Mineral Components, Organic Matter Quality and Soil Enzymatic Activity under the Influence of Differentiated Farmyard Manure and Nitrogen Fertilisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Methods
2.2.1. Physicochemical Properties of Soil
- Granulometric composition by laser diffraction, using a Mastersizer MS 2000 analyser;
- pH, potentiometrically in 1 M KCl extract [32];
- Hydrolytic acidity (Hh) and total exchangeable base cations (TEB), by using the Kappen method. Based on TEB and Hh, the cation exchange capacity (CEC) was calculated, and the sorption complex’s degree of saturation with bases (BS) was calculated from CEC and TEB;
- Electrical conductivity of 1:5 soil–water extract (EC1:5), by using the conductometric method [33].
2.2.2. Properties of Organic Matter
2.2.3. Content of Available Macroelements
- Available phosphorus (P) [36] and potassium (K) [37] were determined by using the Egner–Riehm (DL) method [38]. The method involves the extraction of phosphorus and potassium from the soil with a calcium lactate solution buffered to a pH of approximately 3.6. Available magnesium (Mg) was determined according to PN-R-04020 [39] by using the Schachtschabel [40] method, which involves extracting magnesium from the soil with a solution of 0.0125 M CaCl2 and a soil-to-solution ratio of 1:10.
2.2.4. Activity of Enzymes
- The total enzyme activity index (TEI) [45]:
- The geometric mean of enzyme activities (GMea) [46]:
2.3. Statistical Analyses
3. Results and Discussion
3.1. Selected Physical and Chemical Properties of Soils
3.2. Properties of Organic Matter
3.3. Content of Available Macroelements in Soil
3.4. Soil Enzyme Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lemanowicz, J.; Bartkowiak, A.; Zielińska, A.; Jaskulska, I.; Rydlewska, M.; Klunek, K.; Polkowska, M. The effect of enzyme activity on carbon sequestration and the cycle of available macro- (P, K, Mg) and microelements (Zn, Cu) in Phaeozems. Agriculture 2023, 13, 172. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Dębska, B.; Lamparski, R.; Michalska, A.; Pobereżny, J.; Wszelaczyńska, E.; Bartkowiak, A.; Szczepanek, M.; Banach-Szott, M.; Knapowski, T. Influence of plant growth retardants and nitrogen doses on the content of plant secondary metabolites in wheat, the presence of pests, and soil quality parameters. Agriculture 2023, 13, 1121. [Google Scholar] [CrossRef]
- Hofman, G.; van Cleemput, O. Soil and plant nitrogen, international fertilizer industry association (IFA), Paris. Sci. Res. 2004.
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Polyak, Y.; Gubelit, Y.; Bakina, L.; Shigaeva, T.; Kudryavtseva, V. Impact of macroalgal blooms on biogeochemical processes in estuarine systems: A case study in the eastern Gulf of Finland, Baltic Sea. J. Soils Sediments 2024, 24, 1854–1866. [Google Scholar] [CrossRef]
- Jaskulska, I.; Lemanowicz, J.; Dębska, B.; Jaskulski, D.; Breza-Boruta, B. Changes in soil organic matter and biological parameters as a result of long-term strip-till cultivation. Agriculture 2023, 13, 2188. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Dukes, J.S.; Paul, E.A.; Wallenstein, M.D. Microbial responses to multi-factor climate change: Effects on soil enzymes. Front. Microbiol. 2013, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, Q.; Wen, Y.; Wang, D.; Sun, X.; Hill, P.W.; Macdonald, A.; Chadwick, D.R.; Wu, L.; Jones, D.L. Farmyard manure applications stimulate soil carbon and nitrogen cycling by boosting microbial biomass rather than changing its community composition. Soil Biol. Biochem. 2020, 144, 107760. [Google Scholar] [CrossRef]
- Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of Differentiated nitrogen fertilization on the enzymatic activity of the soil for sweet potato (Ipomoea batatas L. [Lam.]) Cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- Reay, D.; Davidson, E.; Smith, K.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nature Clim. Change 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Gu, B.; Ge, Y.; Chang, S.X.; Luo, W.; Chang, J. Nitrate in groundwater of China: Sources and driving forces. Glob. Environ Chang. 2013, 23, 1112–1121. [Google Scholar] [CrossRef]
- Du, Y.; Cui, B.; Zhang, Q.; Wang, Z.; Sun, J.; Niu, W. Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis. Catena 2020, 193, 104617. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, N.; Xu, M.; Li, Z.; Lou, Y.; Chen, Y.; Wu, C.; Wang, Z.-L. 23-year manure and fertilizer application increases soil organic carbon sequestration of a rice–barley cropping system. Biol. Fertil. Soils 2015, 51, 583–591. [Google Scholar] [CrossRef]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Simansky, V.; Juriga, M.; Jonczak, J.; Uzarowicz, Ł.; Stepień, W. How relationships between soil organic matter parameters and soil structure characteristics are affected by the long-term fertilization of a sandy soil. Geoderma 2019, 342, 75–84. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, K.; Shi, L.; Li, C.; Wen, L.; Li, W.; Sun, M.; Sun, G.; Long, Z. Effects of long-term organic matter application on soil carbon accumulation and nitrogen use efficiency in a double-cropping rice field. Environ. Res. 2022, 213, 113700. [Google Scholar] [CrossRef] [PubMed]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The effect of application of organic manures and mineral fertilizers on the state of soil organic matter and nutrients in the long-term field experiment. J. Soils Sediments 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Gong, Q.; Chen, P.; Shi, R.; Gao, Y.; Zheng, S.-A.; Xu, Y.; Shao, C.; Zheng, X. Health assessment of trace metal concentrations in organic fertilizer in northern China. Int. J. Environ. Res. Public Health 2019, 16, 1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Li, G.; Ding, J.; Shen, Y.; Liu, D.; Cheng, H.; Zhang, Y.; Li, R. Speciation analysis method of heavy metals in organic fertilizers: A Review. Sustainability 2022, 14, 16789. [Google Scholar] [CrossRef]
