Exploring the Composition of Egyptian Faience
Abstract
:1. Introduction
2. Historical Framework
 . Wood determinative could be related to the word shabti if we consider that they have the same root, the difference being that the latter has a mummiform figure as determinative
. Wood determinative could be related to the word shabti if we consider that they have the same root, the difference being that the latter has a mummiform figure as determinative  . The term SAwAbty (shawabty)
. The term SAwAbty (shawabty)  identified wood figurines during the 17th Dynasty. In the Third Intermediate Period, the term wSbty
identified wood figurines during the 17th Dynasty. In the Third Intermediate Period, the term wSbty  (the who that responds) emerged, emphasising the role of the figurines in responding to their owner’s needs in the afterlife [12]. SAb (food) also links shabti to sustenance, depicting it as the one who provides sustenance. In the First Prophet Pinedjem II’s figurines, the term “ushabti” first appears in hieroglyphs of Chapter 6 in the Book of the Dead, replacing “shabti” in the Late Period. Statuettes of Masaharta, Pinedjem I’s son, feature a distinctive headband known as a “seshed”, a characteristic of figurines from the 21st and 22nd dynasties. This feature provides dating. Shabtis have simplified forms and material uniformity in his period with ordinary terracotta and Faience. Shabti inscriptions become shorter and are written in a single column with the name and title of the deceased. The figurines turn into amulets with magical properties after this age. Table 1 is the summary of the principal features and evolution of shabtis.
(the who that responds) emerged, emphasising the role of the figurines in responding to their owner’s needs in the afterlife [12]. SAb (food) also links shabti to sustenance, depicting it as the one who provides sustenance. In the First Prophet Pinedjem II’s figurines, the term “ushabti” first appears in hieroglyphs of Chapter 6 in the Book of the Dead, replacing “shabti” in the Late Period. Statuettes of Masaharta, Pinedjem I’s son, feature a distinctive headband known