Distribution and Enrichment of Au, Hg, and Tl in the Lanmuchang Deposit, Guizhou, China
Abstract
1. Introduction
2. Geological Background
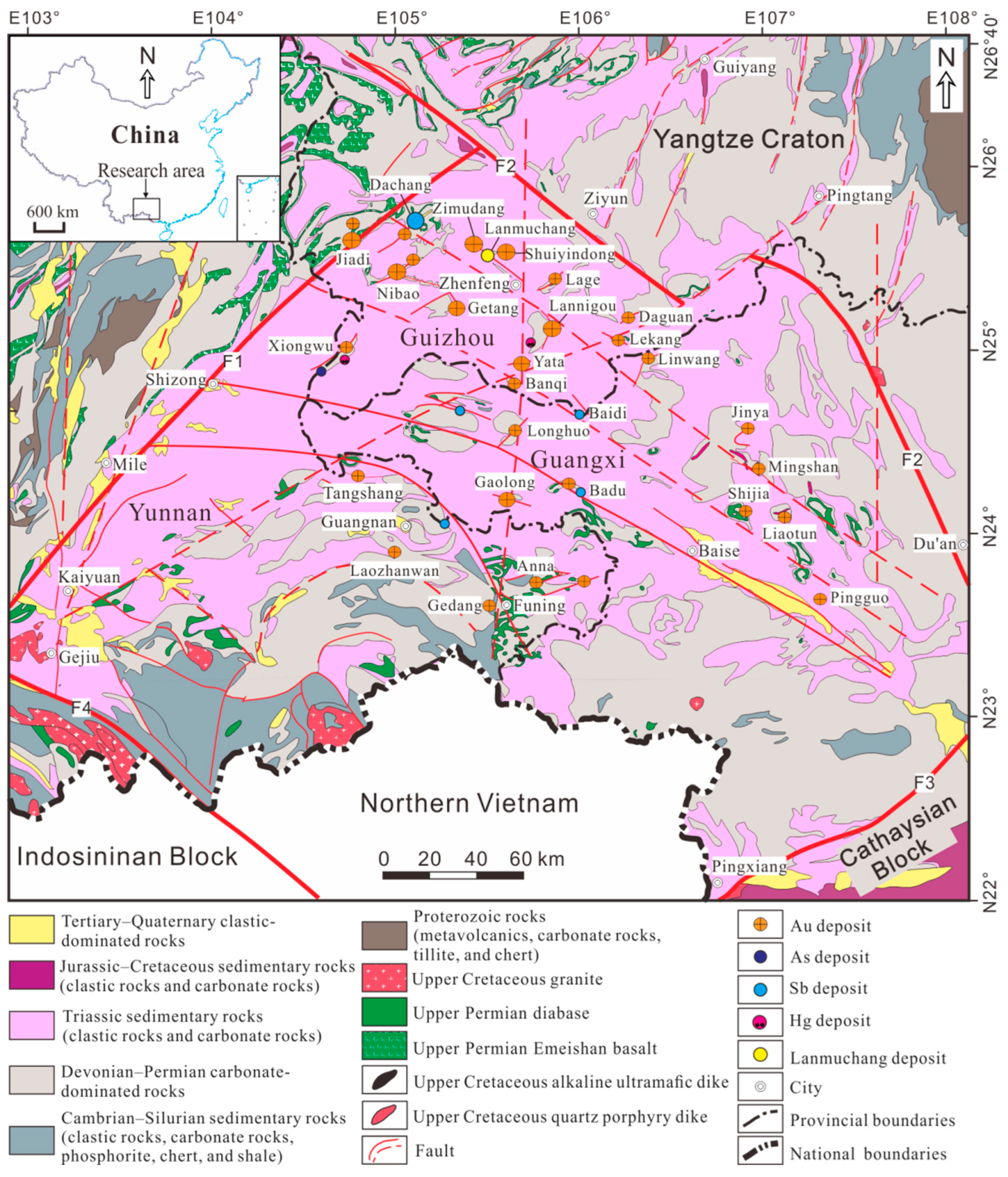
2.1. Regional Geology
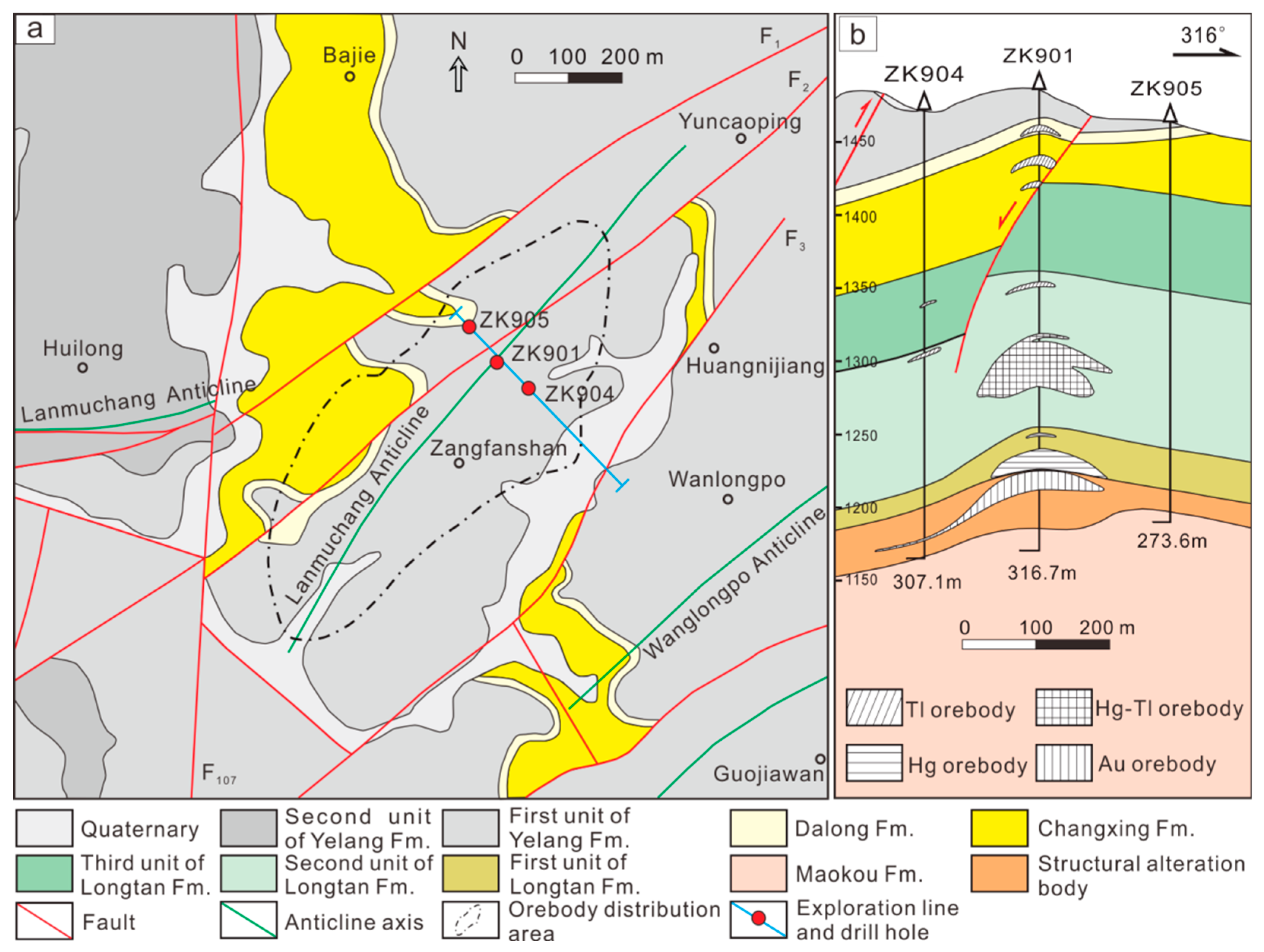
2.2. Deposit Geology
3. Sampling and Analytical Methods
3.1. SEM
3.2. TIMA
3.3. EPMA
4. Analytical Results
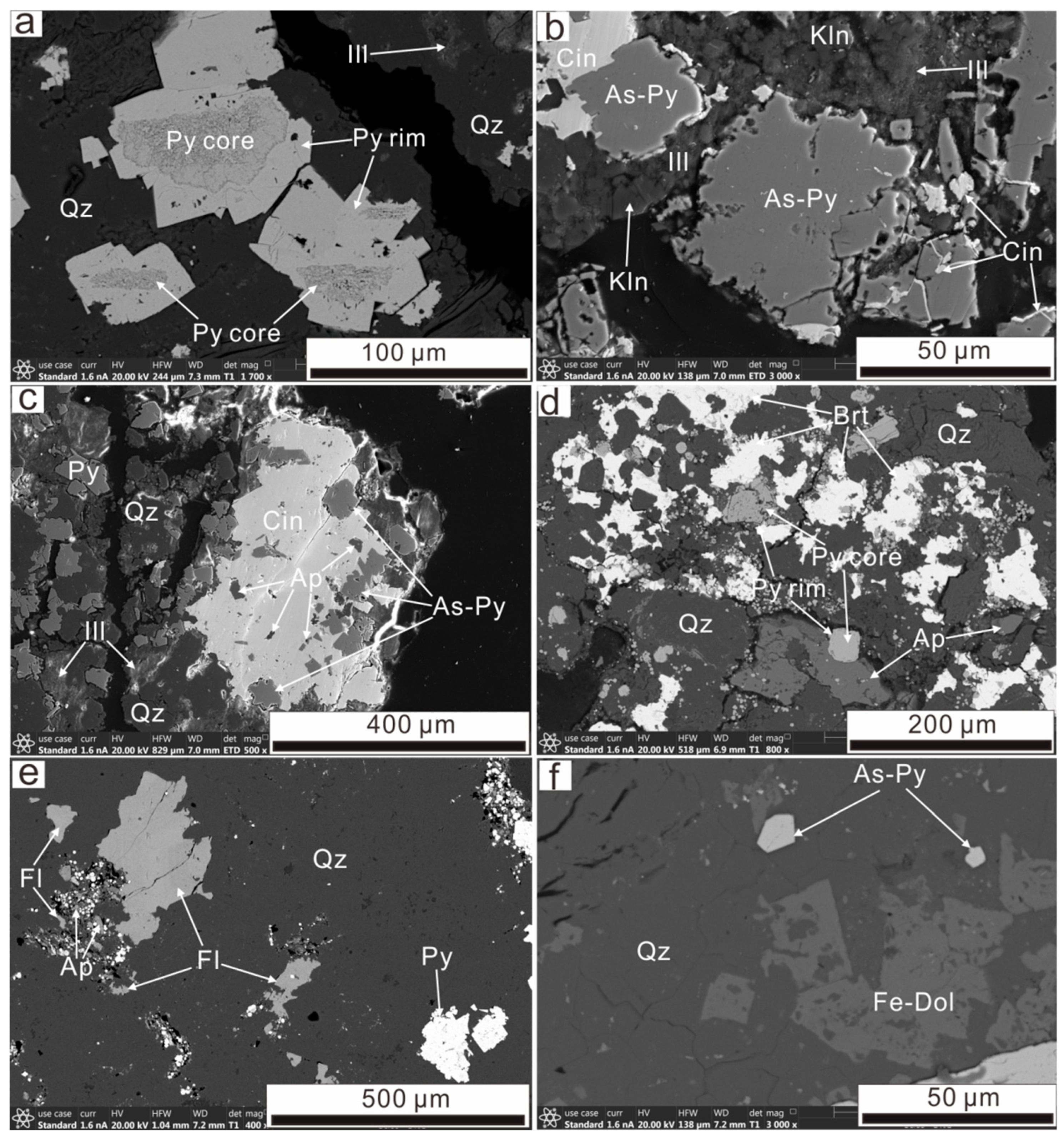
4.1. Ore Paragenesis and Their Textures
4.2. Mineral Abundance
4.3. Mineral Chemistry
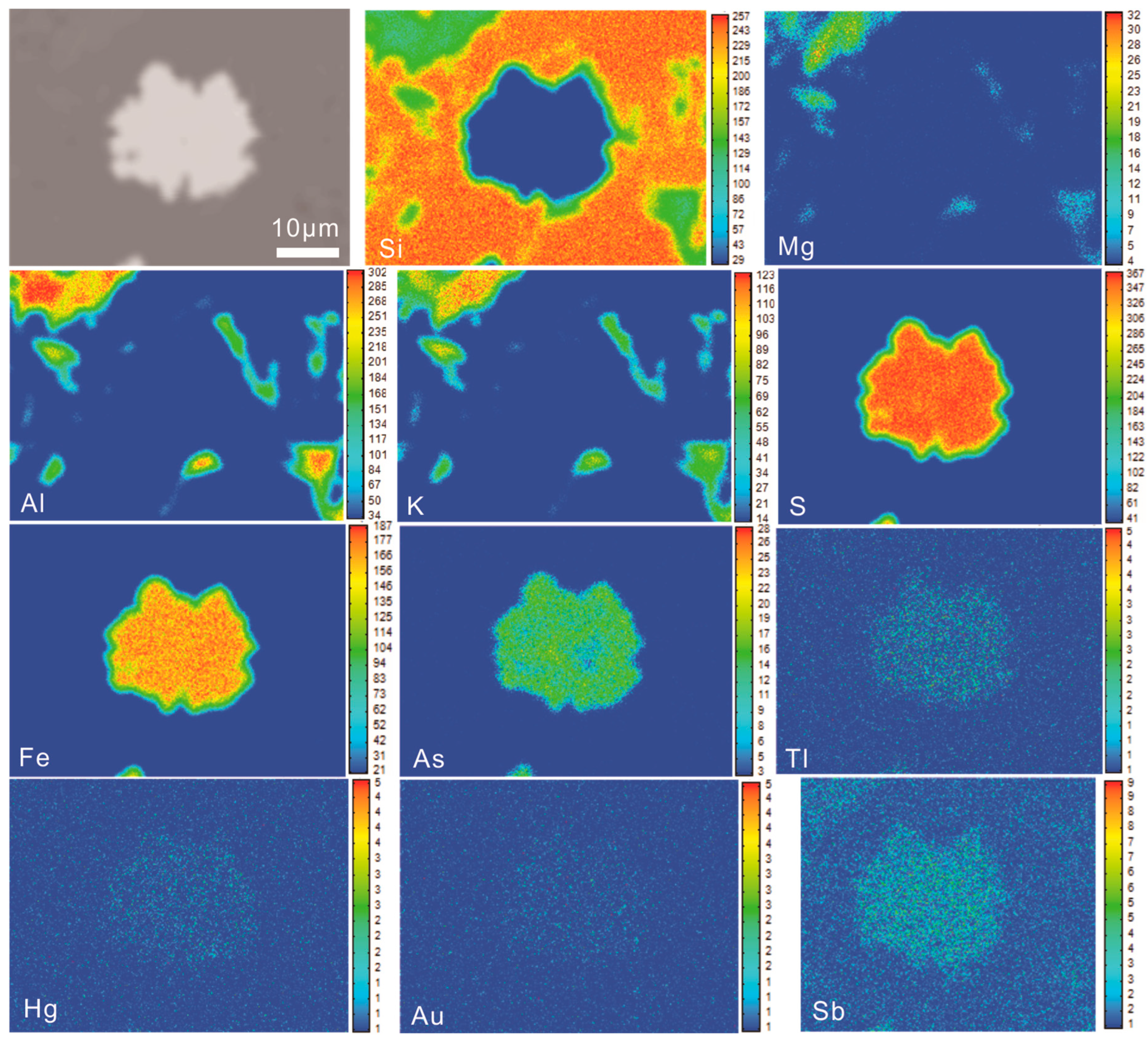
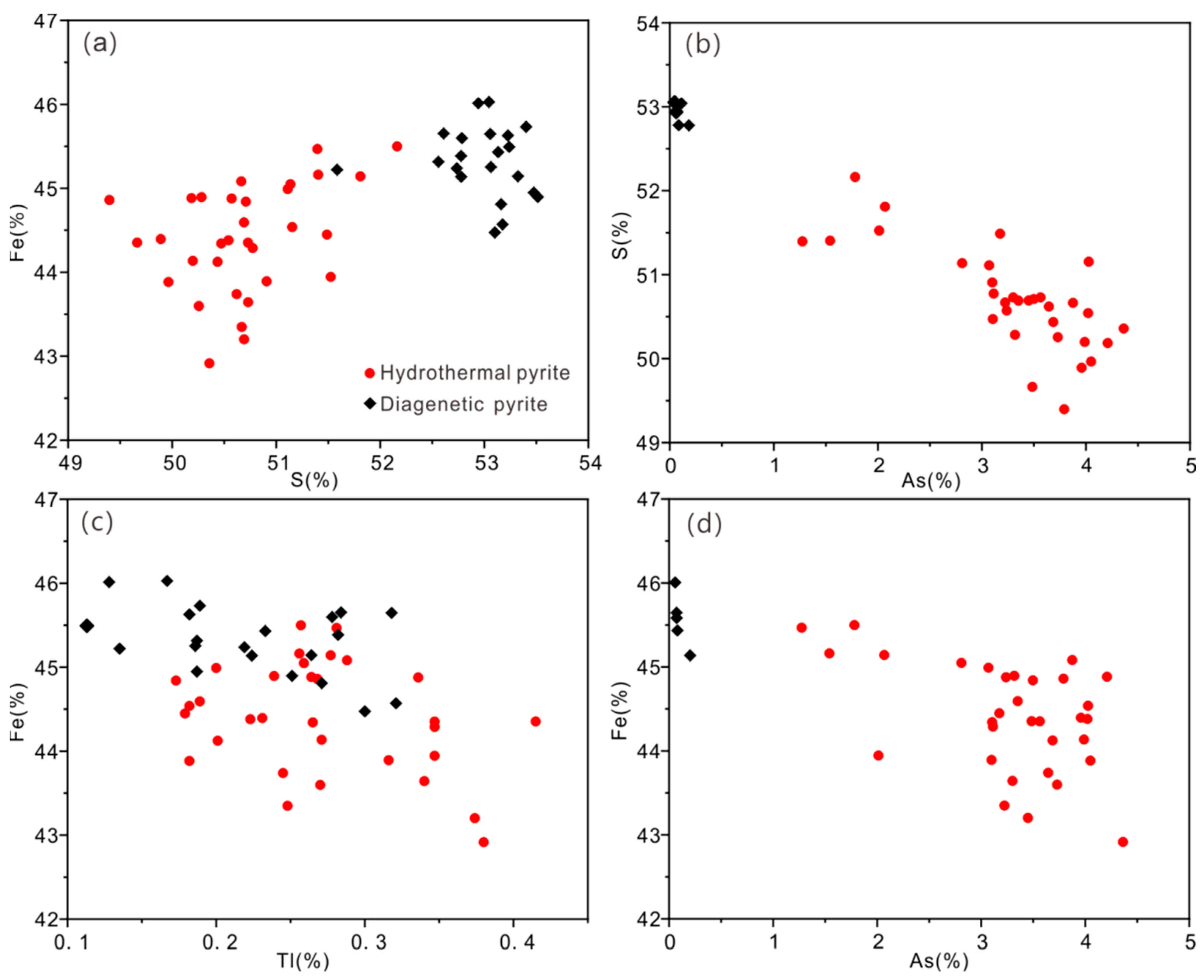
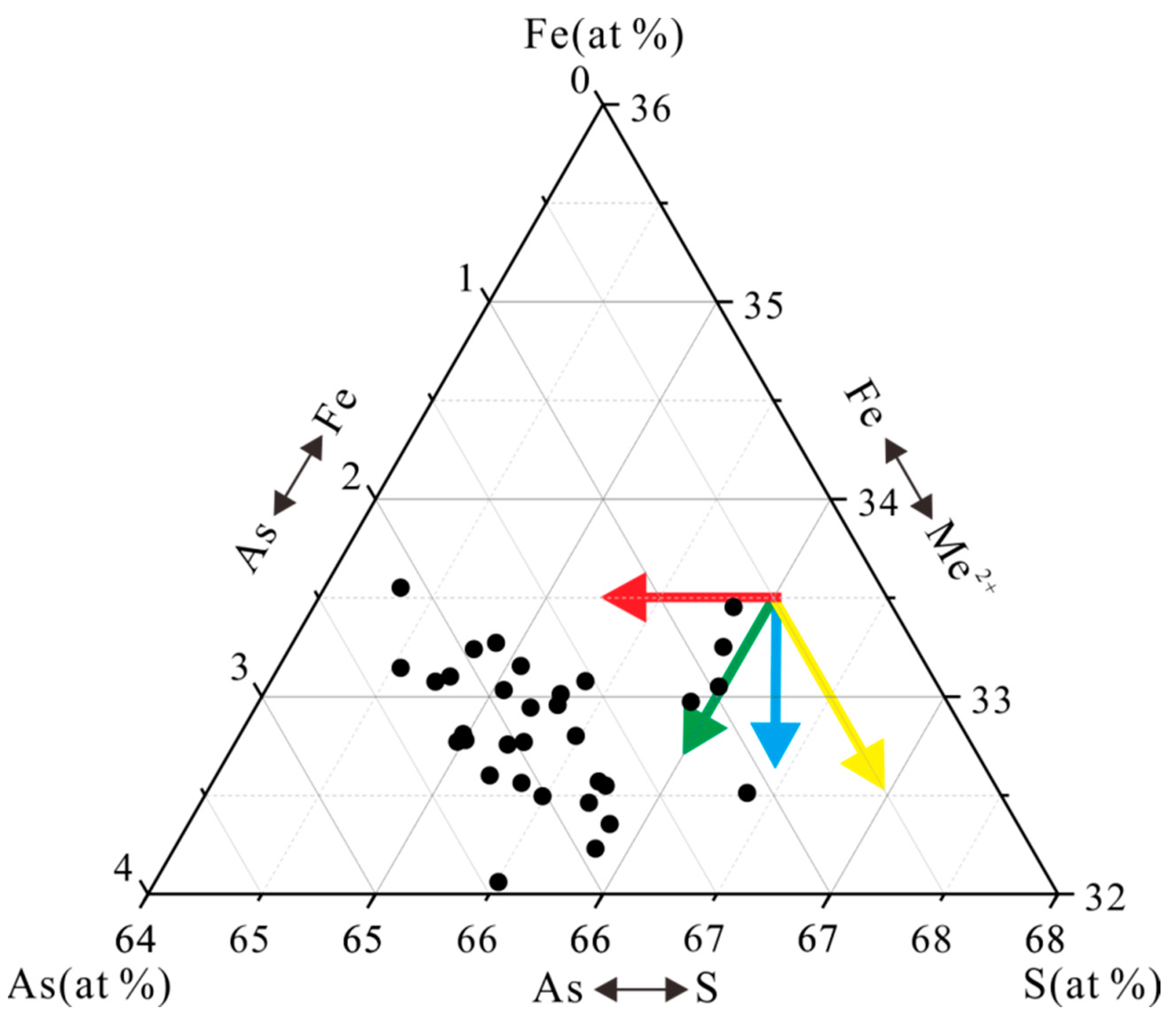
4.3.1. Pyrite
4.3.2. Cinnabar
4.3.3. Quartz
4.3.4. Barite
4.3.5. Kaolinite
4.3.6. Illite
5. Discussion
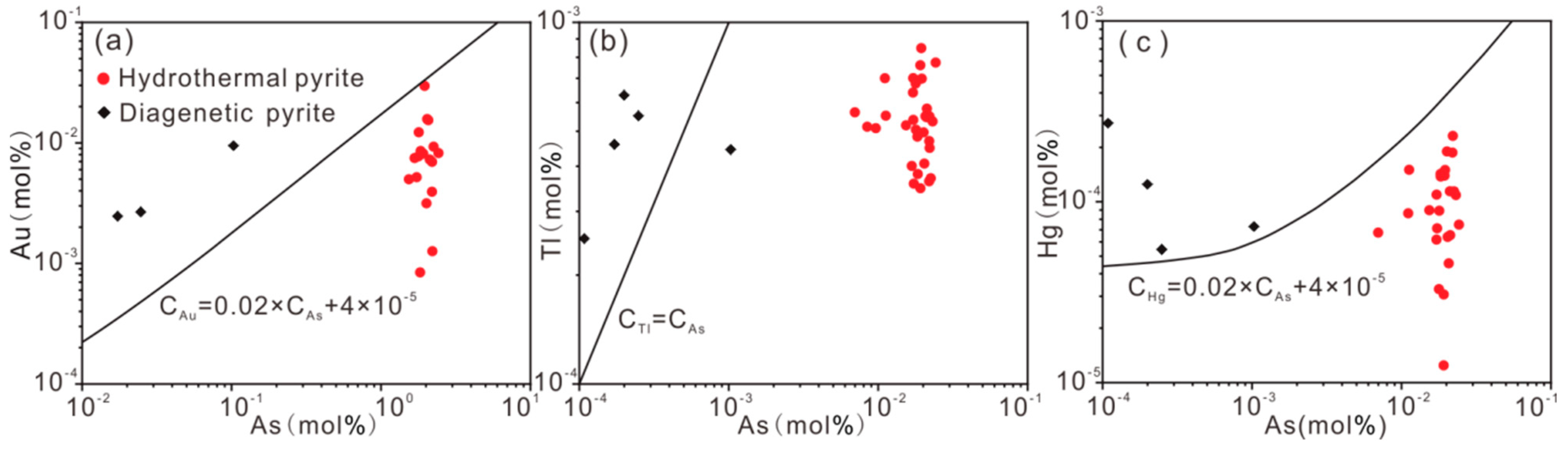
5.1. Occurrence of Ore-Forming Elements
5.2. Occurrence Mechanism of Ore-Forming Elements
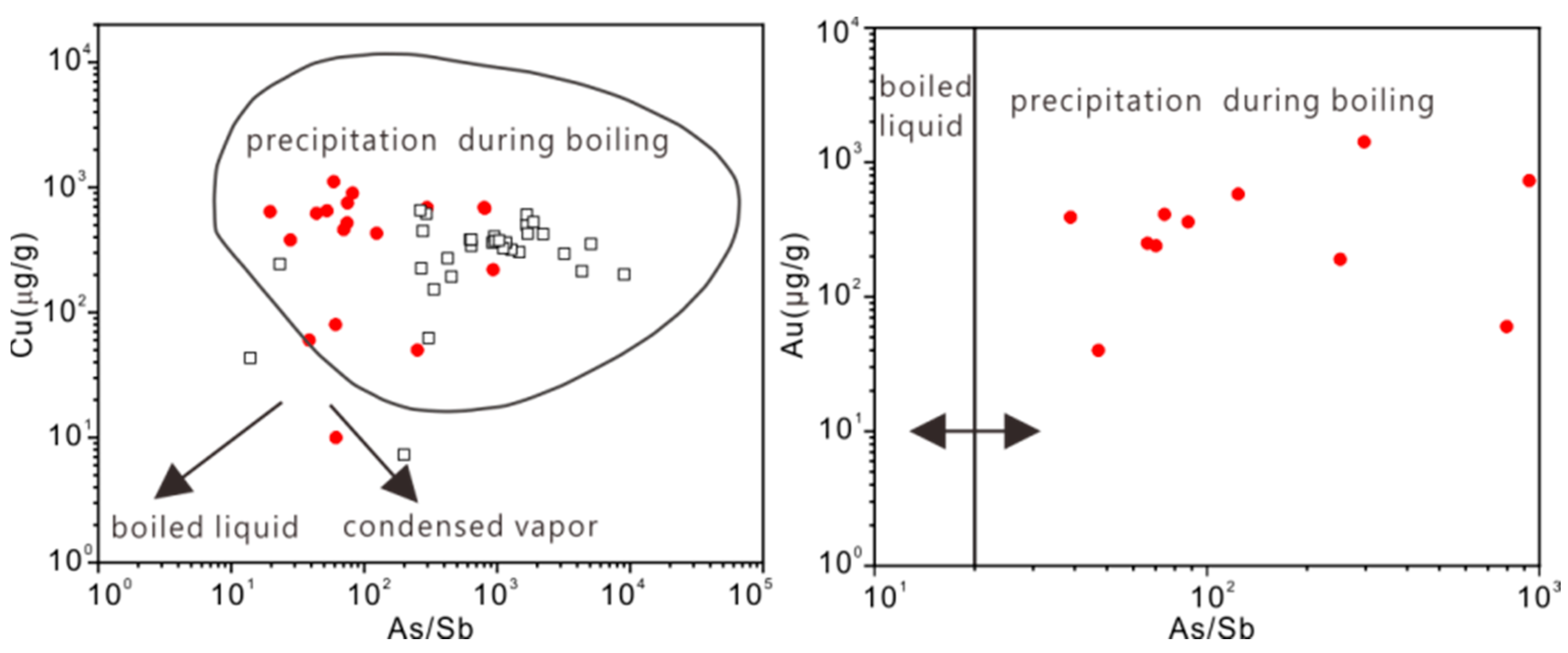
5.3. Mineralization Conditions and Mechanisms
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muntean, J.L.; Cline, J.S. Diversity of Carlin-style gold deposits. Rev. Econ. Geol. 2018, 20, 1–6. [Google Scholar]
- Su, W.C.; Dong, W.D.; Zhang, X.C.; Shen, N.P.; Hu, R.Z.; Hofstra, A.H.; Cheng, L.Z.; Xia, Y.; Yang, K.Y. Carlin-type gold deposits in the Dian-Qian-Gui “golden triangle” of Southwest China. Rev. Econ. Geol. 2018, 20, 157–185. [Google Scholar]
- Li, S.T.; Xu, L.Y.; Wang, Z.P.; Yang, C.F.; Tan, L.J.; Nie, R.; Meng, M.H.; Li, J.H.; Zhang, B.Q.; Liu, J.Z. Application of tectono-geochemistry method for weak information extraction of Carlin-type gold deposits in Yunnan–Guizhou–Guangxi, SW China. Ore Geol. Rev. 2023, 163, 105813. [Google Scholar] [CrossRef]
- Hu, R.Z.; Fu, S.L.; Huang, Y.; Zhou, M.F.; Fu, S.H.; Zhao, C.H.; Wang, Y.J.; Bi, X.W.; Xiao, J.F. The giant South China Mesozoic low-temperature metallogenic domain: Reviews and a new geodynamic model. J. Asian Earth Sci. 2017, 137, 9–34. [Google Scholar] [CrossRef]
- Xie, Z.J.; Xia, Y.; Cline, J.S.; Pribil, M.J.; Koenig, A.; Tan, Q.P.; Wei, D.T.; Wang, Z.P.; Yan, J. Magmatic origin for sediment-hosted Au deposits, Guizhou Province, China: In situ chemistry and sulfur isotope composition of pyrites, Shuiyindong and Jinfeng deposits. Econ. Geol. 2018, 113, 1627–1652. [Google Scholar] [CrossRef]