- Lopes, C.; Herva, M.; Franco-Uría, A.; Roca, E. Inventory of heavy metal content in organic waste applied as fertilizer in agriculture: Evaluating the risk of transfer into the food chain. Environ. Sci. Pollut. Res. 2011, 18, 918–939. [Google Scholar] [CrossRef] [PubMed]
- Ström, G.; Albihn, A.; Jinnerot, T.; Boqvist, S.; Andersson-Djurfeldt, A.; Sokerya, S.; Osbjer, K.; San, S.; Davun, H.; Magnusson, U. Manure management and public health: Sanitary and socio-economic aspects among urban livestock-keepers in Cambodia. Sci. Tot. Environ. 2018, 621, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Panainte-Lehadus, M.; Mosnegutu, E.; Tomozei, C.; Chitimus, D.; Irimia, O. Assessment of manure compost used as soil amendment—A Review. Processes 2023, 11, 1167. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Angers, D.A.; Prévost, D.; Simard, R.R.; Chalifour, F.P. Dynamics of soluble organic C and C mineralization in cultivated soils with varying N fertilization. Soil Biol. Biochem. 1999, 31, 543–550. [Google Scholar] [CrossRef]
- Rochette, P.; Gregorich, E.G. Dynamics of soil microbial biomass C, soluble organic C and CO2 evolution after three years of manure application. Can. J. Soil Sci. 1998, 78, 283–290. [Google Scholar] [CrossRef]
- Singh, S.; Dutta, S.; Inamdar, S. Land application of poultry manure and its influence on spectrofluorometric characteristics of dissolved organic matter. Agric. Ecosyst. Environ. 2014, 193, 25–36. [Google Scholar] [CrossRef]
- Jokubauskaite, I.; Slepetiene, A.; Karcauskiene, D. Influence of different fertilization on the dissolved organic carbon, nitrogen and phosphorus accumulation in acid and limed soils. Eurasian J. Soil Sci. 2015, 4, 137–143. [Google Scholar] [CrossRef]
- Orlov, D.S. Humus Acids of Soil, 1st ed.; A.A. Balkema: Rotterdam, The Netherlands, 1986. [Google Scholar]
- Aranda, V.; Oyonarte, C. Characteristics of organic matter in soil surface horizons derived from calcareous and metamorphic rocks and different vegetation types from the Mediterranean high-mountains in SE Spain. Eur. J. Soil Biol. 2006, 42, 247–258. [Google Scholar] [CrossRef]
- Yang, Z.H.; Singh, B.R.; Sitaula, B.K. Soil organic carbon fractions under different land uses in Mardi Watershed of Nepal. Commun. Soil Sci. Plant Anal. 2006, 35, 615–629. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Wang, Y.; Li, J.; Zhang, J.J.; He, N.P. Soil organic carbon contents, aggregate stability, and humic acid composition in different alpine grasslands in Qinghai-Tibet Plateau. J. Mt. Sci. 2016, 13, 2015–2027. [Google Scholar] [CrossRef]
- Debska, B.; Jaskulska, I.; Jaskulski, D. Method of tillage with the factor determining the quality of organic matter. Agronomy 2020, 10, 1250. [Google Scholar] [CrossRef]
- PN-ISO 10390; Chemical and Agricultural Analysis: Determining Soil pH. Polish Standards Committee: Warszawa, Poland, 1997.
- van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; ISRIC: Wageningen, The Netherlands, 2002. [Google Scholar]
- Zhang, W.; Xu, M.; Wang, X.; Huang, Q.; Nie, J.; Li, Z.; Li, S.; Hwang, S.W.; Lee, K.B. Effects of organic amendments on soil carbon sequestration in paddy fields of subtropical China. J. Soils Sediments 2012, 12, 457–470. [Google Scholar] [CrossRef]
- Hayatu, N.G.; Liu, Y.; Han, T.; Daba, N.A.; Zhang, L.; Shen, Z.; Li, J.; Muazu, H.; Lamlom, S.F.; Zhang, H. Carbon sequestration rate, nitrogen use efficiency and rice yield responses to long-term substitution of chemical fertilizer by organic manure in a rice–rice cropping system. J. Integr. Agric. 2023, 22, 2848–2864. [Google Scholar] [CrossRef]
- PN-R-04023; Chemical and Agricultural Analysis—Determination of the Content of Available Phosphorus in Mineral Soils. Polish Standards Committee: Warszawa, Poland, 1996.
- PN-R-04022; Chemical and Agricultural Analysis—Determination of the Content Available Potassium in Mineral Soils. Polish Standards Committee: Warszawa, Poland, 1996.
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen uber die chemische Bodenanalyse als Grundlage fur die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- PN-R-04020; Chemical and Agricultural Analysis. Determination of the Content Available Magnesium. Polish Standards Committee: Warszawa, Poland, 1994.
- Schachtschabel, P. Das pflanzenverfügbare Magnesium des Boden und seine Bestimmung. J. Plant. Nutr. Soil Sci. 1954, 67, 9–23. [Google Scholar] [CrossRef]
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch 1968, 21, 249–258. [Google Scholar]
- Johnson, J.I.; Temple, K.l. Some variables affecting the measurements of catalase activity in soil. Soil Sci. Soci. Am. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p–nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and peptide derivates as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Tan, X.; Xie, B.; Wang, J.; He, W.; Wang, X.; Wei, G. County-scale spatial distribution of soil enzyme activities and enzyme activity indices in agricultural land: Implications for soil quality assessment. Sci. World J. 2014, 2014, 535768. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, M.B.; Garcia-Ruiz, R.; Viñegla, B.; Carreira, J.A. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biol. Biochem. 2004, 36, 1637–1644. [Google Scholar] [CrossRef]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for education and data anlysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- USDA. Keys to Soil Taxonomy, 10th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2006; pp. 1–332.
