Research on Enhancing Copper-Ammonia-Thiosulfate Eco-Friendly Gold Leaching by Magnetization of Lixiviant Solution and Their Kinetic Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experiments
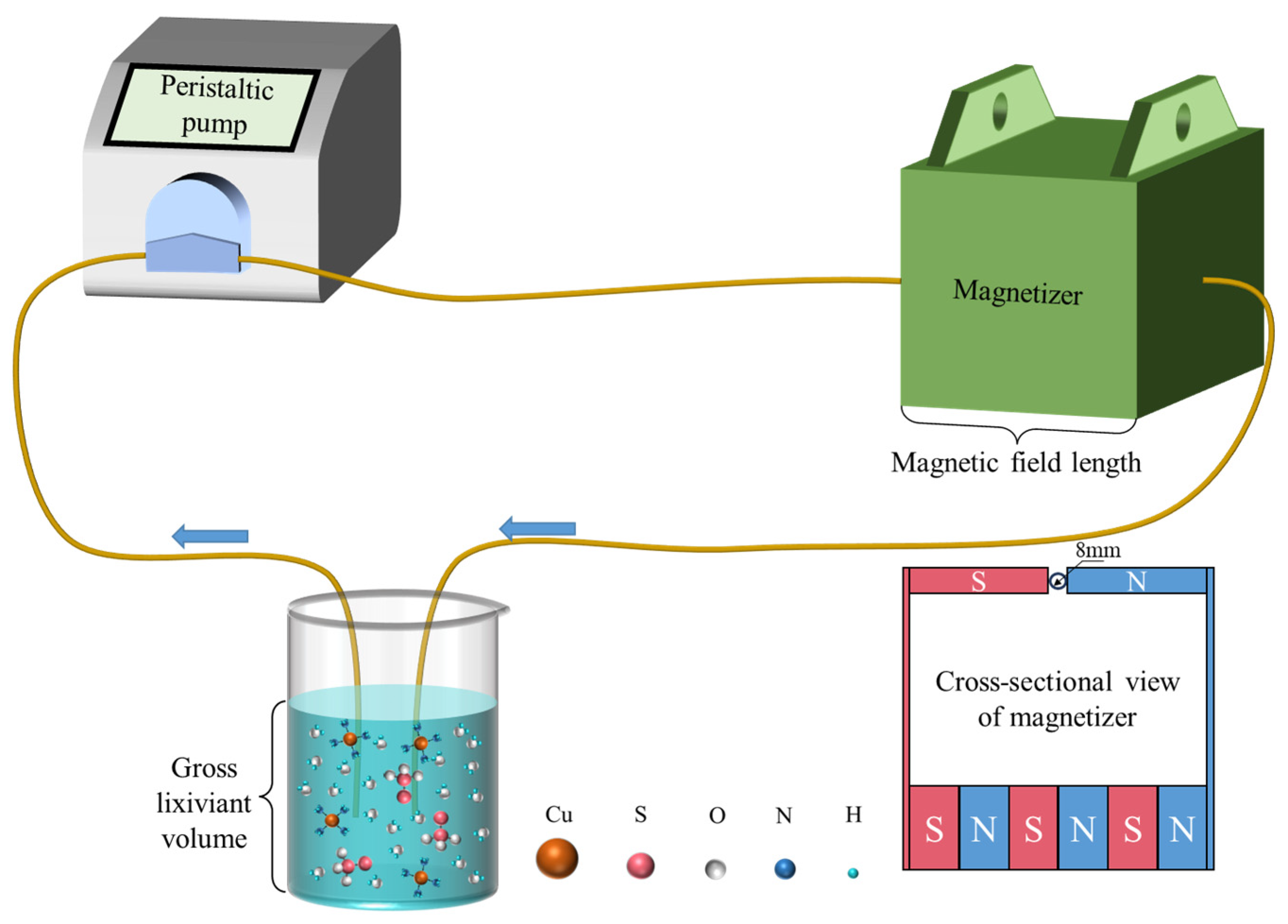
2.2.1. Magnetization of Lixiviant Solution
2.2.2. Column Leaching
2.2.3. Leaching Rate Test Based on QCM-D
2.2.4. Contact Angle Test by Washburn Method
2.3. Analytical Methods
3. Results and Discussion
3.1. Lixiviant Solution Magnetization
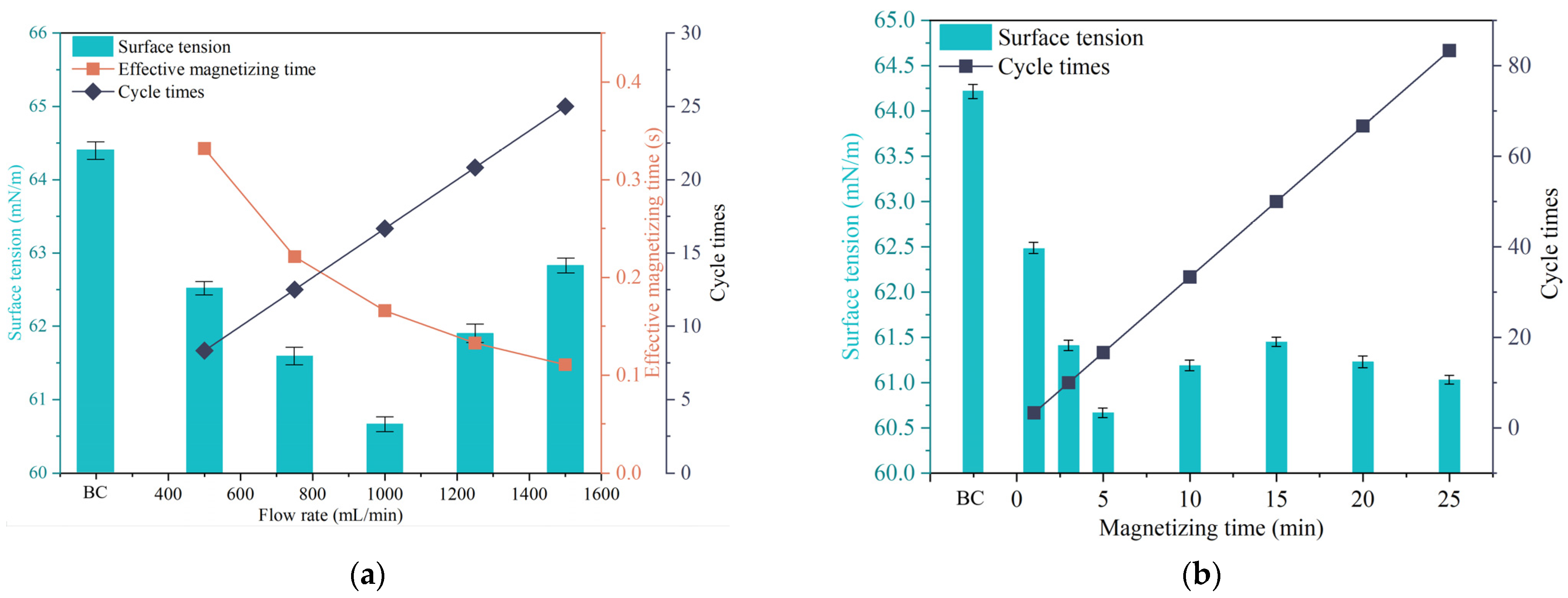