- Yan, J.; Hu, R.Z.; Liu, S.; Lin, Y.T.; Zhang, J.C.; Fu, S.L. NanoSIMS element mapping and sulfur isotope analysis of Au-bearing pyrite from Lannigou Carlin-type Au deposit in SW China: New insights into the origin and evolution of Au-bearing fluids. Ore Geol. Rev. 2018, 92, 29–41. [Google Scholar] [CrossRef]
- Su, W.C.; Heinrich, C.A.; Pettke, T.; Zhang, X.C.; Hu, R.Z.; Xia, B. Sediment-Hosted gold deposits in Guizhou, China: Products of wall-rock sulfidation by deep crustal fluids. Econ. Geol. 2009, 104, 73–93. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.L.; Yang, R.D.; Du, L.J.; Liao, M.Y. Gold and antimony metallogenic relations and ore-forming process of Qinglong Sb (Au) deposit in Youjiang basin.; SW China: Sulfide trace elements and sulfur isotopes. Geosci. Front. 2021, 12, 605–623. [Google Scholar] [CrossRef]
- Gu, X.X.; Zhang, Y.M.; Li, B.H.; Dong, S.Y.; Xue, C.J.; Fu, S.H. Hydrocarbon- and ore-bearing basinal fluids: A possible link between gold mineralization and hydrocarbon accumulation in the Youjiang basin, South China. Miner. Deposita 2012, 47, 663–682. [Google Scholar] [CrossRef]
- Peng, Y.W.; Gu, X.X.; Zhang, Y.M.; Liu, L.; Wu, C.Y.; Chen, S.Y. Ore-forming process of the Huijiabao gold district, southwestern Guizhou Province, China: Evidence from fluid inclusions and stable isotopes. J. Asian Earth Sci. 2014, 93, 89–101. [Google Scholar] [CrossRef]
- Li, S.T.; Xia, Y.; Liu, J.Z.; Xie, Z.J.; Tan, Q.P.; Zhao, Y.M.; Meng, M.H.; Tan, L.J.; Nie, R.; Wang, Z.P.; et al. Geological and geochemical characteristics of the Baogudi Carlin-type gold district (Southwest Guizhou, China) and their geological implications. Acta Geochim. 2019, 38, 587–609. [Google Scholar] [CrossRef]
- Su, W.C.; Zhang, H.T.; Hu, R.Z.; Ge, X.; Xia, B.; Chen, Y.Y.; Zhu, C. Mineralogy and geochemistry of goldbearing arsenian pyrite from the Shuiyindong Carlin-type gold deposit, Guizhou, China: Implications for gold depositional processes. Miner. Deposita 2012, 47, 653–662. [Google Scholar] [CrossRef]
