Synthesis of an Antipyrine-Based Fluorescent Probe with Synergistic Effects for the Selective Recognition of Zinc Ion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Synthesis and Characterization of Probe P2
2.3. Spectral Analysis
3. Results and Discussion
3.1. Synthesis of Probe P2
3.2. Influence of Solvents on Fluorescence Detection
3.3. Influence of Water Content on Fluorescence Detection
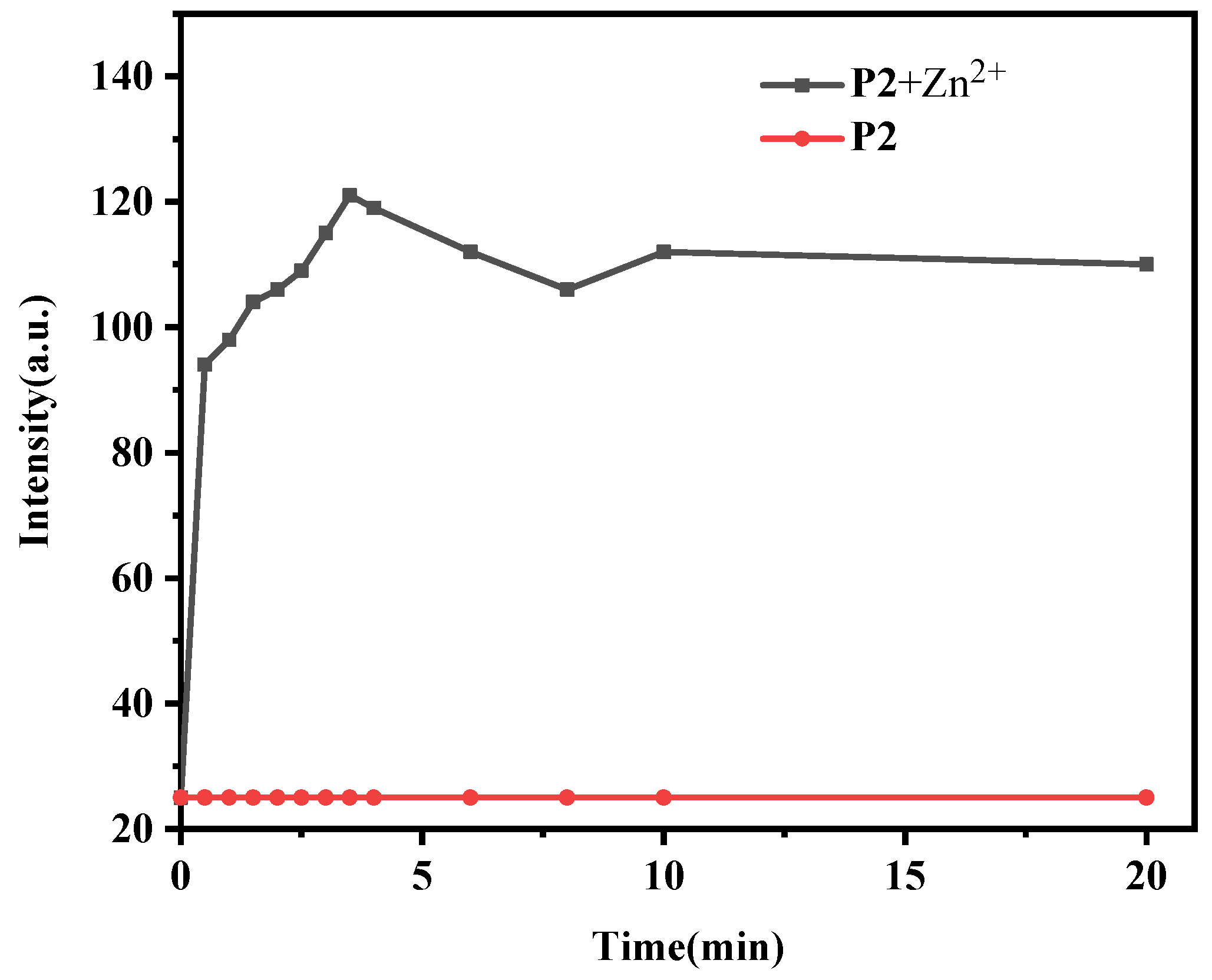
3.4. Influence of Incubation Time on Fluorescence Detection
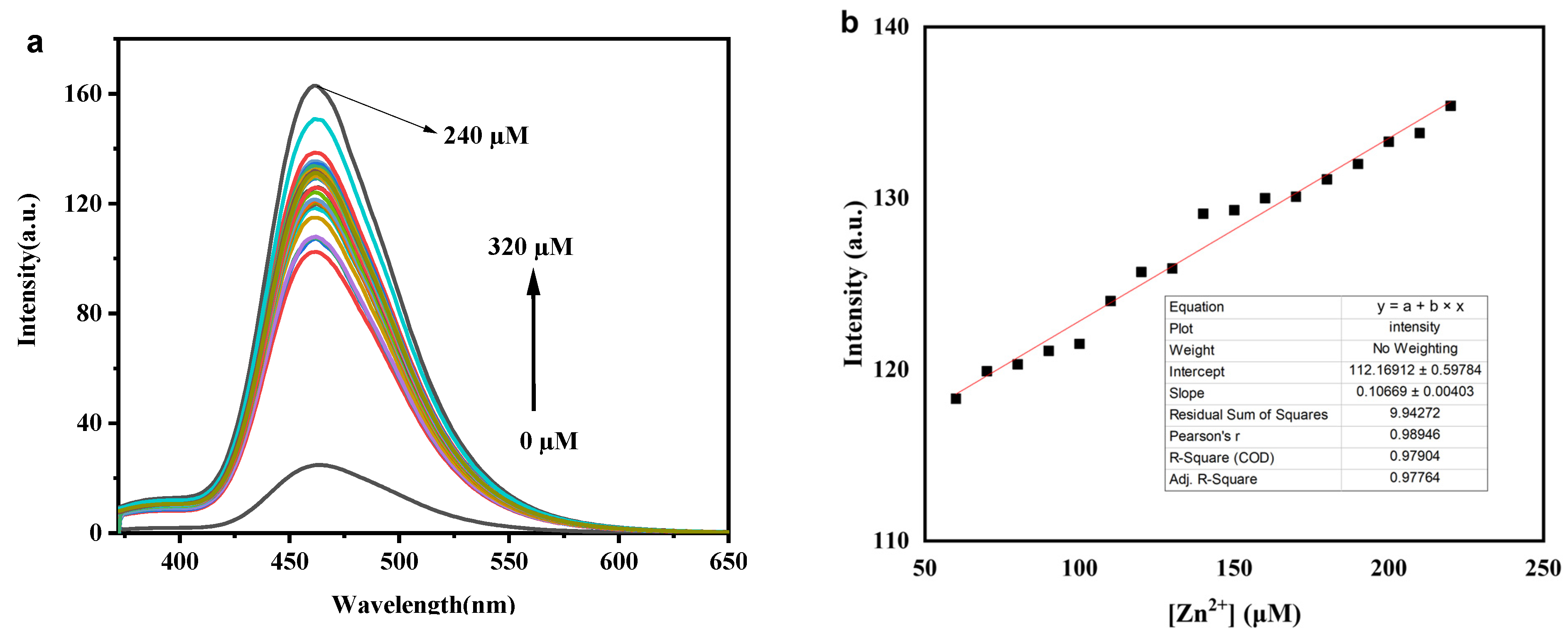
3.5. Fluorescence Titration
3.6. Anti-Interference Experiments
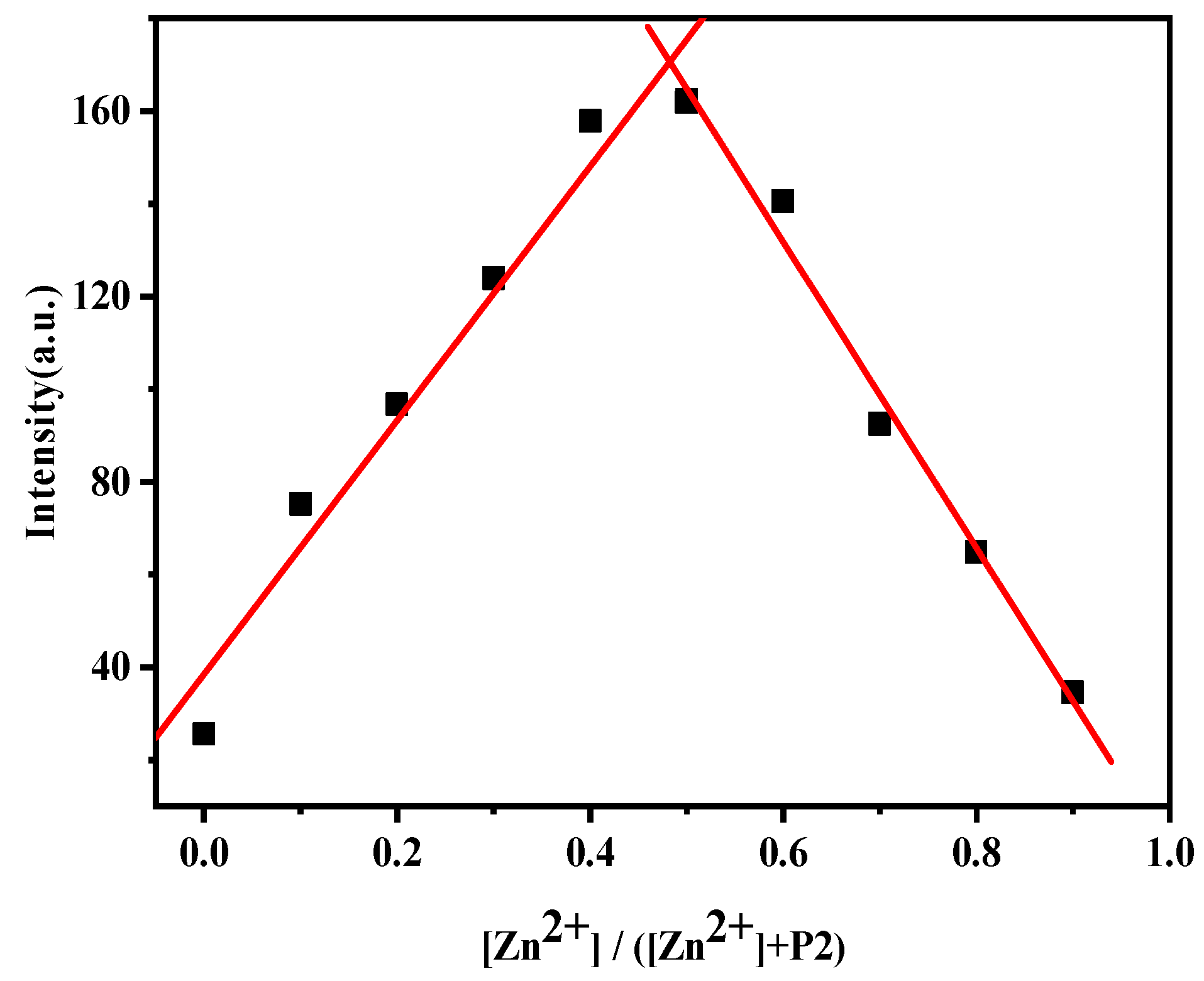
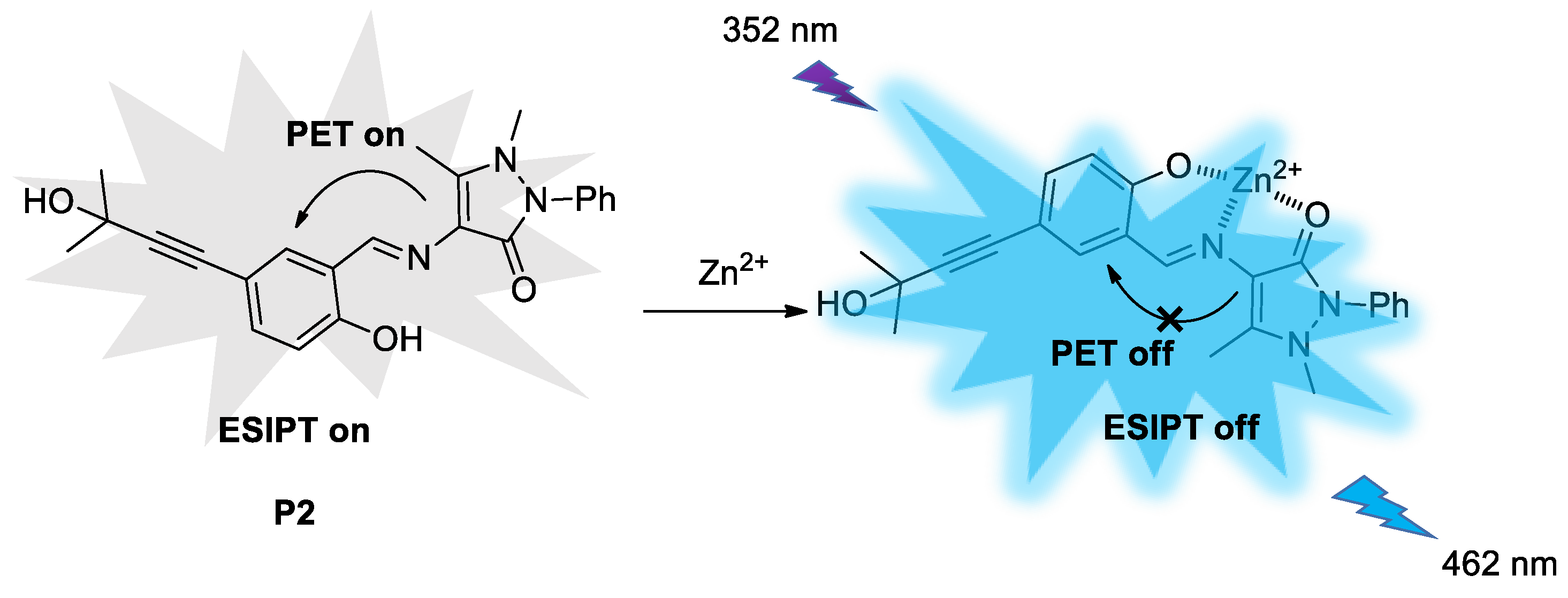
3.7. Job’s Plot Curve and Recognition Mechanism
3.8. Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Aulakh, S.K.; Varma, S.J.; Ralser, M. Metal ion availability and homeostasis as drivers of metabolic evolution and enzyme function. Curr. Opin. Genet. Dev. 2022, 77, 101987. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Teng, Y.-F.; Ji, C.-L.; Wu, H.-F.; Li, F. Critical target identification and human health risk ranking of metal ions based on mechanism-driven modeling. Chemosphere 2022, 301, 134724. [Google Scholar] [CrossRef]
- Zhai, J.-Y.; Xie, X.-J.; Cherubini, T.; Bakker, E. Ionophore-Based Titrimetric Detection of Alkali Metal Ions in Serum. ACS Sens. 2017, 2, 606–612. [Google Scholar] [CrossRef]
- Narin, I.; Soylak, M.; Elçi, L.; Doğan, M. Determination of trace metal ions by AAS in natural water samples after preconcentration of pyrocatechol violet complexes on an activated carbon column. Talanta 2000, 52, 1041–1046. [Google Scholar] [CrossRef]
