 125, 223–230. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.W.; Cai, M.F.; Li, Y. A review on pyrrhotite oxidation. J. Geochem. Explor. 2004, 84, 65–76. [Google Scholar] [CrossRef]
- Gordon, S.C.; McDonald, A.M. A study of the composition, distribution, and genesis of pyrrhotite in the copper cliff offset, sudbury, Ontario, Canada. Can. Mineral. 2015, 53, 859–878. [Google Scholar] [CrossRef]
- Becker, M.; De Villiers, J.; Bradshaw, D. The mineralogy and crystallography of pyrrhotite from selected nickel and PGE ore deposits. Econ. Geol. 2010, 105, 1025–1037. [Google Scholar] [CrossRef]
- Becker, M.; Bradshaw, D.; De Villiers, J. The mineralogy of pyrrhotite from Sudbury CCN and Phoenix nickel ores and its effect on flotation performance. Can. Metall. Q. 2011, 50, 10–19. [Google Scholar] [CrossRef]
- Becker, M.; De Villiers, J.; Bradshaw, D.J.M.E. The flotation of magnetic and non-magnetic pyrrhotite from selected nickel ore deposits. Miner. Eng. 2010, 23, 1045–1052. [Google Scholar] [CrossRef]
- Schrader, D.L.; Davidson, J.; McCoy, T.J.; Zega, T.J.; Russell, S.S.; Domanik, K.J.; King, A.J. The Fe/S ratio of pyrrhotite group sulfides in chondrites: An indicator of oxidation and implications for return samples from asteroids Ryugu and Bennu. Geochim. Cosmochim. Acta. 2021, 303, 66–91. [Google Scholar] [CrossRef]
