Geochemical Characteristics of Soil Rare Earth Elements within Spontaneous Combustion Coalfields of Rujigou Coal Mine
Abstract
:1. Introduction
2. Overview of the Study Area and Explanation of Materials
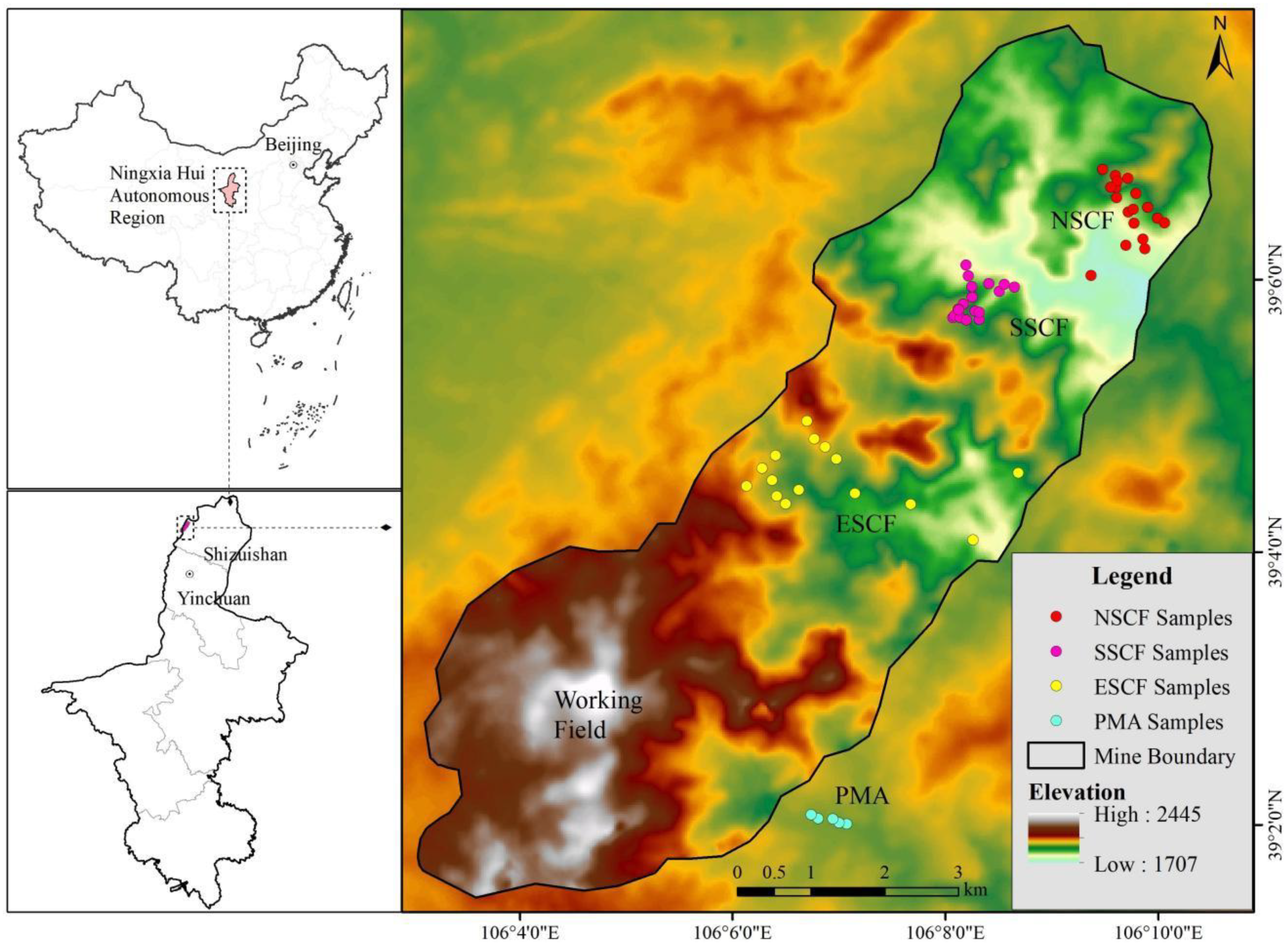
2.1. Overview of the Rujigou Mining Area