- Zengin, H.B.; Gürkan, R. Application of a novel poly(SMAm)-Tris-Fe3O4 nanocomposite for selective extraction and enrichment of Cu(I)/Cu(II) from beer, soft drinks and wine samples, and speciation analysis by micro-volume UV–Vis spectrophotometry. Talanta 2021, 224, 121789. [Google Scholar] [CrossRef]
- Smirnova, S.V.; Ilin, D.V.; Pletnev, I.V. Extraction and ICP-OES determination of heavy metals using tetrabutylammonium bromide aqueous biphasic system and oleophilic collector. Talanta 2021, 221, 121485. [Google Scholar] [CrossRef]
- Ali, T.A.; Abd-Elaal, A.A.; Mohamed, G.G. Screen printed ion selective electrodes based on self-assembled thiol surfactant-gold-nanoparticles for determination of Cu(II) in different water samples. Microchem. J. 2021, 160, 105693. [Google Scholar] [CrossRef]
- Bereza-Malcolm, L.T.; Mann, G.; Franks, A.E. Environmental sensing of heavy metals through whole cell microbial biosensors: A synthetic biology approach. ACS Synth. Biol. 2015, 4, 535–546. [Google Scholar] [CrossRef]
- Jiang, D.-D.; Zheng, M.-H.; Ma, X.-F.; Zhang, Y.-Z.; Jiang, S.-H.; Li, J.-H.; Zhang, C.-M.; Liu, K.-M.; Li, L.-Q. Rhodamine-anchored polyacrylamide hydrogel for fluorescent naked-eye sensing of Fe3+. Molecules 2023, 28, 6572. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, M.-H.; Liu, H.-Y.; Xiao, J.; Gong, T.-H.; Liu, K.-M.; Li, J.-H.; Liu, J.-B. A novel HBI-based ratiometric fluorescent probe for rapid detection of trifluoroborate. RSC Adv. 2023, 13, 23812. [Google Scholar]