- Li, S.T.; Zhou, G.H.; Liu, J.Z.; Yuan, S.F.; Wang, Z.P.; Yang, C.F.; Wang, X.Y.; Long, C.X.; Zhang, B.Q.; Li, J.H.; et al. Geological and geochemical characteristics of the Lanmuchang gold–mercury–thallium deposit in Southwestern Guizhou Province and their geological implications. Geol. Explor. 2022, 58, 475–488, (In Chinese with English abstract). [Google Scholar]
- An, S.R.; An, X.G.; Li, X.L. Discovery and study of rare natural christite in Guizhou. Guizhou Geol. 1988, 4, 377–379+383, (In Chinese with English abstract). [Google Scholar]
- Duan, H.Y.; Wang, C.M. Geochemistry and mineralization of a critical element: Thallium. Acta Petrol. Sin. 2022, 6, 1771–1794. [Google Scholar]
- Li, G.Z. A study of ore compositions and thallium occurrence in mercury-thallium deposit at Lanmuchang of Xingren County in southwestern Guizhou. Guizhou Geol. 1996, 1, 24–37, (In Chinese with English abstract). [Google Scholar]
- Chen, D.Y.; Wang, G.X.; Zou, Z.X.; Chen, Y.M. The discovery and study of Lanmuchangite, the First New Thallium Mineral, in China. J. Guizhou Univ. Technol. 2001, 5, 5–7+12, (In Chinese with English abstract). [Google Scholar]
- Ren, D.Y.; Chen, D.Y. The features of tectonics-controlling metallogenesis and its experimental study on Lanmuchang thallium deposit. Guizhou province. Geol. Geochemi. 2001, 3, 201–205, (In Chinese with English abstract). [Google Scholar]
- Yuan, S.F.; Liu, J.Z.; Song, W.F.; Su, C.P.; Yang, C.F.; Xu, L.Y.; Zhang, M.; Long, C.X.; Wang, X.Y.; He, J.P. Geologic and Tectono-geochemical Characteristics of Lanmuchang Au-Hg-Tl Deposit in Xingren, Guizhou. Guizhou Geol. 2018, 1, 7–13, (In Chinese with English abstract). [Google Scholar]
- Chen, D.Y.; Zou, Z.X. Studying Situation about Lanmuchang Type Thallium (Mercury) Ore Deposits in Southwestern Guizhou. Guizhou Geol. 2000, 4, 236–241, (In Chinese with English abstract). [Google Scholar]
- Zhang, Z.; Zhang, B.G.; Hu, J.; Yao, L.B.; Tian, Y.F. A Preliminary discussion on the bio-metallogenesis of Tl deposits in the Low-temperature minerogenetic province of southwestern China. Sci. China Ser. D Earth Sci. 2007, 50, 359–370. [Google Scholar] [CrossRef]
- Deng, F. The Geological Characteristics and Elemental Geochemistry Research about Xingren Lanmuchang Thallium (Mercury) Ore Deposits. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2010. [Google Scholar]