2.2. Sample Preparation and Chemical Assay
3. Results
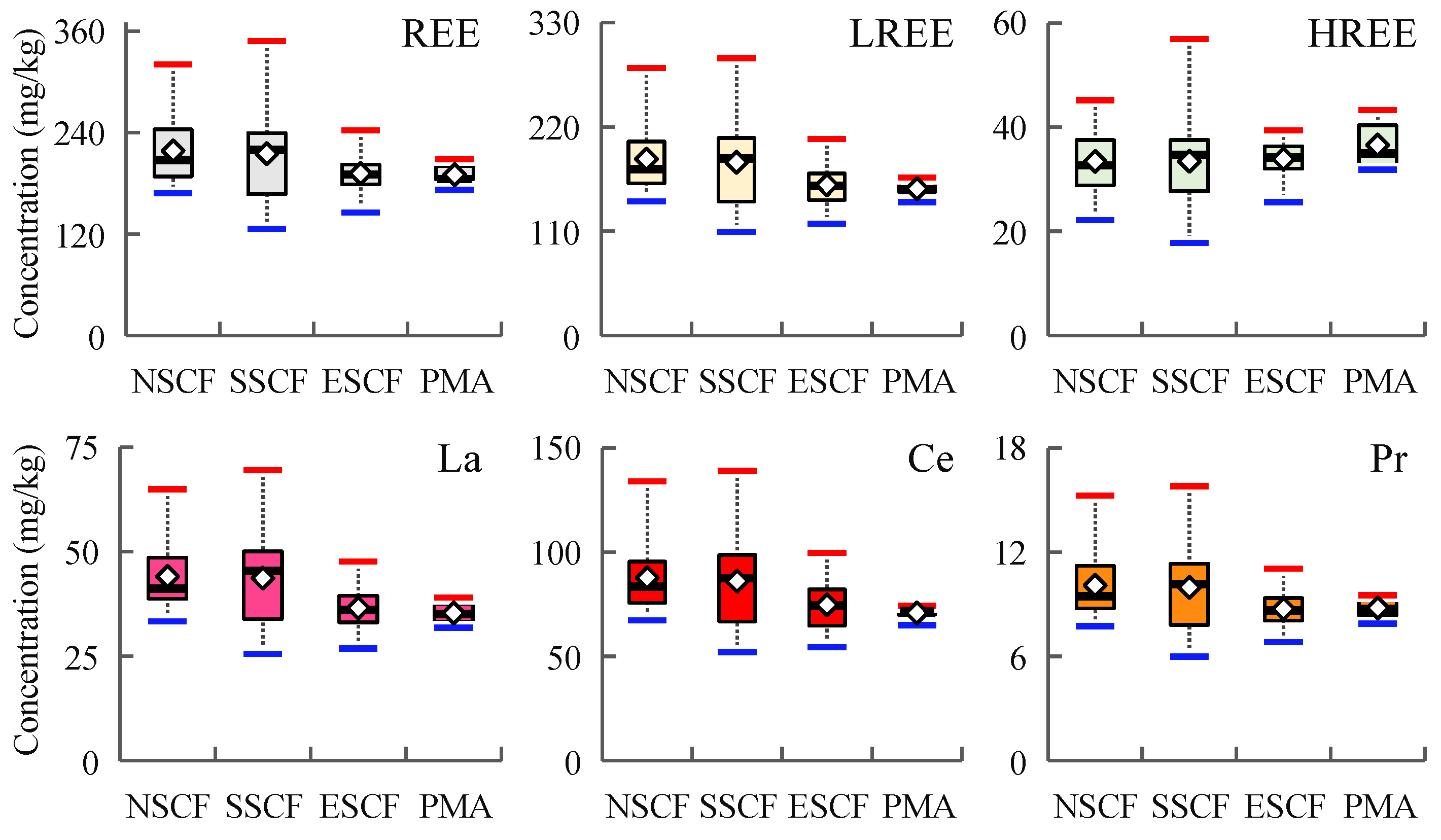
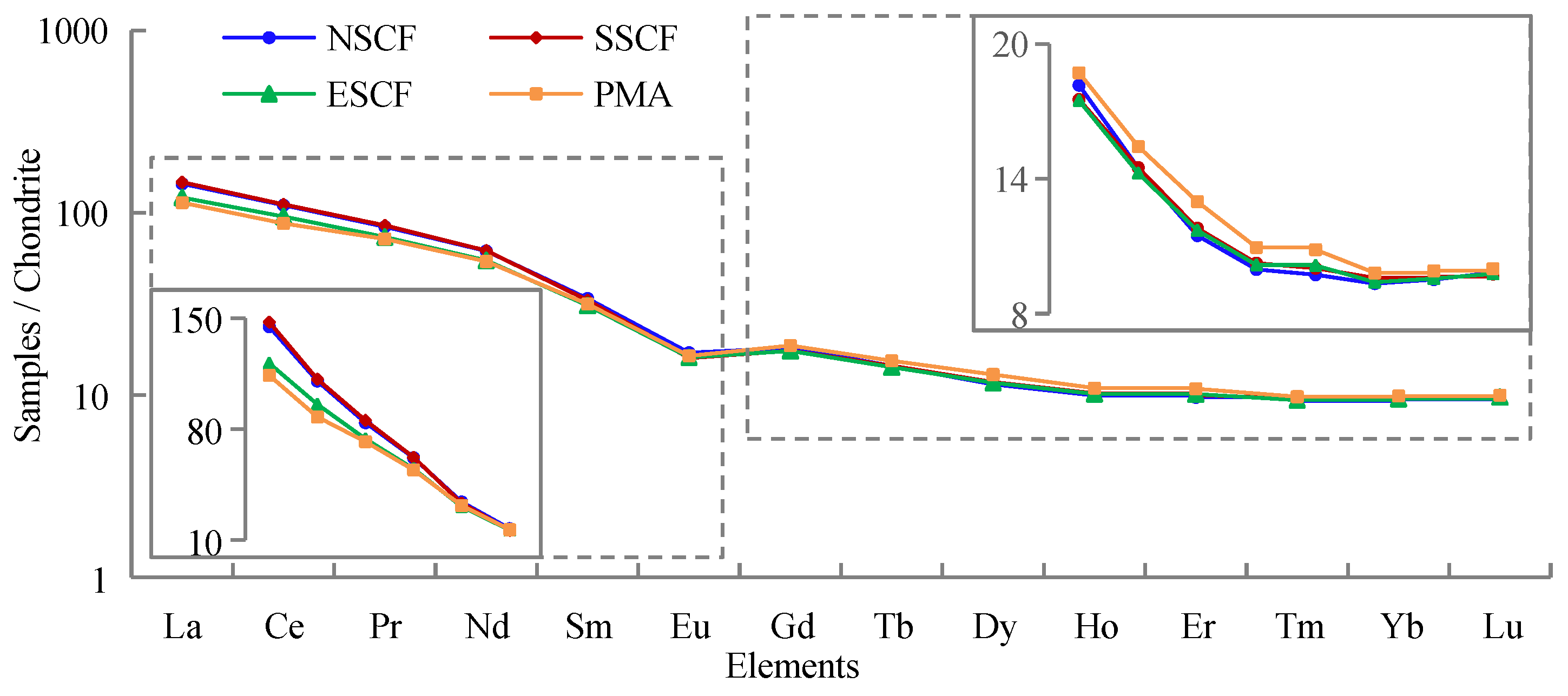
3.1. REE Concentration of Mine Area and Its Periphery
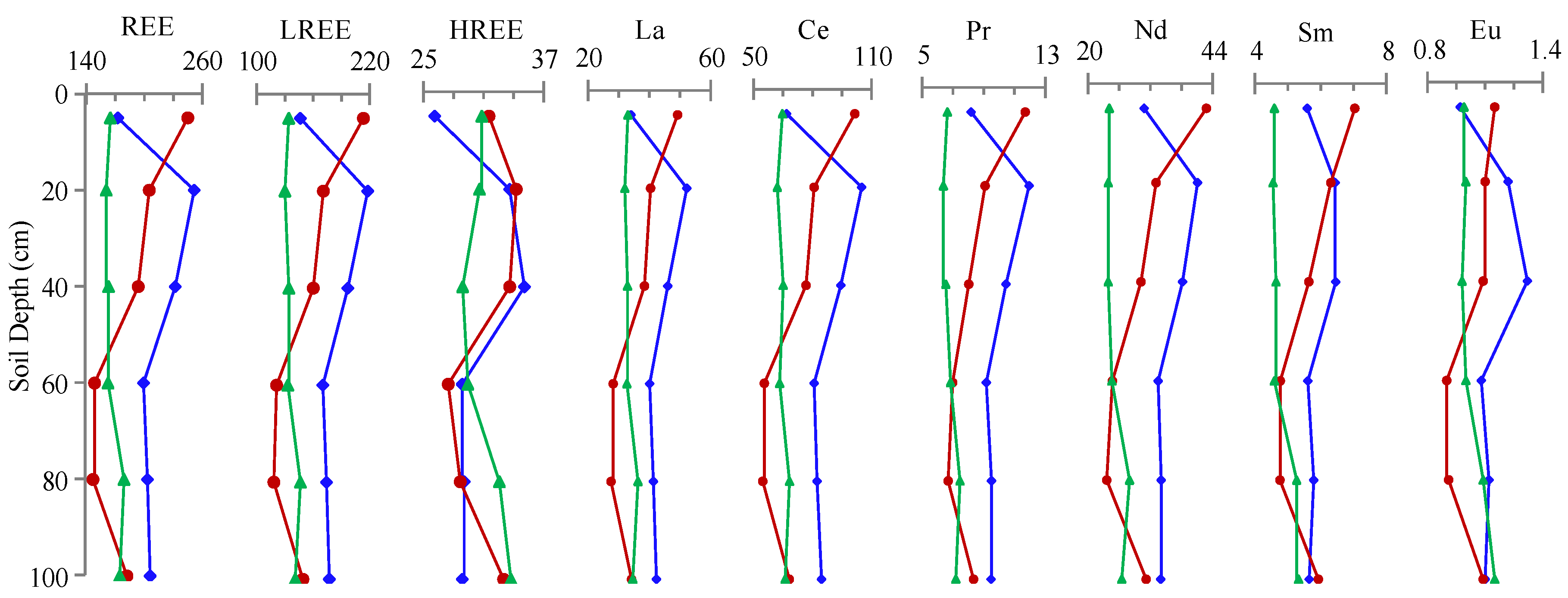
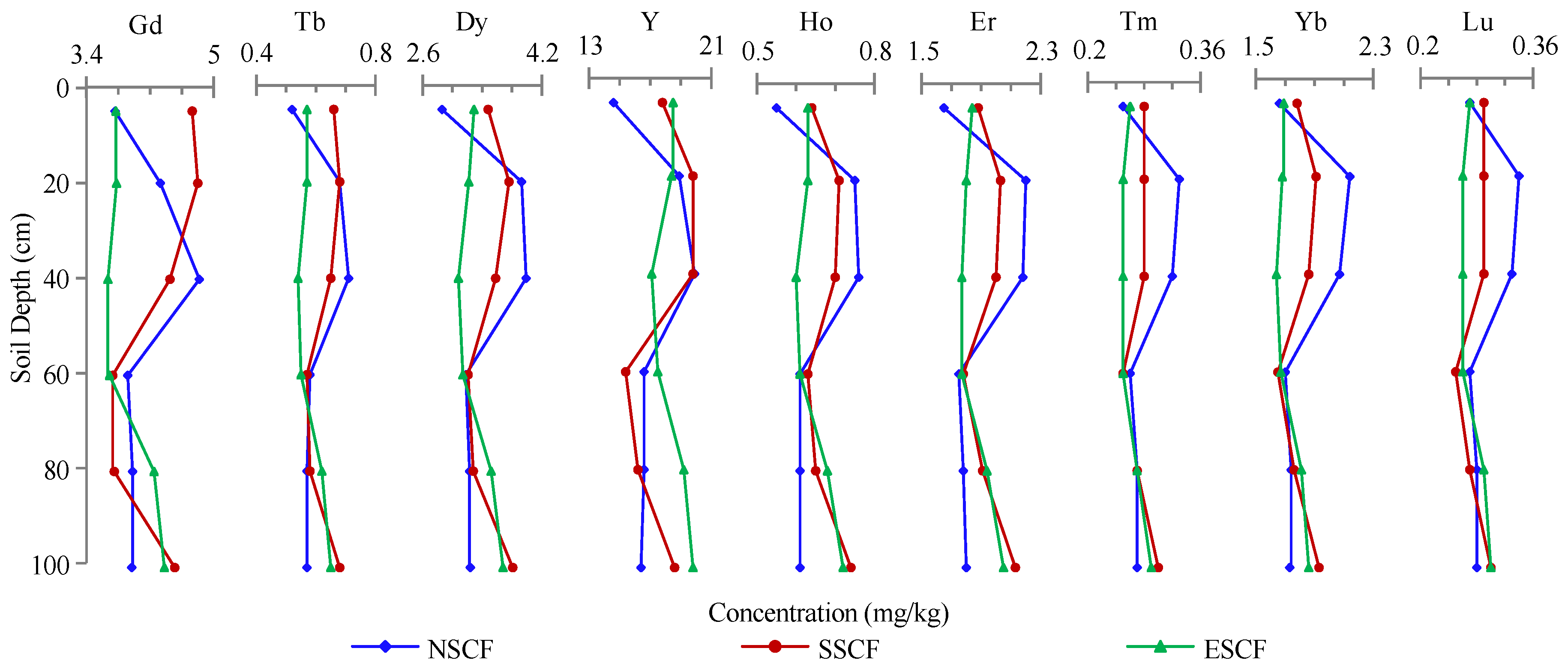
3.2. REE Concentration of the Soil Profiles
