Experimental Investigation on Hydrophobic Alteration of Mining Solid Waste Backfill Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Raw Materials
2.2. Preparation
2.3. Experimental Methods
2.3.1. Rheological Test
2.3.2. Setting Time Test
2.3.3. Uniaxial Compressive Strength (UCS) Test
2.3.4. XRD and SEM Analyses
2.3.5. Water Contact Angle Test
2.3.6. FTIR Spectroscopy Analysis
3. Results and Discussion
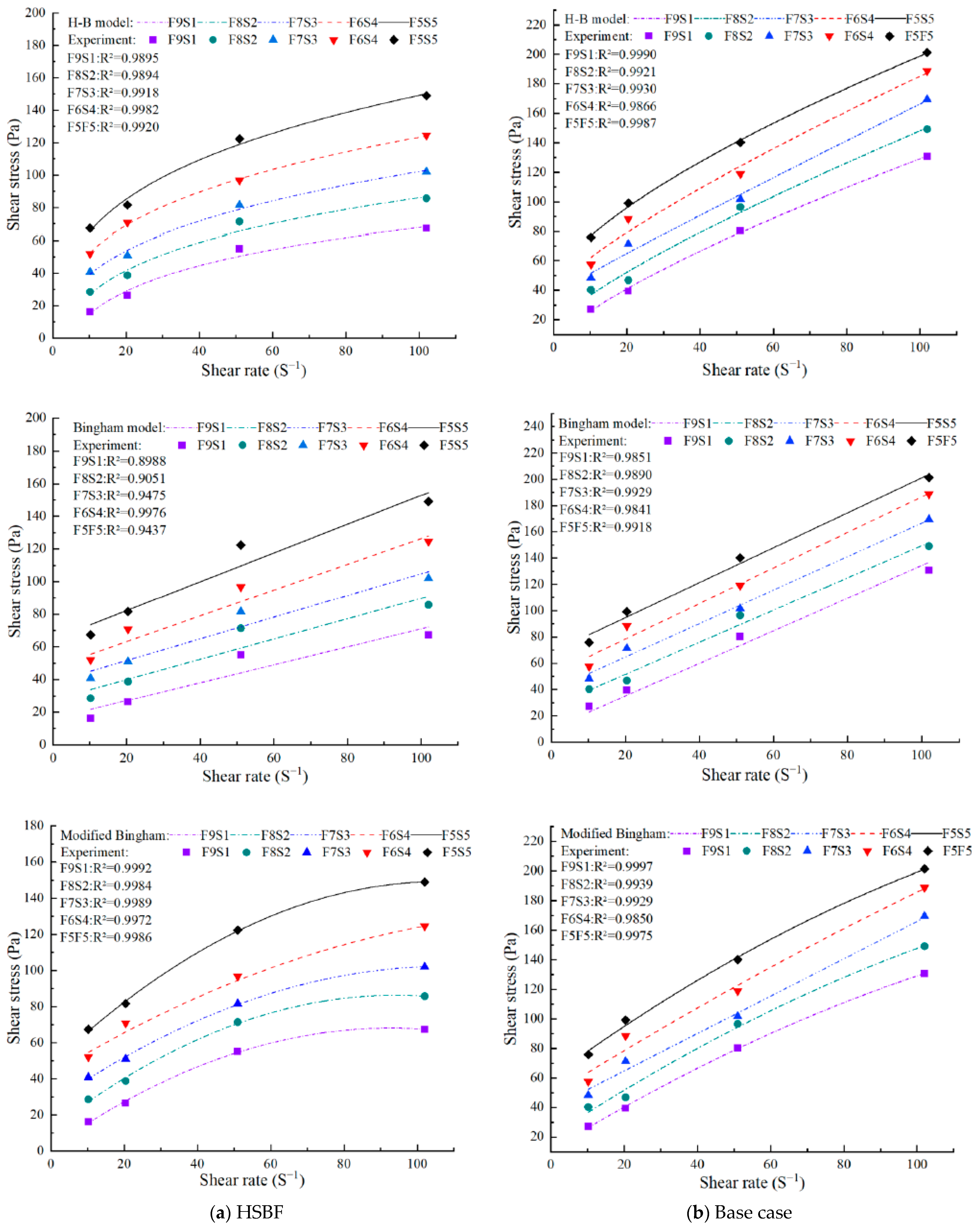
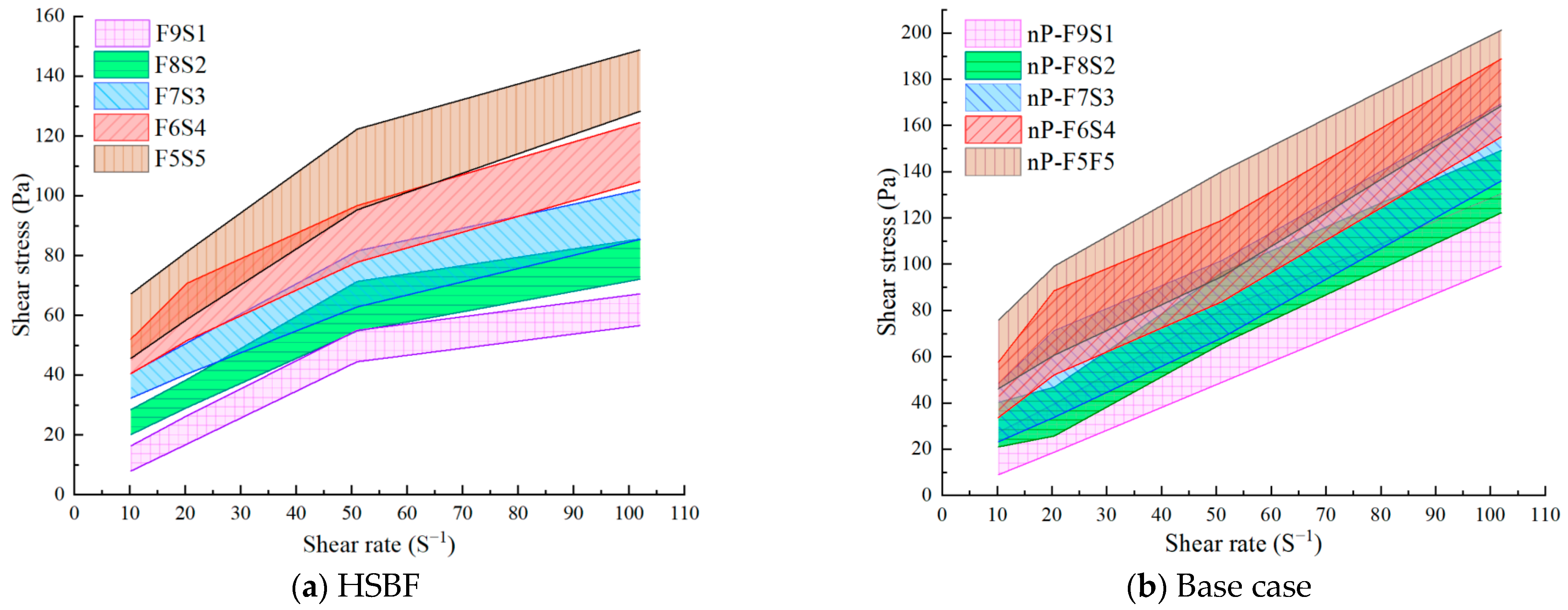
3.1. Rheological Properties
3.1.1. Yield Stress and Viscosity
3.1.2. Thixotropy Analysis
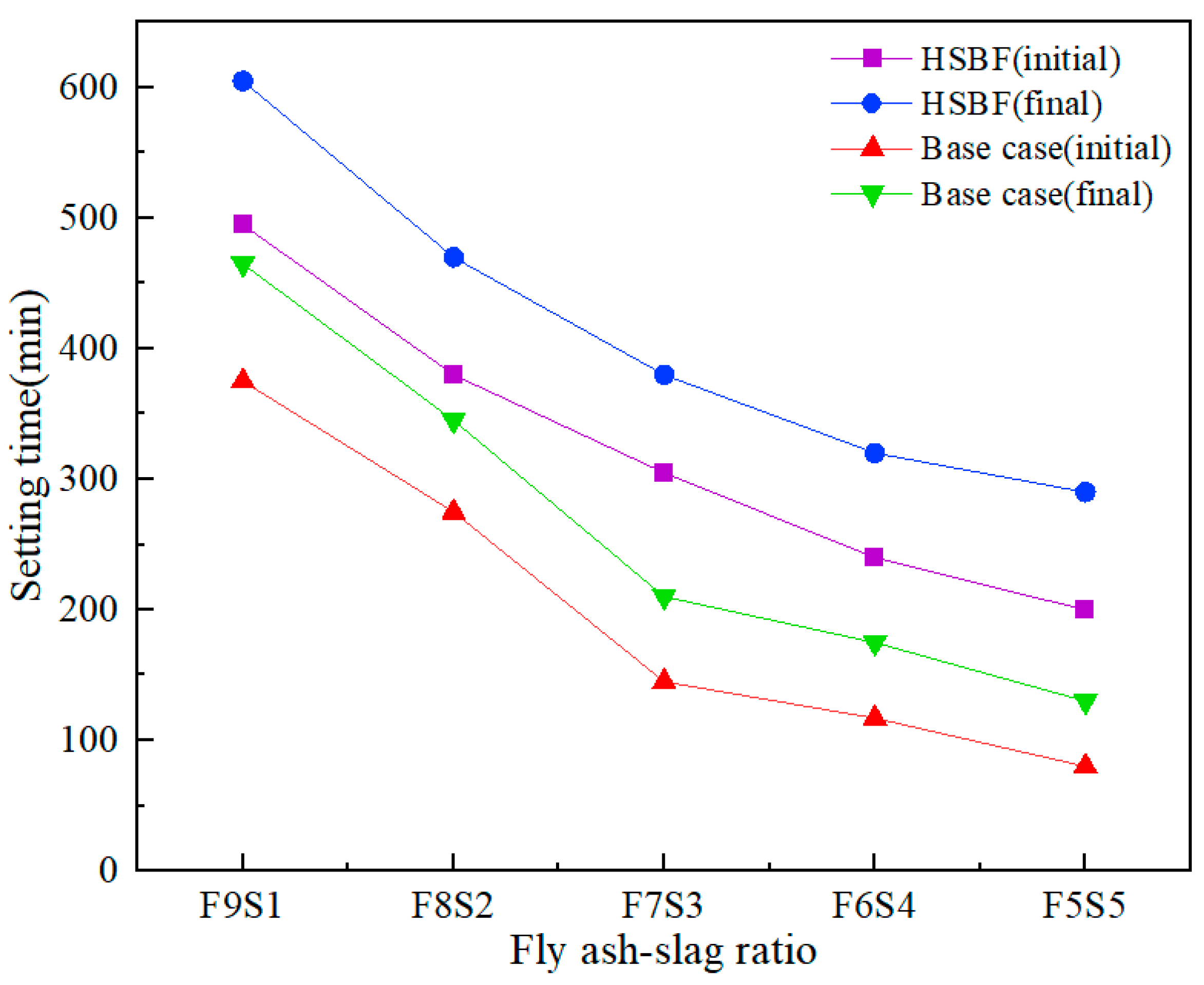
3.2. Setting Time
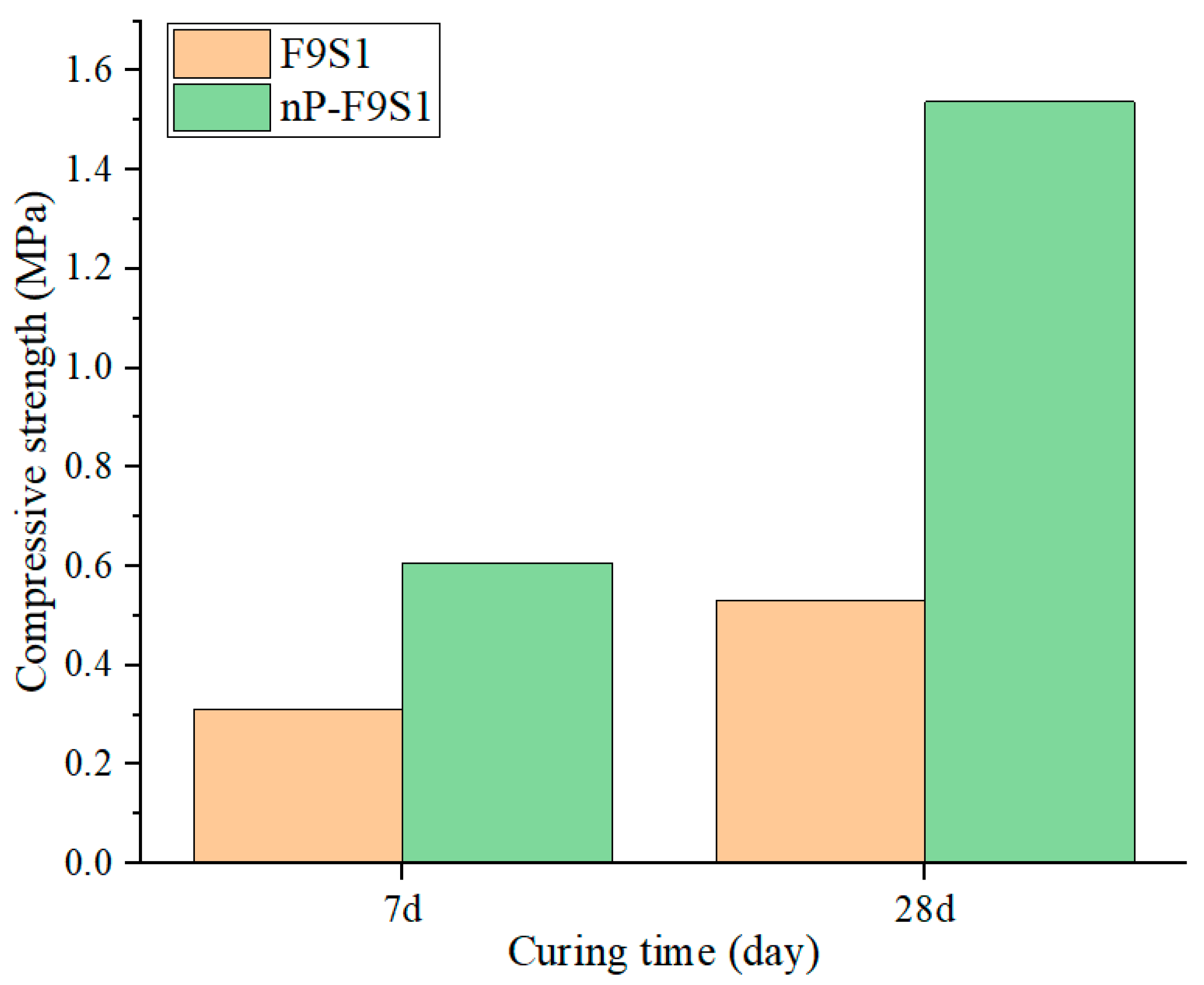
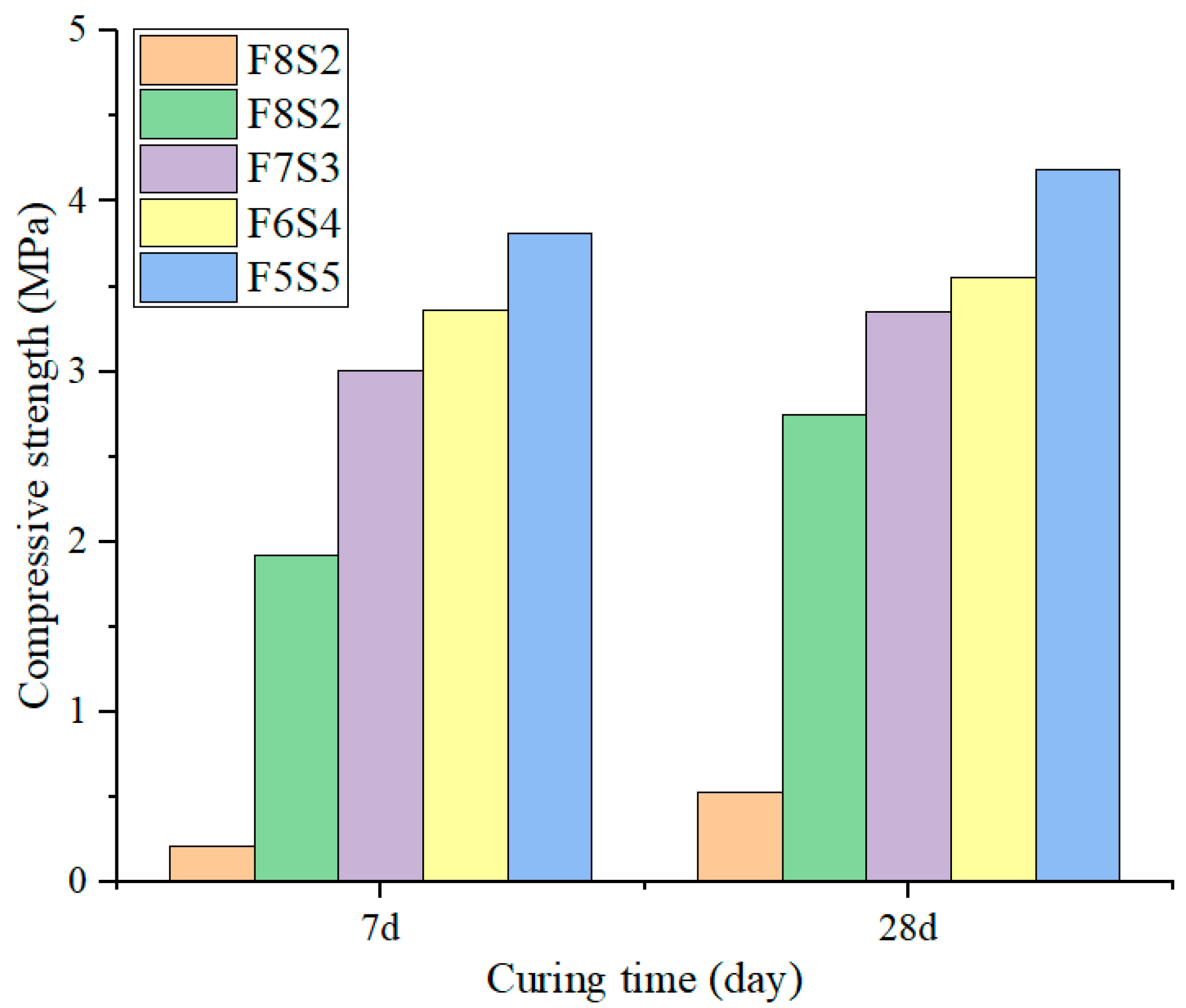