- Haines, G.L.; Carpenter, M. Magnetoelastic coupling associated with vacancy ordering and ferrimagnetism in natural pyrrhotite, Fe7S8. J. Phys. Condens. Matter. 2020, 32, 385401. [Google Scholar] [CrossRef]
- de Villiers, J.P.; Liles, D.C. The crystal-structure and vacancy distribution in 6 C pyrrhotite. Am. Mineral. 2010, 95, 148–152. [Google Scholar] [CrossRef]
- Multani, R.S.; Waters, K.E. A review of the physicochemical properties and flotation of pyrrhotite superstructures (4C–Fe7S8/5C–Fe9S10) in Ni-Cu sulphide mineral processing. Can. J. Che. Eng. 2018, 96, 1185–1206. [Google Scholar] [CrossRef]
- Wang, H.; Salveson, I. A review on the mineral chemistry of the non-stoichiometric iron sulphide, Fe1-x S (0 ≤ x ≤ 0.125): Polymorphs, phase relations and transitions, electronic and magnetic structures. Phase Transit. 2005, 78, 547–567. [Google Scholar] [CrossRef]
- Janzen, M.P.; Nicholson, R.V.; Scharer, J.M. Pyrrhotite reaction kinetics: Reaction rates for oxidation by oxygen, ferric iron, and for nonoxidative dissolution. Geochim. Cosmochim. Acta. 2000, 64, 1511–1522. [Google Scholar] [CrossRef]
- Multani, R.S.; Waters, K.E. Flotation recovery–by–size comparison of pyrrhotite superstructures with and without depressants. Miner. Eng. 2019, 130, 92–100. [Google Scholar] [CrossRef]

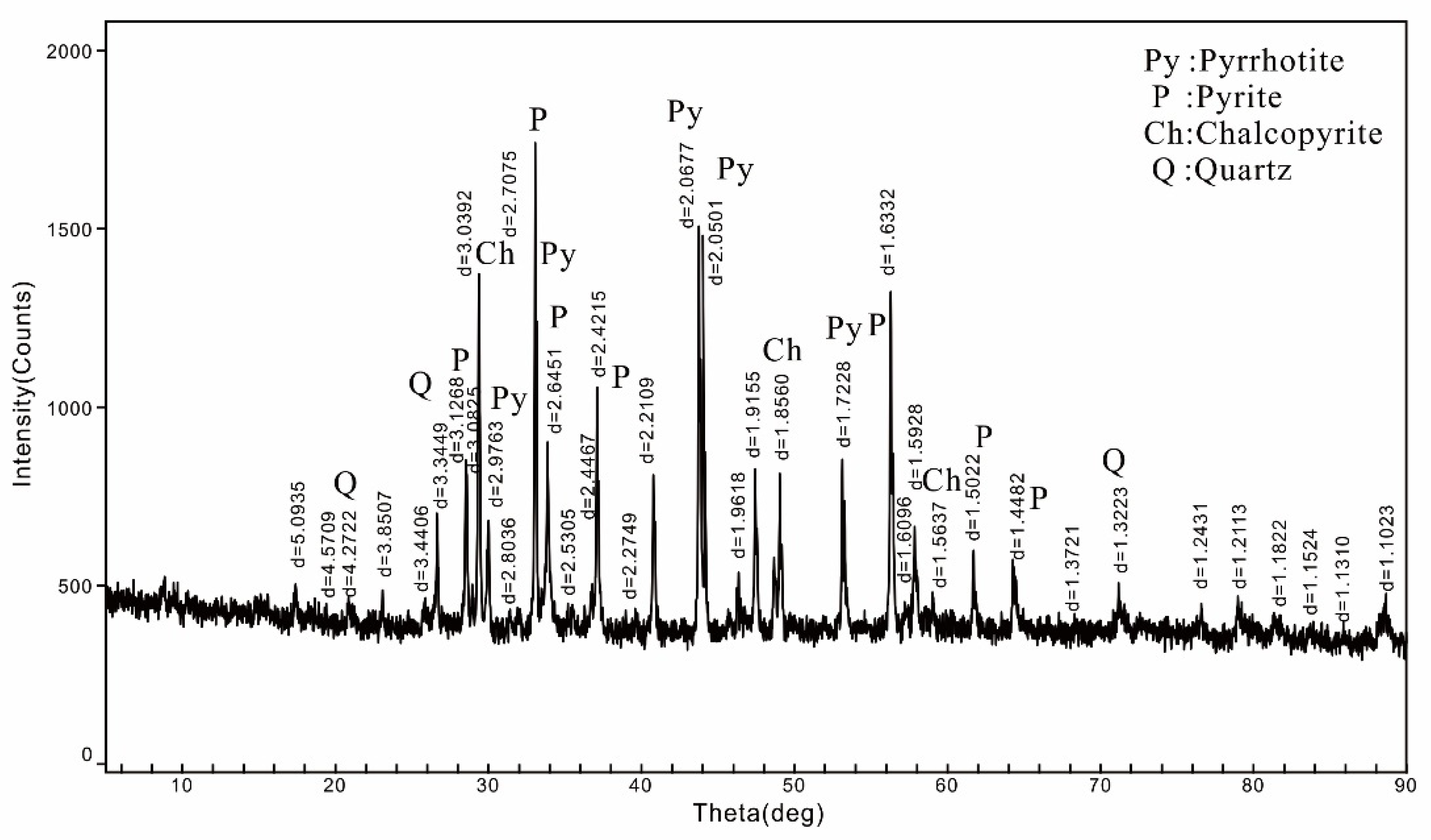