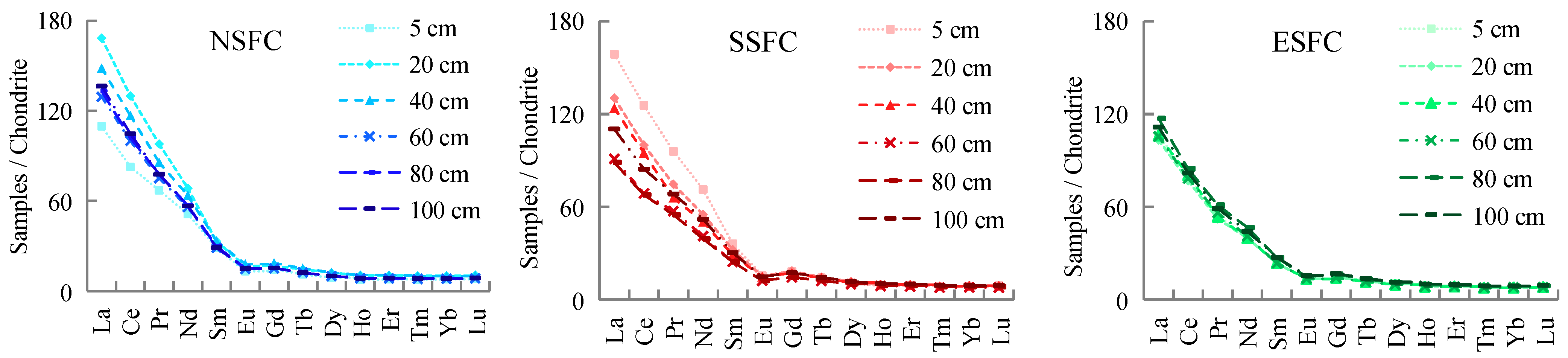
3.3. Distribution Patterns of Light and Heavy Rare Earth Elements
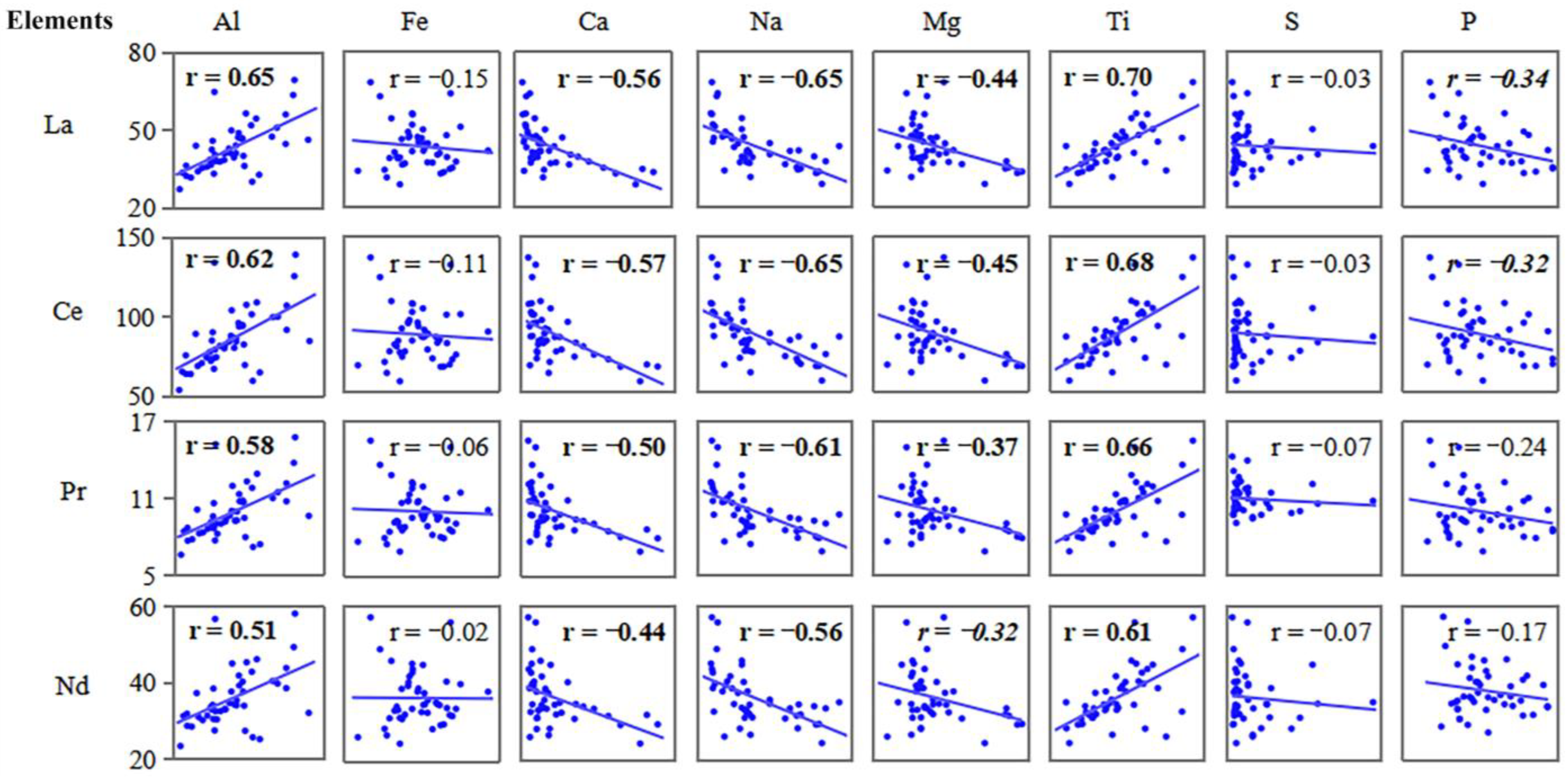
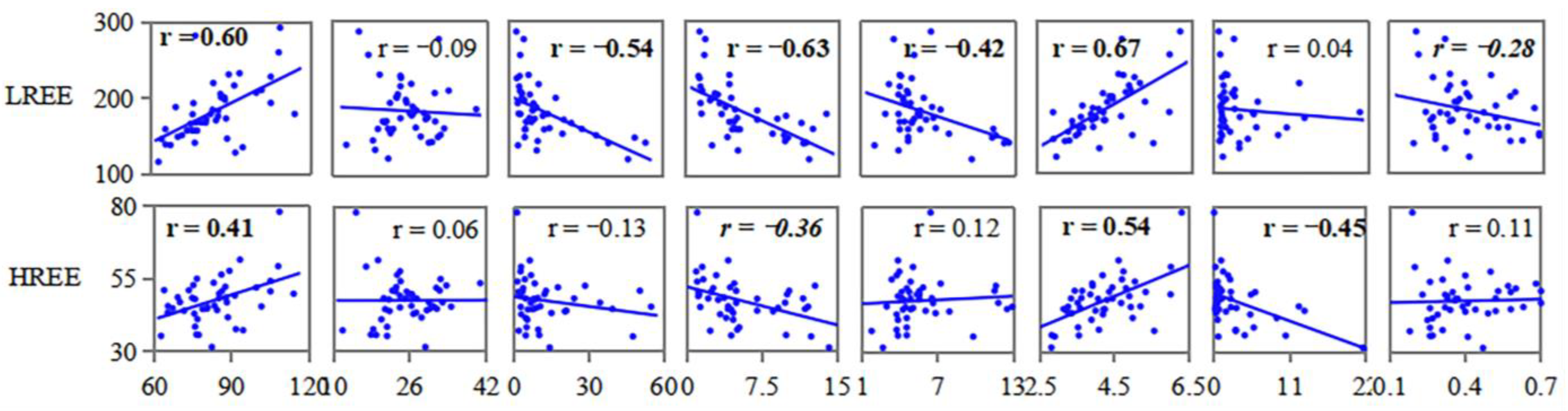
3.4. Relationships between Common Elements and REE
4. Discussion