3.1.1. Surface Tension
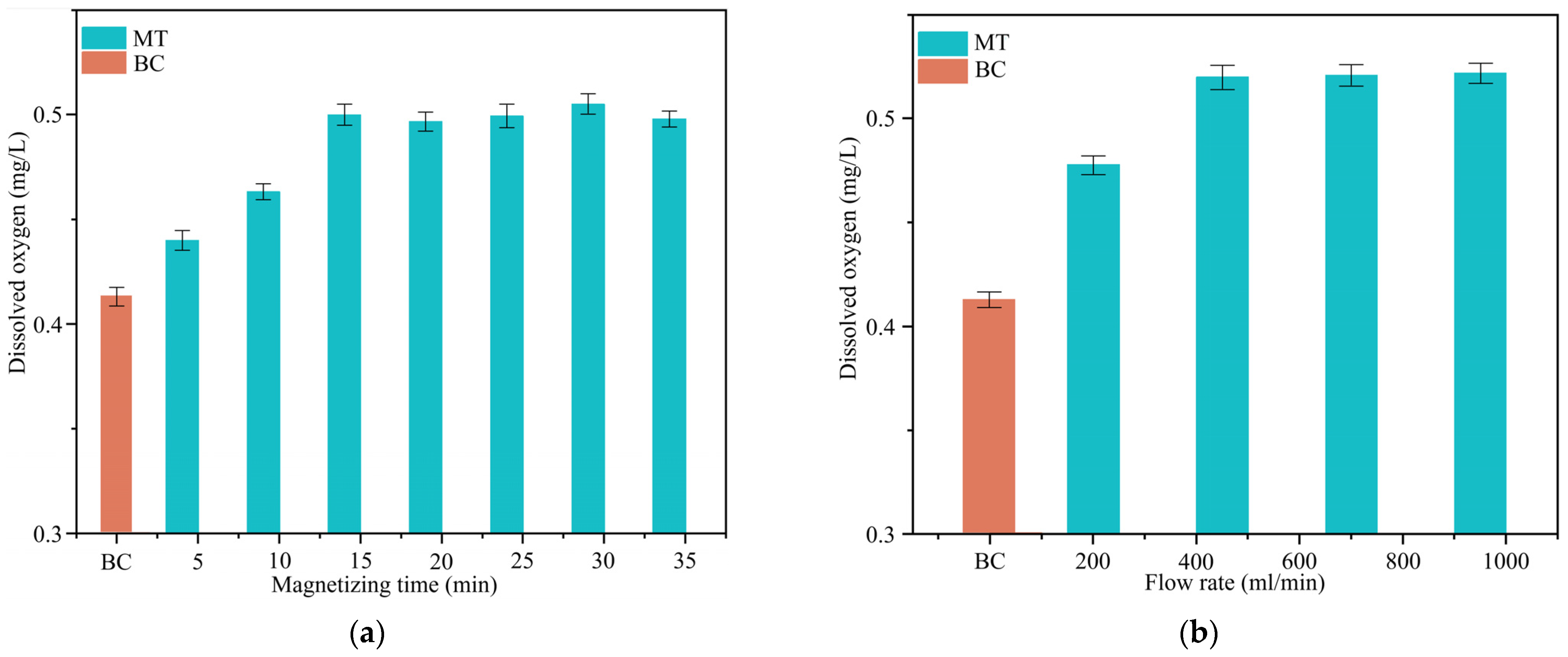
3.1.2. DO
3.2. Mechanism
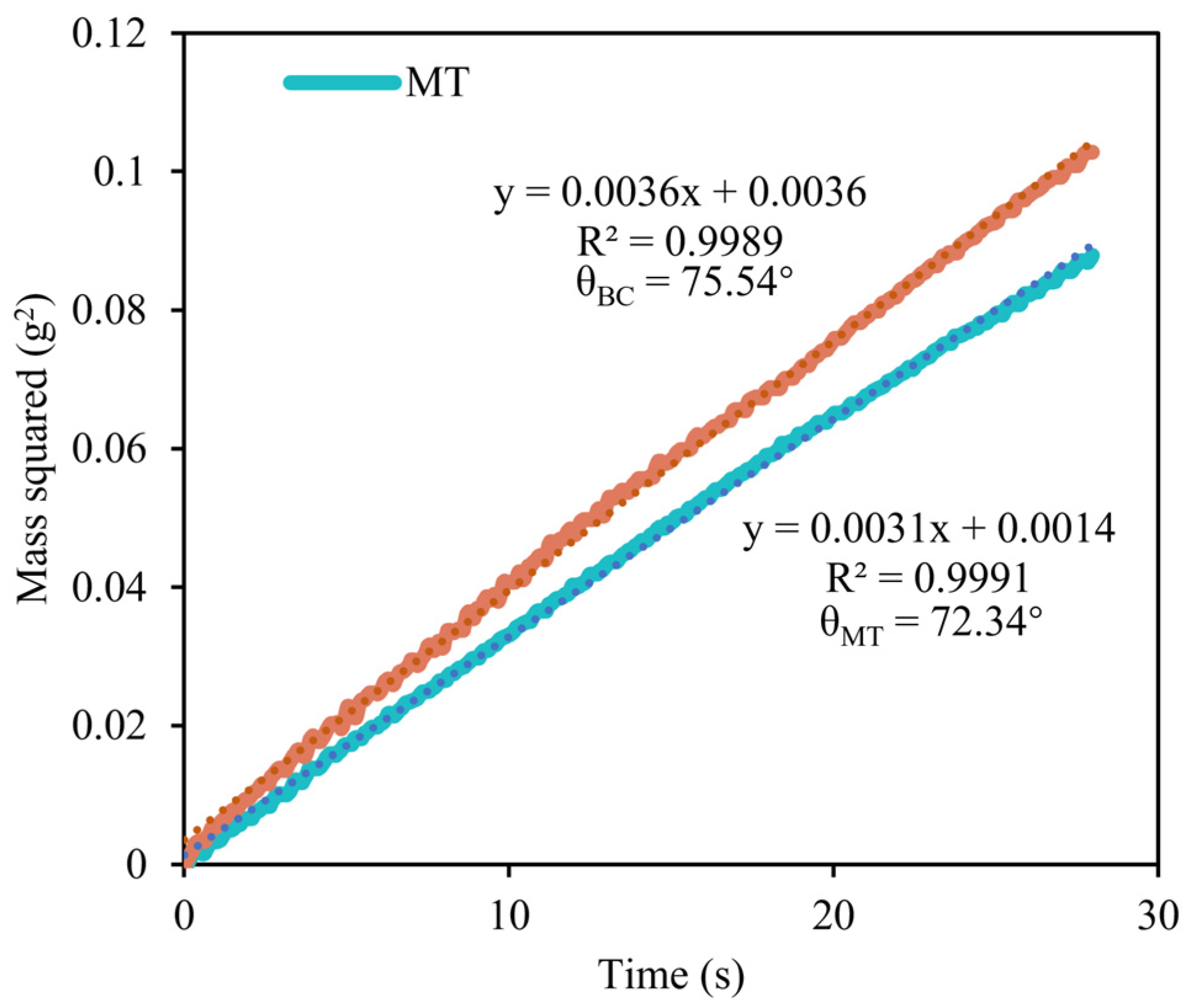
3.2.1. Contact Angle
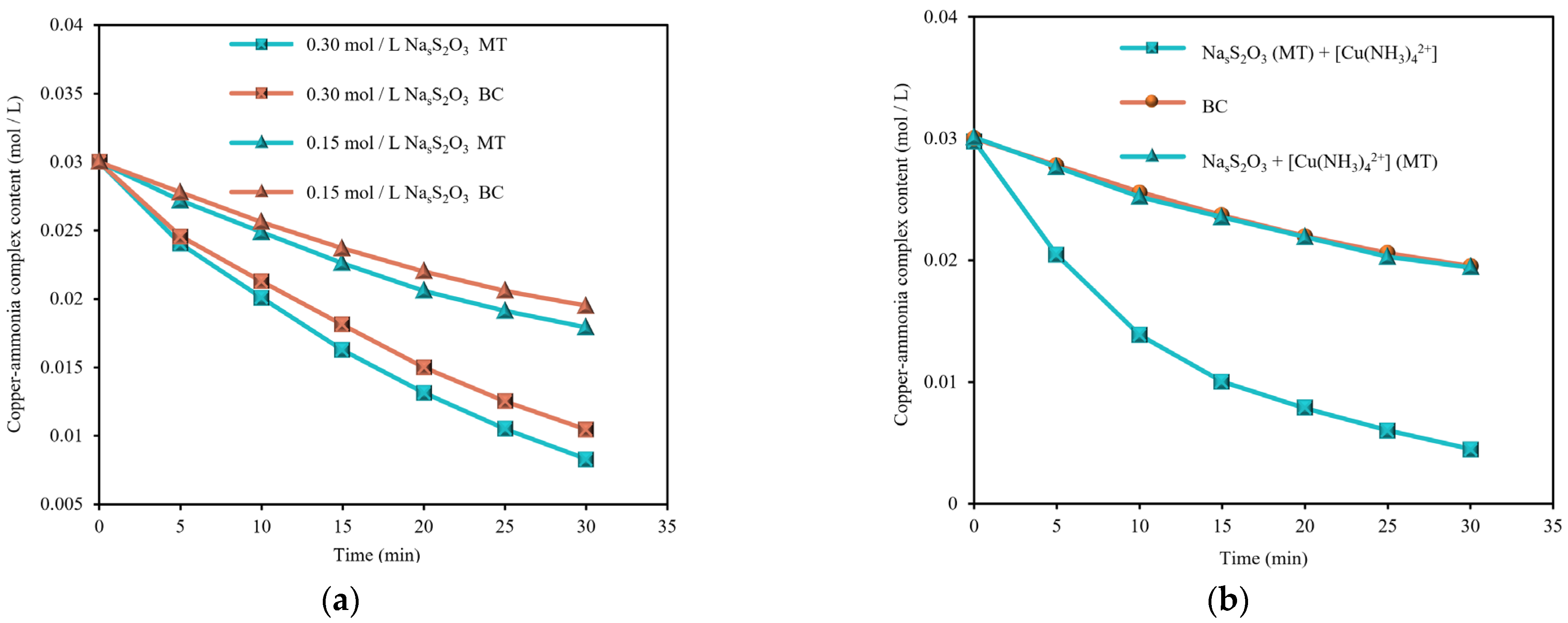
3.2.2. Chemical Reactivity
3.2.3. Leaching Rate
3.2.4. Contact Angle
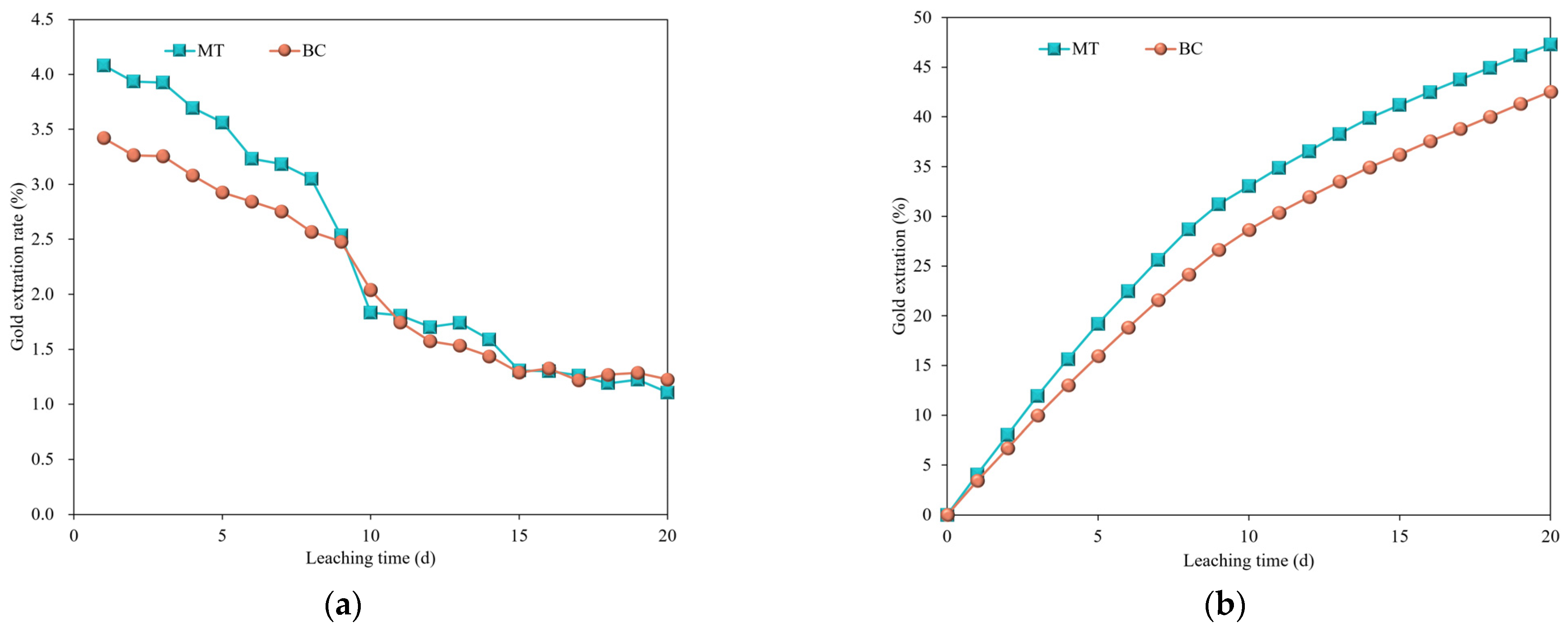
3.3. Column Leaching
4. Conclusions
- (1)
- Under optimized conditions including a magnetic field strength of 1.5 T, magnetization time of 5 min, and liquid flow rate of 1000 