- PTG. Particle size distribution and textural classes of soil and mineral materials—Classification of Polish Society of Soil Sciences 2008. Soil Sci. Ann. 2009, 60, 5–16. [Google Scholar]
- Jaskulska, I.; Jaskulski, D. Influence of many years’ fertilization on the dynamics of soil properties. Adv. Agric. Sci. 2003, 4, 21–35. [Google Scholar]
- Murawska, B.; Spychaj-Fabisiak, E. Degree of soil acidity and the content of available forms of Zinc and copper as an effect of 35-year nitrogen and potassium fertilisation. Sci. Agric. UPWr 2010, 47, 85–96. [Google Scholar]
- Tang, Y.; Garvin, D.F.; Kochian, L.V.; Sorrells, M.E.; Carver, B.F. Physiological genetics of aluminum tolerance in the wheat cultivar Atlas 66. Crop Sci. 2002, 42, 1541–1546. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Q.; Zeng, M.; Shen, J.; Shi, X.; Liu, X.; de Vries, W. Impacts of nitrogen fertilizer type and application rate on soil acidification rate under a wheat-maize double cropping system. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.L.B.; Antonangelo, J.A.; Zhang, H.; Reed, V. Impact of long-term fertilization in no-till on the stratification of soil acidity and related parameters. Soil Tillage Res. 2023, 228, 105624. [Google Scholar] [CrossRef]
- Kariuki, S.K.; Zhang, H.; Schroder, J.L.; Edwards, J.; Payton, M.; Carver, B.F.; Raun, W.R.; Krenzer, E.G. Hard red winter wheat cultivar responses to a pH and aluminum concentration gradient. Agron. J. 2007, 99, 88–98. [Google Scholar] [CrossRef]
- Chien, S.H.; Gearhart, M.M.; Collamer, D.J. The effect of different ammonical nitrogen sources on soil acidification. Soil Sci. 2008, 173, 544–551. [Google Scholar] [CrossRef]
- Krzywy, E.; Krupa, J.; Wołoszyk, C. Wpływ wieloletniego nawożenia organicznego i mineralnego na niektóre wskaźniki żyzności gleby. Zeszyty Naukowe Akademii Rolniczej w Szczecinie 172. Rolnictwo 1996, 62, 259–264. [Google Scholar]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salt-Affected Soils and Their Management; FAO Soils Bulletin 39; FAO: Rome, Italy, 1988. [Google Scholar]
- Li, J.; Fan, X.; Zhu, Y.; Rao, G.; Chen, R.; Duan, T. Effects of irrigation and nitrogen fertilization on mitigating salt-induced Na+ toxicity and sustaining sea rice growth. Open Life Sci. 2022, 17, 1165–1173. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ. Sci. Pollut. Res. 2015, 22, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Zsolnay, A.; Gorlitz, H. Water extractable organic matter in arable soils effects of drought and long-term fertilization. Soil Biol. Biochem. 1994, 26, 1257–1261. [Google Scholar] [CrossRef]
- Liu, Z.J.; Clay, S.A.; Clay, D.E.; Harper, S.S. Ammonia fertilizer influences atrazine adsorption–desorption characteristics. J. Agric. Food. Chem. 1995, 43, 815–819. [Google Scholar] [CrossRef]
- Homann, P.S.; Grigal, D.F. Molecular weight distribution of soluble organics from laboratory-manipulated soils. Soil Sci. Soc. Am. J. 1992, 56, 1305–1310. [Google Scholar] [CrossRef]