3.3. UCS Development of the Backfill Samples
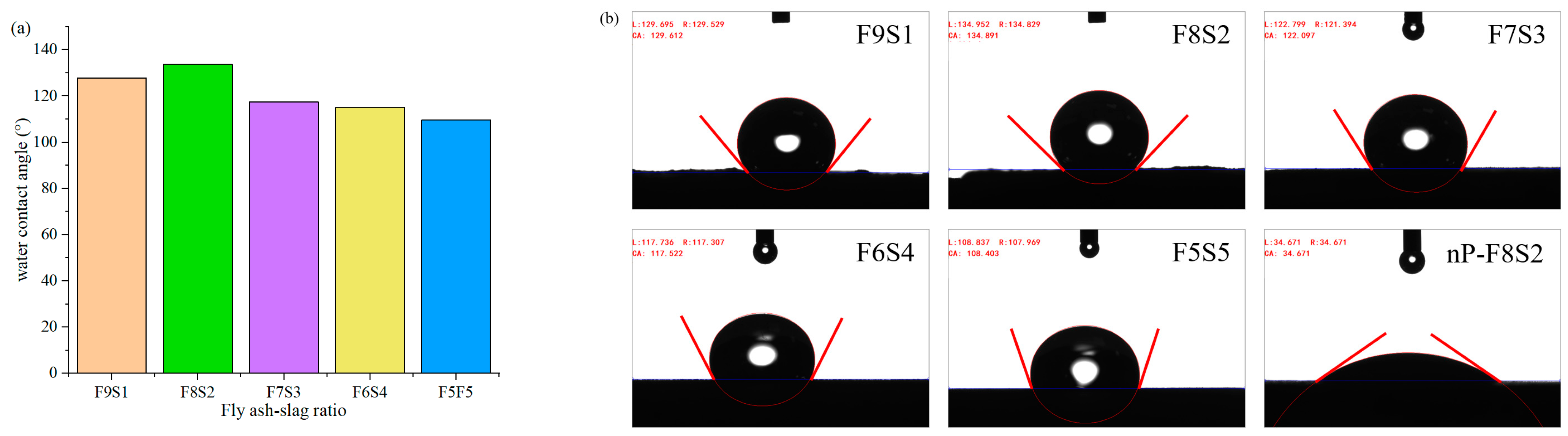
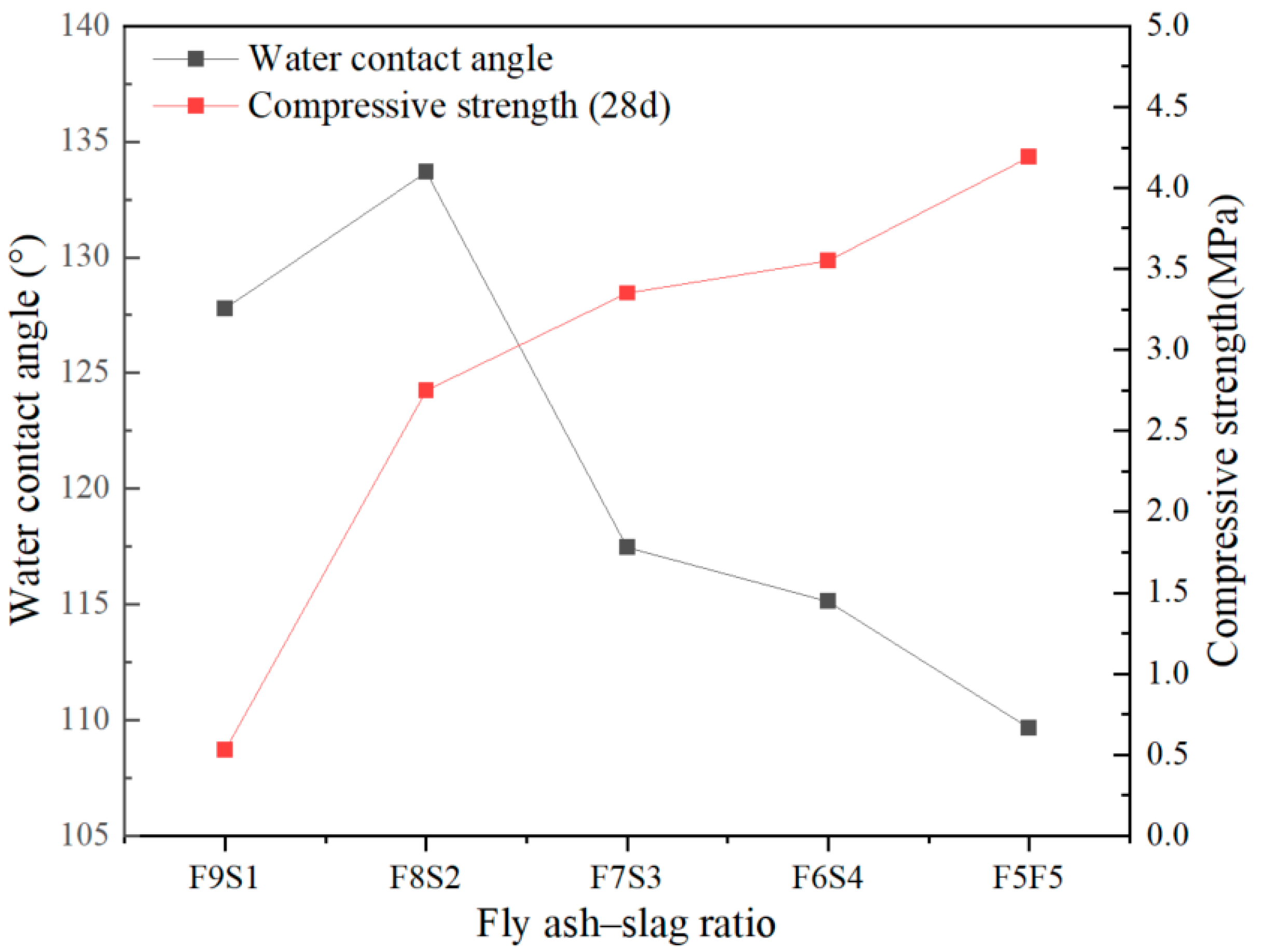
3.4. Water Contact Angle
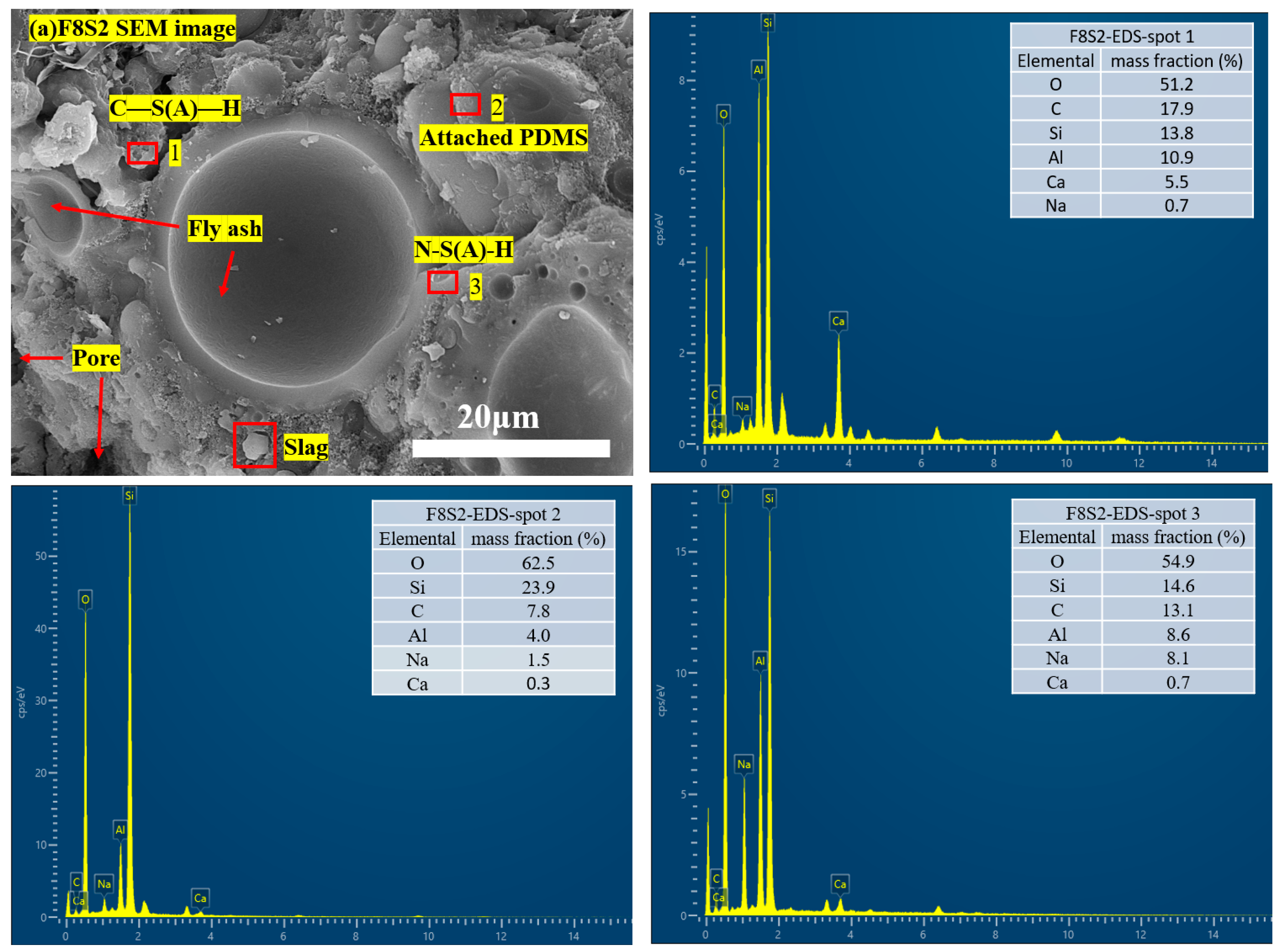
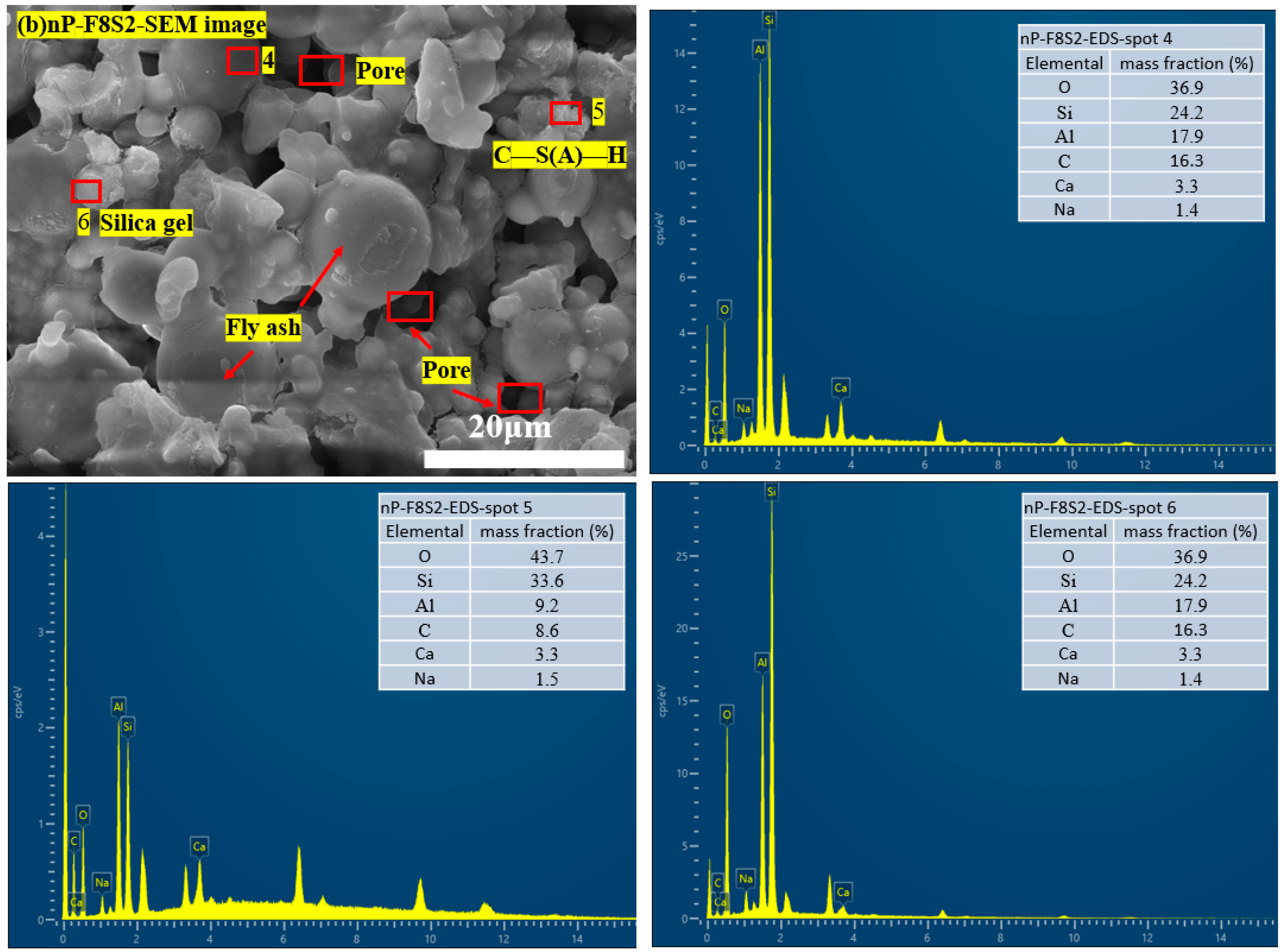
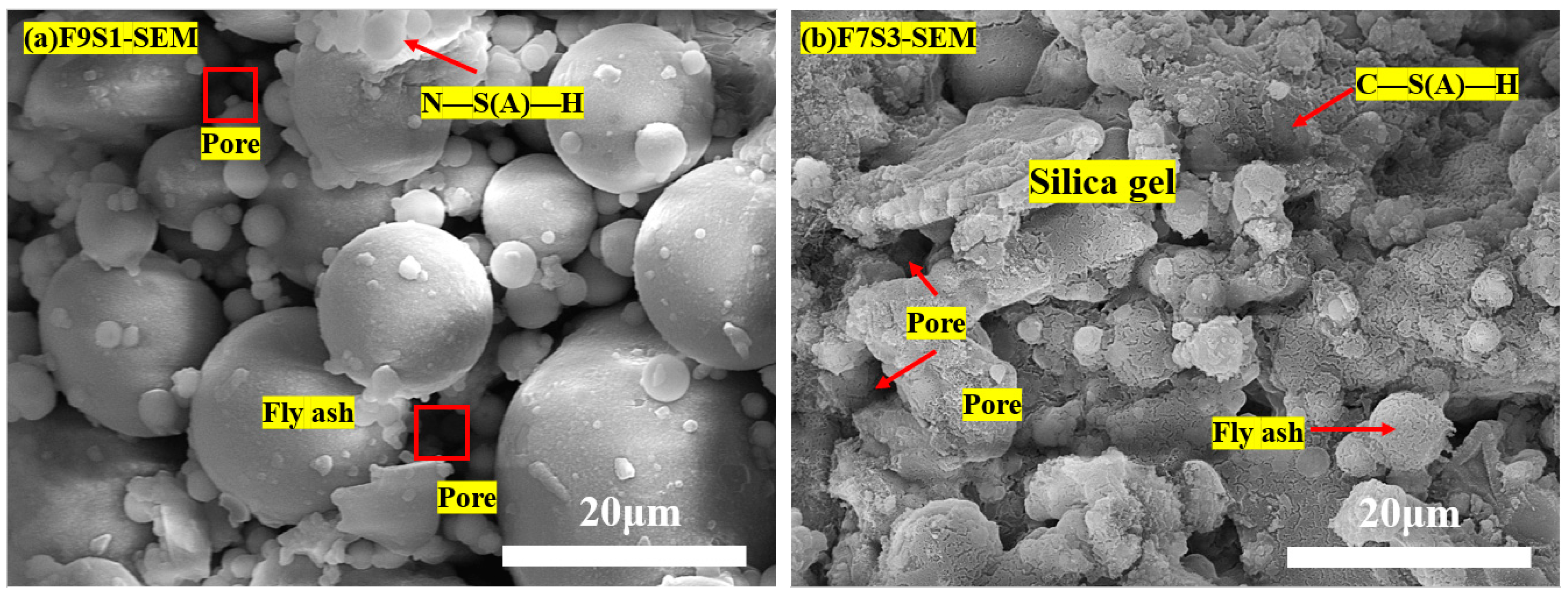
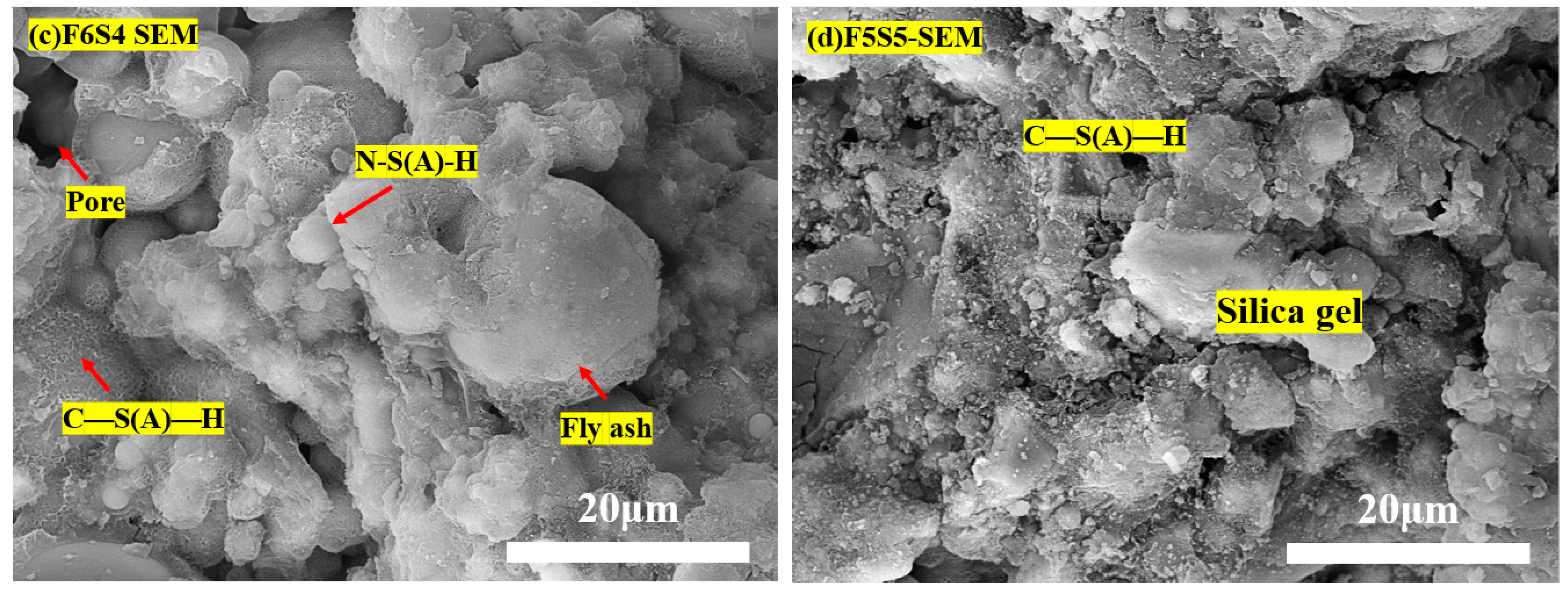
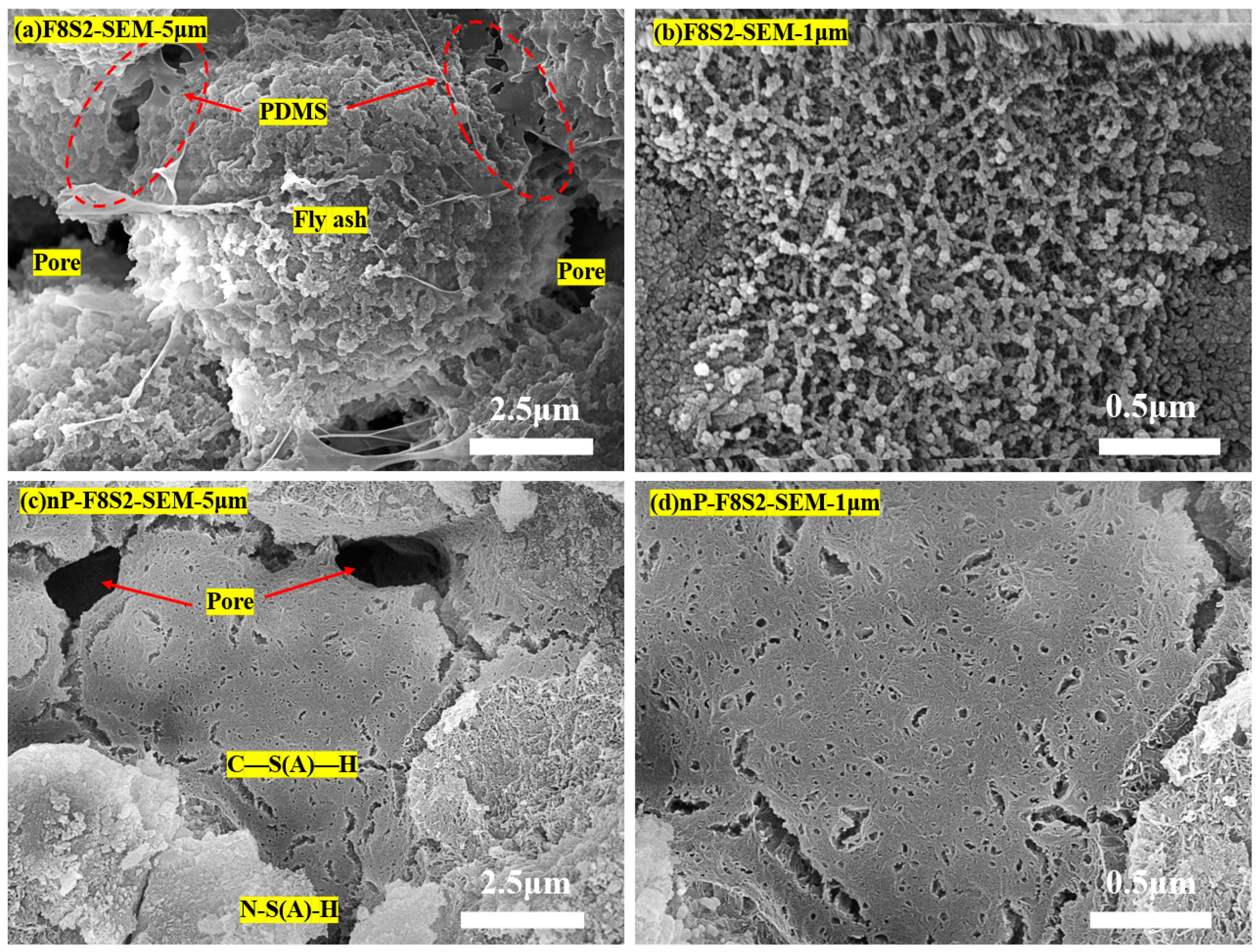
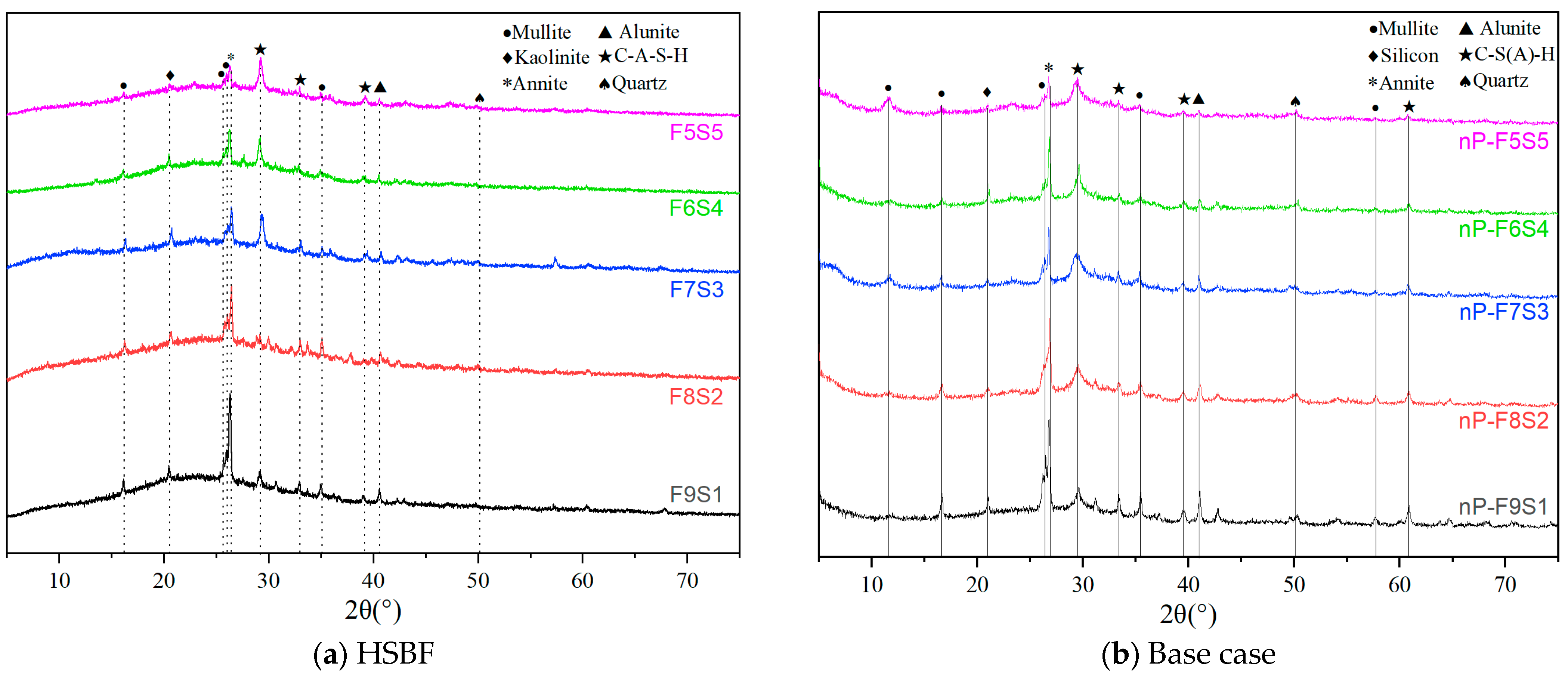