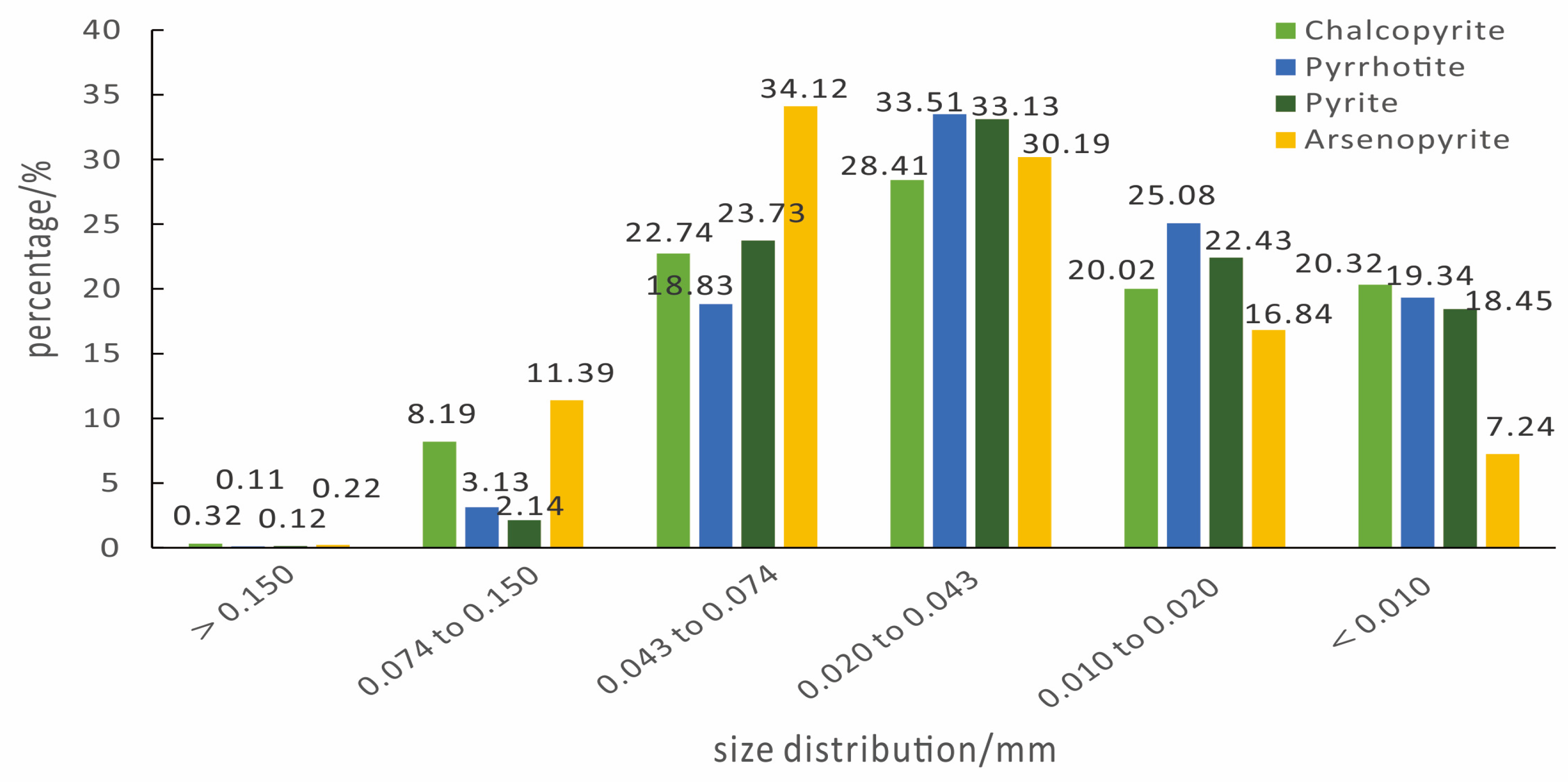

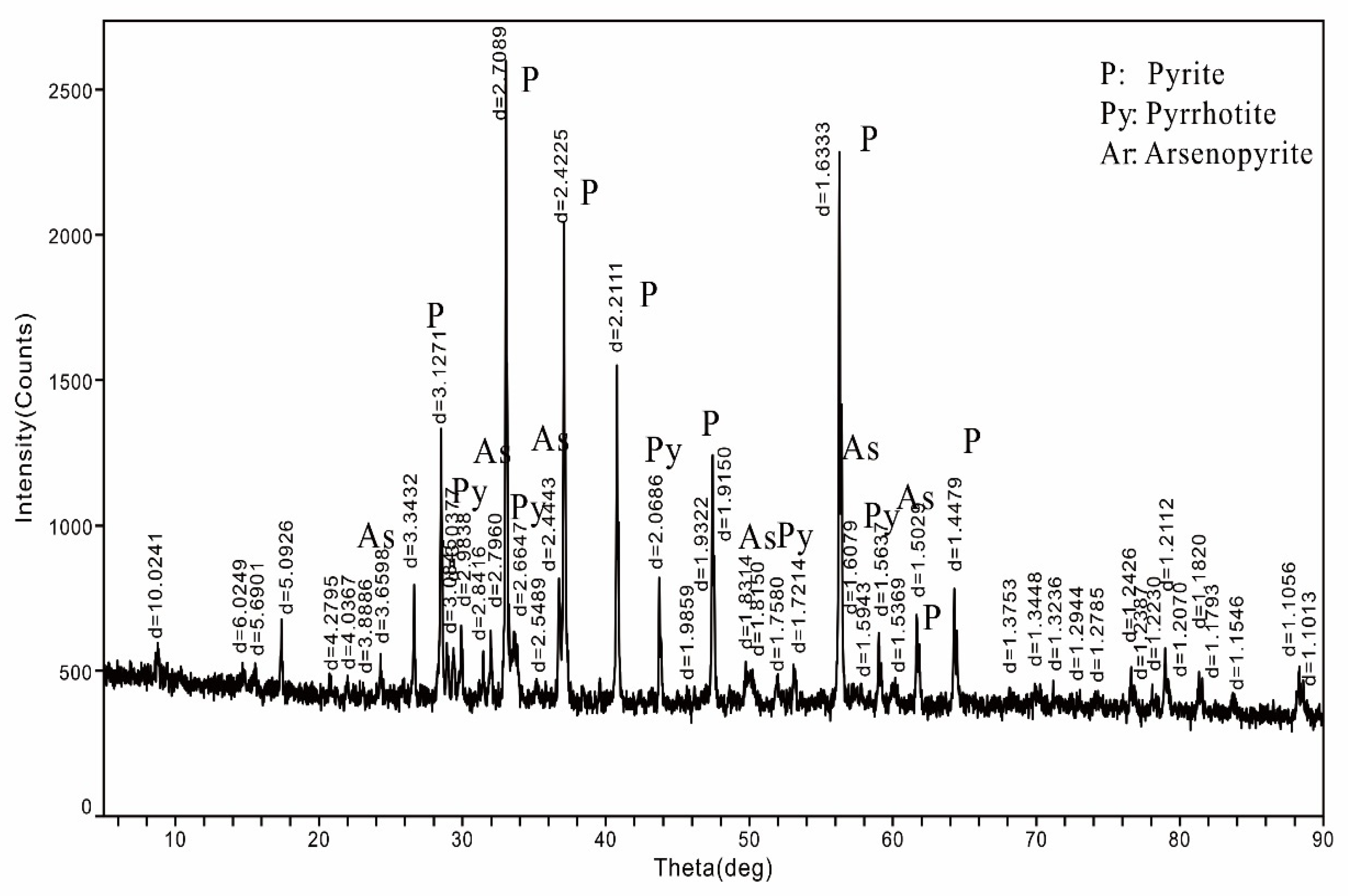
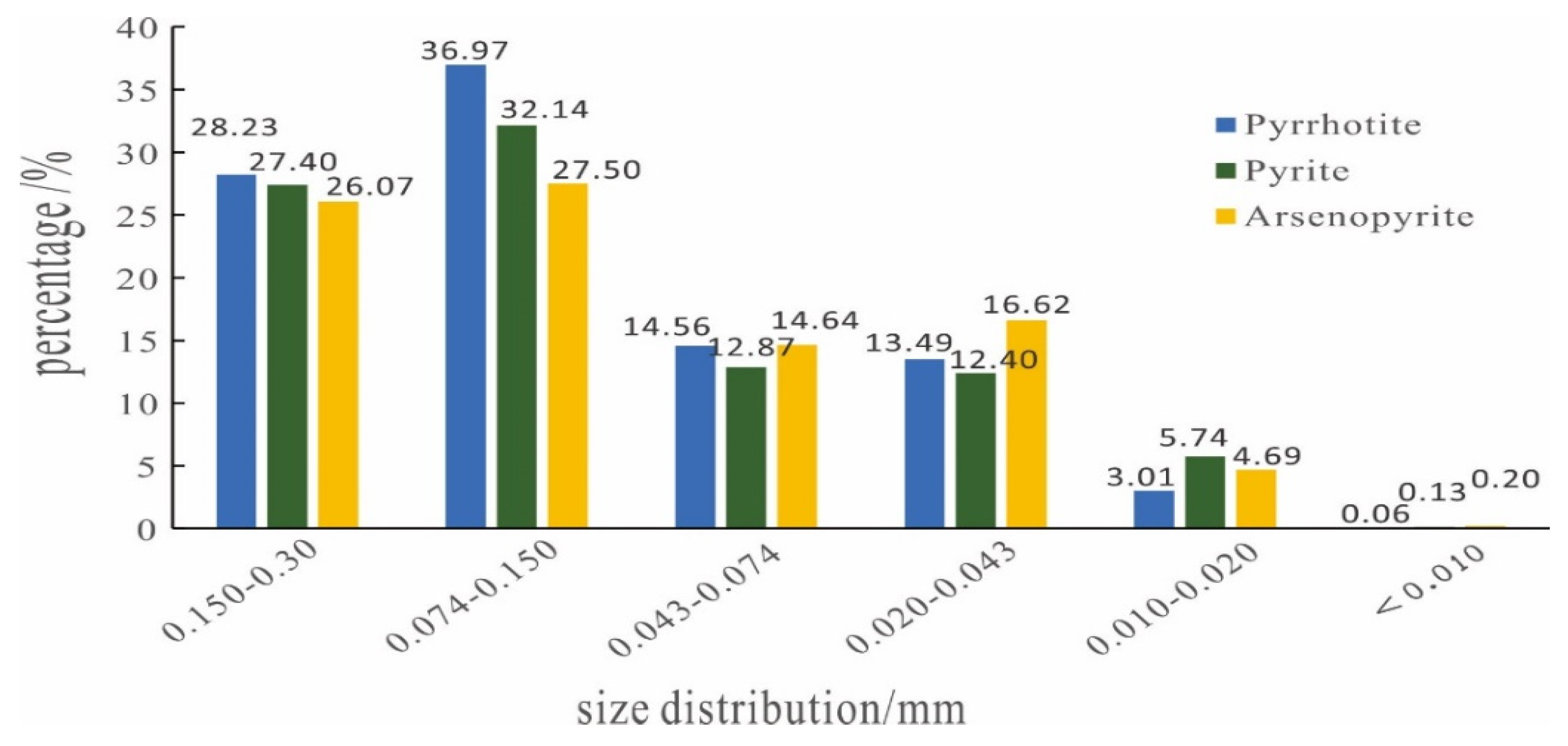



| Element | Cu | Sn | S | Fe | Bi | As |
|---|---|---|---|---|---|---|
| Content, % | 3.62 | 0.13 | 37.16 | 47.07 | 0.21 | 2.97 |
| Element | SiO2 | CaO | MgO | Al2O3 | K2O | Na2O |
| Content, % | 3.72 | 1.07 | 0.67 | 0.65 | 0.23 | 0.56 |
| Mineral | Content (%) | Mineral | Content (%) |
|---|---|---|---|
| Pyrrhotite | 39.05 | Dolomite | 0.88 |
| Pyrite | 31.83 | Hornblende | 0.72 |
| Arsenopyrite | 6.58 | Calcite | 0.64 |
| Chalcopyrite | 9.90 | Chlorite | 0.52 |
| Tetrahedrite | 0.23 | Muscovite | 0.50 |
| Covellite | 0.15 | Essonite | 0.42 |
| Cassiterite | 0.17 | Limonite | 0.27 |
| Siderite | 2.07 | Andradite | 0.27 |
| Albite | 2.11 | Sphene | 0.17 |
| Quartz | 1.11 | Hematite | 0.14 |
| K-feldspar | 1.01 | Others | 0.35 |
| Diopside | 0.90 |
| Fe | S | Fe | S | Fe | S | Fe | S | Fe | S | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60.15 | 39.42 | 11 | 60.17 | 39.52 | 21 | 60.25 | 39.34 | 31 | 60.16 | 39.47 | 41 | 61.46 | 38.22 |
| 2 | 60.22 | 39.76 | 12 | 60.19 | 39.26 | 22 | 60.24 | 39.34 | 32 | 60.34 | 39.11 | 42 | 61.28 | 38.18 |
| 3 | 60.36 | 39.49 | 13 | 60.35 | 39.17 | 23 | 60.13 | 39.46 | 33 | 60.23 | 39.23 | 43 | 61.43 | 38.35 |