mL/min, the surface tension of sodium thiosulphate lixiviant solution decreased by 3.71 mN/m, representing a 5.5% reduction. Magnetization can increase the dissolved oxygen in the sodium thiosulphate lixiviant solution by up to 38%.
- (2)
- The magnetization treatment significantly improved the infiltration characteristics of the leaching solution on gold ore, reducing the contact angle from 75.54° to 73.24°. Moreover, it enhanced the reactivity of copper-ammonia complexes and improved the leaching reaction. Based on QCMD leaching rate tests, the magnetization treatment increased the reaction rate of copper-ammonia sodium thiosulphate leaching from 1561.39 ng/cm2-min to 1637.53 ng/cm2-min, a 4.88% increase.
- (3)
- Leaching kinetics studies reveal that the reaction rate of gold leaching with sodium copper-ammonia thiosulphate is determined by external diffusion and chemical reaction processes. Magnetization improves the infiltration characteristics of the leaching solution, aiding the diffusion of molecules/ions onto gold surfaces and enhancing external diffusion rates. Additionally, it boosts the activity of copper-ammonia complexes and DO in the leaching solution, thereby improving the chemical reaction process of gold leaching. Hence, magnetization treatment effectively increases the leaching reaction rate, although further research is needed to elucidate the detailed mechanisms, particularly regarding the enhancement of copper-ammonia complex activity post-magnetization.