- Li, L.-Q.; Zheng, M.-H.; Yan, X.-Y.; Huang, H.; Cao, S.-X.; Liu, K.-M.; Liu, J.-B. Quantitative detection of H2O2 with a composite fluorescent probe of 8-quinoline boronic acid-Al(III). J. Photoch. Photobio. A Chem. 2022, 432, 114069. [Google Scholar] [CrossRef]
- Jiang, D.-D.; Zheng, M.-H.; Yan, X.-Y.; Huang, B.; Huang, H.; Gong, T.-H.; Liu, K.-M.; Liu, J.-B. A “turn-on” ESIPT fluorescence probe of 2-(aminocarbonyl)phenylboronic acid for the selective detection of Cu(II). RSC Adv. 2022, 12, 31186. [Google Scholar] [CrossRef]
- Ye, Q.-X.; Ren, S.-F.; Huang, H.; Duan, G.-G.; Liu, K.-M.; Liu, J.-B. Fluorescent and Colorimetric Sensors Based on the Oxidation of o-Phenylenediamine. ACS Omega 2020, 5, 20698–20706. [Google Scholar] [CrossRef]
- Arumugam, A.; Shanmugam, R.; Munusamy, S.; Muhammad, S.; Algarni, H.; Sekar, M. Study of the crystal architecture, optoelectronic characteristics, and nonlinear optical properties of 4-amino antipyrine Schiff bases. ACS Omega 2023, 8, 15168–15180. [Google Scholar] [CrossRef]
- Hassanien, A.M.; Altalhi, T.A.; Atta, A.A.; AlHazaa, A.N.; Alsawat, M.; Mersal, G.A.M.; Adam, A.M.A.; Refat, M.S. Studying spectroscopic, cyclic voltammetry, and electrical properties of novel 4-amino antipyrine derivative for photonic applications. J. Mol. Struct. 2023, 1272, 134201. [Google Scholar] [CrossRef]
- Manjunath, M.; Kulkarni, A.D.; Bagihalli, G.B.; Malladi, S.; Patil, S.A. Bio-important antipyrine derived Schiff bases and their transition metal complexes: Synthesis, spectroscopic characterization, antimicrobial, anthelmintic and DNA cleavage investigation. J. Mol. Struct. 2017, 1127, 314–321. [Google Scholar] [CrossRef]