| 4 | 60.17 | 39.26 | 14 | 60.42 | 39.14 | 24 | 60.34 | 39.57 | 34 | 60.45 | 39.17 | 44 | 61.74 | 38.19 |
| 5 | 60.23 | 39.02 | 15 | 60.28 | 39.53 | 25 | 60.23 | 39.16 | 35 | 61.23 | 38.42 | 45 | 61.33 | 38.23 |
| 6 | 60.34 | 39.38 | 16 | 60.34 | 39.31 | 26 | 60.45 | 39.21 | 36 | 61.36 | 38.37 | 46 | 61.63 | 38.25 |
| 7 | 60.26 | 39.55 | 17 | 60.13 | 39.23 | 27 | 60.23 | 39.22 | 37 | 61.42 | 38.15 | 47 | 61.29 | 38.23 |
| 8 | 60.35 | 39.61 | 18 | 60.26 | 39.58 | 28 | 60.56 | 39.12 | 38 | 61.23 | 38.53 | 48 | 61.18 | 38.26 |
| 9 | 60.34 | 39.11 | 19 | 60.64 | 39.29 | 29 | 60.15 | 39.44 | 39 | 61.32 | 38.45 | 49 | 61.67 | 38.27 |
| 10 | 60.43 | 39.15 | 20 | 60.66 | 39.15 | 30 | 60.67 | 39.12 | 40 | 61.45 | 38.43 | 50 | 61.65 | 38.27 |
| Element | Fe | S | Sn | Cu | Bi | As |
|---|---|---|---|---|---|---|
| Content (%) | 45.95 | 39.49 | 0.46 | 0.92 | 0.089 | 6.84 |
| Element | SiO2 | CaO | MgO | Al2O3 | K2O | Na2O |
| Content (%) | 3.95 | 0.62 | 0.46 | 0.38 | 0.11 | <0.005 |
| Mineral | Content (%) | Mineral | Content (%) |
|---|---|---|---|
| Pyrite | 38.53 | Chlorite | 0.54 |
| Arsenopyrite | 14.86 | Hematite | 0.48 |
| Pyrrhotite | 35.23 | Diopside | 0.37 |
| Chalcopyrite | 1.43 | Andradite | 0.35 |
| Cassiterite | 0.51 | Sphene | 0.34 |
| Stannite | 0.22 | Essonite | 0.25 |
| Quartz | 2.09 | Calcite | 0.18 |
| Siderite | 1.58 | K-feldspar | 0.15 |