as a “seshed”, a characteristic of figurines from the 21st and 22nd dynasties. This feature provides dating. Shabtis have simplified forms and material uniformity in his period with ordinary terracotta and Faience. Shabti inscriptions become shorter and are written in a single column with the name and title of the deceased. The figurines turn into amulets with magical properties after this age. Table 1 is the summary of the principal features and evolution of shabtis.3. Materials and Methods
3.1. Materials
3.2. Methods
Optical Microscope (OM)
3.3. Scanning Electron Microscopy with Energy Dispersive Spectrometry (SEM-EDS)
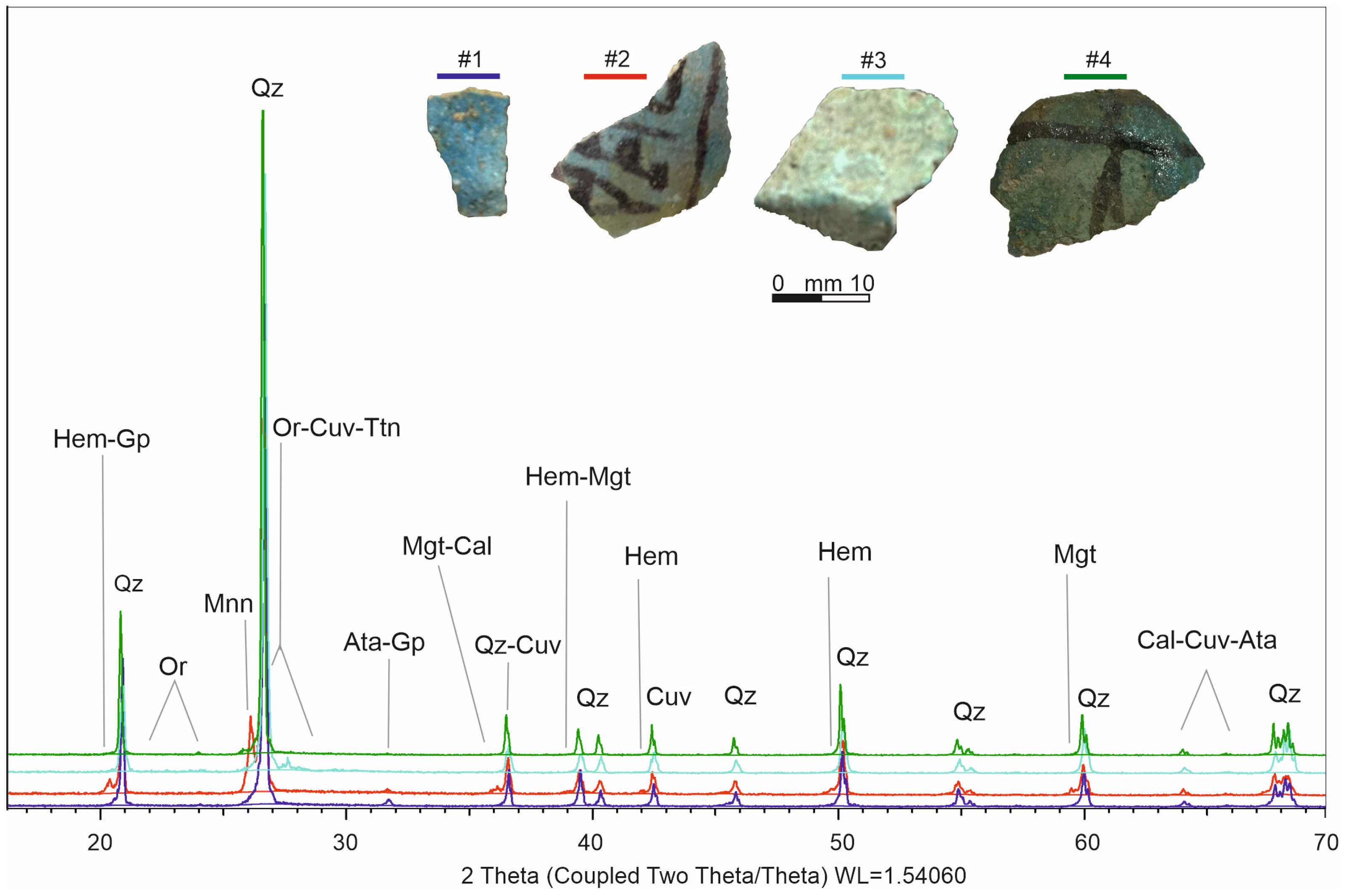
3.4. X-ray Powder Diffraction (XRPD)
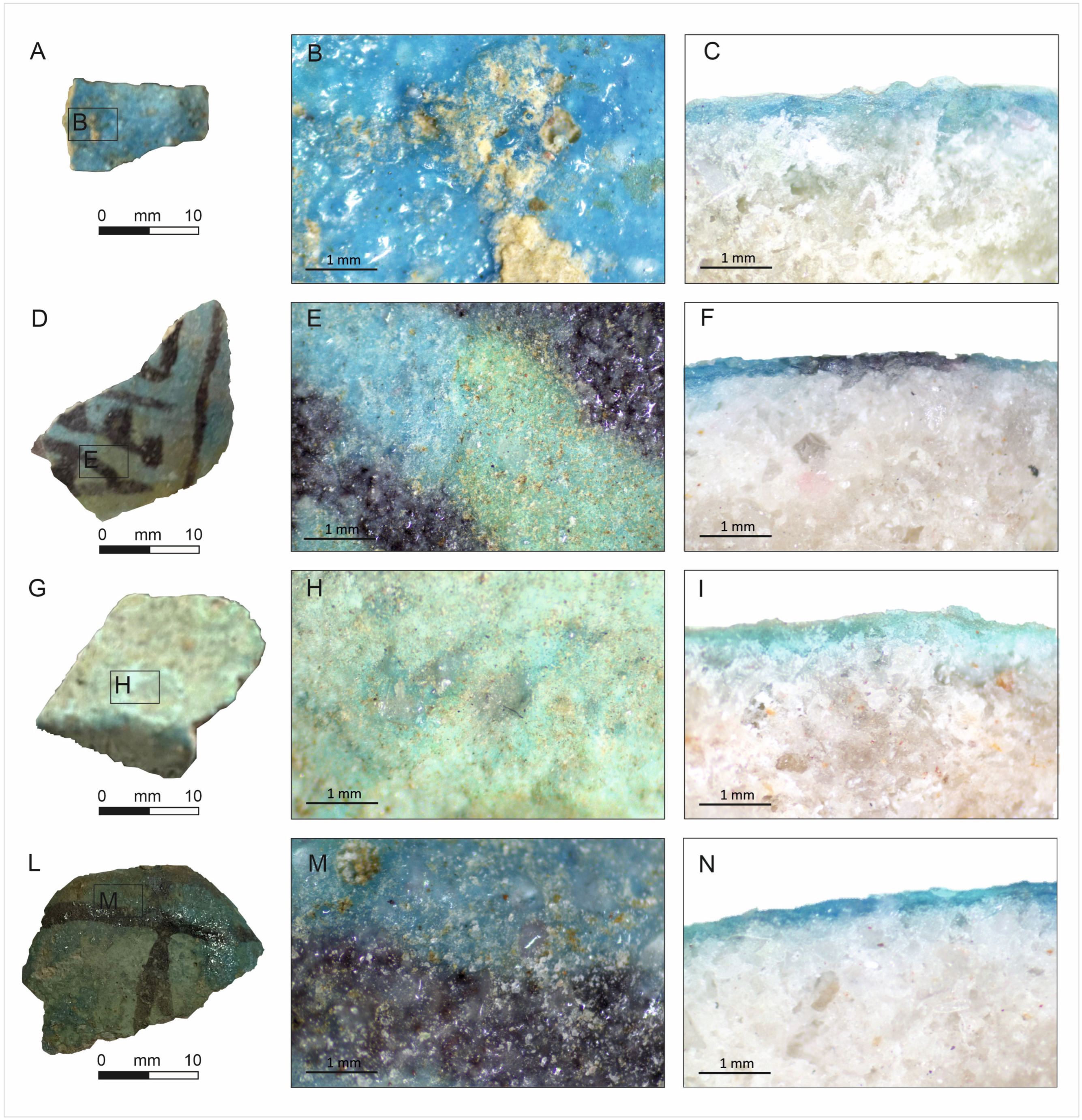
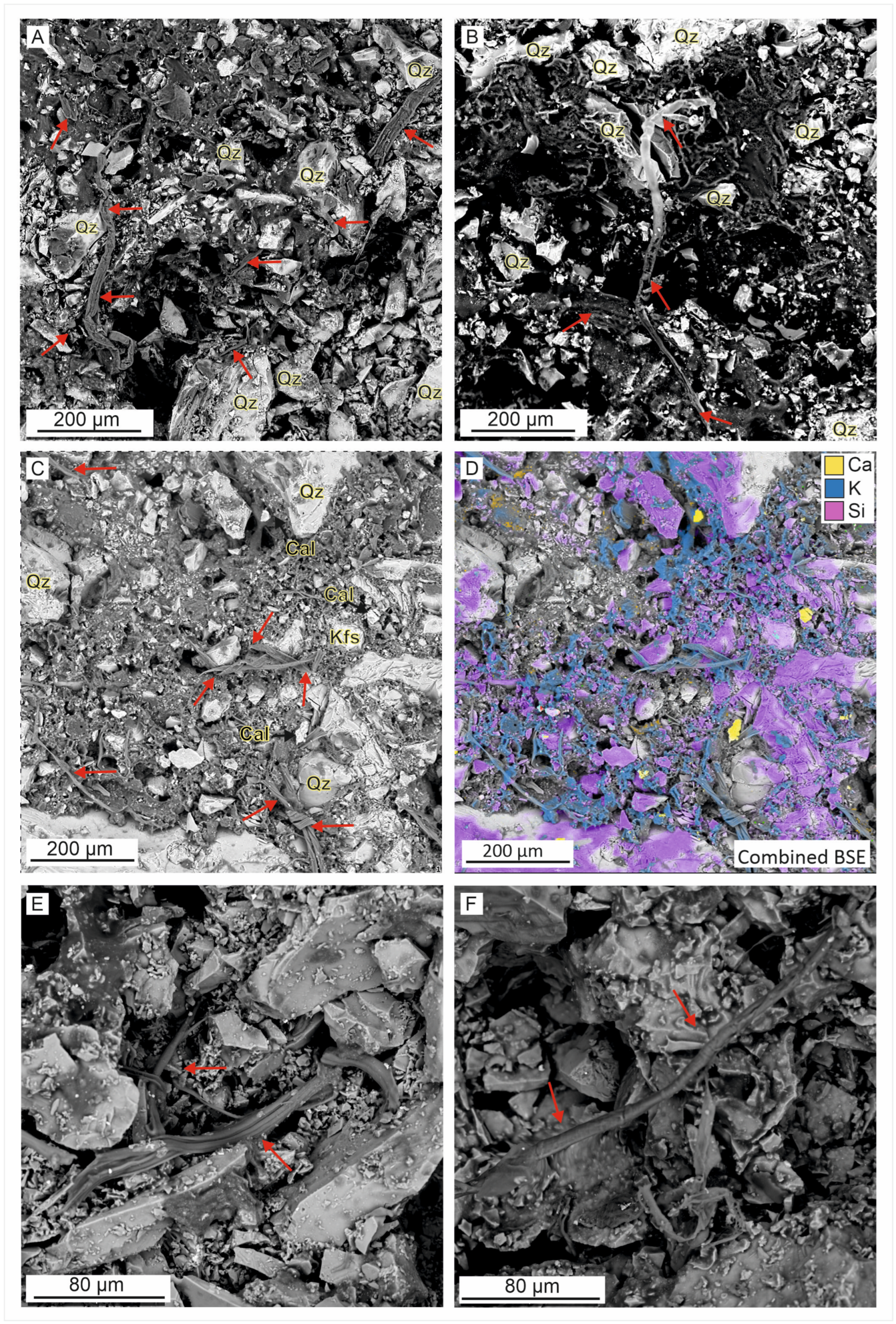
4. Descriptions and Results
4.1. Scanning Electron Microscopy with Energy Dispersive Spectrometry (SEM-EDS) and XRPD
Matrix
4.2. Minerals by XRPD and SEM-EDS
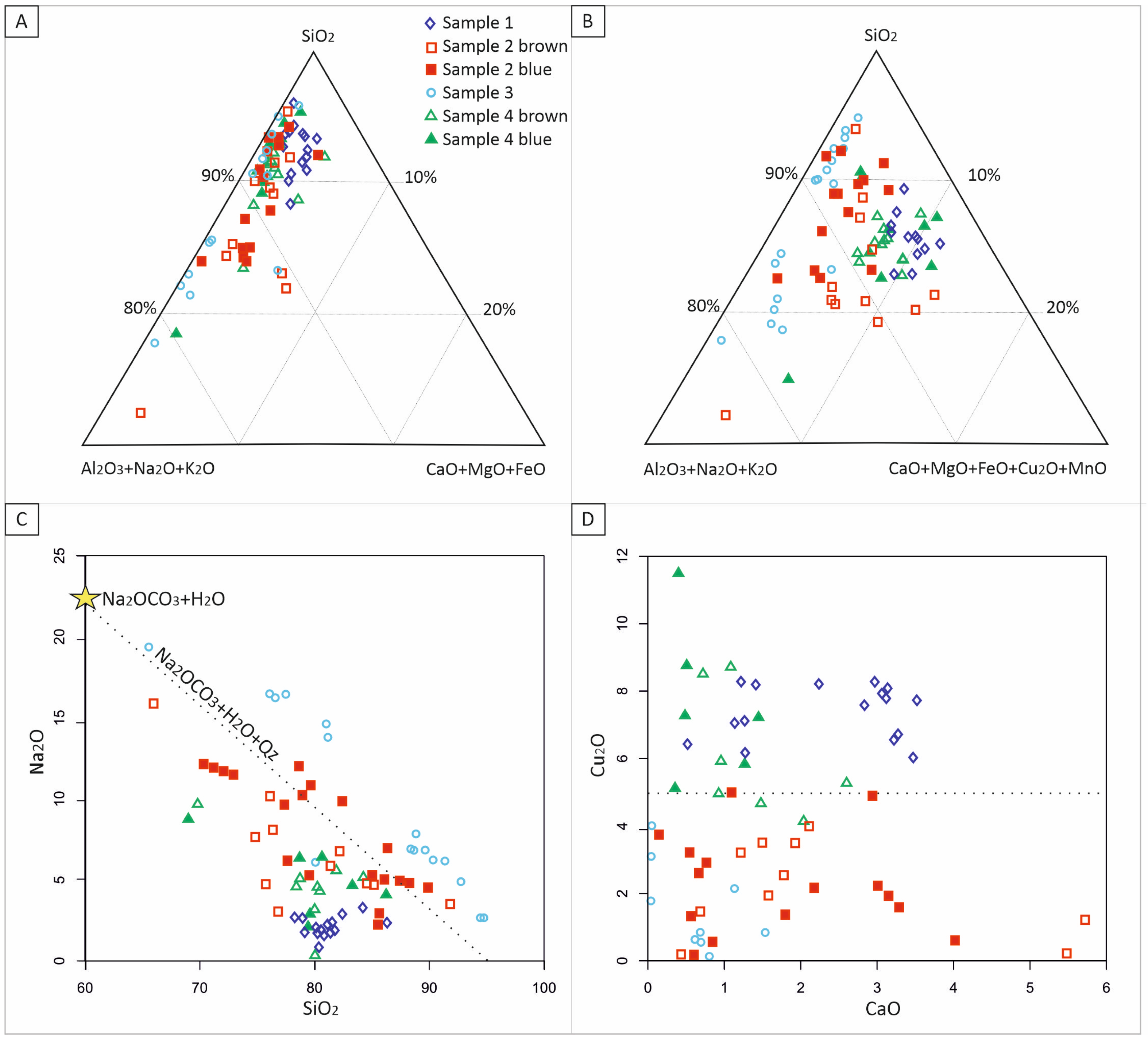
4.3. Glaze Glass
5. Discussion
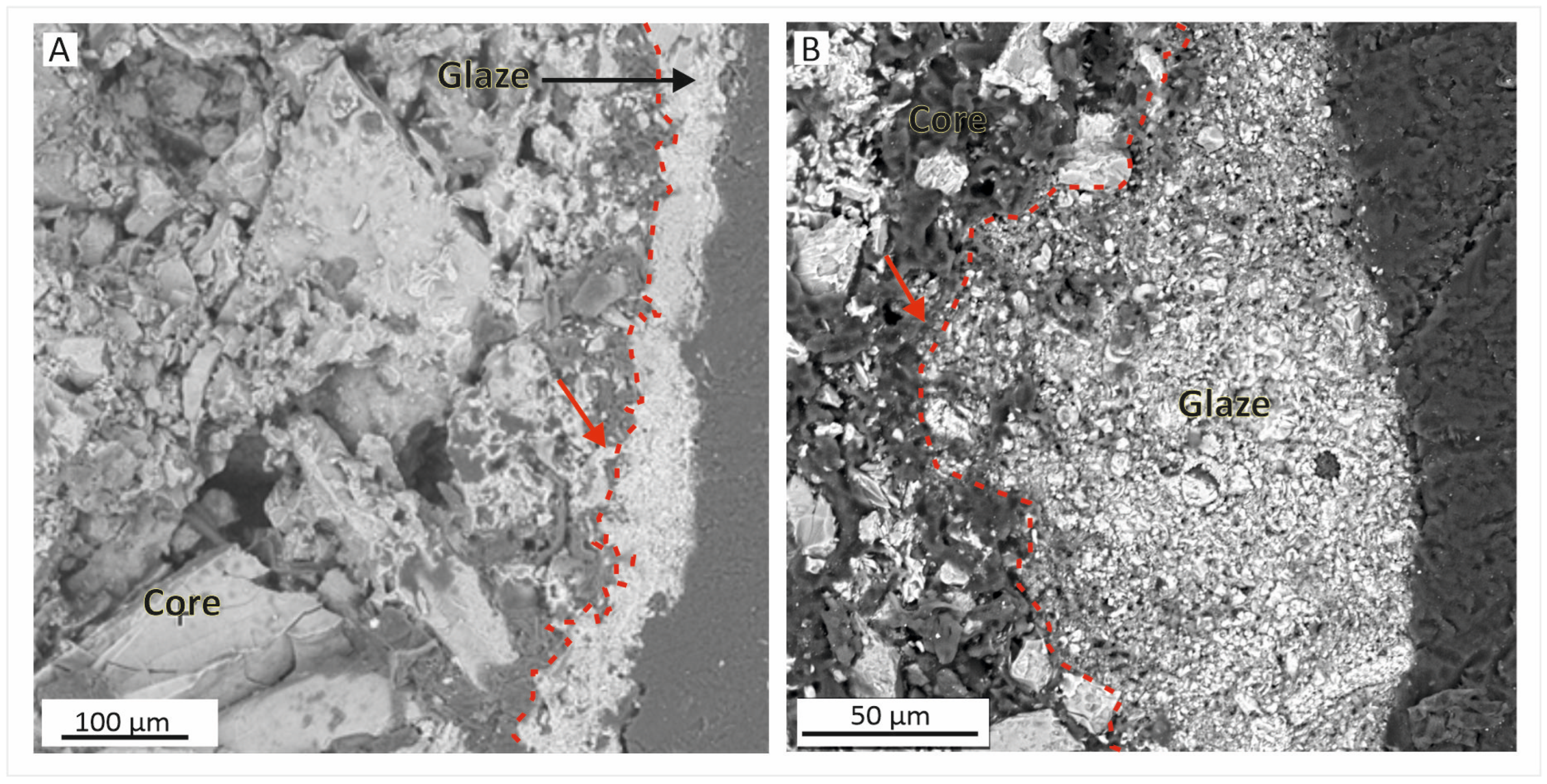
5.1. Matrix–Core
5.2. Glaze
6. Technology of Production
7. Conclusions
- Our study centred on a modest yet compositionally interesting collection of shabti fragments and aimed to delve deeper into the glaze’s material components and shabtis’ inner core. Through chemistry and mineralogy examination, we discovered that the core comprises a silica-dominant silt mixture blended with organic substances, vegetable fibres, and natron additives. The identification of cotton fibres highlights the hitherto rarely detected Egyptian Faience associated with linen. This intricate blend facilitated the creation of ushabti figurines that underwent a series of processes, including modelling, drying, colouring with glaze, and firing.
- Furthermore, examination of the shabti revealed a single firing event with minimal impact on the body, suggesting a relatively short firing time and insufficient flux salts in the matrix. Glaze composition analysis confirmed the presence of sodic silica glass with low potassium content, indicating a firing temperature between 920 °C and 1050 °C.