- (4)
- Magnetization significantly enhanced heap leaching with copper-ammonia sodium thiosulphate, increasing the leaching rate by approximately 0.6%. The reaction rate in the early stage is primarily driven by external diffusion and chemical reaction processes, with bare gold as the primary reactant. In the middle and late stages, the increase in leaching reaction by-products obstructs the external diffusion process, leading to a sharp decrease in reaction rate. The cumulative extraction rate for the copper-ammonia sodium thiosulphate magnetized group reached 47.27%, with magnetization treatment enhancing the gold leaching rate by 4.74% and 3.67% compared to the blank control group and cyanide leaching, respectively, indicating the potential of magnetization treatment in promoting the industrial use of sodium thiosulphate as an alternative to cyanide for gold leaching.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bas, A.D.; Koc, E.; Yazici, E.Y.; Deveci, H. Treatment of copper-rich gold ore by cyanide leaching, ammonia pretreatment and ammoniacal cyanide leaching. Trans. Nonferrous Met. Soc. 2015, 25, 597–607. [Google Scholar] [CrossRef]
- Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Chen, C.; Gu, X. Efficient destruction of sodium cyanide by thermal decomposition with addition of ferric oxide. Trans. Nonferrous Met. Soc. 2021, 31, 1113–1126. [Google Scholar] [CrossRef]
- Li, K.; Li, Q.; Jiang, T.; Yang, Y.; Xu, B.; Xu, R.; Zhang, Y. Thiourea leaching of gold in presence of jarosite and role of oxalate. Trans. Nonferrous Met. Soc. 2024, 34, 322–335. [Google Scholar] [CrossRef]
- Yin, S.; Wang, L.; Chen, X.; Wu, A. Effect of ore size and heap porosity on capillary process inside leaching heap. Trans. Nonferrous Met. Soc. 2016, 26, 835–841. [Google Scholar] [CrossRef]
- Li, G.; Kou, J.; Xing, Y.; Hu, Y.; Han, W.; Liu, Z.; Sun, C. Gold-leaching performance and mechanism of sodium dicyanamide. Int. J. Min. Met. Mater. 2021, 28, 1759–1768. [Google Scholar] [CrossRef]
- Ou, Y.; Yang, Y.; Li, K.; Gao, W.; Wang, L.; Li, Q.; Jiang, T. Eco-friendly and low-energy innovative scheme of self-generated thiosulfate by atmospheric oxidation for green gold extraction. J. Clean. Prod. 2023, 387, 135818. [Google Scholar] [CrossRef]
- Örgül, S.; Atalay, Ü. Reaction chemistry of gold leaching in thiourea solution for a Turkish gold ore. Hydrometallurgy 2022, 67, 71–77. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Y.; Li, H.; Li, H.; Ju, J. Effect of sodium carbonate on alkaline self-leaching of gold from flotation gold ore. Sep. Purif. Technol. 2021, 256, 117499. [Google Scholar] [CrossRef]
- Liu, W.; Yang, T.; Xia, X. Behavior of silver and lead in selective chlorination leaching process of gold-antimony alloy. Trans. Nonferrous Met. Soc. 2010, 20, 322–329. [Google Scholar] [CrossRef]
- Konyratbekova, S.S.; Baikonurova, A.; Ussoltseva, G.A.; Erust, C.; Akcil, A. Thermodynamic and kinetic of iodine–iodide leaching in gold hydrometallurgy. Trans. Nonferrous Met. Soc. 2015, 25, 3774–3783. [Google Scholar] [CrossRef]
- Yin, X.; Liu, R.; Cheng, M.; Sun, Q.; Yang, Y. Efficient leaching and recovery of metallic gold and copper from integrated circuits with the novel bromotrihalide ionic liquids based on the redox mechanism. Sep. Purif. Technol. 2023, 313, 123456. [Google Scholar] [CrossRef]
- Sarvar, M.; Shafaei, Z.T.; Noaparast, M.; Badiei, A.R.; Amiri, A. Application of amino acids for gold leaching: Effective parameters and the role of amino acid structure. J. Clean. Prod. 2023, 391, 136123. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, T.; Zi, F.; Zang, H.; Li, G. Study on Synergistic Strengthening of Gold Extraction with Copper Ethylenediamine Thiosulfate Using Pyrite and Nickel Ions. Minerals 2024, 14, 2. [Google Scholar] [CrossRef]