| Albite | 0.98 | Muscovite | 0.12 |
| Dolomite | 0.89 | Others | 0.16 |
| Hornblende | 0.74 |
| Fe | S | Fe | S | Fe | S | Fe | S | Fe | S | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60.18 | 39.59 | 11 | 60.21 | 39.37 | 21 | 60.87 | 39.08 | 31 | 61.02 | 38.92 | 41 | 60.92 | 38.83 |
| 2 | 60.12 | 39.53 | 12 | 60.22 | 39.37 | 22 | 60.89 | 39.07 | 32 | 61.05 | 38.92 | 42 | 61.02 | 38.87 |
| 3 | 60.18 | 39.56 | 13 | 60.33 | 39.42 | 23 | 60.86 | 39.05 | 33 | 61.05 | 38.92 | 43 | 61.02 | 38.87 |
| 4 | 60.26 | 39.58 | 14 | 60.21 | 39.34 | 24 | 60.91 | 39.03 | 34 | 60.88 | 38.81 | 44 | 60.98 | 38.84 |
| 5 | 60.23 | 39.56 | 15 | 60.22 | 39.34 | 25 | 60.92 | 39.01 | 35 | 60.96 | 38.86 | 45 | 60.91 | 38.79 |
| 6 | 60.29 | 39.55 | 16 | 60.27 | 39.36 | 26 | 60.91 | 38.97 | 36 | 61.04 | 38.91 | 46 | 60.92 | 38.75 |
| 7 | 60.39 | 39.58 | 17 | 60.11 | 39.24 | 27 | 61.01 | 38.95 | 37 | 61.04 | 38.91 | 47 | 61.03 | 38.79 |
| 8 | 60.13 | 39.39 | 18 | 60.17 | 39.25 | 28 | 61.02 | 38.94 | 38 | 61.04 | 38.91 | 48 | 60.98 | 38.73 |
| 9 | 60.35 | 39.48 | 19 | 60.42 | 39.39 | 29 | 61.02 | 38.94 | 39 | 61.01 | 38.89 | 49 | 61.08 | 38.72 |
| 10 | 60.22 | 39.39 | 20 | 60.15 | 39.21 | 30 | 61.02 | 38.93 | 40 | 61.01 | 38.89 | 50 | 61.24 | 38.71 |
| Element | Fe | S | Sn | Cu | Bi | As |
|---|---|---|---|---|---|---|
| Content (%) | 45.95 | 39.49 | 0.46 | 0.92 | 0.089 | 6.84 |