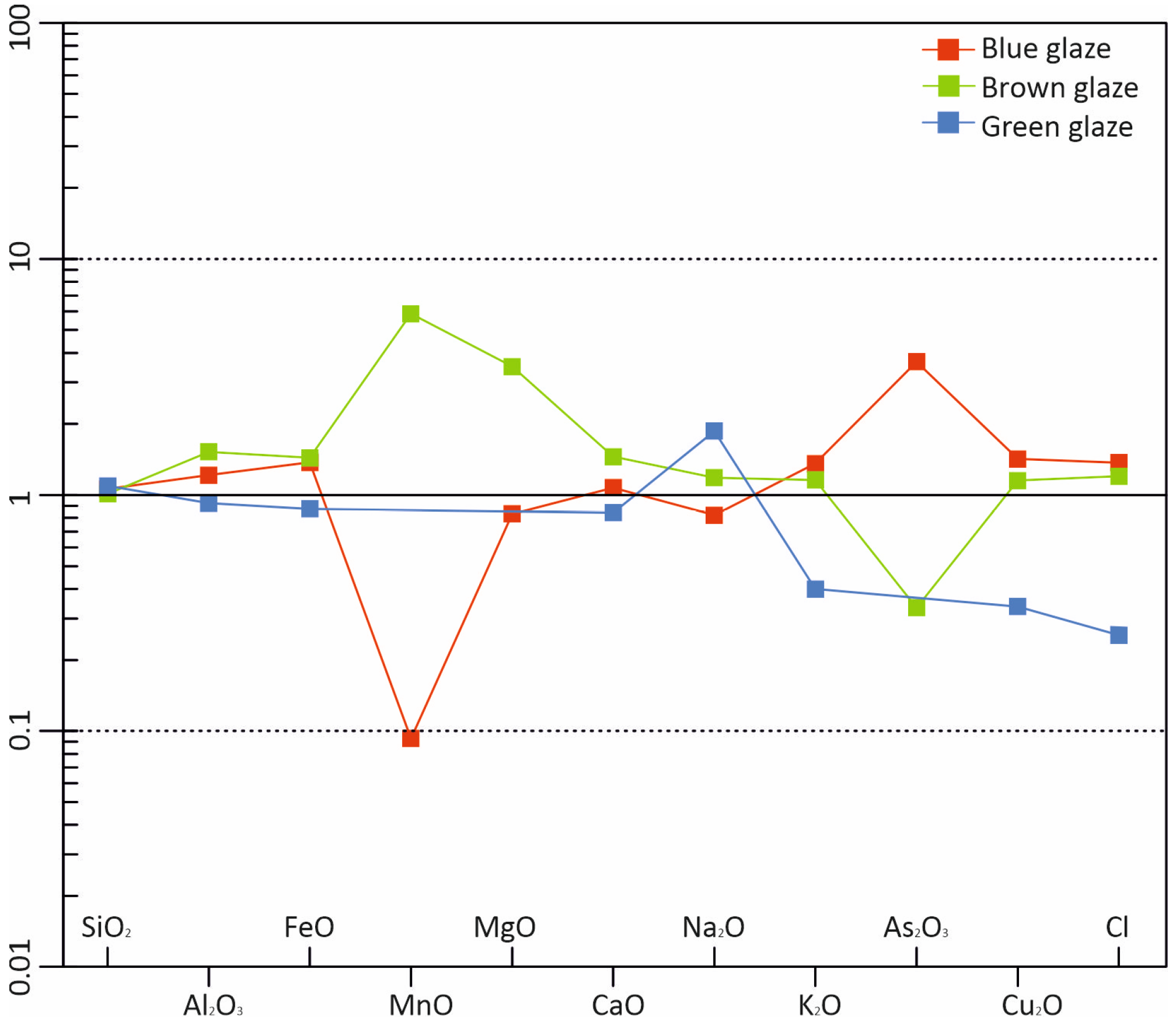
- The pigments predominantly consisted of manganese (Mn) and copper (Cu) compounds, with the potential addition of Mg and As for specific colour variations. The Cu/Ca ratio is crucial to give a blue or green colour. Upon contact with the silica matrix, sodium metasilicate and sulphate compounds form a protective shell, ensuring cohesion within the fragile matrix.
- Our study gives new chemical and mineralogical data and unravels some oddities in shabti production techniques, shedding light on the multifaceted nature of Egyptian crafting and its significance in ancient Egyptian civilisation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandiver, P.B.; Kingery, W.D. Egyptian Faience, The First High-Tech Ceramic. In Ceramics and Civilisation III: High-Technology Ceramics. Past, Present and Future; Kingery, W.D., Ed.; American Ceramics Society: Columbia, OH, USA, 1987; pp. 19–34. [Google Scholar]
- Nicholson, P.T.; Henderson, J. Ancient Egyptian Materials and Technology; Cambridge University Press: Cambridge, UK, 2000; pp. 195–224. ISBN 0521452570. [Google Scholar]
- Kiefer, C.; Allibert, A. Pharaonic Blue Ceramics: The Process of Self-Glazing. Am. J. Archaeol. 1974, 24, 107–117. [Google Scholar]
- Kaczmarczyk, A.; Hedges, R.E.M. Ancient Egyptian Faience: An Analytical Survey of Egyptian Faience from Predynastic to Roman Times; Aris & Phillips: Warminster, UK, 1983. [Google Scholar]
- Shortland, A.J.; Tite, M.S. Technological study of Ptolemaic—Early Roman Faience from Memphis, Egypt. Archaeometry 2005, 47, 31–46. [Google Scholar] [CrossRef]
- Nicholson, P.T. Egyptian Faience and Glass; Shire Publications LTD.: Buckinghamshire, UK, 1993. [Google Scholar]
- Delfa, S.L.; Formisano, V.; Ciliberto, E. Laboratory Production of Egyptian Faiences and Their Characterisation. J. Cult. Herit. 2008, 9, e113–e116. [Google Scholar] [CrossRef]
- Kakoulli, I. Egyptian blue in Greek painting between 2500 and 50 BC. In From Mine to Microscope: Advances in the Study of Ancient Technology; Shortland, A., Freestone, I., Rehren, T., Eds.; Oxbow Books: Oxford, UK, 2009; pp. 101–112. [Google Scholar]
- Siddall, R. Mineral Pigments in Archaeology: Their Analysis and the Range of Available Materials. Minerals 2018, 8, 201. [Google Scholar] [CrossRef]
- Frame, L.; DeSordao, D.B.; Chiang, Y.C.; Vandiver, P. Methods of Faience Manufacture in Antiquity: Investigation of Colorants and Technological Processes. In Materials Issues in Art and Archaeology VIII; Vandidver, P.B., McCarthy, B., Tykot, R.H., Ruvalcaba-Sil, J.L., Casadio, F., Eds.; MRS Proceedings; Cambridge University: Cambridge, UK, 2011; Volume 1319, p. 43. [Google Scholar]
- Schneider, H. Shabtis: An Introduction to the History of Ancient Egyptian Funerary Statuettes with a Catalogue of the Collection of Shabtis in the National Museum of Antiquities at Leiden; National Museum of Antiquities: Leiden, The Netherlands, 1977. [Google Scholar]
- Rosmorduc, S. Sesh Documentation. Available online: http://jseshdoc.qenherkhopeshef.org (accessed on 12 June 2014).