- Kumawat, L.K.; Mergu, N.; Asif, M.; Gupta, V.K. Novel synthesized antipyrine derivative based “Naked eye” colorimetric chemosensors for Al3+ and Cr3+. Sens. Actuators B Chem. 2016, 231, 847–859. [Google Scholar] [CrossRef]
- Chakraborty, N.; Chakraborty, A.; Das, S. A pyrene based fluorescent turn on chemosensor for detection of Cu2+ ions with antioxidant nature. J. Lumin. 2018, 199, 302–309. [Google Scholar] [CrossRef]
- Yu, J.-N.; Ye, W.-C.; Jin, Y.-Y.; Park, K.; Chang, P.; Kim, C. Dual-channel detection of Cu2+ and F− with a simple Schiff-based colorimetric and fluorescent sensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1649–1657. [Google Scholar]
- Leyana, K.S.; Kumar, S.K.A. Antipyrine derived Schiff’s base as a colorimetric probe for the rapid and selective detection of Cu2+ ions. Inorg. Chem. Commun. 2021, 134, 109037. [Google Scholar]
- Luxami, V.; Gupta, A.S.; Paul, K. Dansyl–antipyrine dyad as a fluorescent sensor for Cu2+ and F−: Sequential XNOR logic operation. New J. Chem. 2014, 38, 2841–2846. [Google Scholar] [CrossRef]
- Rani, B.K.; John, S.A. Pyrene–antipyrine based highly selective and sensitive turn-on fluorescent sensor for Th(IV). New J. Chem. 2017, 41, 12131. [Google Scholar] [CrossRef]
- Maity, S.; Shyamal, M.; Maity, R.; Mudi, N.; Hazra, P.; Giri, P.K.; Samanta, S.S.; Pyne, S.; Misra, A. An antipyrine based fluorescent probe for distinct detection of Al3+ and Zn2+ and its AIEE behaviour. Photochem. Photobiol. Sci. 2020, 19, 681. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Ahmmed, E.; Ball, B.; Chattopadhyay, P. Rational design of a new AIE-coupled ESIPT-based multi-chromic state depend organo-luminophore with turn-on emissive response to Zn(II) in aqueous and solid-state. ChemistrySelect 2022, 7, e202103857. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, W.-J.; Yang, L.-L.; Li, G.; Ning, D.-H.; Yu, Y. Synthesis and properties of a new 4-aminoantipyrine Schiff-base for selective recognition of Cu2+. Chin. J. Lumin. 2013, 34, 1144–1148. [Google Scholar] [CrossRef]
- Lohar, S.; Sahana, A.; Banerjee, A.; Banik, A.; Mukhopadhyay, S.K.; Matalobos, J.S.; Das, D. Antipyrine based arsenate selective fluorescent probe for living cell imaging. Anal. Chem. 2013, 85, 1778–1783. [Google Scholar] [CrossRef]
- Chang, D.Z.; Li, J.H.; Gao, Y.; Liu, S.H.; Gen, C.C.; Yun, X.J.; Liu, H.Z.; Zhang, Y.M.; Wang, Y.R.; Guo, F.F.; et al. Compound and its Preparation Method, Application. Chinese Patent CN 117586185 A, 23 February 2024. [Google Scholar]
- Arsenyan, P.; Vasiljeva, J.; Domracheva, I.; Kanepe-Lapsa, I.; Gulbe, A. Selenopheno [2,3-f]coumarins: Novel scaffolds with antimetastatic activity against melanomaand breast cancer. New J. Chem. 2019, 43, 11851. [Google Scholar] [CrossRef]
- Gupta, V.K. Optical and electrochemical selective sensor for Zn2+ ions based on 4-(furan-2-ylmethyleneamino)-2,3-dimethyl-1-phenyl-1,2-dihydropyrazol-5-one. Electrochem. Sci. 2016, 11, 1640–1650. [Google Scholar] [CrossRef]
- Gogoi, A.; Samanta, S.; Das, G. A benzothiazole containing CHEF based fluorescence turn-ON sensor for Zn2+ and Cd2+ and subsequent sensing of H2PO4− and P4O74− in physiological pH. Sens. Actuators B 2014, 202, 788–794. [Google Scholar] [CrossRef]