- Hu, R.Z.; Gao, W.; Fu, S.L.; Su, W.C.; Peng, J.T.; Bi, X.W. Mesozoic intraplate metallogenesis in South China. Earth Sci. Front. 2024, 31, 226–238. [Google Scholar]
- Pi, Q.H.; Hu, R.Z.; Xiong, B.; Li, Q.L.; Zhong, R.C. In situ SIMS U-Pb dating of hydrothermal rutile, reliable age for the Zhesang Carlin-type gold deposit in the golden triangle region, SW China. Miner. Deposita 2017, 52, 1179–1190. [Google Scholar] [CrossRef]
- Chen, M.H.; Bagas, L.; Liao, X.; Zhang, Z.Q.; Li, Q.L. Hydrothermal apatite SIMS Th-Pb dating: Constraints on the timing of low-temperature hydrothermal Au deposits in Nibao, SW China. Lithos 2019, 324, 418–428. [Google Scholar] [CrossRef]
- Jin, X.Y.; Zhao, J.X.; Feng, Y.X.; Hofstra, A.H.; Deng, X.D.; Zhao, X.F.; Li, X.W. Calcite U-Pb dating unravels the age and hydrothermal history of the giant Shuiyindong Carlin-type gold deposit in the golden triangle, South China. Econ. Geol. 2021, 116, 1253–1265. [Google Scholar] [CrossRef]
- Fleet, M.E.; Mumin, A.H. Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis. Am. Mineral. 1997, 82, 182–193. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Palinkaš, S.S.; Hofstra, A.H.; Percival, T.J.; Šoštari´c, S.B.; Palinkaš, L.; Bermanec, V.; Pecskay, Z.; Boev, B. Comparison of the Allchar Au-As-Sb-Tl Deposit, Republic of Macedonia, with Carlin-Type Gold Deposits. Rev. Econ. Geol. 2018, 20, 335–363. [Google Scholar]
- Gopon, P.; Douglas, J.O.; Auger, M.A.; Hansen, L.; Wade, J.; Cline, J.S.; Robb, L.J.; Moody, M.P. A nanoscale investigation of Carlin-type gold deposits: An atom-scale elemental and isotopic perspective. Econ. Geol. 2019, 114, 1123–1133. [Google Scholar] [CrossRef]
- Palenik, C.S.; Ustunomiya, S.; Reich, M.; Kesler, S.E.; Wang, L.; Ewing, R.C. “Invisible” gold revealed: Direct imagining of gold nanoparticles in a Carlin-type deposit. Am. Miner. 2004, 89, 1359–1366. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Kesler, S.E.; Reich, M.; Ewing, R.C. Trace elements nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kokh, M.A.; Proux, O.; Hazemann, J.L.; Bazarkina, E.F.; Testemale, D.; Escoda, C.; Boiron, M.C.; Blanchard, M.; Aigouy, T.; et al. The nature and partitioning of invisible gold in the pyrite-fluid system. Ore Geol. Rev. 2019, 109, 545–563. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Escoda, C.; Blanchard, M.; Testemale, D.; Hazemann, J.L.F.; Gouy, S.; Kokh, M.A.; Boiron, M.C.; de Parseval, F.; Aigouy, T.; et al. An arsenic-driven pump for invisible gold in hydrothermal systems. Geochem. Persp. Let. 2021, 17, 39–44. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, Y.; Sha, G.; Brugger, J.; Pring, A.; Ni, P.; Qian, G.; Luo, Z.; Zhang, Y.; Tan, W. Effects of arsenic on the distribution and mode of occurrence of gold during fluid-pyrite interaction: A case study of pyrite from the Qiucun gold deposit, China. Am. Miner. 2022, 107, 914–929. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M. Constraints on the solid solubility of Hg, Tl, and Cd in arsenian pyrite. Am. Miner. 2016, 101, 1451–1459. [Google Scholar] [CrossRef]
- Chouinard, A.; Paquette, J.; Williams-Jones, A.E. Crystallographic controls on trace-element incorporation in auriferous pyrite from the Pascua epithermal highsulfidation deposit, Chile-Argentina. Can. Miner. 2005, 43, 951–963. [Google Scholar] [CrossRef]
- Liu, Y.H.; Xue, C.J.; Zhao, Y.; Zhao, X.B.; Chu, H.X.; Liu, C.X.; Yu, L.; Wang, L.; Wu, D.H. Research on Auriferous Pyrite in Hydrothermal Gold Deposits, China. Geoscience 2020, 34, 1–12, (In Chinese with English abstract). [Google Scholar]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Qian, G.; Brugger, J.; Testamale, D.; Skiner, W.; Pring, A. Formation of As(II)-pyrite during experimental replacement of magnetite under hydrothermal conditions. Geochim. Cosmochim. Acta 2012, 100, 1–10. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Jenkin, G.R.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- D’Orazio, M.; Biagioni, C.; Dini, A.; Vezzoni, S. Thallium-rich pyrite ores from the Apuan Alps, Tuscany, Italy: Constraints for their origin and environmental concerns. Miner. Deposita 2017, 52, 687–707. [Google Scholar] [CrossRef]
- George, L.L.; Biagioni, C.; D’Orazio, M.; Cook, N.J. Textural and trace element evolution of pyrite during greenschist facies metamorphic recrystallization in the southern Apuan Alps (Tuscany, Italy): Influence on the formation of Tl-rich sulfosalt melt. Ore Geol. Rev. 2018, 102, 59–105. [Google Scholar] [CrossRef]