- Debska, B.; Dlugosz, J.; Piotrowska-Dlugosz, A.; Banach-Szott, M. The impact of a bio- fertilizer on the soil organic matter status and carbon sequestration—Results from a field-scale study. J. Soils Sediments 2016, 16, 2335–2343. [Google Scholar] [CrossRef]
- Guimaraes, D.V.; Gonzaga, M.I.S.; da Silva, T.O.; da Silva, T.L.; da Silva Dias, N.; Silva Matias, M.I. Soil organic matter pools and carbon fractions in soil under different land uses. Soil Tillage Res. 2012, 126, 177–182. [Google Scholar] [CrossRef]
- Lemanowicz, J. Mineral fertilisation as a factor determining selected sorption properties of soil against the activity of phosphatases. Plant Soil Environ. 2013, 59, 439–445. [Google Scholar] [CrossRef]
- Wang, T.; Bauke, S.L.; von Sperbera, C.; Tamburini, F.; Guigue’a, J.; Winkler, P.; Kaisera, K.; Honermeiera, B.; Amelunga, W. Soil phosphorus cycling is modified by carbon and nitrogen fertilization in a long-term field experiment. J. Plant Nutr. Soil Sci. 2021, 184, 282–293. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Till. Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Nong, C.; Gao, P.; Wang, B.; Lin, W.; Jiang, N.; Cai, K. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; Zhang, L.; Gao, S. Nitrification and acidification from urea application in red soil (Ferralic Cambisol) after different long-term fertilization treatments. J. Soils Sediments 2014, 14, 1526–1536. [Google Scholar] [CrossRef]
- Samuel, A.D.; Bungau, S.; Tit, D.M.; Melinte, C.E.; Purza, L.; Badea, G.E. Effects of long term application of organic and mineral fertilizers on soil enzymes. Rev. Chim. 2018, 69, 2608–2612. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Lemanowicz, J.; Rydlewska, M.; Drabińska, O.; Ewert, K. Enzymatic activity of soil after applications distillery stillage. Agriculture 2022, 12, 652. [Google Scholar] [CrossRef]
- Naga Raju, M.; Golla, N.; Vengatampalli, R. Soil Protease. In Soil Enzymes. Springer Briefs in Environmental Science; Springer: Cham, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Vasbieva, M.T. Changes in the agrochemical properties of soddy-podzolic soil under the impact of long-term application of fertilizers. Eurasian Soil Sc. 2021, 54, 108–116. [Google Scholar] [CrossRef]
- Padhan, K.; Bhattacharjya, S.; Sahu, A.; Manna, M.C.; Sharma, M.P.; Singh, M.; Wanjari, R.H.; Sharma, R.P.; Sharma, G.K.; Patra, A.K. Soil N transformation as modulated by soil microbes in a 44 years long term fertilizer experiment in a sub-humid to humid Alfisol. App. Soil Ecol. 2020, 145, 103355. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme activities in soil. In Nucleic Acids and Proteins in Soil; Springer: Berlin/Heidelberg, Germany, 2006; pp. 257–311. [Google Scholar] [CrossRef]