- Baghalha, M. Leaching of an oxide gold ore with chloride/hypochlorite solutions. Int. J. Min. Process 2007, 82, 178–186. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; Panitantum, N. Enhanced and selective gold recovery from phone waste: Use of thiosulfate, dissolved oxygen, and an agriculture-based, low-cost adsorbent. Hydrometallurgy 2023, 220, 106108. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, H.; Zhang, Q.; Tong, L.; Jin, Z.; Chen, G. Research status of factors influence on leaching of gold with thiosulfate. Gol. Sci. Technol. 2018, 26, 105–114. [Google Scholar]
- Zhang, X.; Senanayake, G. A Review of Ammoniacal Thiosulfate Leaching of Gold: An Update Useful for Further Research in Non-cyanide Gold Lixiviants. Miner. Process. Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Senanayake, G. Analysis of reaction kinetics, speciation and mechanism of gold leaching and thiosulfate oxidation by ammoniacal copper(II) solutions. Hydrometallurgy 2004, 75, 55–75. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Min. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching in non-cyanide lixiviant systems: Critical issues on fundamentals and applications. Min. Eng. 2004, 17, 785–801. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, X.; Zi, F.; Chen, Y.; Chen, S.; Li, X.; Zhao, L.; Li, Y. Synergistical thiourea and thiosulfate leaching gold from a gold concentrate calcine with copper-ammonia catalysis. Sep. Purif. Technol. 2023, 327, 124928. [Google Scholar] [CrossRef]
- Jeffrey, M.I. Kinetic aspects of gold and silver leaching in ammonia–thiosulfate solutions. Hydrometallurgy 2001, 60, 7–16. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, L.; Chen, P.; Zhang, Q.; Chen, Y.; Zainiddinovich, N.Z.; Wu, C.; Alejandro, L.V.; Jia, F. Efficient and stable leaching of gold in a novel ethydiaminedhephen acetic-thiosulfate system. Min. Eng. 2024, 209, 108639. [Google Scholar] [CrossRef]
- Mahandra, H.; Faraji, F.; Ghahreman, A. Novel Extraction Process for Gold Recovery from Thiosulfate Solution Using Phosphonium Ionic Liquids. Acs Sustain. Chem. Eng. 2021, 9, 8179–8185. [Google Scholar] [CrossRef]
- Guo, H.; Wang, S.; Nie, Y.; Chen, J.; Wang, Q. Improving the cementation effect of copper on gold in thiosulfate solution by pre-regulating the existing form of Cu2+. J. Ind. Eng. Chem. 2024, 130, 483–493. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Pan, Y.; Liu, F.; Xu, Z. Thermodynamic analysis of gold leaching by copper-glycine-thiosulfate solutions using Eh-pH and species distribution diagrams. Min. Eng. 2022, 179, 107438. [Google Scholar] [CrossRef]
- Chen, J.; Xie, F.; Wang, W.; Fu, Y.; Wang, J. Leaching of a carbonaceous gold concentrate in copper-tartrate-thiosulfate solutions. Miner. Eng. 2022, 183, 107605. [Google Scholar] [CrossRef]
- Moosavi, F.; Gholizadeh, M. Magnetic effects on the solvent properties investigated by molecular dynamics simulation. J. Magn. Magn. Mater. 2014, 354, 239–247. [Google Scholar] [CrossRef]
- Toledo, E.J.L.; Ramalho, T.C.; Magriotis, Z.M. Influence of magnetic field on physical–chemical properties of the liquid water: Insights from experimental and theoretical models. J. Mol. Struct. 2008, 888, 409–415. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Li, Z. Effect of magnetic field on the physical properties of water. Results Phys. 2018, 8, 262–267. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Gong, Z.; Gao, K.; Ou, Y.; Zhang, J. The effect of a static magnetic field on the hydrogen bonding in water using frictional experiments. J. Mol. Struct. 2013, 1052, 102–104. [Google Scholar] [CrossRef]