- Das, B.; Dolai, M.; Dhara, A.; Ghosh, A.; Mabhai, S.; Misra, A.; Dey, S.; Jana, A. Solvent-regulated fluorimetric differentiation of Al3+ and Zn2+ using an AIE-active single sensor. J. Phys. Chem. A 2021, 125, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Maity, A.C.; Dias, A.K.; Bhattacharyya, N. Dual-mode chemosensor for the fluorescence detection of zinc and hypochlorite on a fluorescein backbone and its cell-imaging applications. Anal. Methods 2022, 14, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, Y.; Kim, G.; Jun, E.J.; Swamy, K.M.K.; Kim, Y.; Kim, S.-J.; Yoon, J. Pyrene based fluorescent probes for detecting endogenous zinc ions in live cells. Dye. Pigment. 2015, 113, 72–377. [Google Scholar] [CrossRef]
- Jacobson, A.R.; Dousset, S.; Andreux, F.; Baveye, P.C. Electron microprobe and synchrotron X-ray fluorescence mapping of the heterogeneous distribution of copper in high-copper vineyard soils. Environ. Sci. Technol. 2007, 41, 6343–6349. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Li, J.-H.; Gao, Y.; Yang, Y.; Gao, Y.; Xu, Z.-F. Removal of aluminum from rare-earth leaching solutions via a complexation-precipitation process. Hydrometallurgy 2020, 191, 105220. [Google Scholar] [CrossRef]




| Entry | Probe | Solvent System | LOD (μM) | Linear Range (μM) | Ref. |
|---|---|---|---|---|---|
| 1 | 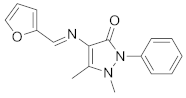 | CH3OH | 0.84 | 0–40 | [31] |
| 2 | 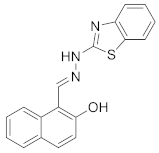 | CH3CN/H2O (7/3) | 0.65 | - | [32] |
| 3 |  | DMSO/H2O (9/1) | 3.65 | - | [33] |