- Aubert, J.F.; Aubert, L. Statuettes funéraires égyptiennes du département des Monnaies, Médailles et Antiques; Éditions de la Bibliothèque Nationale de France; Bibliothèque Nationale de France-BNF: Paris, France, 2005. [Google Scholar] [CrossRef]
- Janes, G. The Shabti collections 5. In A Selection from the Manchester Museum England; The Amadeus Press: Huddersfield, UK, 2012; 500p, ISBN 978-0-9566271-5-5. [Google Scholar]
- Gama, A. Os Servidores Funerários da Coleção Egípcia do Museu Nacional: Catálogo e Interpretação. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Janes, G. Shabtis a Private View. In Ancient Egyptian Funerary Statuettes in European Private Collections; Éditions Cybèle: Paris, France, 2002. [Google Scholar]
- Aubert, J.F. Statuettes Égyptiennes: Chaouabtis, Ouchebtis; Librairie d’Amérique et d’Orient Adrien Maisonneuve: Paris, France, 1974. [Google Scholar]
- Stewart, H.M. Egyptian Shabtis Buckinghamshire; Shire Publications: London, UK, 1995. [Google Scholar]
- Brancaglion, J. Manual de Arte e Arqueologia do Egito Antigo II; Sociedade dos Amigos do Museu Nacional: Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Hornung, E.; Krauss, R.; Warburton, D.A. Ancient Egyptian Chronology; Brill: Boston, MA, USA, 2006. [Google Scholar]
- Ahmed, E.H. Brief of Natural Fibers in Historical Textiles Manufacturer. Trends Text. Eng. Fash. Technol. 2022, 6, 765–767. [Google Scholar] [CrossRef]
- Carbonaro, A.B.; Caruso, R.; Greco, V.; El-Aguizy, O.; Ali, M.F.; Gambino, G.L.; Giuffrida, A.; Ciliberto, E. First evidence of fractionation on stable organic carbon isotopes in ancient Egyptian linen textile fibress during degradation. J. Cult. Heritage 2023, 63, 194–200. [Google Scholar] [CrossRef]
- El Messiry, M.; Mohsen, S.A. Characterisation of Egyptian cotton fibres. J. Fibres Text. Res. 2013, 38, 109–113. [Google Scholar]
- Lee, L.; Quirke, S. Painting Materials. In Ancient Egyptian Materials and Technology; Nicholson, P.T., Shaw, I., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 104–120. [Google Scholar]
- Moorey, P.R.S. Materials and Manufacture in Ancient Mesopotamia: The Evidence of Archaeology and Art. Metals and Metalwork, Glazed Materials and Glass; BAR International Series 237; Bar Publications: Oxford, UK, 1985. [Google Scholar]
- Miller, A.; Johnson, B.; Garcia, C. Enhancing Cob Material Properties with Fibres and Lye Treatments. J. Sustain. Constr. 2000, 15, 124–137. [Google Scholar]
- Henderson, J. Ancient Glass: An Interdisciplinary Exploration; Cambridge University Press: Cambridge, UK, 2013; ISBN 9781139021883. [Google Scholar] [CrossRef]
- Stocks, A. Derivation of Ancient Egyptian Faience Core and Glaze Materials. Antiquity 1997, 71, 179–182. [Google Scholar] [CrossRef]
- Tite, M.S.; Freestone, J.C.; Rimson, M. Egyptian Faience: An investigation of production methods. Archaeometry 1983, 25, 17–27. [Google Scholar] [CrossRef]
- Aramaki, S.; Roy, R. Revised phase diagram for the system Al2O3—SiO2. J. Am. Ceram. Soc. 1962, 45, 229–242. [Google Scholar] [CrossRef]
- Kilikoglou, V.; Vekinis, G.; Maniatis, Y.; Day, P.M. Mechanical performance of quartz-tempered ceramics: Part I. Strength and toughness. Archaeometry 1998, 40, 261–279. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Egyptian blue. In The Pigment Compendium: Optical Microscopy of Historical Pigments; Elsevier Butterworth Heinemann: Oxford, UK, 2004; pp. 147–148. [Google Scholar]
- Kaczmarczyk, A.; Lahanier, C. Ancient Egyptian Frits and Coloured Faience Bodies: Problems of Classification. In Application of Science in Examination of Works of Art; England, P.A., Van Zelst, L., Eds.; Museum of Fine Arts: Boston, MA, USA, 1985. [Google Scholar]
- Ibrahima, M.M.; Hassib, A.A.; Omara, S. Deterioration Aspects of the Egyptian Faience Ushabti. Statuette of the King Aspelta kept in Atfih Magazine, Egypt. Adv. Res. Conserv. Sci. 2022, 3, 1–14. [Google Scholar] [CrossRef]
- Matin, M. Egyptian Faience glazing by the cementation method part 1: An investigation of the glazing powder composition and glazing mechanism. J. Archaeol. Sci. 2012, 39, 763–776. [Google Scholar] [CrossRef]