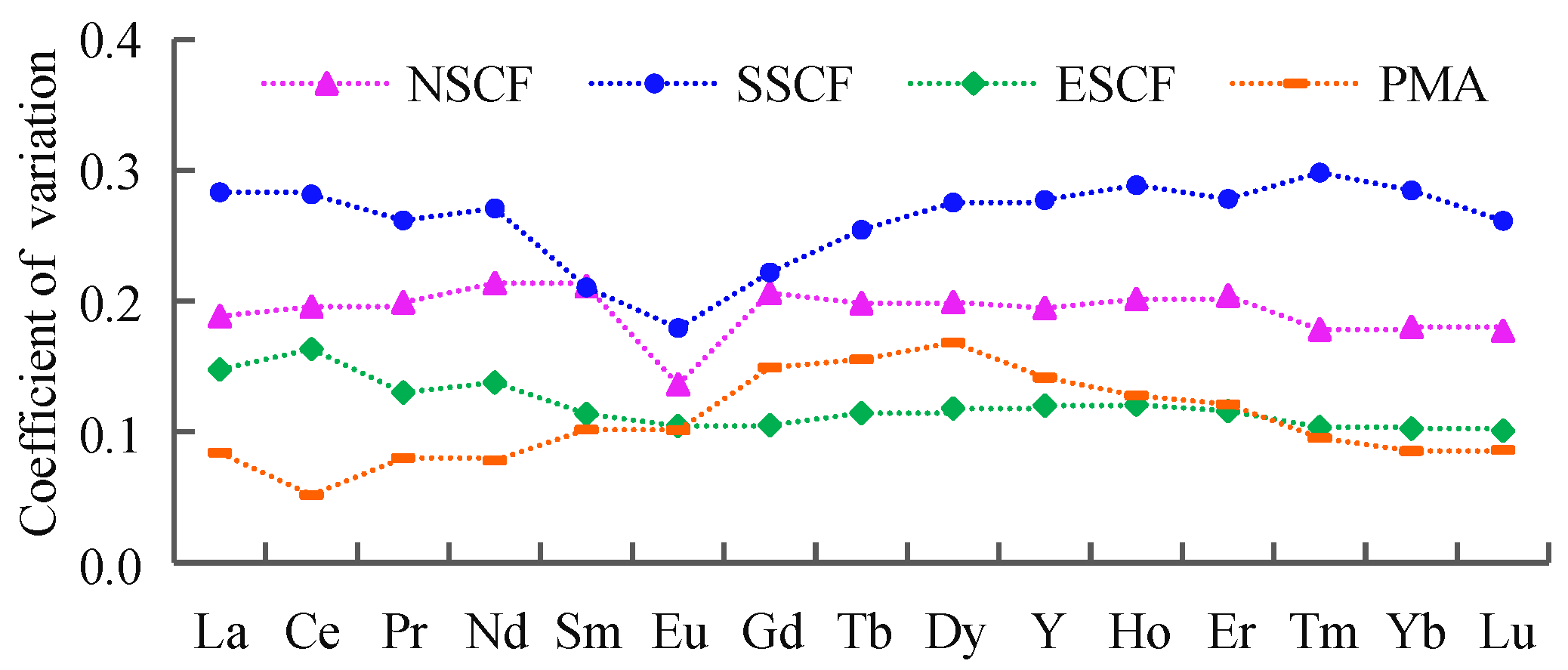
4.1. REE Differences among Each Coalfield
4.2. REE Differences in Soil Profiles
4.3. REE Distribution Patterns
4.4. Impact Factors of REE
5. Conclusions
- (1)
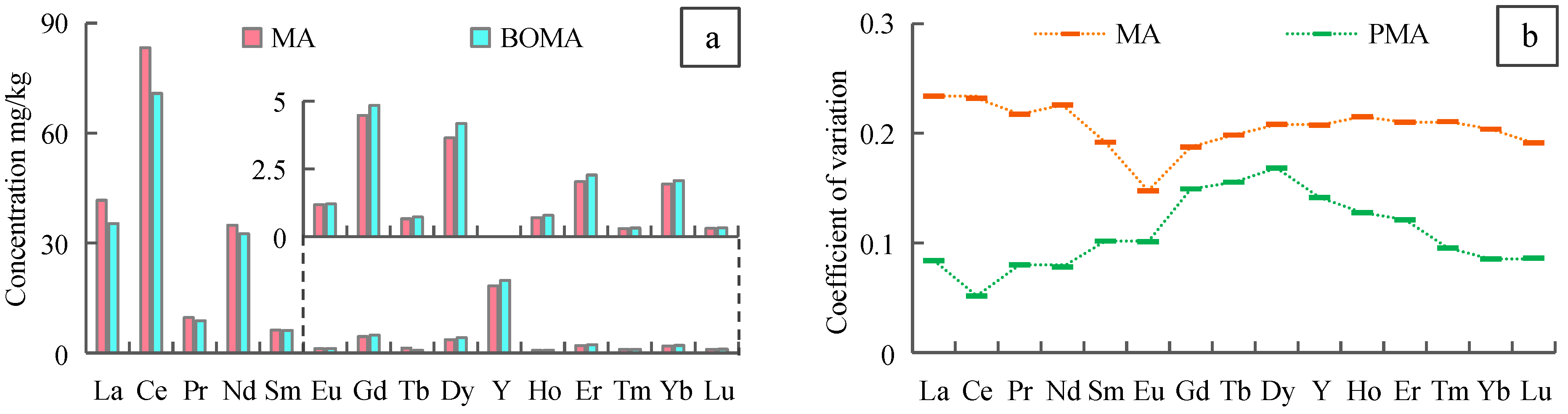
- The REE concentration in the soil of the Rujigou Coal Mine was about 216.09 mg/kg, of which LREE is 84.55%. The sum of REEs and LREEs were lower in PMA than MA, but ΣHREE is higher. The sum of REE in SSCF was similar to NSCF and higher than ESCF. Coal combustion can contribute to LREE enrichment to some extent, while HREE may be depleted in the process.