3.5. Microstructure and Hydration Products
3.6. Hydration and Carbonation Products in the HSBF Sample
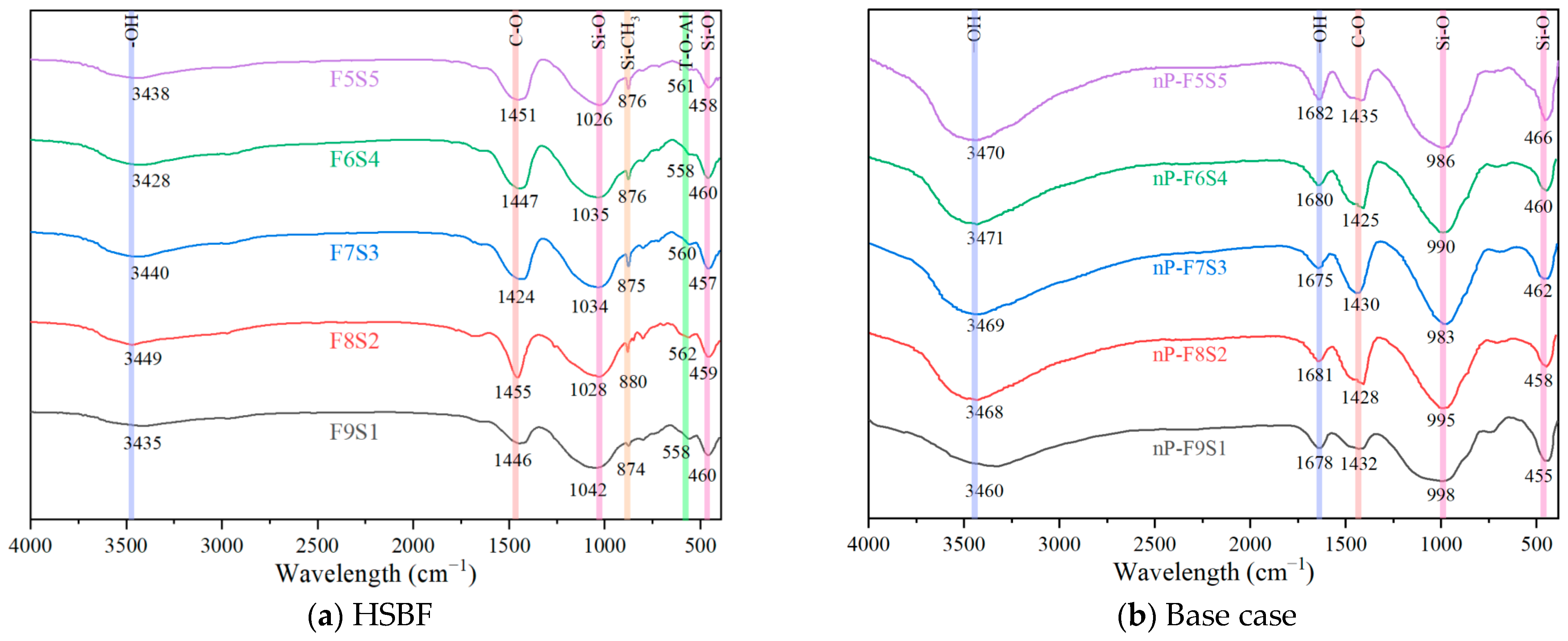
3.7. Infrared Spectroscopy Analysis
3.8. Strengths and Applications
4. Conclusions
- (1)
- The HSBF paste conforms to the Modified Bingham model and exhibits shear-thinning behavior. The thixotropy of the HSBF paste was consistent with the yield stress.
- (2)
- After PDMS hydrophobic modification, the water contact angle of the backfill material increased up to 134.9°, exhibiting excellent hydrophobic properties. SEM, XRD, and FTIR tests showed that the PDMS adhered to the surface of inorganic molecules such as fly ash and slag through the bridging action of the coupling agent to form a dense capillary structure, which gave the backfill a hydrophobic effect.
- (3)
- The overall strength of HSBF increased up to 98% by increasing the slag content from 20 to 50% through the fly ash–slag ratio optimization. The strength enhancement was obtained by the greater development in C-S(A)-H gel, which was achieved in the case of high slag content.