- Abubakr, M.; Fouad, A.M. Color Alteration of Ancient Egyptian Blue Faience. Int. J. Archit. Herit. 2013, 7, 261–274. [Google Scholar] [CrossRef]
- Helmi, F.; Abdel-Rehim, N. Study of Color Conversion By Time In Ancient Egyptian Faience Artifacts. Sci. Cult. 2016, 2, 17–23. [Google Scholar] [CrossRef]
- Pradell, T.; Salvado, N.; Hatton, G.D.; Tite, M.S. Physical Processes Involved in Production of the Ancient Pigment, Egyptian Blue. J. Am. Ceram. Soc. 2006, 89, 1426–1431. [Google Scholar] [CrossRef]
- Mao, Y. Lead-Alkaline Glazed Egyptian Faience: Preliminary Technical Investigation of Ptolemaic Period Faience Vessels in the Collection of the Walters Art Gallery. J. Am. Inst. Conserv. 2000, 39, 185–204. [Google Scholar] [CrossRef]
- Nicholson, P.T. Working in Memphis, The Production of Faience at Roman Period Kom Helul; Egypt Exploration Society: London, UK, 2013. [Google Scholar]
- Pradell, T.; Salvado, N.; Hatton, G.D.; Tite, M.S. Painting the Palace of Apries I: Ancient binding media and coatings of the reliefs from the Palace of Apries, Lower Egypt. Herit. Sci. 2018, 6, 1–20. [Google Scholar] [CrossRef]
- Riccardelli, C. Egyptian Faience: Technology and Production. In Heilbrunn Timeline of Art History; The Metropolitan Museum of Art: New York, NY, USA, 2017; Available online: http://www.metmuseum.org/toah/hd/egfc/hd_egfc.htm (accessed on 1 April 2024).
- Jaksch, H.; Seipel, W.; Weiner, K.L. Egyptian blue—Cuprorivaite a win-dow to ancient Egyptian technology. Naturwissenschaften 1983, 70, 525–535. [Google Scholar] [CrossRef]
- Casolino, C.; Falcone, F.; Perna, M.G.; Metalla, E.; Rosatelli, G.; Stoppa, F.; Antonelli, S. Exploring Durrës between East and West: Discovery of a protostonepaste—Archaeological context and archaeometric analysis. Heritage Sci. 2024, 12, 84. [Google Scholar] [CrossRef]
- Perna, M.G.; Falcone, F.; Casolino, C.; Metalla, E.; Rosatelli, G.; Antonelli, S.; Stoppa, F. Analysing the glaze of a medieval ceramic fragment from the Durres Amphitheater in Albania. Herit. Sci. 2024, 12, 82. [Google Scholar] [CrossRef]
- Belousov, M.V.; Selivanov, E.N.; Gulyaeva, R.I.; Tyushnyakov, S.N.; Rakipov, D.F. Thermodynamics and kinetics of dolomite. Izvestiya. Non-Ferr. Metall. 2016, 57, 18–25. [Google Scholar] [CrossRef]
- Tite, M.S.; Shortland, A.J.; Nicholson, P.T.; Jackson, C.M. The Use of Copper and Cobalt Colourants in Vitreous Materials in Ancient Egypt. In La Couleur Dans La Peinture et L’Émaillage de L’Égypte Ancienne; Colinart, S., Menu, M., Eds.; Edipuglia: Bari, Italy, 1998; pp. 112–113. [Google Scholar]
- Colomban, P.; Kırmızı, B.; Simsek Franci, G. Cobalt and Associated Impurities in Blue (and Green) Glass, Glaze and Enamel: Relationships between Raw Materials, Processing, Composition, Phases and International Trade. Minerals 2021, 11, 633. [Google Scholar] [CrossRef]


| Period | Dynasty | Materials | Inscriptions | Characteristics and Functions |
|---|---|---|---|---|
| Middle Kingdom ca. 1980–1760 BC | 11–12th Dyn. | Stone, less frequently, wood. | Rare inscriptions, some bear the name and title of the owner. Occasionally, the shabti spell. | Mummiform appearance: transition to shabtis as labourers in the afterlife, guided by the shabti spell [11,13]. |
| Second Intermediate Period | 17th Dyn. | Wood. | It may contain an offer formula. | The variant shawabty, made from Persea wood, is called a stick and has a simple and raw shape [14]. |
| New Kingdom ca. 1539–1077 BC | 18th Dyn. | Hard rock-type granite and later alabaster were used. | Name and titles of the owner. Shabti spell. | The objects are crafted in the shape of mummies (mummiform) and often feature agricultural tools such as hoes, baskets, and sacks in their painted or embossed hands as part of their iconography, symbolising the shabti’s role as a servant in the afterlife. [14,15]. |
| End of 18th–19th Dyn. | Wood and stone were replaced by ceramic and Faience. | Name and titles of the owner. Shabti spell. | The deceased wore the dress of daily life [16]. | |
