Abstract
The significance of froth rheology in affecting flotation performance is widely acknowledged. Clays could deteriorate flotation performance by altering froth rheology. The presence of cations further complicates the flotation system. Thus far, the interaction between clay minerals and cations and their impact on froth rheology remains unclear. The present work selected three typical clays and cations with two valences (Na+ and Ca2+) to investigate their interacting influences on froth rheology. The results indicate that clays exhibit diverse froth rheological behaviors, with increasing cation strength from 0 to 0.1 mol/L. For montmorillonite, the froth viscosity initially decreased and subsequently increased. For kaolinite, upon the addition of cations, there was a significant decrease in froth viscosity; nevertheless, froth viscosity barely changed as the valency and concentration of the cations increased. Talc produced a considerably more viscous froth, and froth viscosity continued to rise with increasing concentrations of cations. The underlying mechanisms of the different responses in froth rheology were also investigated. The findings of this work have the potential to advance the optimization of flotation for complex ores containing clay minerals in high-salt processing water.
1. Introduction
With the depletion of readily processable ores, addressing ores of complex composition has become an urgent challenge, notably those comprising clay minerals. Generally, clays exert adverse effects on froth flotation by diminishing flotation recovery and concentrate grade [1,2,3,4,5,6]. Furthermore, due to the scarcity of freshwater resources, there is a growing demand in flotation plants for the recycling of processing water. Hence, the dissolution of ions from minerals leads to a gradual increase in ion concentration in the flotation system, especially for cations such as Na+, K+, Ca2+, and Mg2+ [7,8,9]. The presence of a high cation concentration could impact both the pH of flotation slurry and thus flotation performance [10,11,12,13,14].
In froth flotation, the influence of clay minerals on flotation efficiency could be affected by the presence of cations. Wang et al. [15] noted that the presence of montmorillonite contributed to a decline in copper flotation recovery, attributable to increased pulp viscosity. Later, Wang et al. [16] pointed out that the introduction of cations can diminish the viscosity of the pulp in the presence of montmorillonite and consequently enhance copper recovery. The influence of divalent cations (Mg2+ and Ca2+) on pulp viscosity is more pronounced than that of monovalent cations (Na+ and K+) [17]. Du et al. [18] systematically characterized the reticular structure of montmorillonite in both deionized water and calcium-containing saline water, finding that the stability of montmorillonite association structures firstly increased and then gradually decreased as the concentration of calcium ions escalated. In the flotation of a copper-gold ore, Zhang et al. [19] reported that the presence of kaolinite led to substantial entrainment, consequently decreasing both the copper and gold concentrate grades, which became worse when fresh water was substituted with seawater [20]. Farrokhpay et al. [21] reported that talc influenced both copper flotation recovery and concentrate grade by altering froth stability, attributable to its inherent hydrophobicity. As mentioned above, the influence of clay on flotation performance seemed to be closely related to the clay characteristics and cations in the slurry.
Flotation encompasses both the pulp and froth phases. It is known that the interaction between clay minerals and cations could impact flotation performance by varying pulp rheology [22,23,24]. In addition, the effect of froth rheology on flotation is well established. Shi et al. [25] noted a linear correlation between copper grade and froth viscosity during copper ore flotation. Zhang et al. [26] found a significant correlation between froth rheology and froth setting, with the introduction of cations leading to an augmented volume of froth on the water surface. Li et al. [27] concluded that froth rheology could also impact the entrainment of hydrophilic minerals by modulating the dynamics of froth movement. Wang et al. [28] argued that the effect of clays on froth rheology was closely related to their crystal structure. However, the impact of the interaction between clay minerals and cations on froth rheology remains ambiguous, which impedes flotation optimization in the processing of ores containing clays.
This study aims to investigate the influence of the interaction between clay and cations on froth rheology by focusing on three typical clays in flotation (montmorillonite, kaolinite, and talc), as well as common cations with different valences (Na+ and Ca2+). The concentration of clays remained at 3 wt%. For each cation, the ionic concentration was tested at 0, 0.001 mol/L, 0.01 mol/L, 0.1 mol/L, respectively. The study investigated the response of froth rheology to the interaction between clay minerals and cations, the underlying mechanisms of which was also studied. The findings of this work will contribute to the flotation optimization of complex ores containing clays in high-salt processing water.
2. Experimental Methods
2.1. Experimental Materials
The clay minerals used in the flotation experiments were obtained from China: montmorillonite (Inner Mongolia), kaolinite (Jiangsu Province), and talc (Shandong Province). The particle size measurement showed that the three clays had close size distribution with P80 all less than 35 μm. The X-ray diffraction (XRD) and X-ray fluorescence (XRF) analyses revealed that the purity of montmorillonite was 90.87% with minor quartz. It was 97.82% and 95.50% for kaolinite and talc, respectively. A contact angle measurement instrument (Biolin Scientific, Gothenburg, Sweden) was used to measure the clay hydrophobicity. The montmorillonite and kaolinite exhibited a hydrophilic nature with a contact angle of 13.18° and 19.11°, whilst the talc showed a hydrophobic nature with a contact angle of 67.88°. More details of the material characterizations can be seen elsewhere [28].
For flotation, analytical grade (AR) NaCl and CaCl2 were used as the source of Na+ and Ca2+. Methyl isobutyl carbinol (MIBC) (>98.0% purity, Macklin, Shanghai, China) was employed as a frother, while deionized water was utilized for the duration of the study.
2.2. Experimental Set-Up
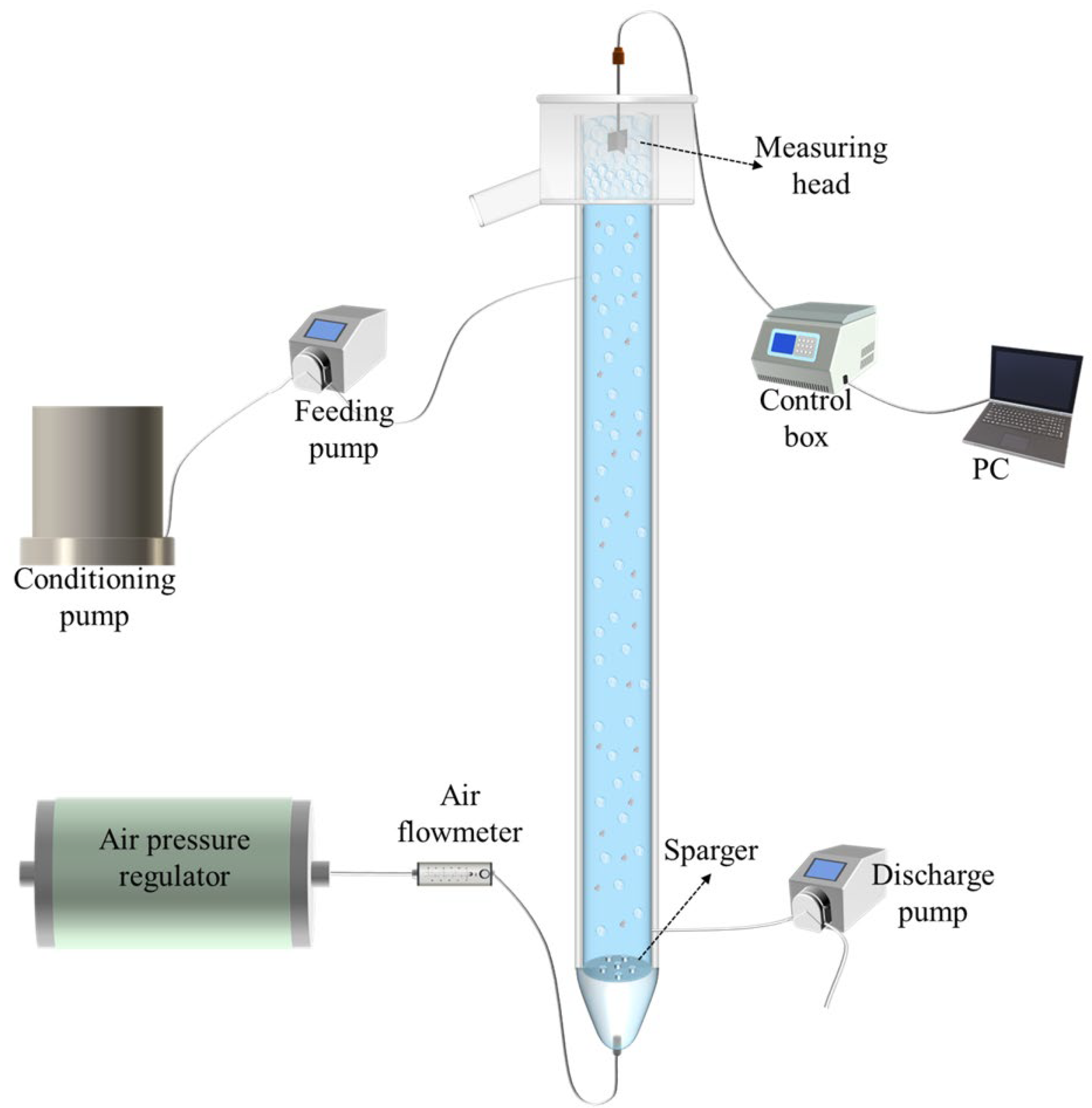
Figure 1 presents the rheometer and the flotation rig employed in the experimental investigation. The flotation experiments were conducted in a column with 150 cm in height and a diameter of 5 cm. A porous-plate sparger with a 30 μm aperture size was positioned near the column bottom. Feed slurry was agitated first within a 20 L conditioning tank and then introduced into the column at 130 cm above the sparger. The tailing was discharged from the bottom of the column through a peristaltic pump. Meanwhile, the pulp level was regulated by adjusting the tailing’s flowrate. Additionally, the flowrate and pressure of air introduced to the flotation column were regulated by an air flowmeter and an air pressure regulator, respectively.