- (4)
- This research demonstrates that HSBF with various slag ratios (F8S2, F7S3, F6S4, F5F5) exhibits excellent mechanical performance and hydrophobic characteristics. The novel developed HSBF materials are feasible to implement in underground construction with active water interaction.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.B.; Sun, X.H.; Liu, Z.; Wang, D.M.; Zhu, L. Study on preparation, working performance and microstructure of coal mine filling material with large amount of fly ash. J. China Coal Soc. 2023, 48, 4536–4548. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, K.Y.; Ren, L. Evaluation and coupling coordination analysis of high quality development of China’s coal industry: Empirical research based on data from 2000 to 2019. J. Min. Sci. Technol. 2021, 6, 764–776. [Google Scholar] [CrossRef]
- Jiang, L. Comprehensiveutilization situation of fly ash in coal—Fired power plants and its development suggestions. Clean Coal Technol. 2020, 26, 31–39. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zhang, Q.; Zhou, N.; Li, M.; Huang, P.; Li, B. Research progress and prospect of coal based solid waste backfilling mining technology. J. China Coal Soc. 2022, 47, 4167–4181. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, X.Y.; He, X.; Wei, Z. Basic theory and key technology of multi-source coal-based solid waste for green backfilling. J. China Coal Soc. 2022, 47, 4201–4216. [Google Scholar] [CrossRef]
- Yin, S.; Yan, Z.; Chen, X.; Yan, R.; Chen, D.; Chen, J.; Li, G. Active roof-contact: The future development of cemented paste backfill. Constr. Build. Mater. 2023, 370, 130657. [Google Scholar] [CrossRef]
- Feng, Y.; Qi, W.; Zhao, Q.; Huang, Y.; Ren, Q.; Qi, W.; Kong, F. Synthesis and characterization of cemented paste backfill: Reuse of multiple solid wastes. J. Clean. Prod. 2023, 383, 135376. [Google Scholar] [CrossRef]
- Wang, Y.Y. Study on Strengthening Mechanism and Application of CO2 Mineralized Solid Waste Backfill Materials; China University of Mining and Technology: Xuzhou, China, 2023. [Google Scholar] [CrossRef]
- Ding, J.; Feng, Z.; Sun, D.; You, K.; Yan, M.; Xun, Y. Analysis of Influencing Factors of Silt Solidified Soil in Flowing State. World J. Eng. Technol. 2019, 7, 455–464. [Google Scholar] [CrossRef]
- Shao, L.; Li, P.Q.; Wang, B.J. Analysis of the strength characteristics of soft soil reinforced by composite excited blast furnace slag. China Water Transp. 2021, 21, 154–156. [Google Scholar]
- Luo, H.G.; Liu, H.S.; Li, P.; Zhang, P.H.; Wu, R. Optimisation Test of Ratio of Sand-based Paste Filling Materials in Shendong Mine. Coal Tech-Nology 2023, 42, 45–49. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, G.; Qi, Y.; Zeng, Q. In-situ assessment of the water-penetration resistance of polymer modified cement mortars by μ-XCT, SEM and EDS. Cem. Concr. Compos. 2020, 114, 103821. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Xu, W.; Liu, H.; Lu, Y. Inexpensive and non-fluorinated superhydrophobic concrete coating for anti-icing and anti-corrosion. J. Colloid Interface Sci. 2019, 541, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Guo, Z.; Liu, W.; Zhang, Y. Mechanical properties and meso-structure response of cemented gangue-fly ash backfill with cracks under seepage- stress coupling. Constr. Build. Mater. 2020, 250, 118863. [Google Scholar] [CrossRef]
- Ngo, I.; Ma, L.; Zhai, J.; Wang, Y.; Wei, T. Durability of CO2-fly ash-based backfill materials in cation water deterioration. Int. J. Min. Reclam. Environ. 2023, 1–24. [Google Scholar] [CrossRef]
- Guo, Y.X.; Ran, H.; Feng, G.; Wang, P. Strength and creep characteristics of cemented gangue backfill in acid environment. J. Min. Saf. Eng. 2021, 38, 361–369. [Google Scholar] [CrossRef]
- Sidhu, J.; Kumar, P. Comprehensive review on hydrophobic modification of concrete: Progress and perspectives. Adv. Civ. Arch. Eng. 2023, 14, 155–180. [Google Scholar] [CrossRef]
- Konstantin, G.S.; Batrakov, V.G. Effect of a polyethylhydrosiloxane admixture on the durability of concrete with supplementary cementitious materi-als. J. Mater. Civ. Eng. 2007, 19, 809–819. [Google Scholar]
- Wong, H.S.; Barakat, R.; Alhilali, A.; Saleh, M.; Cheeseman, C.R. Hydrophobic concrete using waste paper sludge ash. Cem. Concr. Res. 2015, 70, 9–20. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, C.; Sun, S.; Kalulu, M.; Chen, L.; Zhou, X.; Jiang, Y. Durable superhydrophobic coating based on inorgan-ic/organic double-network polysiloxane and functionalized nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123550. [Google Scholar] [CrossRef]
- Flores-Vivian, I.; Hejazi, V.; Kozhukhova, M.I.; Nosonovsky, M.; Sobolev, K. Self-assembling particle-siloxane coatings for superhydrophobic concrete. ACS Appl. Mater. Interfaces 2013, 5, 13284–13294. [Google Scholar] [CrossRef]
- Wang, F.; Lei, S.; Ou, J.; Xue, M.; Li, C.; Li, W. Superhydrophobic calcium aluminate cement with super mechanical stability. Ind. Eng. Chem. Res. 2019, 58, 10373–10382. [Google Scholar] [CrossRef]
- Shao, Y. Immobilization Mechanisms of Solidified Municipal Solid Waste Incineration Fly Ash Using Blast Furnace Slag Based Cementitious Material; Wu Han University: Wuhan, China, 2014. [Google Scholar]
- Zhou, W. Study of the Properties and Preparation of PDMS Coating Material; Wuhan University of Technology: Wuhan, China, 2007. [Google Scholar]
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). China Cement Standardization Technical Committee (SAC/TC 184): Beijing, China, 2021.
- ASTM C150; Standard Specification for Portland Cement. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2017.
- Ma, L.; Zhai, J.; NGO Ichhuy. Experimental study on preparation of negative carbon filling material forwater protection mining by CO2mineralization of coal-based solid waste. J. China Coal Soc. 2022, 47, 4228–4236. [Google Scholar] [CrossRef]
- Ngo, I.; Ma, L.; Zhai, J.; Wang, Y. Enhancing fly ash utilization in backfill materials treated with CO2 carbonation under ambient conditions. Int. J. Min. Sci. Technol. 2023, 33, 323–337. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Ihle, C.F.; Tamburrino, A. Analytical solutions for the flow depth of steady laminar, Bingham plastic tailings down wide channels. Miner. Eng. 2018, 128, 284–287. [Google Scholar] [CrossRef]
- Ettehadi, A.; Altun, G. Functional and practical analytical pressure surges model through herschel bulkley fluids. J. Pet. Sci. Eng. 2018, 171, 748–759. [Google Scholar] [CrossRef]
- Saramito, P. A new elastoviscoplastic model based on the Herschel–Bulkley viscoplastic model. J. Non-Newton. Fluid Mech. 2009, 158, 154–161. [Google Scholar] [CrossRef]
- de Larrard, F.; Ferraris, C.F.; Sedran, T. Fresh concrete: A Herschel-Bulkley material. Mater. Struct. 1998, 31, 494–498. [Google Scholar] [CrossRef]
- Zhaidarbek, B.; Tleubek, A.; Berdibek, G.; Wang, Y. Analytical predictions of concrete puming: Extending the Khatib–Khayat model to Herschel–Bulkley and modified Bingham fluids. Cem. Concr. Res. 2023, 163, 107035. [Google Scholar] [CrossRef]
- Palacios, M.; Banfill, P.; Puertas, F. Rheology and setting of alkali-activated slag pastes and mortars: Effect of organ admixture. ACI Mater. J. 2008, 105, 140–148. [Google Scholar]
- Kashani, A.; Provis, J.L.; Qiao, G.G.; van Deventer, J.S. The interrelationship between surface chemistry and rheology in alkali activated slag paste. Constr. Build. Mater. 2014, 65, 583–591. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS func-tional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the CASH gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F.; Sobrados, I.; Sanz, J. Structure of calcium silicate hydrates formed in alkaline-activated slag: Influence of the type of alkaline activator. J. Am. Ceram. Soc. 2003, 86, 1389–1394. [Google Scholar] [CrossRef]
- Gonzalez-Taboada, I.; Gonzalez-Fonteboa, B.; Martinez-Abella, F.; Seara-Paz, S. Thixotropy and interlayer bond strength of self-compacting recycled concrete. Constr. Build. Mater. 2018, 161, 479–488. [Google Scholar] [CrossRef]