- (2)
- The presence of ground cracks in the spontaneous combustion zone not only acts as a smoke evacuation channel but also disturbs the uniform distribution of REE. The vertical distribution of REE varies slowly in bedrock debris and soils with a uniform texture.
- (3)
- There was an apparent differentiation of REE in the mine that resulted in LREE enrichment and HREE deficiency, especially in the coalfield. All coalfields showed an LREE distribution pattern with a moderately negative Eu anomaly (δEu ranged from 0.64 to 0.67) and no significant Ce anomaly (δCe ranged from 0.95 to 0.98). The source of REE material in this area may come from soil-forming clastic rocks such as acid or neutral rock, which are weakly controlled by reduction.
- (4)
- Aluminum and Ti might have the same material source as REEs, especially LREEs, because they have similar geochemical behavior. Sulfur and Fe had a negligible relation with the enrichment and distribution of REEs, while Ca, Na, and Mg had a negative correlation with LREEs.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, L.F.O.; Crissien, T.J.; Tutikian, B.F.; Sampaio, C.H. Rare Earth Elements and carbon nanotubes in coal mine around spontaneous combustions. J. Clean. Prod. 2020, 253, 120068. [Google Scholar] [CrossRef]
- Singh, R.N.; Shonhardt, J.A.; Terezopoulos, N. A new dimension to studies of spontaneous combustion of coal. Miner. Resour. Eng. 2002, 11, 147–163. [Google Scholar] [CrossRef]
- Zhai, X.; Wu, S.; Wang, K. Environment influences and extinguish technology of spontaneous combustion of coal gangue heap of Baijigou coal mine in China. Int. Conf. Energy Environ. Res. 2017, 163, 66–72. [Google Scholar] [CrossRef]
- Zhuo, H.; Qin, B.T.; Qin, Q.H. The impact of surface air leakage on coal spontaneous combustion hazardous zone in gob of shallow coal seams: A case study of Bulianta mine, China. Fuel 2021, 295, 120636. [Google Scholar] [CrossRef]
- Huo, Y.; Zhu, H.; He, X.; Fang, S.; Wang, W. Quantum chemical calculation of the effects of H2O on oxygen functional groups during coal spontaneous combustion. ACS Omega 2021, 6, 25594–25607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, Y.; Xu, D.; Jiang, Q.; Xin, H.; Fu, C.; He, W. Study on the reaction path of -CH3 and -CHO functional groups during coal spontaneous combustion: Quantum chemistry and experimental research. Energies 2022, 15, 4891. [Google Scholar] [CrossRef]
- Wang, K.; Guo, L.W.; Zhai, X.W.; Deng, J.; Li, Y. Hydrogen abstraction reaction mechanism of oil-rich coal spontaneous combustion. Fuel 2024, 367, 131538. [Google Scholar] [CrossRef]
- Wessling, S.; Kuenzer, C.; Kessels, W.; Wuttke, M.W. Numerical modeling for analyzing thermal surface anomalies induced by underground coal fires. Int. J. Coal Geol. 2008, 74, 175–184. [Google Scholar] [CrossRef]
- Li, L.; Qin, B.; Liu, J.; Leong, Y.-K.; Li, W.; Zeng, J.; Ma, D.; Zhuo, H. Influence of airflow movement on methane migration in coal mine goafs with spontaneous coal combustion. Process Saf. Environ. Prot. 2021, 156, 405–416. [Google Scholar] [CrossRef]
- Rosema, A.; Guan, H.; Veld, H. Simulation of spontaneous combustion, to study the causes of coal fires in the Rujigou Basin. Fuel 2001, 80, 7–16. [Google Scholar] [CrossRef]
- Kuenzer, C.; Stracher, G.B. Geomorphology of coal seam fires. Geomorphology 2012, 138, 209–222. [Google Scholar] [CrossRef]
- Qin, B.T.; Lu, Y. Experimental research on inorganic solidified foam for sealing air leakage in coal mines. Int. J. Min. Sci. Technol. 2013, 23, 151–155. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Tang, G.X.; Yang, Y.X.; Shao, S.Z.; Ding, Y.W. Research and prevention of upper remaining coal spontaneous combustion induced by air leakage in multi-inclination regenerated roof: A case study in the Luwa coal mine, China. Energy 2023, 275, 127484. [Google Scholar] [CrossRef]
- Ide, S.T.; Orr, F.M. Comparison of methods to estimate the rate of CO2 emissions and coal consumption from a coal fire near Durango, CO. Int. J. Coal Geol. 2011, 86, 95–107. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B. A review of research on spontaneous combustion of coal. Int. J. Min. Sci. Technol. 2020, 3, 303–311. [Google Scholar] [CrossRef]
- Riyas, M.J.; Syed, T.H.; Kumar, H.; Kuenzer, C. Detecting and analyzing the evolution of subsidence due to coal fires in jharia coalfield, India using sentinel-1 SAR data. Remote Sens. 2021, 13, 1521. [Google Scholar] [CrossRef]
- Deng, J.; Ge, S.; Qi, H.; Zhou, F.; Shi, B. Underground coal fire emission of spontaneous combustion, sandaoba coalfield in xinjiang, china: Investigation and analysis. Sci. Total Environ. 2021, 777, 146080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.Q.; Hower, J.C.; Hu, S.R. Mineralogical and geochemical characteristics of pyrometamorphic rocks induced by coal fires in junggar basin, xinjiang, china. J. Geochem. Explor. 2020, 213, 106511. [Google Scholar] [CrossRef]
- Zhang, D.; Cen, X.X.; Wang, W.F.; Deng, J.; Wen, H.; Xiao, Y.; Shu, C.M. The graded warning method of coal spontaneous combustion in Tangjiahui Mine. Fuel 2021, 288, 119635. [Google Scholar] [CrossRef]