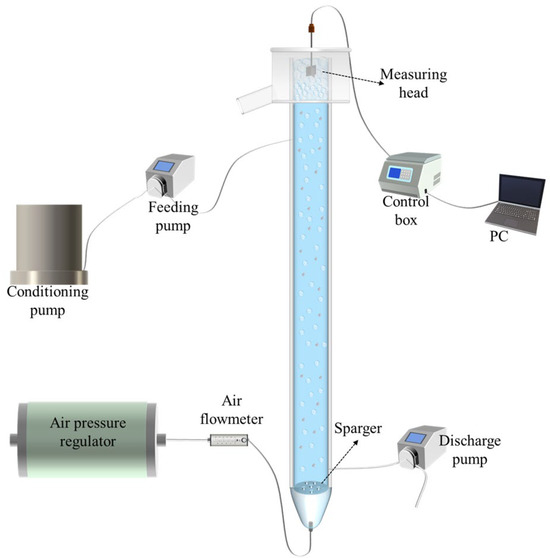
Figure 1.
Schematic of the flotation rig and the rheometer.
This study conducted in situ froth rheology measurement using a blade method with a rheometer (Anton Paar DSR502, Graz, Austria), by which froth structure was maintained under dynamic equilibrium [29]. The rheometer has the capability to measure torques ranging from 0.01 μNm to 200.0 mNm, providing a high resolution of 0.1 nNm, enabling the precise rheological characterization of samples. A four-blade vane, measuring 16 mm in diameter and 25 mm in height, was affixed to the rheometer for the measurement. The rheometer was linked to a computer running the measurement software, which regulated the rotational speed of the vane. At the end, a Zetasizer Nano (Malvern Instruments, Almelo, The Netherlands) was used to test the zeta potential of the three clays.
2.3. Experimental Procedure
In this study, the flotation was carried out in a continuous mode. For each condition, the required amount of clay minerals and NaCl/CaCl2 were introduced into the conditioning tank. Then, water was added to make a 10 L of slurry and agitated to ensure thorough mixing. MIBC was added at a dosage of 15 ppm and stirred for 10 min. Subsequently, the prepared slurry was fed into the column at a constant flow rate of 0.80 L/min, while the froth depth remained at 10 cm by controlling the discharge flowrate. Air was conveyed into the column under a superficial gas velocity of 1.0 cm/s and an air pressure of 200 kPa. After concentrically positioning and installing the vane within the flotation column, the rotational speed of the blades was increased from 2 rpm to 10 rpm in 2 rpm increments, repeating each vane speed five times at 5 s intervals. The previous study has proved that the vane operated within this range imposed no impact on froth characteristics [23]. When the flotation system reached a steady state, the froth rheology measurement was commenced. As the blade method is not the standard geometry for the rheometer, the obtained vane speed and torque were converted to shear rate and shear stress. The detailed conversion is shown in the Supplementary Materials.
For the measurement of water holdup and solids concentration within the froth by volume, the overflowing froth was amassed for a period of 2 min after vane removal. The collected samples were then measured for volume prior to and subsequent to oven-drying. The calculation of water holdup and the solids volumetric concentration in the froth is detailed below [28]:
where εw represents the water holdup (%), εs denotes the solids volumetric concentration within the froth (%), Qw is the volumetric flow rate of water spilling over the discharge lip (cm3/min), Qs signifies the volumetric flow rate of solids spilling over the discharge lip (cm3/min), and Qa represents the volumetric air flow rate entering the column (cm3/min).
To test the zeta potential of montmorillonite, kaolinite, and talc, clay samples were mixed with NaCl/CaCl2 electrolyte solutions with different concentrations and stirred for 20 min. The mineral suspensions were settled naturally for 1 h, after which the supernatant was carefully decanted for zeta potential test. Each zeta potential measurement was replicated three times, and the average value was used.
Settling tests can indirectly provide information on the association of clay platelets. Montmorillonite and kaolinite were used to prepare suspensions under identical experimental conditions and then transferred to a 100 mL graduated cylinder. The cylinder was then stoppered and inverted four times to ensure the slurry was well mixed before the settling tests. Note that the pH value for each condition was measured but not controlled. The pH values for the montmorillonite, kaolinite, and talc suspensions were 8.4, 4.5, and 9.7, respectively. For the same clay type, the pH value remained unchanged under the different concentrations of Na+, and there was a slight change over the different concentrations of Ca2+ in this study.