- Ruan, S.; Chen, S.; Lu, J.; Zeng, Q.; Liu, Y.; Yan, D. Waterproof geopolymer composites modified by hydrophobic particles and polydimethylsiloxane. Compos. Part B Eng. 2022, 237, 109865. [Google Scholar] [CrossRef]
- Sellgren, A.; Addie, G.; Whitlock, L. Technical-economical feasibility of using centrifugal pumps in high-density thickened tailings slurry systems. In Proceedings of the Paste2005: Proceedings of the International Seminar on Paste and Thickened Tailings, Santiago, Chile, 20–22 April 2005; Australian Centre for Geomechanics: Crawley, Australia, 2005; pp. 195–204. [Google Scholar] [CrossRef]
- Khayat, K.H.; Saric-Coric, M.; Liotta, F. Influence of thixotropy on stability characteristics of cement grout and concrete. Mater. J. 2002, 99, 234–241. [Google Scholar]
- Khayat, K.H.; Omran, A.; Magdi, W.A. Evaluation of thixotropy of self-consolidating concrete and influence on concrete per-formance. In Proceedings of the 3rd Iberian Congress on Self Compacting Concrete, Madrid, Spain, 8–10 December 2012; pp. 3–16. [Google Scholar]
- Wardhono, A.; Gunasekara, C.; Law, D.W.; Setunge, S. Comparison of long term performance between alkali activated slag and fly ash geopolymer concretes. Constr. Build. Mater. 2017, 143, 272–279. [Google Scholar] [CrossRef]
- Long, L. Mechanism Study on Solidification and Stabilization of Municipal Solid Waste Incineration Fly Ash via Coal Gangue and Blast Furnace Slag; Zhejiang University: Hangzhou, China, 2023. [Google Scholar]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.; Hussein, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Qin, Z.; Dong, Z.; Pu, J.; Zeng, Y.; Lan, X. Study and Application of a Fly Ash-slag Bonding Reinforcement Material. Drill. Fluid Complet. Fluid 2023, 40, 527–534. [Google Scholar]
- Zhu, Z.; Wang, Z.; Zhou, Y.; Wei, Y.; She, A. Synthesis and structure of calcium silicate hydrate (CSH) modified by hydroxyl-terminated polydime-thylsiloxane (PDMS). Constr. Build. Mater. 2021, 267, 120731. [Google Scholar] [CrossRef]
- Jang, K.P.; Kwon, S.H.; Choi, M.S.; Kim, Y.J.; Park, C.K.; Shah, S.P. Experimental observation on variation of rheological properties during concrete pumping. Int. J. Concr. Struct. Mater. 2018, 12, 79. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag(Si + Ca) and metakaolin(Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Wang, X.-M.; Li, J.-X.; Fan, P.-Z. Applied technique of the cemented fill with fly ash and fine-sands. J. Cent. South Univ. Technol. 2001, 8, 189–192. [Google Scholar] [CrossRef]
- De Vries, J.; Polder, R.B. Hydrophobic treatment of concrete. Constr. Build. Mater. 1997, 11, 259–265. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, H.; Wu, Q.; Yang, T.; Yin, Z.; Tian, L. Investigation of the hydrophobicity and microstructure of fly ash-slag geopolymer modified by polydimethylsiloxane. Constr. Build. Mater. 2023, 369, 130540. [Google Scholar] [CrossRef]
- Saha, S.; Rajasekaran, C. Enhancement of the properties of fly ash based geopolymer paste by incorporating ground granulated blast furnace slag. Constr. Build. Mater. 2017, 146, 615–620. [Google Scholar] [CrossRef]
- Ruan, S.; Chen, S.; Zhu, X.; Zeng, Q.; Liu, Y.; Lai, J.; Yan, D. Matrix wettability and mechanical properties of geopolymer cement-polydimethylsiloxane (PDMS) hybrids. Cem. Concr. Compos. 2021, 124, 104268. [Google Scholar] [CrossRef]
- Mosquera, M.J.; de los Santos, D.M.; Rivas, T. Surfactant-synthesized ormosils with application to stone restoration. Langmuir 2010, 26, 6737–6745. [Google Scholar] [CrossRef]
- Feng, H.; Le, H.T.N.; Wang, S.; Zhang, M.-H. Effects of silanes and silane derivatives on cement hydration and mechanical properties of mortars. Constr. Build. Mater. 2016, 129, 48–60. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, K.; Wang, J.; Guo, J.; Ling, Y. Macroscopic and microscopic analyses on mechanical performance of metakaolin/fly ash based geopolymer mortar. J. Clean. Prod. 2021, 294, 126193. [Google Scholar] [CrossRef]
- Ngo, I.; Ma, L.; Zhai, J.; Wang, Y.; Xu, Y.; Wei, T.; Yu, K. Effect of the co-activation of sodium silicate and CO2 on setting and mechanical properties of coal gangue-fly ash backfill (CGFB). Environ. Earth Sci. 2023, 82, 190. [Google Scholar] [CrossRef]
- Ozer, I.; Soyer-Uzun, S. Relations between the structural characteristics and compressive strength in metakaolin based geopolymers with different molar Si/Al ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Qiu, J.; Wu, P.; Zhao, Y. Alkali activation of blast furnace slag using a carbonate-calcium carbide residue alkaline mixture to prepare cemented paste backfill. Constr. Build. Mater. 2022, 320, 126234. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in CASH gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T. How to Interpret Infrared (IR) Spectra. Univ. Chem. 2016, 31, 90–97. [Google Scholar] [CrossRef]
- Partyka, J.; Leśniak, M. Raman and infrared spectroscopy study on structure and microstructure of glass–ceramic materials from SiO2–Al2O3–Na2O–K2O–CaO system modified by variable molar ratio of SiO2/Al2O3. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 82–91. [Google Scholar] [CrossRef]
- Mozgawa, W.; Sitarz, M.; Rokita, M. Spectroscopic studies of different aluminosilicate structures. J. Mol. Struct. 1999, 511, 251–257. [Google Scholar] [CrossRef]


| Property | Fly Ash | Slag |
|---|---|---|
| D10 a | 31.73 | 33.9 |
| D30 | 67.54 | 91.68 |
| D50 | 77.78 | 99.46 |
| D60 | 81.83 | 99.86 |
| D90 | 86.93 | 100 |
| Cu b | 2.58 | 2.95 |
| Cc c | 1.76 | 2.48 |
| U d | 0.12 | 0.001 |
| Specific surface area | 0.88 | 0.99 |
| Oxides | Fly Ash | Slag |
|---|---|---|
| CaO | 2.08 | 39.99 |
| SiO2 | 53.43 | 26.92 |
| Al2O3 | 31.93 | 16.26 |
| Fe2O3 | 3.36 | 0.99 |
| K2O | 1.29 | 0.51 |
| TiO2 | 1.18 | 1.14 |
| MgO | 0.53 | 9.93 |
| SO3 | 0.53 | 2.12 |
| P2O3 | 0.18 | 0.05 |
| Others | 5.16 | 2.62 |
| Minerals | Mullite, quartz, and hematite | Melilite, pyroxene, and plagioclase |
| Solid-Liquid Ratio | Solid Ratio (Fly Ash–Slag) | Na2SiO3 and NaOH Solution Concentration | PDMS Main Agent | KBM-403 SCA |
|---|---|---|---|---|
| 5:2 | 9:1 | SS 10 wt%/SH 5 wt% | 1 wt% of solid mass | 2 wt% of PMDS |
| 8:2 | ||||
| 7:3 | ||||
| 6:4 | ||||
| 5:5 |
| Model | Fitting Result | τ0 (Pa) | K | n | R2 | |
|---|---|---|---|---|---|---|
| F9S1 | H-B | −125.88 | 102.09 | 0.14 | 0.9895 | |
| F8S2 | −64.31 | 55.12 | 0.22 | 0.9894 | ||
| F7S3 | −24.51 | 31.84 | 0.3 | 0.9918 | ||
| F6S4 | −27.92 | 42.41 | 0.28 | 0.9991 | ||
| F5S5 | −32.77 | 52.5 | 0.27 | 0.9920 | ||
| nP-F9S1 | 3.99 | 3.8 | 0.76 | 0.9990 | ||
| nP-F8S2 | 15.8 | 3.29 | 0.8 | 0.9921 | ||
| nP-F7S3 | 37.22 | 1.57 | 0.96 | 0.9930 | ||
| nP-F6S4 | 38.57 | 3.7 | 0.8 | 0.9866 | ||
| nP-F5S5 | 45.1 | 6.5 | 0.69 | 0.9987 |
| Model | Fitting Result | τ0 (Pa) | η (Pa·s) | c | R2 | |
|---|---|---|---|---|---|---|
| F9S1 | M-B | 1.76 | 1.43 | −0.008 | 0.9987 | |
| F8S2 | 12.09 | 1.57 | −0.008 | 0.9972 | ||
| F7S3 | 26.29 | 1.41 | −0.007 | 0.9989 | ||
| F6S4 | 40.37 | 1.44 | −0.006 | 0.9932 | ||
| F5S5 | 47.81 | 1.92 | −0.009 | 0.9992 | ||
| nP-F9S1 | 11.25 | 1.52 | −0.003 | 0.9996 | ||
| nP-F8S2 | 20.41 | 1.63 | −0.004 | 0.9939 | ||
| nP-F7S3 | 39.41 | 1.27 | −0.005 | 0.9930 | ||
| nP-F6S4 | 48.13 | 1.55 | −0.002 | 0.9850 | ||
| nP-F5S5 | 60.23 | 1.81 | −0.004 | 0.9975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Ma, L.; Ngo, I.; Yu, K.; Xu, Y.; Zhai, J.; Gao, Q.; Peng, C.; Wang, D.; Alarifi, S.S.; et al. Experimental Investigation on Hydrophobic Alteration of Mining Solid Waste Backfill Material. Minerals 2024, 14, 580. https://doi.org/10.3390/min14060580
Zhao Z, Ma L, Ngo I, Yu K, Xu Y, Zhai J, Gao Q, Peng C, Wang D, Alarifi SS, et al. Experimental Investigation on Hydrophobic Alteration of Mining Solid Waste Backfill Material. Minerals. 2024; 14(6):580. https://doi.org/10.3390/min14060580
Chicago/Turabian StyleZhao, Zhiyang, Liqiang Ma, Ichhuy Ngo, Kunpeng Yu, Yujun Xu, Jiangtao Zhai, Qiangqiang Gao, Chengkun Peng, Dangliang Wang, Saad S. Alarifi, and et al. 2024. "Experimental Investigation on Hydrophobic Alteration of Mining Solid Waste Backfill Material" Minerals 14, no. 6: 580. https://doi.org/10.3390/min14060580