- Shi, Q.L.; Qin, B.; Liang, H.; Gao, Y.; Bi, Q.; Qu, B. Effects of igneous intrusions on the structure and spontaneous combustion propensity of coal: A case study of bituminous coal in daxing mine, china. Fuel 2018, 216, 181–189. [Google Scholar] [CrossRef]
- Dijk, P.V.; Zhang, J.Z.; Wang, J.; Kuenzer, C.; Wolf, K.H. Assessment of the contribution of in-situ combustion of coal to greenhouse gas emission; based on a comparison of chinese mining information to previous remote sensing estimates. Int. J. Coal Geol. 2011, 86, 108–119. [Google Scholar] [CrossRef]
- Melody, S.M.; Johnston, F.H. Coal mine fires and human health: What do we know? Int. J. Coal Geol. 2015, 152, 1–14. [Google Scholar] [CrossRef]
- Wang, H.Y.; Fan, C.; Li, J.L.; Zhang, Y.W.; Sun, X.D.; Xing, S.Y. Dynamic characteristics of near-surface spontaneous combustion gas flux and its response to meteorological and soil factors in coal fire area. Environ. Res. 2022, 217, 114817. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Graedel, T.E. Uncovering the global life cycles of the rare earth elements. Sci. Rep. 2011, 1, 145. [Google Scholar] [CrossRef] [PubMed]
- Laveuf, C.; Cornu, S. A review on the potentiality of rare earth elements to trace pedogenetic processes. Geoderma 2009, 154, 1–12. [Google Scholar] [CrossRef]
- Su, C.; Li, Y.; Wang, Y. REE geochemistry as an indicator of activity of faults. In Proceedings of the International Symposium on Water Resources and the Urban Environment, Wuhan, China, 9–10 November 2003. [Google Scholar]
- Anne, E.T.; Susan, A.W.; Jillian, F.B. Geomicrobiological controls on light rare earth element, y and ba distributions during granite weathering and soil formation. J. Alloys Compd. 2000, 303–304, 30–36. [Google Scholar]
- Battsengel, A.; Batnasan, A.; Narankhuu, A.; Haga, K.; Shibayama, A. Recovery of light and heavy rare earth elements from apatite ore using sulphuric acid leaching, solvent extraction and precipitation. Hydrometallurgy 2018, 179, 100–109. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Zhang, J.J.; Hu, Y.; Shi, C.Q.; Zhao, T.N.; Wu, C.; Liu, X.Y.; Liu, B.R. Changes of vegetation and soil water, temperature and nutrient under different remediation modes in a dump. Sci. Soil Water Conserv. 2022, 20, 88–93. [Google Scholar]
- Zhang, H.; Jin, X.L.; Zhang, H.; Li, X.Y.; Wu, C.R. Quartz vein and its geological significance Rujigou mining area in the west of Ordos Basin. Acta Petrol. Sin. 2006, 80, 768–774+784. [Google Scholar]
- Su, C.Q.; Yang, X.K.; Liu, J.Q.; Zhang, Z.Y. A study of foreland basins in the light of Triassic-Jurassic strata of the Helan Mountain. Acta Petrol. Mineral. 2004, 4, 318–326. [Google Scholar]
- Wang, W.F.; Qin, Y.; Sang, S.X.; Jiang, B.; Guo, Y.H.; Zhu, Y.M.; Fu, X.H. Partitioning of minerals and elements during preparation of taixi coal, china. Fuel 2006, 85, 57–67. [Google Scholar] [CrossRef]
- Wang, X.P. Study on Temporal and Spatial Variation Characteristics of Coal Fires in Rujigou Coalfield of Ningxia Based on Landsat Data during the Past 30 Years. Master’s Thesis, Sun Yat-Sen University, Guangzhou, China, 2022; pp. 28–29. [Google Scholar]
- Nikanorov, S.P.; Osipov, V.N.; Korchunov, B.N.N.; Averkin, A.I. The effect of strontium on the mechanical properties of aluminum-silicon alloy. Tech. Phys. Lett. Lett. Russ. J. Appl. Phys. 2016, 42, 201–203. [Google Scholar]
- Boynton, W.V. Cosmochemistry of the Rare Earth Elements: Meteorite Studies. In Developments in Geochemistry; Elsevier Science Publishing Company: Amsterdam, The Netherlands, 1984; Volume 2, pp. 63–114. [Google Scholar]
- Wang, X.Q.; Zhou, J.; Chi, Q.H.; Wang, W.; Zhang, B.M.; Nie, L.S.; Lli, D.S.; Xu, S.F.; Wu, H.; Gao, Y.F. Geochemical Background and Distribution of Rare Earth Elements in China: Implications for Potential Prospects. Acta Geosci. Sin. 2020, 41, 747–758. [Google Scholar]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. Treatise Geochem. 2003, 3, 1–64. [Google Scholar]
- Dai, S.F.; Jiang, Y.F.; Ward, C.R.; Gu, L.D.; Seredin, V.V.; Liu, H.D.; Zhou, D.; Wang, X.B.; Sun, Y.S.; Zou, J.H.; et al. Mineralogical and geochemical compositions of the coal in the Guanbanwusu Mine, Inner Mongolia, China: Further evidence for the existence of an Al (Ga and REE) ore deposit in the Jungar Coalfield. Int. J. Coal Geol. 2012, 98, 10–40. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimation of Clarkes for carbonaceous biolithes: World average for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Seredin, V.V.; Shpirt, M.Y.B. Metalliferous coals: A new potential source of valuable trace elements as by-products. Coal Sci. Technol. 1995, 24, 1649–1652. [Google Scholar]
- Xu, J.; Sun, Y.Z.; Kalkreuth, W. Characteristics of trace elements of the No.06 Coal in the Guanbanwusu Mine, Junger Coalfield, Inner Mongolia. Energy Explor. Exploit. 2011, 29, 827–842. [Google Scholar] [CrossRef]
- Seredin, V.V. Rare earth element-bearing coals from the Russian Far East deposits. Int. J. Coal Geol. 1996, 30, 101–129. [Google Scholar] [CrossRef]