- Manceau, A.; Merkulova, M.; Murdzek, M.; Batanova, V.; Baran, R.; Glatzel, P.; Saikia, B.K.; Paktunc, D.; Lefticariu, L. Chemical forms of mercury in pyrite: Implications for predicting mercury releases in acid mine drainage settings. Environ. Sci. Technol. 2018, 52, 10286–10296. [Google Scholar] [CrossRef] [PubMed]
- Voute, F.; Hagemann, S.G.; Evans, N.J.; Villanes, C. Sulfur isotopes, trace element, and textural analyses of pyrite, arsenopyrite and base metal sulfides associated with gold mineralization in the Pataz-Parcoy district, Peru: Implication for paragenesis, fluid source, and gold deposition mechanisms. Miner. Deposita 2019, 54, 1077–1100. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Doyle, K.; Holwell, D.A.; Jenkin, G.R.; Barry, T.L.; Becker, J.; Rampe, J. Pyrite chemistry: A new window into Au-Te ore-forming processes in alkaline epithermal districts, Cripple Creek, Colorado. Geochim. Cosmochim. Acta 2020, 274, 172–191. [Google Scholar] [CrossRef]
- Gregory, D.D.; Cracknell, M.J.; Large, R.R.; McGoldrick, P.; Kuhn, S.; Maslennikov, V.V.; Baker, M.J.; Fox, N.; Belousov, I.; Figueroa, M.C.; et al. Distinguishing ore deposit type and barren sedimentary pyrite using laser ablation-inductively coupled plasma-mass spectrometry trace element data and statistical analysis of large data sets. Econ. Geol. 2019, 114, 771–786. [Google Scholar] [CrossRef]
- Babedi, L.; Heyden, B.P.; Tadie, M.; Mayne, M. Trace elements in pyrite from five different gold ore deposit classes: A review and meta-analysis. In Magmatism in the Ocean Basins; Special Publications; Saunders, A.D., Norry, M.J., Eds.; Geological Society: London, UK, 2022; Volume 516, pp. 47–83. [Google Scholar]
- Keith, M.; Haase, K.M.; Chivas, A.R.; Klemd, R. Phase separation and fluid mixing revealed by trace element signatures in pyrite from porphyry systems. Geochim. Cosmochim. Acta 2022, 329, 185–205. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Gu, S.Y.; Mao, Q.; Deng, Z.X.; Guo, Z.D. LA-ICP-MS trace element characteristics of pyrites from the Lanmuchang Hg-Tl deposit in Xingren County, Guizhou Province and the Tl mineralization of the deposit. Acta Mineral. Sin. 2022, 42, 692–704, (In Chinese with English abstract). [Google Scholar]