3. Results and Discussion
3.1. Rheology of the Froths Generated with Clays in the Presence of Cations
3.1.1. The Froth Rheology of Clays in the Presence of Na+
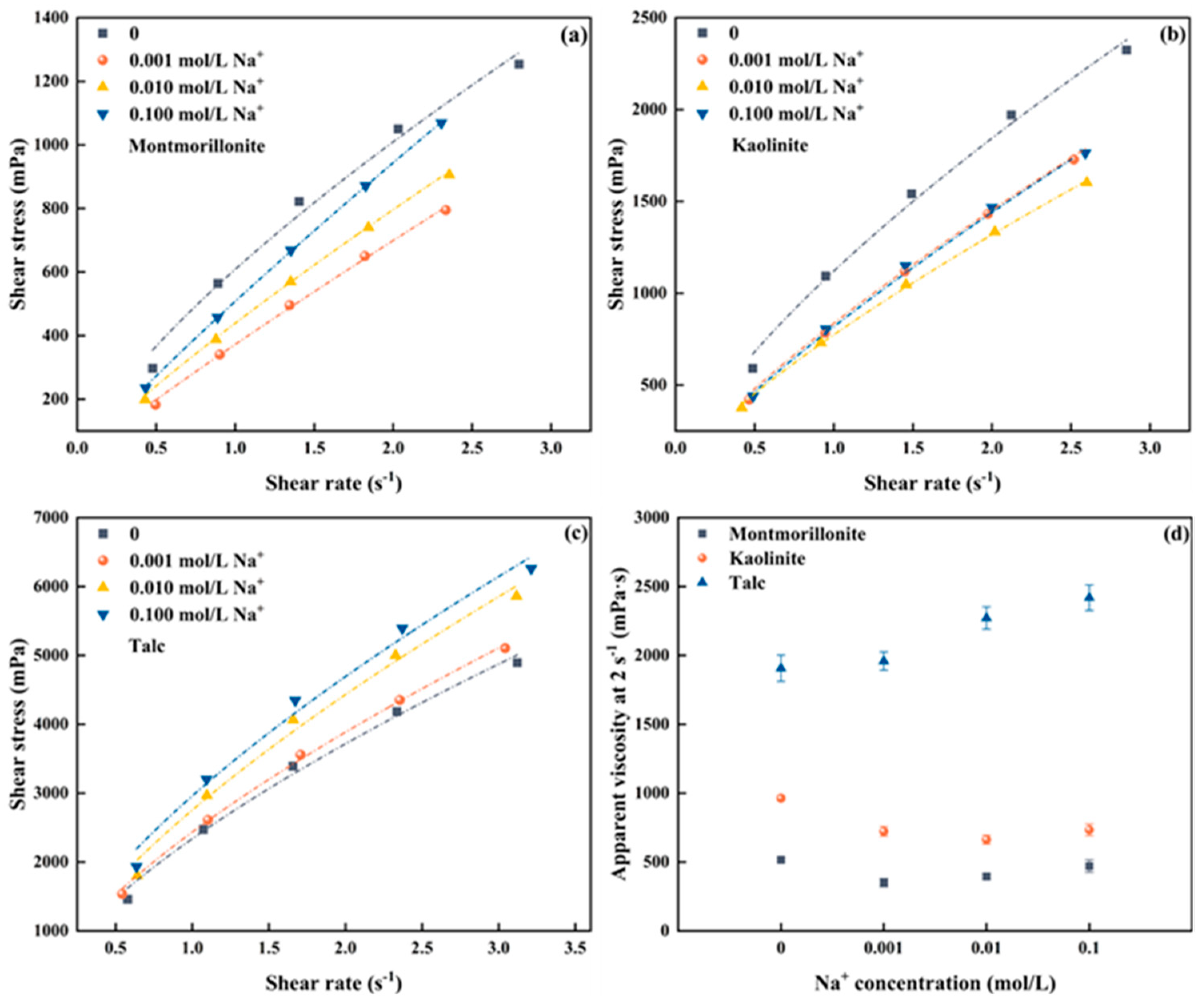
Figure 2a–c shows the rheological change of the froths produced by montmorillonite, kaolinite, and talc at different Na+ concentrations. The measured raw data for the three clays can be seen in Figure S1a–c. The froths generated with the three clays in the presence of Na+ exhibit pseudo-plastic rheological behavior. To further study the correlation between froth viscosity and clay type as well as Na+ concentration, Figure 2d depicts the local apparent viscosity at a shear rate of 2 s−1 to represent the rheological properties of the froths for comparison.
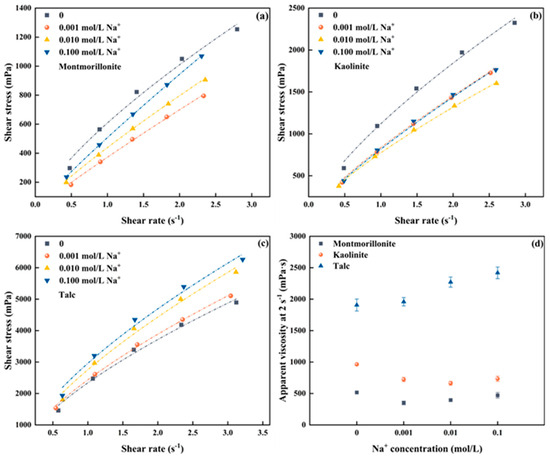
Figure 2.
Froth rheology of three clay minerals under different Na+ concentrations (a) Montmorillonite, (b) Kaolinite, (c) Talc, (d) apparent viscosity at 2 s−1.
As shown in Figure 2d, a nonlinear trend in the froth apparent viscosity of montmorillonite is observed with increasing Na+ concentrations in the pulp. Specifically, the froth apparent viscosity decreases first when the Na+ concentration in the pulp increases from 0 to 0.001 mol/L, and then gradually increases as the Na+ concentration continues to rise. However, even when the Na+ concentration reaches 0.1 mol/L, the froth apparent viscosity remains lower than that measured with the absence of Na+. For kaolinite, it is evident that the froth apparent viscosity dramatically drops in the presence of Na+ at a concentration of 0.001 mol/L. However, with the Na+ concentration further increasing, the froth apparent viscosity barely changes and is much lower than the froth viscosity without the addition of Na+. The finding indicates that the addition of Na+ in kaolinite slurry can substantially reduce the froth viscosity. For talc, its behavior deviates from that observed for montmorillonite and kaolinite. The froth apparent viscosity shows a gradually increase with the increase of Na+ concentration in the pulp.
In addition, Figure 2d shows that, under the same Na+ concentration, the froth produced by talc exhibits the highest apparent viscosity owing to its inherent hydrophobic nature, followed by kaolinite and montmorillonite in sequence. For instance, when the Na+ concentration in the pulp is 0.01 mol/L, the apparent viscosities of talc, kaolinite, and montmorillonite at 2 s−1 are 2271.80 mPa·s, 662.95 mPa·s, and 395.46 mPa·s, respectively.
3.1.2. The Froth Rheology of Clays in the Presence of Ca2+
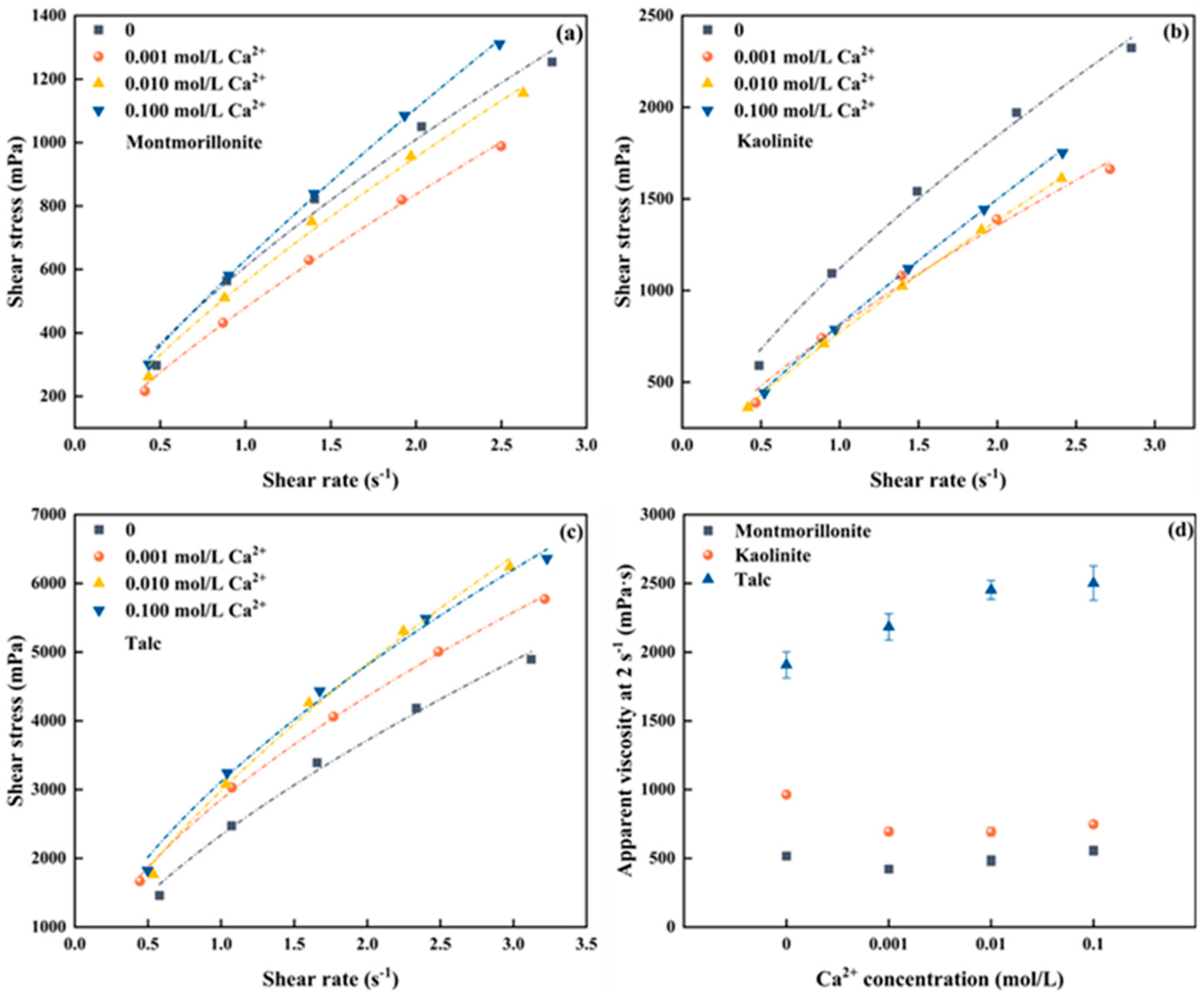
The influence of Ca2+ on the flotation froths of clay minerals was also investigated. Figure S2a–c shows the raw data of froths produced by three clay minerals at different Ca2+ concentrations, the rheological curves converted from which are shown in Figure 3a–c. Similarly, the froths generated with the three clays in the presence of Ca2+ also exhibit pseudo-plastic rheological behavior. The apparent viscosity at a shear rate of 2 s−1 for each clay is selected as the representative for comparison, as demonstrated in Figure 3d.