- Picariello, E.; Baldantoni, D.; Muniategui-Lorenzo, S.; Concha-Grana˜, E.; De Nicola, F. A synthetic quality index to evaluate the functional stability of soil microbial communities after perturbations. Ecol. Indic. 2021, 128, 107844. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A.; Tobiasova, E. The application of biochar from waste biomass to improve soil fertility and soil enzyme activity and increase carbon sequestration. Energies 2023, 16, 380. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutierrez, B.; Mendez, A. Soil biochemical activities and geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Chmiel, M.J.; Dziedzic, K.; Taras, H. Assessment of soil quality after biochar application based on enzymatic activity and microbial composition. Int. Agrophys. 2019, 33, 331–336. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T. Trace elements composition and enzymes activity of soil amended with municipal sewage sludge at three locations in Kentucky. Int. J. Appl. Agric. Sci. 2020, 6, 89–95. [Google Scholar] [CrossRef]
- Maphuhla, N.G.; Oyedeji, O.O. Effects of clay minerals on enzyme activity as a potential biosensor of soil pollution in Alice Township. Waste 2024, 2, 85–101. [Google Scholar] [CrossRef]
- Olagoke, F.K.; Kalbitz, K.; Vogel, C. Control of soil extracellular enzyme activities by clay minerals-perspectives on microbial responses. Soil Syst. 2019, 3, 64. [Google Scholar] [CrossRef]
- Campdelacreu Rocabruna, P.; Domene, X.; Preece, C.; Peñuelas, J. Relationship among soil biophysicochemical properties, agricultural practices and climate factors influencing soil phosphatase activity in agricultural land. Agriculture 2024, 14, 288. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Haddad, S.A.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P. The role of an urban park’s tree stand in shaping the enzymatic activity, glomalin content and physicochemical properties of soil. Sci. Tot. Environ. 2020, 741, 140446. [Google Scholar] [CrossRef] [PubMed]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Parham, J.; Deng, S.; Raun, W.; Johnson, G. Long-term cattle manure application in soil. Biol. Fertil. Soils 2002, 35, 328–337. [Google Scholar] [CrossRef]
- Hok, L.; de MoraesSá, J.C.; Reyes, M.; Boulakia, S.; Tivet, F.; Leng, V.; Kong, R.; Briedis, C.; da Cruz Hartman, D.; Ferreira, L.A.; et al. Enzymes and C pools as indicators of C build up in short-term conservation agriculture in a savanna ecosystem in Cambodia. Soil Till. Res. 2018, 177, 125–133. [Google Scholar] [CrossRef]
- Datta, A.; Gujre, N.; Gupta, D.; Agnihotri, R.; Mitra, S. Application of enzymes as a diagnostic tool for soils as affected by municipal solid wastes. J. Environ. Managem. 2021, 286, 112169. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, E.K.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Li, J.; Xie, T.; Zhu, H.; Zhou, J.; Li, C.; Xiong, W.; Xu, L.; Wu, Y.; He, Z.; Li, X. Alkaline phosphatase activity mediates soil organic phosphorus mineralization in a subalpine forest ecosystem. Geoderma 2021, 404, 115376. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes. A meta-analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Jusheng, G.; Lei, W.; Mustafa, A.; Waqas, A.; Aziz, T.; Khan, W.; Rehman, S.; Hussain, B.; Farooq, M.; et al. Soil microbial biomass and extracellular enzyme–mediated mineralization potentials of carbon and nitrogen under long-term fertilization (> 30 years) in a rice–rice cropping system. J. Soils Sediments 2021, 21, 3789–3800. [Google Scholar] [CrossRef]