- Cao, G.S.; Zhang, Y.; Chen, H.Y. Trace elements in pyrite from orogenic gold deposits: Implications for metallogenic mechanism. Acta Petrol. Sin. 2023, 39, 2330–2346, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Chen, L.A. A discussion on geochemical mechanism of mineralization of Hg, Sb, As and Au in carbonate formation in Guizhou. Guizhou Geol. 1990, 24, 196–203, (In Chinese with English abstract). [Google Scholar]
- Xiong, Y.L. The aqueous geochemistry of thallium: Speciation and solubility of thallium in low temperature systems. Environ. Chem. 2009, 6, 441. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]
- Román, N.; Reich, M.; Leisen, M.; Morata, D.; Barra, F.; Deditius, A.P. Geochemical and micro-textural fingerprints of boiling in pyrite. Geochim. Cosmochim. Acta 2019, 246, 60–85. [Google Scholar] [CrossRef]
- Schaarschmidt, A.; Haase, K.M.; Klemd, R.; Keith, M.; Voudouris, P.C.; Alfieris, D.; Strauss, H.; Wiedenbeck, M. Boiling effects on trace element and sulfur isotope compositions of sulfides in shallow-marine hydrothermal systems: Evidence from Milos Island, Greece. Chem. Geol. 2021, 583, 120457. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, S.Y.; Frimmel, H.E.; Zhu, L.Y. In situ chemical and isotopic analyses and element mapping of multiple-generation pyrite: Evidence of episodic gold mobilization and deposition for the Qiucun epithermal gold deposit in Southeast China. Am. Miner. 2022, 107, 1133–1148. [Google Scholar] [CrossRef]
- Kesler, S.E.; Fortuna, J.; Ye, Z.; Alt, J.C.; Core, D.P.; Zohar, P.; Borhauer, J.; Chryssoulis, S.L. Evaluation of the role of sulfidation in deposition of gold, Screamer section of the Betze-Post Carlin-type deposit, Nevada. Econ. Geol. 2003, 98, 137–1157. [Google Scholar] [CrossRef]

| Sample/Mineral | ZK901-10 | ZK901-13 | ||
|---|---|---|---|---|
| Mass % of Phase | Volume % of Phase | Mass % of Phase | Volume % of Phase | |
| Quartz | 36.42 | 31.92 | 56.45 | 61.20 |
| Pyrite | 11.01 | 18.45 | 15.80 | 8.96 |
| Kaolinite | 20.55 | 17.88 | 7.11 | 7.77 |
| Illite | 18.43 | 16.03 | 17.29 | 18.88 |
| Si, Al, S, O, Fe, Ti, K | 4.05 | 3.66 | 1.18 | 1.24 |