- Lee, H.S.; Yap, A.C.W.; Ng, C.C.; Mohd, N.S.; Loo, J.L. Increased electron density and dissolved oxygen level in water through magnetic effect. In Proceedings of the 9th International Conference on Future Environment and Energy, Osaka, Japan, 9–11 January 2019. [Google Scholar]
- Zheng, Y.; Hao, Z.; Wang, X.; Qiao, J.; Li, X. Study on effect of magnetization on wetting characteristics of water with different ions to coal dust. Fuel 2024, 367, 131441. [Google Scholar] [CrossRef]
- Al-Akhras, M.A.H.; Al-Quraan, N.A.; Abu-Aloush, Z.A.; Mousa, M.S.; AlZoubi, T.; Makhadmeh, G.N.; Donmez, O.; Aljarrah, K. Impact of magnetized water on seed germination and seedling growth of wheat and barley. Results Eng. 2024, 22, 101991. [Google Scholar] [CrossRef]
- Keshta, M.M.; Elshikh, M.M.Y.; Kaloop, M.R.; Hu, J.; Elmohsen, I.A. Effect of magnetized water on characteristics of sustainable concrete using volcanic ash. Constr. Build. Mater. 2022, 361, 129640. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, Y.; Li, J.; Lu, N.; Yin, Y.; Jia, H.; Wang, J. Magnetization coupled reverse osmosis (RO): Enhanced inhibition scaling mechanisms and operation optimization. Chem. Eng. Sci. 2024, 286, 119650. [Google Scholar] [CrossRef]
- Pang, J.; Xie, J.; Zhao, Z.; Hao, Y.; Han, Q.; Liang, L. Study on improving the wettability of anthracite dust by surfactant-magnetized water. Trans. Beijing Inst. Technol. 2021, 44, 83–90. [Google Scholar]
- Zhou, Q.; Qin, B.; Huang, H. Research on the formation mechanism of magnetized water used to wet coal dust based on experiment and simulation investigation on its molecular structures. Powder Technol. 2021, 391, 69–76. [Google Scholar] [CrossRef]
- Amiri, M.C.; Dadkhah, A.A. On reduction in the surface tension of water due to magnetic treatment. Colloids Surf. A 2006, 278, 252–255. [Google Scholar] [CrossRef]
- Sronsri, C.; U-yen, K.; Sittipol, W. Analyses of vibrational spectroscopy, thermal property and salt solubility of magnetized water. J. Mol. Liq. 2023, 323, 114613. [Google Scholar] [CrossRef]
- Rong, X.; Li, J.; Dan, H.; Xue, C.; Gao, M.; Li, M.; Liu, Y. Characteristics, Mechanism and Applications of Magnetized Water: A Review. Mater. Rep. 2022, 36, 21020032-7. [Google Scholar]
- Huminic, G.; Huminic, A.; Vărdaru, A.; Fleacă, C.; Dumitrache, F.; Morjan, I. Surface tension of Ag NPs-rGO based hybrid nanofluids. J. Mol. Liq. 2023, 390, 123002. [Google Scholar] [CrossRef]
- GB/T20899.1-2019; Methods for Chemical Analysis of Gold Ores—Part 1: Determination of Gold Content. National Public Service Platform for Standards Information: Beijing, China, 2019.


| Gold Phase | Gross Gold | Bare and Semi-Bare Gold | Carbonate-Encapsulated Gold | Sulfides-Encapsulated Gold | Hematite-Encapsulated Gold | Quartz and Silicates-Encapsulated Gold |
|---|---|---|---|---|---|---|
| Content (g/t) | 0.48 | 0.31 | 0.13 | <0.1 | <0.1 | <0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Kou, J.; Fan, L.; Zhang, W.; Tian, J.; Sun, C.; Li, Q.; Liu, J.; Xing, C.; Li, G. Research on Enhancing Copper-Ammonia-Thiosulfate Eco-Friendly Gold Leaching by Magnetization of Lixiviant Solution and Their Kinetic Mechanism. Minerals 2024, 14, 697. https://doi.org/10.3390/min14070697
Liu Z, Kou J, Fan L, Zhang W, Tian J, Sun C, Li Q, Liu J, Xing C, Li G. Research on Enhancing Copper-Ammonia-Thiosulfate Eco-Friendly Gold Leaching by Magnetization of Lixiviant Solution and Their Kinetic Mechanism. Minerals. 2024; 14(7):697. https://doi.org/10.3390/min14070697
Chicago/Turabian StyleLiu, Zhengyu, Jue Kou, Lipeng Fan, Weibin Zhang, Jie Tian, Chunbao Sun, Qiang Li, Jiubo Liu, Chengjun Xing, and Guanhua Li. 2024. "Research on Enhancing Copper-Ammonia-Thiosulfate Eco-Friendly Gold Leaching by Magnetization of Lixiviant Solution and Their Kinetic Mechanism" Minerals 14, no. 7: 697. https://doi.org/10.3390/min14070697