| 4 |  | CH3CN/HEPES (1/1) | 1.79 | 0–40 | [34] |
| 5 | 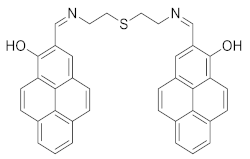 | DMSO/H2O (1/1) | 1.24 | - | [35] |
| This work | 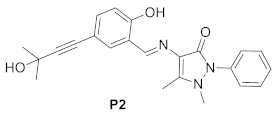 | EtOH/H2O (7/3) | 0.63 | 60–220 |
| Samples | pH | Added Amount (μM) | Found Amount (μM) | Std Dev (μM) | RSD (%, n = 3) | Recovery (%, n = 3) |
|---|---|---|---|---|---|---|
| Tap water | 6.47 | 100.00 | 95.72 | 0.35 | 0.91 | 95.72 |
| Ganjiang river water | 7.22 | 100.00 | 87.77 | 0.54 | 1.02 | 87.77 |
| Rainwater | 5.84 | 100.00 | 96.30 | 0.36 | 0.88 | 96.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Chang, D.; Luo, Y.; Yu, H.; Li, J.; Liu, K. Synthesis of an Antipyrine-Based Fluorescent Probe with Synergistic Effects for the Selective Recognition of Zinc Ion. Minerals 2024, 14, 649. https://doi.org/10.3390/min14070649
Gao Y, Chang D, Luo Y, Yu H, Li J, Liu K. Synthesis of an Antipyrine-Based Fluorescent Probe with Synergistic Effects for the Selective Recognition of Zinc Ion. Minerals. 2024; 14(7):649. https://doi.org/10.3390/min14070649
Chicago/Turabian StyleGao, Yan, Dezheng Chang, Yuyang Luo, Haojie Yu, Jinhui Li, and Kunming Liu. 2024. "Synthesis of an Antipyrine-Based Fluorescent Probe with Synergistic Effects for the Selective Recognition of Zinc Ion" Minerals 14, no. 7: 649. https://doi.org/10.3390/min14070649
APA StyleGao, Y., Chang, D., Luo, Y., Yu, H., Li, J., & Liu, K. (2024). Synthesis of an Antipyrine-Based Fluorescent Probe with Synergistic Effects for the Selective Recognition of Zinc Ion. Minerals, 14(7), 649. https://doi.org/10.3390/min14070649