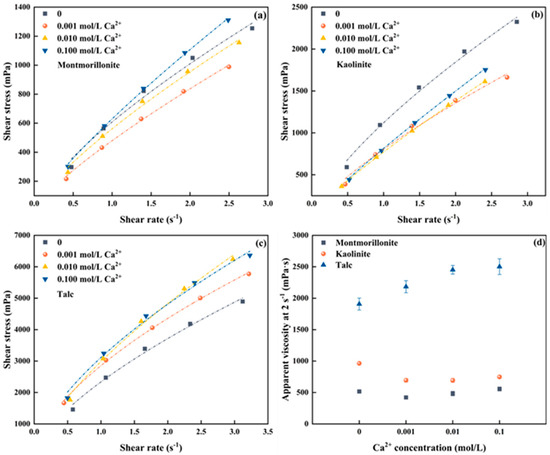
Figure 3.
Froth rheology of three clay minerals under different Ca2+ concentrations (a) Montmorillonite, (b) Kaolinite, (c) Talc, (d) apparent viscosity at 2 s−1.
As shown in Figure 3d, the froth apparent viscosity of montmorillonite decreased first and then increased over the Ca2+ concentrations, achieving the smallest apparent viscosity at a Ca2+ concentration of 0.001 mol/L. Remarkably, when the Ca2+ concentration reaches 0.1 mol/L, the froth apparent viscosity surpasses that measured in the absence of montmorillonite. Contrarily, the froth apparent viscosity of kaolinite significantly decreases with the presence of Ca2+ but remains barely changed over the Ca2+ concentrations, which aligns with the trend observed for Na+. For talc, the froth apparent viscosity gradually increases with the increase of Ca2+ concentration in the pulp, which is consistent with the trend observed in the presence of Na+. Similarly, the froth produced by talc exhibits the highest apparent viscosity, followed by kaolinite and montmorillonite. For instance, when the Ca2+ concentration in the slurry is 0.1 mol/L, the apparent viscosities of montmorillonite, kaolinite, and talc at a shear rate of 2 s−1 are measured at 2501.70 mPa∙s, 747.76 mPa∙s, and 556.21 mPa∙s, respectively.
The results above show that the strength of metallic cations has a significant impact on the froth rheological properties of clay minerals, but the valence state of the metallic cations imposes a relatively less significant effect.
3.2. Effect of Cations on Froth Properties of Clay Minerals
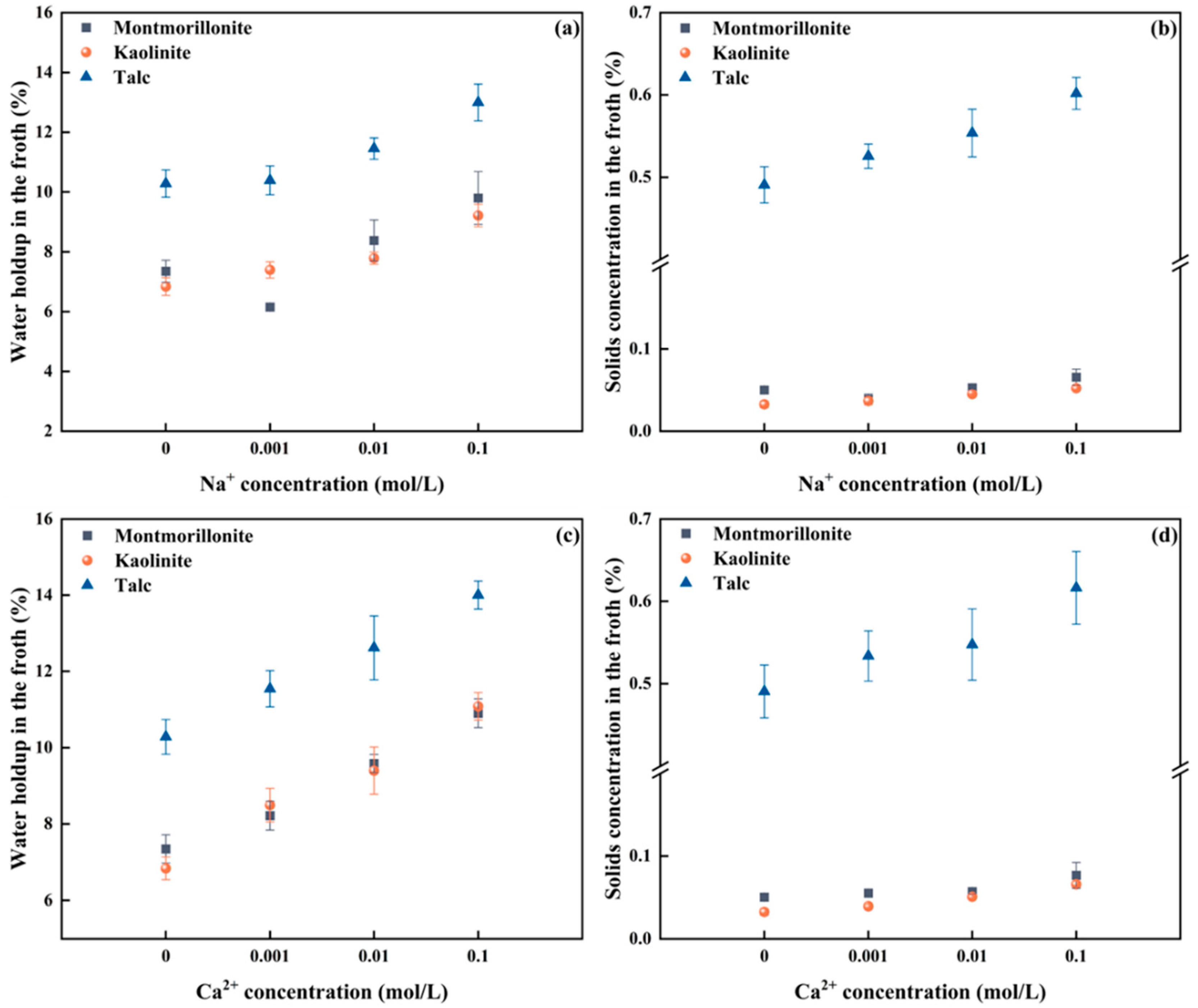
Given the intrinsic relationship between froth rheology and froth composition, an exploration into the impact of cation type and concentration on the water holdup and solids volumetric concentration in the froth for the three clays was undertaken. The results are summarized in Figure 4. The error bars represent one standard deviation obtained from three independent experimental runs.
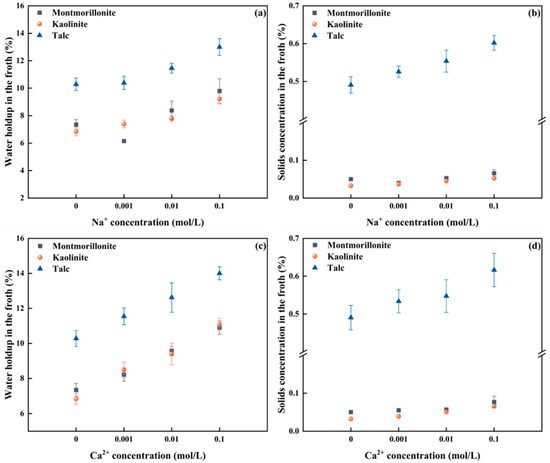
Figure 4.