| Element | SiO2 | CaO | MgO | Al2O3 | K2O | Na2O |
| Content (%) | 3.95 | 0.62 | 0.46 | 0.38 | 0.11 | <0.005 |
| Mineral | Content (%) | Mineral | Content (%) |
|---|---|---|---|
| Pyrrhotite | 60.56 | Albite | 1.18 |
| Pyrite | 7.35 | Quartz | 1.04 |
| Arsenopyrite | 3.11 | Chlorite | 0.89 |
| Chalcopyrite | 1.17 | Muscovite | 0.58 |
| Cassiterite | 5.12 | K-feldspar | 0.54 |
| Stannite | 0.22 | Dolomite | 0.32 |
| Essonite | 6.98 | Hornblende | 0.31 |
| Calcite | 3.48 | Sphene | 0.31 |
| Diopside | 3.18 | Hematite | 0.21 |
| Andradite | 1.83 | Others | 0.28 |
| Siderite | 1.34 |
| Fe | S | Fe | S | Fe | S | Fe | S | Fe | S | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60.33 | 39.65 | 11 | 60.94 | 39.02 | 21 | 60.92 | 38.89 | 31 | 61.03 | 38.89 | 41 | 60.97 | 38.71 |
| 2 | 60.15 | 39.41 | 12 | 60.95 | 39.02 | 22 | 61.02 | 38.95 | 32 | 61.02 | 38.87 | 42 | 61.02 | 38.73 |
| 3 | 60.07 | 39.34 | 13 | 60.91 | 38.97 | 23 | 61.04 | 38.96 | 33 | 61.02 | 38.82 | 43 | 61.07 | 38.76 |
| 4 | 60.23 | 39.39 | 14 | 60.82 | 38.89 | 24 | 61.01 | 38.94 | 34 | 61.02 | 38.82 | 44 | 61.07 | 38.75 |
| 5 | 60.35 | 39.43 | 15 | 60.98 | 38.99 | 25 | 61.01 | 38.92 | 35 | 60.91 | 38.75 | 45 | 60.98 | 38.69 |
| 6 | 60.29 | 39.38 | 16 | 60.94 | 38.94 | 26 | 61.02 | 38.91 | 36 | 60.96 | 38.78 | 46 | 60.98 | 38.69 |