| Rutile | 1.70 | 2.41 | 0.52 | 0.35 |
| Cinnabar | 0.68 | 1.85 | – | – |
| Baryte | 1.01 | 1.52 | – | – |
| Florencite-(La) | 0.80 | 0.94 | 0.08 | 0.07 |
| Chromite | 0.54 | 0.81 | 0.02 | 0.01 |
| Si, Ti, Al, K, O | 0.50 | 0.45 | 0.47 | 0.50 |
| Apatite | 0.28 | 0.29 | 0.25 | 0.22 |
| Muscovite | 0.31 | 0.29 | 0.34 | 0.35 |
| Schorl | 0.20 | 0.21 | – | – |
| Pyrrhotite | 0.11 | 0.16 | 0.05 | 0.03 |
| Hematite/magnetite | 0.05 | 0.08 | 0.02 | – |
| Biotite | 0.07 | 0.07 | 0.13 | 0.12 |
| Unidentified minerals | 3.27 | 2.95 | 0.26 | 0.27 |
| Total | 99.98 | 99.98 | 99.97 | 99.97 |
| Mineral Type | Mineral Content (%) | Hg Content in Minerals (%) | Hg Distribution in Minerals (%) | Tl Content in Minerals (%) | Tl Distribution in Minerals (%) |
|---|---|---|---|---|---|
| Quartz | 36.42 | 0.02 | 0.007284 | 0.02 | 0.007284 |
| Pyrite | 11.01 | 0.04 | 0.004404 | 0.27 | 0.029727 |
| Cinnabar | 0.68 | 85.53 | 0.581604 | 0.61 | 0.004148 |
| Illite | 18.43 | 0.04 | 0.007372 | 0.02 | 0.003686 |
| Kaolinite | 20.55 | 0.03 | 0.006165 | 0.02 | 0.00411 |
| Barite | 1.01 | 0 | 0 | 0 | 0 |
| Total | 88.1 | 0.606829 | 0.048955 | ||
| The Au content of the ore is less than 0.2%, that of Hg is 0.6149%, and that of Tl is 0.0139%. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, J.; Xia, Y.; Wang, Z.; Yang, C.; Xie, Z.; Tan, Q.; Zhang, B. Distribution and Enrichment of Au, Hg, and Tl in the Lanmuchang Deposit, Guizhou, China. Minerals 2024, 14, 615. https://doi.org/10.3390/min14060615
Li S, Liu J, Xia Y, Wang Z, Yang C, Xie Z, Tan Q, Zhang B. Distribution and Enrichment of Au, Hg, and Tl in the Lanmuchang Deposit, Guizhou, China. Minerals. 2024; 14(6):615. https://doi.org/10.3390/min14060615
Chicago/Turabian StyleLi, Songtao, Jianzhong Liu, Yong Xia, Zepeng Wang, Chengfu Yang, Zhuojun Xie, Qinping Tan, and Bingqiang Zhang. 2024. "Distribution and Enrichment of Au, Hg, and Tl in the Lanmuchang Deposit, Guizhou, China" Minerals 14, no. 6: 615. https://doi.org/10.3390/min14060615
APA StyleLi, S., Liu, J., Xia, Y., Wang, Z., Yang, C., Xie, Z., Tan, Q., & Zhang, B. (2024). Distribution and Enrichment of Au, Hg, and Tl in the Lanmuchang Deposit, Guizhou, China. Minerals, 14(6), 615. https://doi.org/10.3390/min14060615