Correlation between froth properties and cation type, as well as concentration (a) water holdup vs. Na+ concentration, (b) Solids concentration vs. Na+ concentration, (c) water holdup vs. Ca2+ concentration, (d) Solids concentration vs. Ca2+ concentration.
Figure 4a shows that when the Na+ concentration was increased from 0 to 0.001 mol/L, the water holdup in the flotation froth of montmorillonite decreased to 6.15%, after which it increased over the Na+ concentrations and reached 9.79% at an Na+ concentration of 0.1 mol/L. Figure 4b depicts that the solids concentration of montmorillonite in the froth exhibits a similar trend over the Na+ concentrations, achieving the lowest solids concentration in the froth of 0.04% at a Na+ concentration of 0.001 mol/L. The highest solids volumetric concentration in the froth is recorded to be 0.07% at a Na+ concentration of 0.1 mol/L, almost a two-fold increase. For kaolinite, both the water holdup and solids concentration in the froth increase with increasing the Na+ concentration within the varied range. Specifically, the water holdup rises from 6.83% to 9.21% (an increase of nearly 1.5 times), while the solids concentration by volume in the froth increases from 0.033% to 0.052%. However, in the talc system with natural hydrophobicity, the volumetric solids concentration in the froth is significantly higher compared to montmorillonite and kaolinite. It could be attributed to the fact that the presence of Na+ does not affect the hydrophobic groups on the surface of talc, enabling talc to adhere to the bubbles and enter the froth phase. Specifically, as the Na+ concentration in the pulp increases, the solids concentration by volume in the froth rises from 0.49% to 0.60%, and the water holdup increases from 10.28% to 13.02%.
Figure 4c,d shows the correlation between Ca2+ and the properties of flotation froths of different types of clay. As shown in Figure 4c, with increasing Ca2+ concentrations in the pulp, water holdup in the montmorillonite flotation froth increases from 7.14% with deionized water to 10.41% at a Ca2+ concentration of 0.1 mol/L. Correspondingly, Figure 4d shows that the montmorillonite solids volumetric concentration increases from 0.050% to 0.069%. For kaolinite, as the Ca2+ concentration increases, both the water holdup and the solids volumetric concentration in the froth increase. Specifically, water holdup increases from 6.83% to 11.08% (nearly doubled), and the solids concentration by volume in the froth increases from 0.033% to 0.066%. For talc, with the presence of Ca2+, the pH value of the pulp is 9.3, at which point Ca2+ exists in the form of hydroxyl complexes and does not affect the floatability of talc [30]. Hence, the solids volumetric concentration in the froth remains much higher than that of montmorillonite and kaolinite. As the Ca2+ concentration in the pulp increases, water holdup increases from 10.28% to 14.0%, and the solids volumetric concentration in the froth increases from 0.49% to 0.62%.
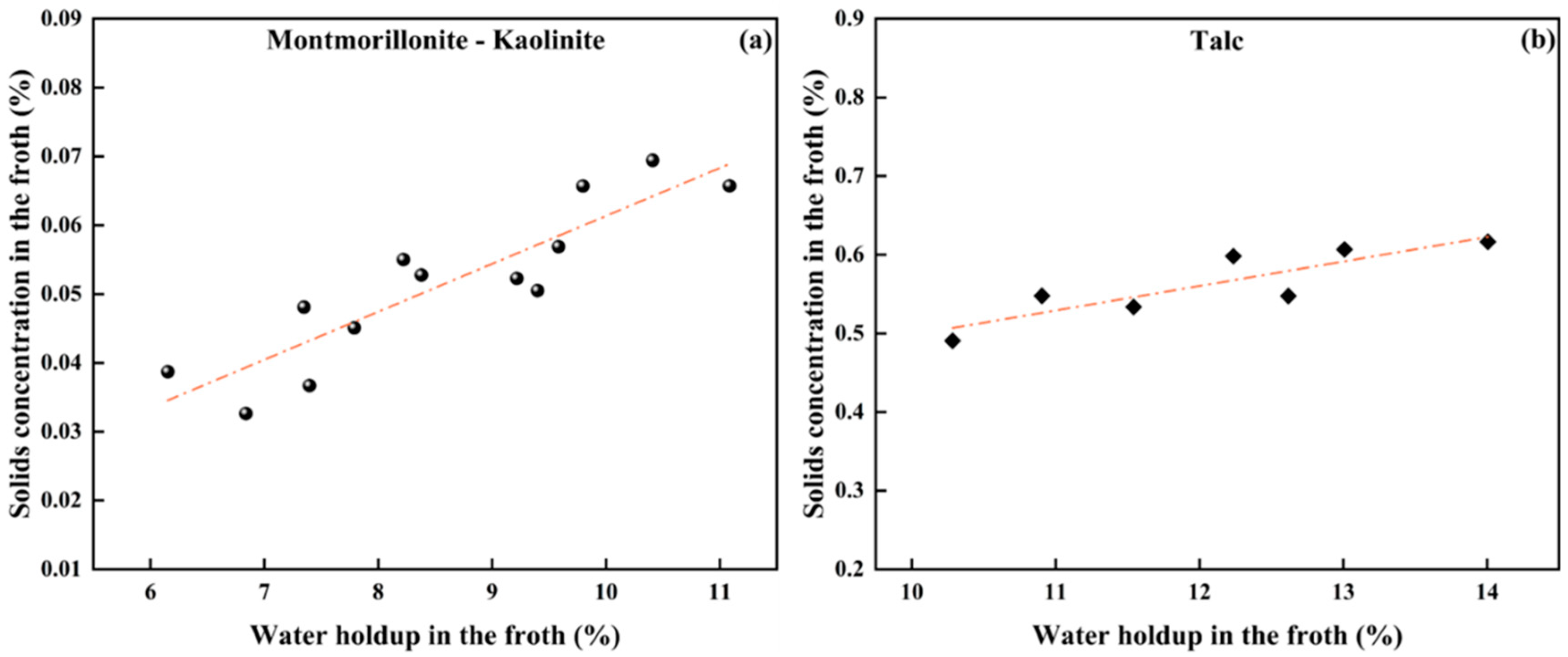
Particles can enter the froth phase by attaching to bubbles or entrained in the up-flowing water dragged by bubbles. Nonselective entrainment is strongly dependent on the water in flotation [31]. For hydrophilic minerals like montmorillonite and kaolinite, solid particles mainly enter the froth phase through entrainment. In contrast, for naturally hydrophobic talc, the particles primarily enter the froth phase through attachment to bubbles. In this work, the correlation between the water holdup and the solids concentration by volume in the froth is plotted in Figure 5. As shown, a linear relationship exists in between, regardless of clay hydrophobicity.
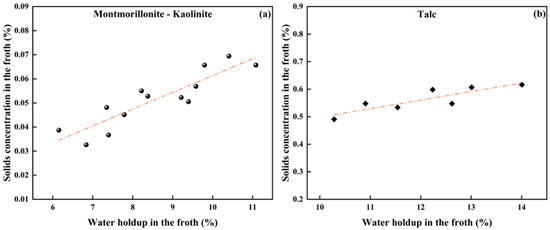
Figure 5.
The correlation between water holdup and solids volumetric concentration in froth: (a) hydrophilic minerals and (b) hydrophobic talc.