- Atoloye, I.A.; Jacobson, A.; Creech, E.; Reeve, J. Variable impact of compost on phosphorus dynamics in organic dryland soils following a one-time application. Soil Sci. Soc. Am. J. 2021, 85, 1122–1138. [Google Scholar] [CrossRef]
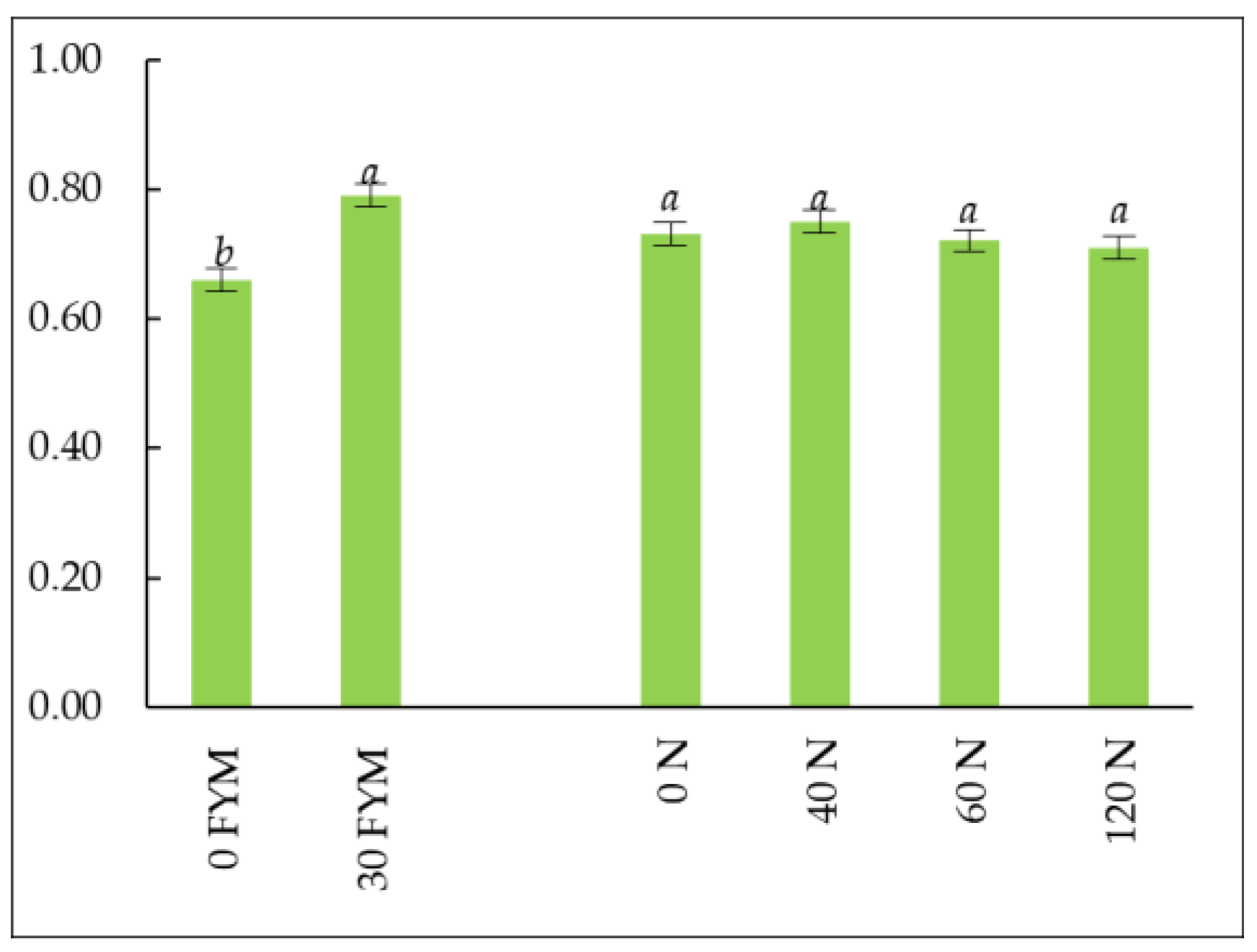


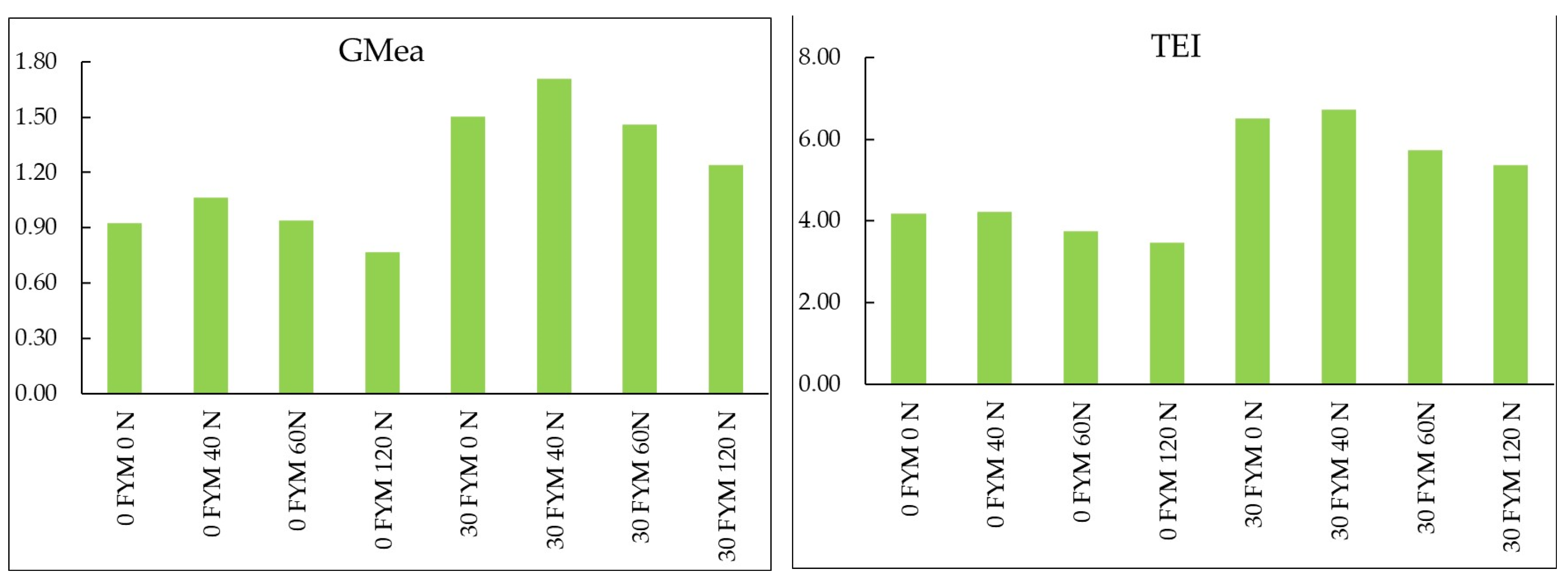
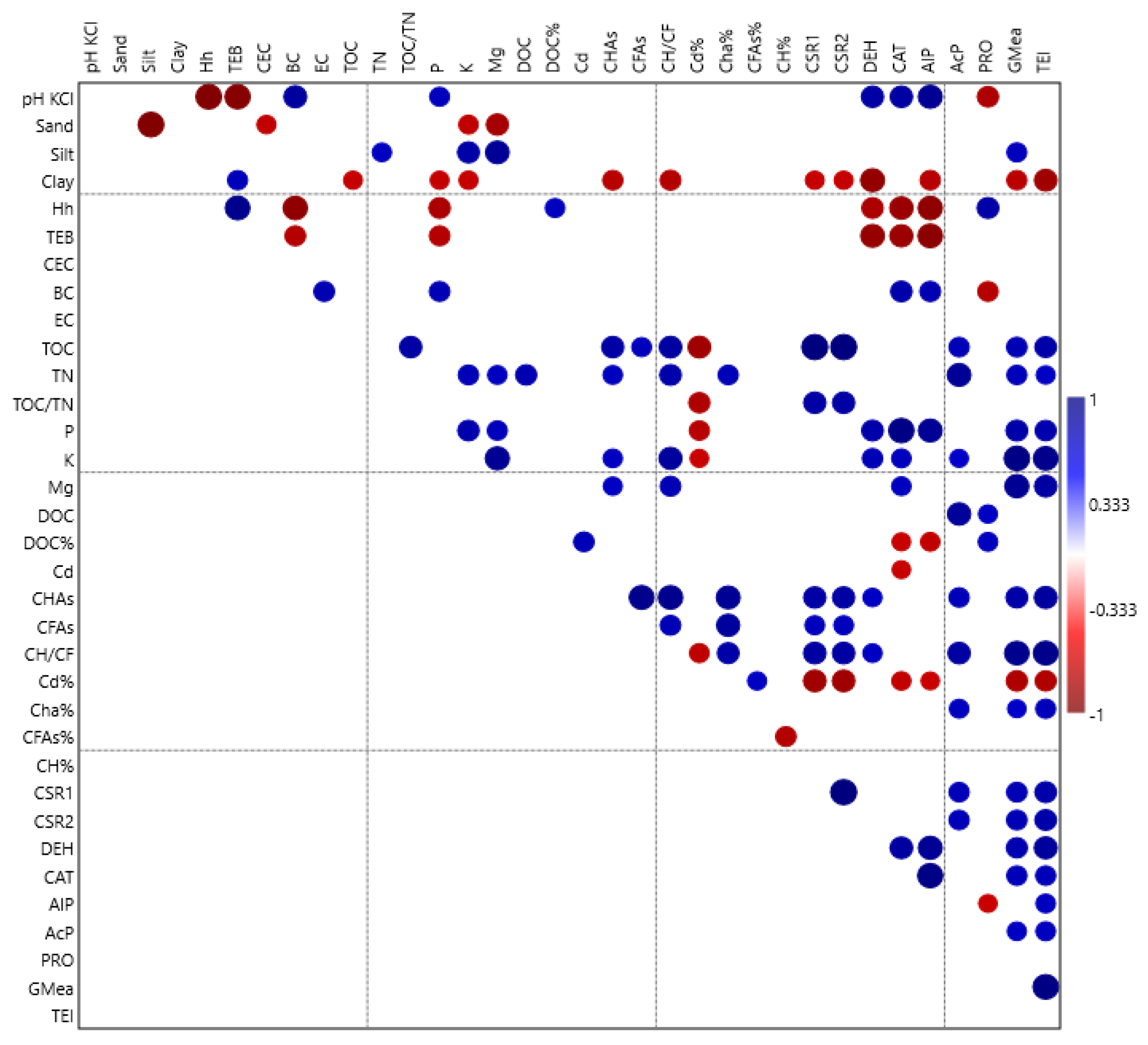
| Nitrogen (kg ha−1) II Factor | FYM (t ha−1) I Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 FYM | 30 FYM | 0 FYM | 30 FYM | 0 FYM | 30 FYM | 0 FYM | 30 FYM | |
| Sand (%) | Silt (%) | Clay (%) | pH KCl | |||||
| 0 N | 55.30 ±9.21 | 55.91 ±8.32 | 39.60 ±5.44 | 39.31 ±3.61 | 5.10 ±1.99 | 4.78 ±1.02 | 5.17 ±0.28 | 5.63 ±0.41 |
| 40 N | 55.40 ±5.32 | 54.49 ±4.86 | 39.47 ±4.62 | 40.64 ±4.11 | 5.13 ±0.83 | 4.87 ±0.75 | 4.94 ±0.34 | 4.88 ±0.22 |
| 60 N | 56.17 ±6.51 | 53.81 ±3.92 | 38.55 ±5.14 | 41.07 ±4.30 | 5.28 ±2.02 | 5.12 ±1.64 | 4.33 ±0.35 | 4.61 ±0.37 |
| 120 N | 56.33 ±4.68 | 55.41 ±5.23 | 38.50 ±4.80 | 39.61 ±3.69 | 5.17 ±0.99 | 4.98 ±1.02 | 4.02 ±0.28 | 4.44 ±0.18 |
| Nitrogen (kg ha−1) II Factor | FYM (t ha−1) I Factor | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | |
| Hh (cmol kg−1) | TEB (cmol kg−1) | CEC (cmol kg−1) | BS (%) | EC (µS cm−1) | |||||||||||
| 0 N | 1.87cA ±0.01 | 1.46cB ±0.22 | 1.67d | 47.70 * ±0.4 | 45.85 ±0.1 | 46.78c | 49.57cA ±0.40 | 47.35cB ±0.45 | 48.46c | 96.23aA ±0.03 | 96.98aA ±0.21 | 96.60a | 657.2bcA ±26.91 | 751.2 aA ±34.05 | 704.2ab |
| 40 N | 1.94cA ±0.03 | 1.93bA ±0.11 | 1.93c | 48.10 ±1.8 | 47.85 ±0.1 | 47.98b | 50.51bA ±0.72 | 49.87bA ±0.30 | 50.19b | 96.72aB ±0.03 | 95.95bB ±0.59 | 96.33a | 961.3aB ±84.86 | 764.3 aB ±30.40 | 862.8a |
| 60 N | 2.46bA ±0.11 | 2.34aA ±0.03 | 2.40b | 49.20 ±1.4 | 48.20 ±1.6 | 48.70ab | 52.44aA ±0.23 | 50.54abB ±0.57 | 51.49a | 95.17bcC ±0.04 | 95.69bC ±0.52 | 95.43b | 576.3cC ±63.80 | 565.1 aC ±47.58 | 570.7b |
| 120 N | 2.74aA ±0.23 | 2.53aB ±0.04 | 2.63a | 49.75 ±0.1 | 48.80 ±0.4 | 49.28a | 52.49aA ±0.33 | 50.90aB ±0.40 | 51.72a | 94.79cD ±0.41 | 95.03cD ±0.04 | 94.91c | 517.5 cD ±35.10 | 777.9 aD ±28.24 | 647.7b |
| Mean | 2.25a * | 2.06b | 48.69a | 47.66a | 51.25a | 49.68a | 95.72a | 95.91a | 678.1a | 714.6a | |||||
| η2 for FYM; N η2 for interaction; error | 5.36%; 89.86%; 3.32%; 1.35% | 20.32%; 69.10%; 6.38%; 3.46% | 21.76%; 72.50%; 3.88%; 1.01% | 1.49%; 79.34%; 14.42%; 2.02% | 1.50%; 51.70%; 31.04%; 12.61% | ||||||||||
| Nitrogen (kg ha−1) II Factor | FYM (t ha−1) I Factor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | |
| TOC | TN | TOC/TN | |||||||