| Third Intermediate Period ca. 1076–723 BC | 21–24th Dyn. | Faience was used frequently, rarely stone and wood. | The inscriptions are brief, with only Osiris’s epithet and the deceased’s title. | The shabti supervisors led groups of ten statuettes: 401 shabtis supervised by 36 reis. The term ushabti appeared for the first time. [12] |
| Late Period ca. 722–332 BC | 25th Dyn-2nd Persian Period. | Faience with inscriptions engraved. A dorsal pillar and trapezoidal base [9]. | The ushabtis are inscribed with Chapter 6 of the Book of the Dead. | The strict rule of 401 figurines per tomb became flexible. The term ushabti was replaced shabti [15,16,17,18,19]. |
| Mineral | Chemical Formula | Mineral Phases Abundance % | |||
|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | ||
| Quartz | SiO2 | 70.5 | 74.7 | 76.4 | 73.9 |
| Cuprorivaite | CaCuSi4O10 | 4.50 | 2.20 | 5.50 | 3.10 |
| Magnetite | Fe3O4 | bdl | 1.30 | bdl | 1.80 |
| Atacamite | ClCu2H3O3 | 2.60 | bdl | 1.10 | 1.30 |
| Gypsum | CaSO4 | 4.60 | 2.90 | 7.10 | 2.40 |
| Calcite | CaCO3 | 4.80 | 1.00 | 2.60 | 1.81 |
| Hematite | Fe2O3 | bdl | 0.90 | bdl | 0.68 |
| Sphene | CaTiSiO5 | 3.20 | 2.50 | 3.90 | 2.80 |
| Manganite | MnO(OH) | bdl | 6.30 | bdl | 5.11 |
| Orthoclase | KAlSi3O8 | 9.70 | 8.20 | 3.40 | 7.30 |
| Point Analysis | Sample | SiO2 | Al2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | As2O3 | Cu2O | Cl | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface glaze | #1Blue | 83.3 | 0.24 | bdl | bdl | 2.59 | 2.26 | 2.10 | 0.09 | 7.48 | 1.97 | 2.59 | bdl |
| #2 Blue | 85.4 | 0.26 | bdl | bdl | 1.79 | 6.51 | 1.51 | 0.14 | 1.89 | 1.91 | 1.79 | 0.20 | |
| #2 Brown | 82.3 | 0.31 | bdl | 3.10 | 2.58 | 6.07 | 1.58 | bdl | 2.20 | 1.07 | 2.58 | bdl | |
| #3 Green | 88.4 | bdl | bdl | bdl | 0.63 | 9.13 | 0.48 | bdl | 1.23 | bdl | 0.63 | 0.17 | |
| #4 Blue | 80.9 | 0.49 | 0.27 | bdl | 0.46 | 5.34 | 1.10 | 0.93 | 5.62 | 1.19 | 0.46 | 0.26 | |
| #4 Brown | 81.9 | 0.38 | 0.26 | 1.00 | 1.39 | 5.24 | 1.21 | 0.11 | 6.02 | 1.12 | 1.39 | bdl | |
| * Alteration | 53.7 | 7.89 | 5.40 | bdl | 1.16 | 5.64 | 14.1 | 3.43 | bdl | 1.59 | 3.59 | 2.70 | |
| Core glaze | #1Blue | 83.7 | 0.22 | bdl | bdl | 0.04 | 2.34 | 2.42 | 1.95 | bdl | 7.39 | 1.94 | bdl |
| #2 Blue | 85.2 | 0.78 | bdl | bdl | 0.18 | 1.67 | 7.20 | 0.99 | bdl | 2.75 | 1.13 | bdl | |
| #2 Brown | 80.4 | 1.13 | bdl | 2.95 | 0.64 | 1.33 | 7.45 | 1.71 | bdl | 2.95 | 1.18 | bdl | |
| #3 Green | 85.6 | 0.20 | bdl | bdl | bdl | 0.91 | 10.6 | 0.49 | bdl | 1.41 | 0.65 | bdl | |
| #4 Blue | 80.9 | 1.52 | 0.31 | bdl | 0.15 | 0.79 | 5.75 | 1.68 | 0.33 | 7.43 | 0.90 | bdl | |
| #4 Brown | 81.8 | 1.24 | 0.47 | 1.46 | 0.23 | 1.61 | 5.77 | 1.03 | bdl | 4.99 | 1.15 | bdl | |
| Average glaze | All types | 83.6 | 0.59 | 0.09 | 0.66 | 0.16 | 1.65 | 6.03 | 1.36 | 0.08 | 4.30 | 1.25 | 0.03 |
| Blue | 83.2 | 0.91 | 0.13 | bdl | 0.13 | 1.61 | 4.91 | 1.55 | 0.25 | 5.43 | 1.51 | 0.08 | |
| Brown | 81.6 | 0.88 | 0.21 | 2.13 | 0.38 | 1.72 | 6.13 | 1.38 | 0.03 | 4.04 | 1.13 | bdl | |
| Green | 87.0 | 0.10 | bdl | bdl | bdl | 0.77 | 9.84 | 0.49 | bdl | 1.32 | 0.33 | 0.08 | |
| Norm *. Values | Blue | 1.06 | 1.22 | 1.38 | 0.09 | 0.83 | 1.08 | 0.82 | 1.36 | 3.67 | 1.42 | 1.37 | 1.50 |
| Brown | 1.01 | 1.52 | 1.44 | 5.86 | 3.50 | 1.46 | 1.18 | 1.16 | 0.33 | 1.15 | 1.20 | bdl | |
| Green | 1.09 | 0.92 | 0.88 | bdl | bdl | 0.84 | 1.87 | 0.40 | bdl | 0.34 | 0.25 | 14.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcone, F.; Aquilino, M.; Stoppa, F. Exploring the Composition of Egyptian Faience. Minerals 2024, 14, 586. https://doi.org/10.3390/min14060586
Falcone F, Aquilino M, Stoppa F. Exploring the Composition of Egyptian Faience. Minerals. 2024; 14(6):586. https://doi.org/10.3390/min14060586
Chicago/Turabian StyleFalcone, Francesca, Maria Aquilino, and Francesco Stoppa. 2024. "Exploring the Composition of Egyptian Faience" Minerals 14, no. 6: 586. https://doi.org/10.3390/min14060586
APA StyleFalcone, F., Aquilino, M., & Stoppa, F. (2024). Exploring the Composition of Egyptian Faience. Minerals, 14(6), 586. https://doi.org/10.3390/min14060586