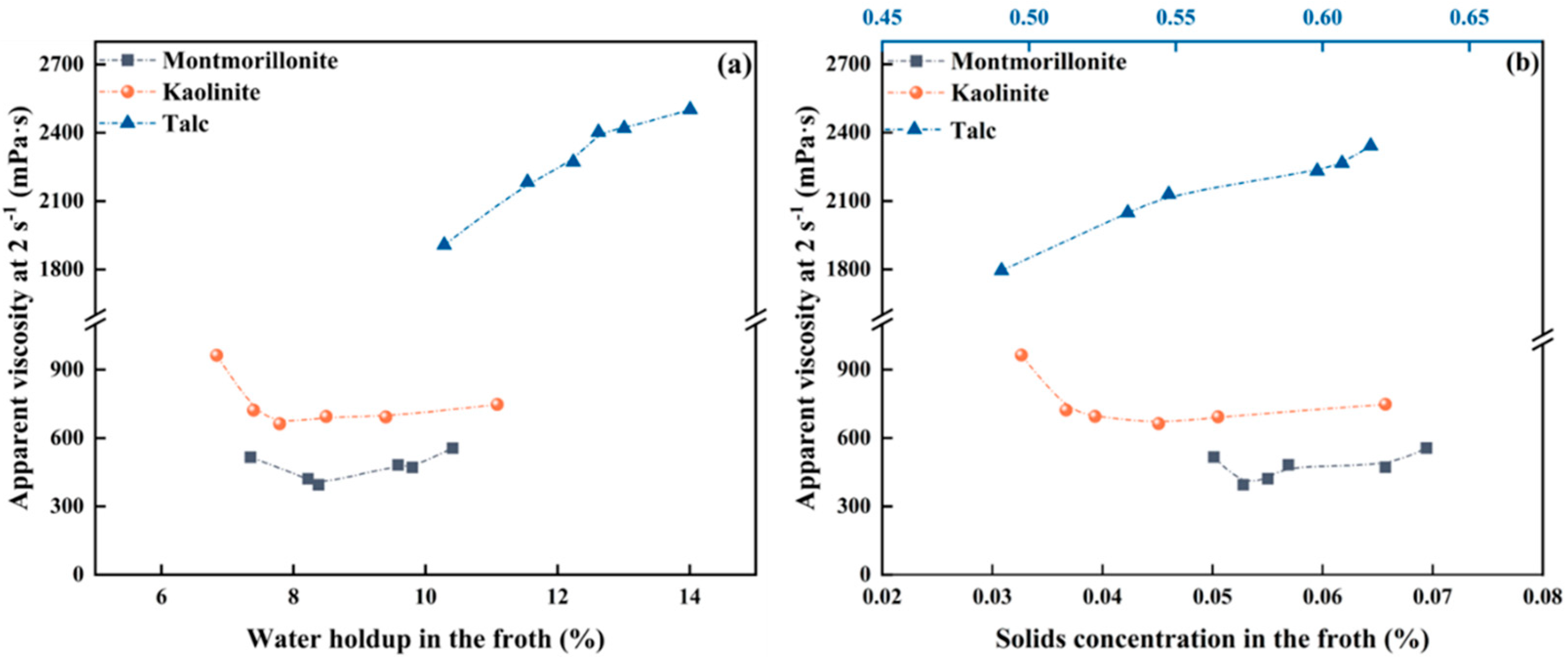
It is well known that flow viscosity is negatively related to water holdup but positively associated with volumetric solids concentration in froth [32,33,34], since varying the cation concentration changes both water holdup and volumetric solids concentration, as shown in Figure 4. Figure 6 comparatively shows the flotation froth apparent viscosity as a function of water holdup and solid concentration by volume within the froth. For hydrophilic montmorillonite and kaolinite, froth apparent viscosity is negatively related to water holdup at low cation concentration but is positively correlated with solids concentration at high cation concentration, which is consistent with the corresponding theory. Hence, it is known that at low cation concentrations, the viscosity of froth is primarily dominated by the water holdup in the froth. With further increase in cation concentration, the froth viscosity begins to be regulated by the solids volumetric concentration in the froth phase. For hydrophobic talc, the solids concentration by volume in the froth phase escalates concomitantly with the augmentation of cation concentrations. Apparently, the apparent froth viscosity is dominated by the solids concentration by volume in the froth within the varied range for the cation concentration, as expected.
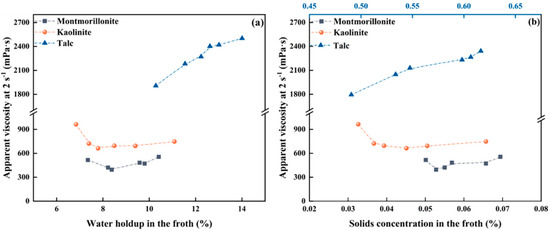
Figure 6.
Froth apparent viscosity in relation to water holdup (a) and solids concentration (b) in the froth.
3.3. Dispersion Behavior of Clay Particles in the Presence of Cations
Figure 4 and Figure 6 together reveal the effect of cations on froth rheology of clay minerals in terms of investigating the variation in froth composition. This section studies the underlying mechanism for the varied froth composition by studying the dispersion behavior of clay particles in the presence of cations. Note that the suspension pHs for montmorillonite and kaolinite are less than 9, at which calcium exists primarily in the form of Ca2+ [12]. Calcium ions exist in the form of hydroxyl complexes in the talc suspension with a pH of 9.3 [30]. Sodium ions exist in the form of Na+ in all the three clay suspensions.
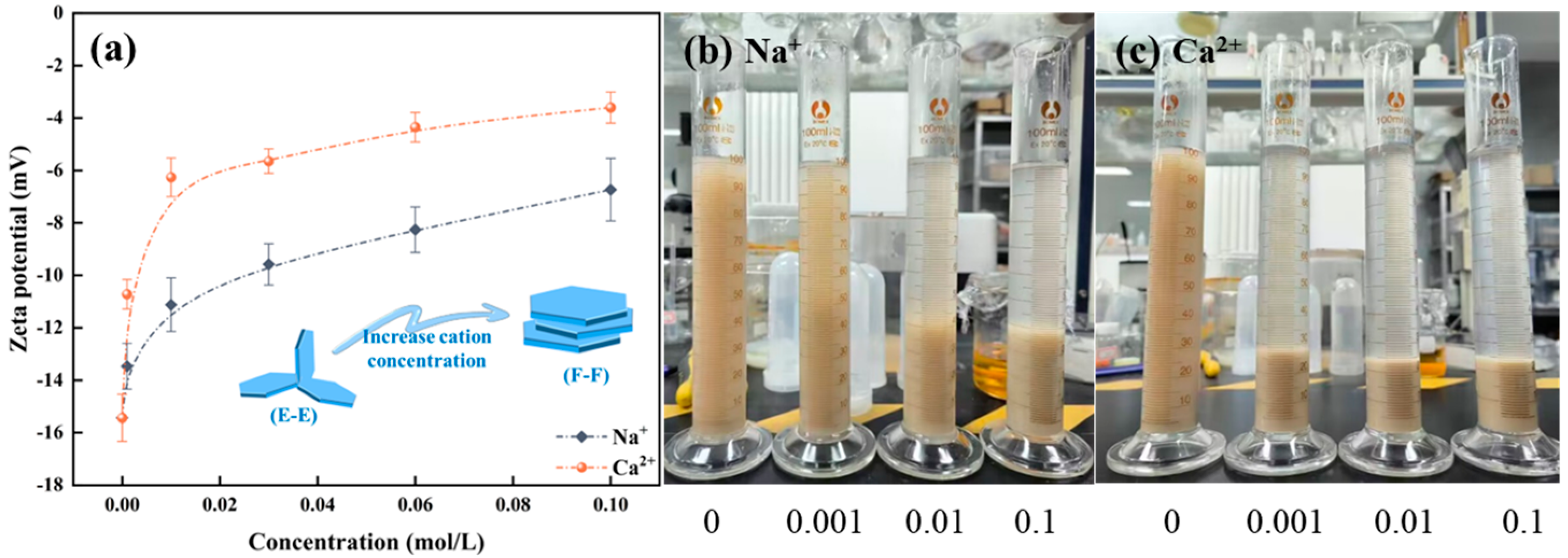
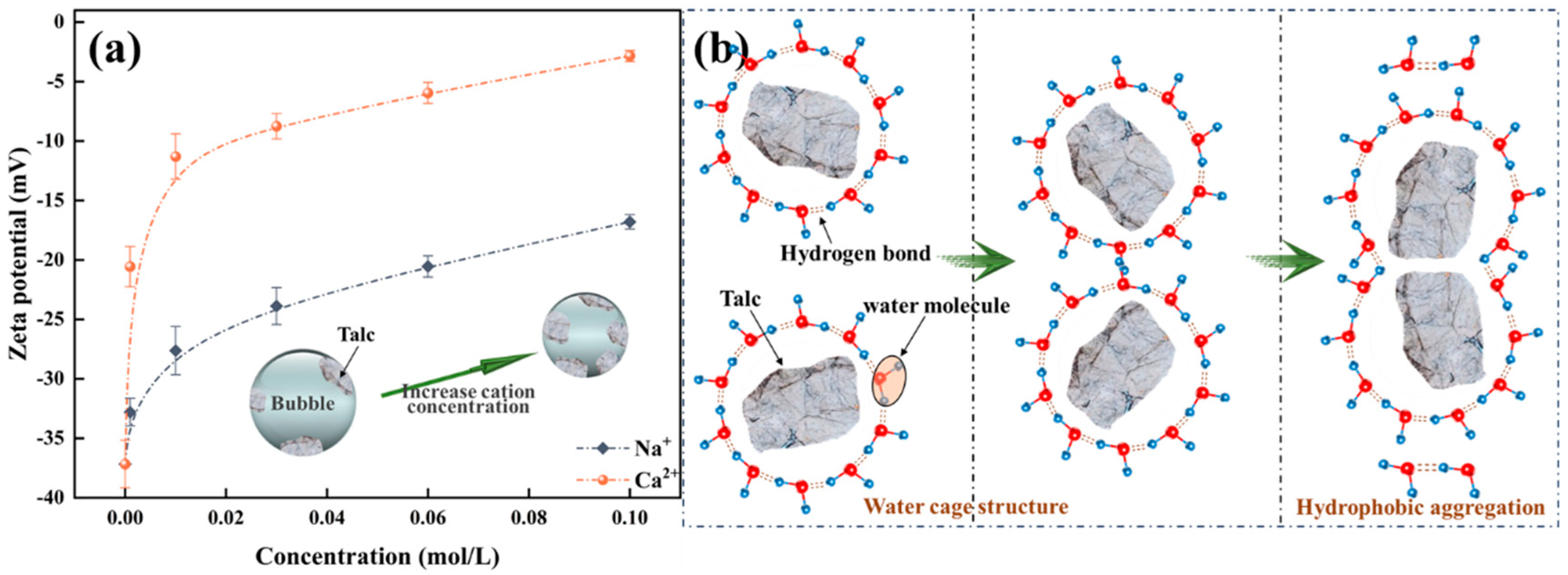
As shown in Figure 7a, Na+/Ca2+ modifies the zeta potential of mineral surfaces by compressing the electric double-layer present on the surface of montmorillonite particles. With the augmentation of ion concentration, the absolute zeta potential value of montmorillonite progressively diminishes, indicating that the compression of the electric double layer intensifies accordingly. The findings further suggest that the impact of ion valence on zeta potential is differential. Na+ will not adsorb into the Stern layer. An elevation in the concentration of positive ions within the diffuse layer induces a shift of the slipping plane towards the particle surface, consequently reducing the zeta potential. Ca2+ can engage in specific adsorption with montmorillonite, react with the surface hydroxyl groups to form hydroxy complexes, and enter the Stern layer. Consequently, the compression of the electric double-layer of montmorillonite exerted by divalent Ca2+ surpasses that induced by monovalent Na+. Similarly, as shown in Figure 7b,c, setting tests indicate that the introduction of cations leads to the aggregation of montmorillonite particles, expediting the sedimentation rate of the formed aggregates, with divalent Ca2+ exerting a more pronounced effect than monovalent Na+. In summary, Na+/Ca2+ effectively compresses the double electric layer of montmorillonite particles, thereby reducing their water absorption and swelling capacity and leading to the collapse of the network structure.