| 0 N | 6.59bB ± 0.10 | 9.13aA ± 0.16 | 7.86a | 0.91 * ± 0.02 | 0.99 ± 0.03 | 0.95b | 7.24abB | 9.22aA | 8.23a |
| 40 N | 6.53bB ± 0.11 | 8.62abA ± 0.26 | 7.58a | 0.98 ±0.02 | 1.06 ±0.02 | 1.02a | 6.65bB | 8.19bA | 7.42b |
| 60 N | 7.33aB ± 0.20 | 8.31abA ± 0.31 | 7.82a | 0.94 ± 0.03 | 1.08 ±0.01 | 1.01ab | 7.78aA | 7.75bA | 7.76ab |
| 120 N | 7.55aA ± 0.22 | 7.89bA ±0.25 | 7.72a | 0.94 ±0.02 | 1.08 ±0.01 | 1.01ab | 8.01aA | 7.33bcB | 7.67b |
| Mean | 7.00b | 8.45a | 0.95a | 1.05a | 7.42b | 8.12a | |||
| η2 for FYM; N η2 for interaction; η2 for error | 59.00%; 1.27% 20.32%; 19.44% | 31.05%; 8.22% 2.28%; 58.44% | 19.49%; 13.60% 46.90%; 20.00% | ||||||
| Nitrogen (kg ha−1) II Factor | FYM (t ha−1) I Factor | |||||
|---|---|---|---|---|---|---|
| 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | |
| DOC (mg kg−1) | DOC (%) | |||||
| 0 N | 75.7bB ± 5.0 | 90.3bA ± 5.2 | 83.0c | 1.15abA ± 0.06 | 0.99bB ± 0.05 | 1.07c |
| 40 N | 76.3bB ± 4.9 | 92.4bA ± 5.8 | 84.3c | 1.17abA ± 0.05 | 1.08bA ± 0.04 | 1.12bc |
| 60 N | 80.5bB ± 5.1 | 105.3aA ± 4.6 | 92.9b | 1.10bcB ± 0.08 | 1.27aA ± 0.05 | 1.18b |
| 120 N | 93.5aB ± 3.8 | 105.5aA ± 5.0 | 99.5a | 1.24aA ± 0.04 | 1.34aA ± 0.05 | 1.29a |
| Mean | 81.5b | 98.4a | 1.17a | 1.17a | ||
| η2 for FYM; N η2 for interaction; error | 56.25%; 35.37% 4.55%; 3.84% | 0.00%; 49.04% 35.03%; 15.92% | ||||
| Nitrogen (kg ha−1) II Factor | FYM (t ha−1) I Factor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | |
| Cd | CHAs | CFAs | |||||||
| 0 N | 106 * ± 3 | 110 ± 8 | 108b | 1175bB ± 40 | 1955A ± 45 | 1565a | 1840 ± 45 | 2437 ± 45 | 2138a |
| 40 N | 112 ± 5 | 102 ± 5 | 107b | 1413aB ± 45 | 1641A ± 40 | 1527a | 2041 ± 55 | 2025 ± 60 | 2033a |
| 60 N | 106 ± 4 | 115 ± 6 | 110b | 1277aB ± 25 | 1735A ± 45 | 1506a | 1901 ± 54 | 2267 ± 57 | 2084a |
| 120 N | 122 ± 4 | 126 ± 7 | 124a | 1273aB ± 28 | 1712A ± 47 | 1493a | 1970 ± 32 | 2211 ± 58 | 2091a |
| Mean | 111a | 113a | 1285b | 1761a | 1938a | 2235a | |||
| η2 for FYM; N η2 for interaction; error | 0.64%; 49.71% 12.20%; 37.46% | 78.11%; 1.04% 13.40%; 7.45% | 29.46%; 1.87% 16.33%; 52.34% | ||||||
| Nitrogen (kg ha−1) II Factor | FYM (t ha−1) I Factor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | |
| P | K | Mg | |||||||
| 0 N | 48.65aB * ± 0.264 | 63.57bA ± 0.587 | 56.11b | 58.40abB ± 0.748 | 65.44bcA ± 0.827 | 61.92b | 40.29abB ± 0.582 | 46.01bA ± 0.448 | 43.15b |
| 40 N | 51.69aB ± 0.304 | 68.14aA ± 0.608 | 59.92a | 60.79aB ± 0.834 | 72.95aA ± 0.869 | 66.87a | 42.62aB ± 0.597 | 49.38aA ± 0.502 | 45.99a |
| 60 N | 43.82bB ± 0.256 | 51.59cA ± 0.782 | 47.71c | 56.05bB ± 0.627 | 66.96bA ± 0.751 | 61.51b | 38.99bA ± 0.429 | 50.77aA ± 0.511 | 44.88ab |
| 120 N | 35.66cB ± 0.185 | 40.52dA ± 0.439 | 38.09d | 54.00bB ± 0.615 | 63.33cA ± 0.722 | 58.67c | 37.21cB ± 0.448 | 41.75cA ± 0.395 | 39.48c |
| Mean | 44.96b | 55.96a | 57.31b | 67.17a | 39.77b | 46.97a | |||
| η2 for FYM; N η2 for interaction; error | 27.99%; 66.77% 4.72%; 0.478% | 71.08%; 25.47% 2.677%; 0.760% | 61.06%; 28.74% 8.97%; 1.12% | ||||||