| 7 | 60.27 | 39.33 | 17 | 60.96 | 38.95 | 27 | 61.04 | 38.92 | 37 | 61.08 | 38.85 | 47 | 60.99 | 38.68 |
| 8 | 60.87 | 39.07 | 18 | 60.87 | 38.89 | 28 | 61.03 | 38.91 | 38 | 60.93 | 38.75 | 48 | 61.09 | 38.65 |
| 9 | 60.91 | 39.02 | 19 | 61.02 | 38.96 | 29 | 60.97 | 38.87 | 39 | 61.04 | 38.81 | 49 | 61.02 | 38.46 |
| 10 | 60.93 | 39.02 | 20 | 60.95 | 38.91 | 30 | 60.92 | 38.83 | 40 | 60.96 | 38.72 | 50 | 61.03 | 38.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Tong, X.; Xie, X. Influence of Mineralogy and Mineralogy Approach to Optimize Processing: A Case Study of Tin–Copper Polymetallic Ore. Minerals 2024, 14, 554. https://doi.org/10.3390/min14060554
Ye X, Tong X, Xie X. Influence of Mineralogy and Mineralogy Approach to Optimize Processing: A Case Study of Tin–Copper Polymetallic Ore. Minerals. 2024; 14(6):554. https://doi.org/10.3390/min14060554
Chicago/Turabian StyleYe, Xiaolu, Xiong Tong, and Xian Xie. 2024. "Influence of Mineralogy and Mineralogy Approach to Optimize Processing: A Case Study of Tin–Copper Polymetallic Ore" Minerals 14, no. 6: 554. https://doi.org/10.3390/min14060554
APA StyleYe, X., Tong, X., & Xie, X. (2024). Influence of Mineralogy and Mineralogy Approach to Optimize Processing: A Case Study of Tin–Copper Polymetallic Ore. Minerals, 14(6), 554. https://doi.org/10.3390/min14060554