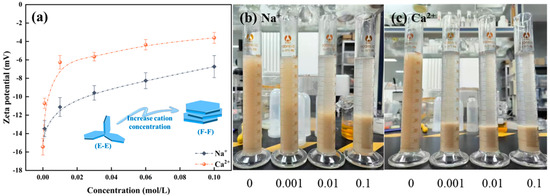
Figure 7.
Influence of cations on the zeta potential of montmorillonite (a) and setting tests (b) Na+, (c) Ca2+.
For the froth properties of montmorillonite, in the context of low salt concentration regimes, due to the ion exchange interaction, cations present in the slurry can be continuously transported to the interlayer space. Concurrently, the compression of the particle electrical double-layer in saline solutions leads to the diminution of repulsive forces and augmentation of the interactions between montmorillonite platelets. Consequently, the hydration of interlayer cations coupled with the augmented montmorillonite interparticle interactions contribute to the stability of the network structure within the slurry [12]. This results in a decrease of free particles within the slurry and a reduction of the bubble rise velocity, which subsequently induces a diminution in the entrained water into the froth phase and a corresponding reduction in the solid particles entering the froth. This explains why the water holdup and solids volumetric concentration in the froth decreases at a Na+ concentration of 0.001 mol/L in comparison to pure water conditions. When the concentration of cations further increases, it strengthens the compression of the electric double-layer and diminishes the water absorption and swelling capacity of montmorillonite, leading to a transformation of the particle association structure from Edge-Edge into Face-Face and a consequent reduction in the obstruction to the ascending flow of bubbles [35]. Correspondingly, both the water and solid particles entrained into the froth phase increases. Apparently, the increase of solids volume in the froth dominated the change in froth rheology.
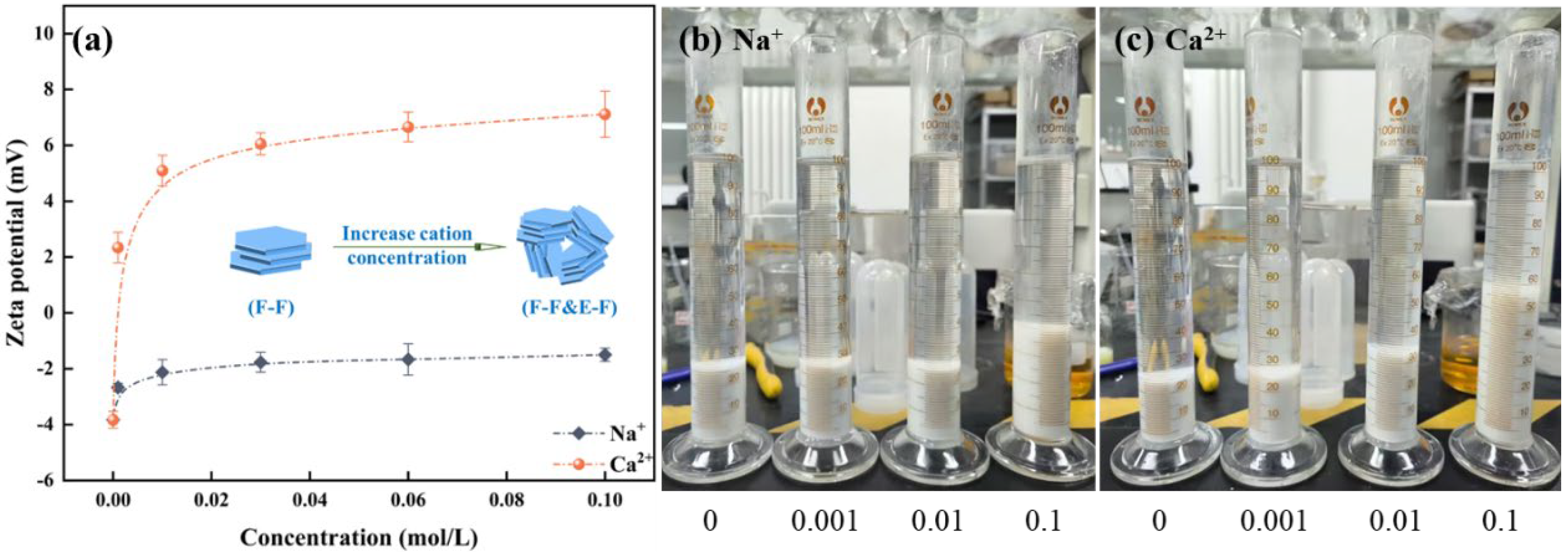
In Figure 8a, zeta potential test results of kaolinite demonstrate that the impact of monovalent Na+ on the surface potential of kaolinite is relatively minimal. With the increase of Ca2+ concentration, the zeta potential undergoes a positive reversal and progressively increases. To clarify the interaction mechanisms between kaolinite particles and their impact on the flotation entrainment process, setting tests on kaolinite suspensions under varying cation concentrations are shown in Figure 8b,c. As shown, the setting rate of kaolinite aggregates diminishes with an increment in the concentration of metallic cations, and the thickness of sediment increases. It suggests that with the increase of cation concentration, kaolinite forms network structure aggregates due to the compression of the electrical double-layer and the adsorption of cations on kaolinite [19]. For the froth properties with kaolinite, the introduction of metallic cations induces kaolinite particles to form aggregates with lower density, which makes it easier for them to enter the froth phase by entrainment water. Meanwhile, the augmented solid particles in the plateau borders of the froth phase hinders the drainage of water, resulting in an increase in the water holdup of the froth phase. It appears that the increases in solids concentration and water holdup offset their impacts on froth rheology. Consequently, with the elevation of Na+/Ca2+ concentration, froth viscosity remains relatively stable following an initial reduction.