| Nitrogen (kg ha−1) II Factor | FYM (t ha−1) I Factor | |||||
|---|---|---|---|---|---|---|
| 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | |
| DEH | CAT | |||||
| 0 N | 0.924 ± 0.030 | 1.258 ± 0.017 | 1.091a | 0.419aB ± 0.031 | 0.624aA ± 0.041 | 0.522a |
| 40 N | 0.772 ± 0.027 | 1.092 ± 0.006 | 0.932b | 0.387bB ± 0.028 | 0.594aA ± 0.038 | 0.491b |
| 60 N | 0.523 ± 0.041 | 0.881 ± 0.031 | 0.702c | 0.280cB ± 0.019 | 0.428bA ± 0.034 | 0.354c |
| 120 N | 0.508 ± 0.022 | 0.869 ± 0.023 | 0.689c | 0.119dB ± 0.014 | 0.209cA ± 0.029 | 0.164d |
| Mean | 0.682b | 1.025a | 0.419b | 0.624a | ||
| η2 for FYM; N η2 for interaction; error | 48.95%; 49.94% 0.554%; 0.554% | 23.59%; 73.70% 2.40%; 0.31% | ||||
| Nitrogen (kg ha−1) II Factor | FYM (t ha−1) I Factor | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | 0 FYM | 30 FYM | Mean | |
| AlP | AcP | PRO | |||||||
| 0 N | 0.599aB ± 0.071 | 0.895aA ± 0.082 | 0.747a | 0.501dB ± 0.035 | 1.132dA ± 0.085 | 0.817c | 5.8dB ± 0.121 | 9.70dA ± 0.259 | 7.75d |
| 40 N | 0.428bB ± 0.065 | 0.722bA ± 0.059 | 0.575b | 0.583cB ± 0.021 | 1.422bA ± 0.081 | 1.003b | 18.2cB ± 0.181 | 21.70bA ± 0.425 | 19.95c |
| 60 N | 0.307cB ± 0.035 | 0.472cA ± 0.051 | 0.390c | 0.711bB ± 0.025 | 1.318cA ± 0.071 | 1.015b | 23.1bB ± 0.358 | 28.21bA ± 0.483 | 25.65b |
| 120 N | 0.173dB ± 0.018 | 0.286dA ± 0.021 | 0.230d | 0.853aB ± 0.058 | 1.572aA ± 0.069 | 1.213a | 29.4aB ± 0.745 | 35.72aA ± 0.688 | 32.55a |
| Mean | 0.377b | 0.594a | 0.662b | 1.361a | 19.13b | 23.83a | |||
| η2 for FYM; N η2 for interaction; η2 for error | 23.00%; 47.00%; 2.80%; 0.20% | 85.27%; 13.35% 1.35%; 0.03% | 5.99%; 93.59%; 0.39%; 0.024% | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemanowicz, J.; Bartkowiak, A.; Dębska, B.; Majcherczak, E.; Michalska, A. Mineral Components, Organic Matter Quality and Soil Enzymatic Activity under the Influence of Differentiated Farmyard Manure and Nitrogen Fertilisation. Minerals 2024, 14, 645. https://doi.org/10.3390/min14070645
Lemanowicz J, Bartkowiak A, Dębska B, Majcherczak E, Michalska A. Mineral Components, Organic Matter Quality and Soil Enzymatic Activity under the Influence of Differentiated Farmyard Manure and Nitrogen Fertilisation. Minerals. 2024; 14(7):645. https://doi.org/10.3390/min14070645
Chicago/Turabian StyleLemanowicz, Joanna, Agata Bartkowiak, Bożena Dębska, Edward Majcherczak, and Agata Michalska. 2024. "Mineral Components, Organic Matter Quality and Soil Enzymatic Activity under the Influence of Differentiated Farmyard Manure and Nitrogen Fertilisation" Minerals 14, no. 7: 645. https://doi.org/10.3390/min14070645
APA StyleLemanowicz, J., Bartkowiak, A., Dębska, B., Majcherczak, E., & Michalska, A. (2024). Mineral Components, Organic Matter Quality and Soil Enzymatic Activity under the Influence of Differentiated Farmyard Manure and Nitrogen Fertilisation. Minerals, 14(7), 645. https://doi.org/10.3390/min14070645