- Kronberg, B.I.; Brown, J.R.; Fyfe, W.S.; Peirce, M.; Winder, C.G. Distributions of trace elements in Western Canadian coal ashes. Fuel 1981, 60, 59–63. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.F. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Marker, A.; Oliveira, J. The formation of rare earth element scavenger minerals in weathering products derived from alkaline rocks of SE-Bahia, Brazil. Chem. Geol. 1990, 84, 373–374. [Google Scholar] [CrossRef]
- Zhou, W.X.; Han, G.L.; Liu, M.; Song, C.; Li, X.Q. Geochemical Distribution Characteristics of Rare Earth Elements in Different Soil Profiles in Mun River Basin, Northeast Thailand. Sustainability 2020, 12, 457. [Google Scholar] [CrossRef]
- Nakada, R.; Takahashi, Y.; Tanimizu, M. Isotopic and speciation study on cerium during its solid–water distribution with implication for Ce stable isotope as a paleo-redox proxy. Geochim. Cosmochim. Acta 2013, 103, 49–62. [Google Scholar] [CrossRef]
- Condie, K.C.; Dengate, J.; Cullers, R.L. Behavior of rare earth elements in a paleoweathering profile on granodiorite in the Front Range, Colorado, USA. Geochim. Cosmochim. Acta 1995, 59, 279–294. [Google Scholar] [CrossRef]
- Dai, S.F.; Zhang, W.G.; Ward, C.R.; Seredin, V.V.; Hower, J.C.; Li, X.; Song, W.J.; Wang, X.B.; Kang, H.; Zheng, L.C.; et al. Mineralogical and geochemical anomalies of late Permian coals from the Fusui Coalfield, Guangxi Province, southern China: Influences of terrigenous materials and hydrothermal fluids. Int. J. Coal Geol. 2013, 105, 60–84. [Google Scholar] [CrossRef]
- Dai, S.F.; Liu, J.J.; Ward, C.R.; Hower, J.C.; French, D.; Jia, S.H.; Hood, M.M.; Garrison, T.M. Mineralogical and geochemical compositions of Late Permian coals and host rocks from the Guxu Coalfield, Sichuan Province, China, with emphasis on enrichment of rare metals. Int. J. Coal Geol. 2016, 166, 71–95. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Li, S.S. Modes of Occurrence of Rare Earth Elements in Some Late Paleozoic Coals of North China. Acta Geosci. Sin. 2003, 24, 273–278. [Google Scholar]
- Cui, X.N.; Huang, W.H.; Ao, W.H.; Zhou, H.P.; Liang, F. Study on the geochemistry of rare carth elcments in the Permian coal from Xiayukou, Weibei. Coalf. Earth Sci. Front. 2016, 23, 90–96. [Google Scholar]
- Qin, S.J.; Gao, K.; Wang, J.X.; Li, Y.H.; Lu, Q.F. Geochemistry of the associated elements in the Late Permian Coal from Huoshaopu and Jinjia Mines, Southwestern Guizhou. J. China Coal Soc. 2016, 41, 1507–1516. [Google Scholar]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloys Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- Luo, Z.G.; Li, C.H.; Wang, S.K.; Liang, H.D. Geochemical characteristics and mining prospects of REE in Lugou coal mine of Xinmi coalfield. J. Min. Sci. Technol. 2020, 5, 140–149. [Google Scholar]
- Yang, S.F.; Fang, W.X.; Hu, R.Z.; Wang, S.D.; Wei, N. Rare Earth Element Enrichment and Its Relationship to Major Elements of Weathering-Basalt Profile in Boloven Plateau, Lao PDR. J. Chin. Soc. Rare Earths 2007, 25, 461–469. [Google Scholar]
- Zhang, L.J.; Li, X.S.; Li, D.C.; Han, Z.Y.; Zhang, G.L. Rare earth elements distribution and its correlation with macro elements and particle-size of basalt-derived soils in Leizhou Peninsula. Acta Pedol. Sin. 2011, 48, 1–9. [Google Scholar]
- Judge, W.D.; Ng, K.L.; Moldoveanu, G.A. Solubilities of heavy rare earth sulfates in water (gadolinium to lutetium) and H2SO4 solutions dysprosium. Hydrometallurgy 2023, 218, 106054. [Google Scholar] [CrossRef]


| Elements | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.310 | 0.808 | 0.122 | 0.600 | 0.195 | 0.0735 | 0.259 | 0.047 | 0.322 | 0.0718 | 0.21 | 0.0324 | 0.209 | 0.0322 |
| Region | δCe | δEu | δCe/δEu | (La/Lu)N | (La/Sm)N | (Gd/Lu)N |
|---|---|---|---|---|---|---|
| NSCF | 0.96 | 0.67 | 1.46 | 14.75 | 4.30 | 1.84 |
| SSCF | 0.95 | 0.64 | 1.52 | 15.34 | 4.42 | 1.83 |
| ESCF | 0.98 | 0.66 | 1.47 | 12.46 | 3.91 | 1.79 |
| MA-Mean | 0.96 | 0.65 | 1.48 | 14.30 | 4.23 | 1.82 |
| PMA | 0.95 | 0.65 | 1.45 | 11.40 | 3.61 | 1.87 |
| Elements | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | 6.4 | 16.6 | 2.09 | 9.5 | 2.93 | 0.45 | 3.07 | 0.43 | 2.42 | 14.8 | 0.45 | 1.18 | 0.15 | 0.87 | 0.14 |
| Burned | 31.5 | 61.4 | 6.75 | 22.8 | 3.38 | 0.33 | 2.16 | 0.32 | 1.90 | 11.4 | 0.38 | 1.17 | 0.19 | 1.22 | 0.19 |
| Multiple | 4.92 | 3.70 | 3.23 | 2.40 | 1.15 | 0.73 | 0.70 | 0.74 | 0.79 | 0.77 | 0.84 | 0.99 | 1.27 | 1.40 | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, B.; Wang, Z.; Xie, P.; Tian, Y. Geochemical Characteristics of Soil Rare Earth Elements within Spontaneous Combustion Coalfields of Rujigou Coal Mine. Minerals 2024, 14, 592. https://doi.org/10.3390/min14060592
Xiao B, Wang Z, Xie P, Tian Y. Geochemical Characteristics of Soil Rare Earth Elements within Spontaneous Combustion Coalfields of Rujigou Coal Mine. Minerals. 2024; 14(6):592. https://doi.org/10.3390/min14060592
Chicago/Turabian StyleXiao, Bei, Zhenghai Wang, Peng Xie, and Yuxin Tian. 2024. "Geochemical Characteristics of Soil Rare Earth Elements within Spontaneous Combustion Coalfields of Rujigou Coal Mine" Minerals 14, no. 6: 592. https://doi.org/10.3390/min14060592