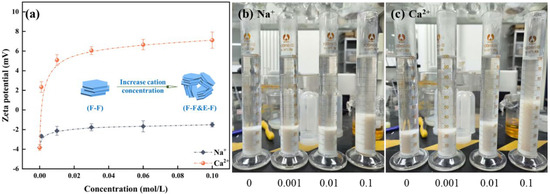
Figure 8.
Influence of cations on the zeta potential of kaolinite (a) and setting tests ((b) Na+, (c) Ca2+).
Talc exhibits negative surfaces charge in a pH range of 2–12, which is attributed to its crystal structure [30]. As seen in Figure 9a, the addition of Na+/Ca2+ induces a positive shift in the surface potential. By compressing the electric double-layer, cations diminish the electrostatic repulsion between talc particles. However, adsorption occurs exclusively at the edges and basal planes of talc particles, with no adsorption taking place on the hydrophobic basal faces. With the increase in the concentration of Na+, their hydration effect intensifies the attraction between water molecules, which diminishes the stability of the water film on the surface of hydrophobic talc particles. This augmentation fosters talc interparticle interactions, leading to hydrophobic aggregation and a concomitant enhancement of talc hydrophobicity. Figure 9b depicts a schematic representation of this hydrophobic interaction process. Consequently, the solids concentration by volume within the talc flotation froth escalates with an increase in Na+ concentration in the solution. The divalent Ca2+ ions form hydroxyl complexes that adhere to the particle surfaces, substantially curtailing electrostatic repulsion between particles by compressing the electrical double-layer, thereby augmenting the likelihood of talc particles adhering to bubbles. Hence, as the Ca2+ concentration in the pulp escalates, the solids concentration by volume in the froth also increases. The solid particles occupy the plateau borders, impeding the drainage of water and thus increasing water holdup in the froth phase. Contrary to hydrophilic clay minerals, hydrophobic talc particles predominantly coat the bubble surfaces, leading to increased energy dissipation on bubble deformation during flow [21]. Consequently, elevating the concentration of talc particles within the froth markedly amplifies its viscosity.
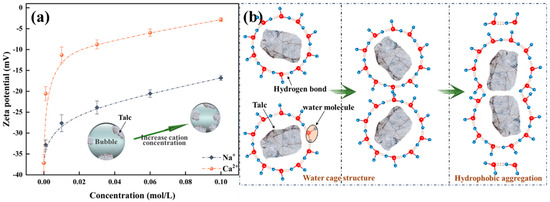
Figure 9.
Influence of cations on the zeta potential (a) and hydrophobic interaction between talc particles (b).
Based on the above, it can be inferred that the type and concentration of cations exert a considerable influence on the rheological characteristics of clay flotation froths, thereby ultimately defining the properties of the froth. Furthermore, this finding also suggests that the impact of cation on the clay interparticle interaction in the pulp should also be investigated, which could assist in elucidating the variation in the froth characteristics of different clays.
4. Conclusions
The effect of the interaction between clays and cations on froth rheology was studied in this work. With increasing concentrations of cations, the apparent froth viscosity of montmorillonite initially decreased and then increased. The viscosity of the kaolinite froth did not exhibit significant variation after the initial substantial decrease. However, the viscosity for talc continued to rise with the increasing cation strength in the feed. The variation in froth viscosity was directly related to the alterations in water holdup and solids volumetric concentration within the froth, which was related to the presence of cations by altering the association structure of clay particles in the pulp phase. The findings show that the particle behavior in the pulp zone directly impacts froth rheology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min14070706/s1, Figure S1: Torque value vs. vane speed under different concentrations of Na+ (a-montmorillonite, b-kaolinite and c-talc); Figure S2: Torque value vs. vane speed under different concentrations of Ca2+ (a-montmorillonite, b-kaolinite and c-talc). References [36,37,38] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, C.L.; funding acquisition, C.L. and Y.C.; investigation, Z.W. (Zhongren Wu) and Z.W. (Zhihang Wu); writing—original draft preparation, Z.W. (Zhongren Wu); validation, X.C.; writing—review and editing, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
Financial supports for this study, provided by the Key R&D and promotion program in Henan Province (242102321176), the Project of Zhongyuan Critical Metals Laboratory (GJJSGFYQ202327), the Zhengzhou Key Project for Collaborative Innovation (21XTZX06027), the Research Fund Program of State Key Laboratory of Rare Metals Separation and Comprehensive Utilization (GK-202301), and the Research Fund Program of Guangdong Provincial Key Laboratory of Development and Comprehensive Utilization of Mineral Resources (GDKT-202301), are gratefully appreciated.
Data Availability Statement
Data are contained within the main text and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, M.; Peng, Y. Effect of clay minerals on pulp rheology and the flotation of copper and gold minerals. Miner. Eng. 2015, 70, 8–13. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y. Managing clay minerals in froth flotation—A critical review. Miner. Process. Extr. Metall. Rev. 2018, 39, 289–307. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Ndlovu, B.; Bradshaw, D. Behaviour of swelling clays versus non-swelling clays in flotation. Miner. Eng. 2016, 96–97, 59–66. [Google Scholar] [CrossRef]
- Jenkins, P.; Ralston, J. The adsorption of a polysaccharide at the talc–aqueous solution interface. Colloids Surf. A Physicochem. Eng. Asp. 1998, 139, 27–40. [Google Scholar] [CrossRef]
- Morris, G.E.; Fornasiero, D.; Ralston, J. Polymer depressants at the talc-water interface- Adsorption isotherm, microflotation and electrokinetic studies. Int. J. Miner. Process. 2002, 67, 211–227. [Google Scholar] [CrossRef]
- Pawlik, M.; Laskowski, J.; Ansari, A. Effect of carboxymethyl cellulose and ionic strength on stability of mineral suspensions in potash ore flotation systems. J. Colloid Interface Sci. 2003, 260, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Gui, X.; Cao, Y. Effect of Calcium Ion on Coal Flotation in the Presence of Kaolinite Clay. Energy Fuels 2016, 30, 1517–1523. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Y.; Zhao, Y.; Chen, T.; Li, H.; Chen, L.; Song, S. Regulation of coal flotation by the cations in the presence of clay. Fuel 2020, 271, 117590. [Google Scholar] [CrossRef]
- Yang, X.; Bu, X.; Xie, G.; Chelgani, S.C. A comparative study on the influence of mono, di, and trivalent cations on the chalcopyrite and pyrite flotation. J. Mater. Res. Technol. 2021, 11, 1112–1122. [Google Scholar] [CrossRef]
- Song, S.; Gu, G.; Huang, W.; Wang, Y. Decoupling the mechanisms in chalcopyrite flotation with high sodium bentonite content when using saline water containing divalent cations. Miner. Eng. 2021, 167, 106902. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C. Considering specific ion effects on froth stability in sulfidic Cu-Ni-PGM ore flotation. Minerals 2022, 12, 321. [Google Scholar] [CrossRef]
- Burdukova, E.; Van Leerdam, G.C.; Prins, F.E.; Smeink, R.G.; Bradshaw, D.J.; Laskowski, J.S. Effect of calcium ions on the adsorption of CMC onto the basal planes of New York talc—A ToF-SIMS study. Miner. Eng. 2008, 21, 1020–1025. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. The Effect of the Ionic Strength of Process Water on the Interaction of Talc and CMC: Implications of Recirculated Water on Floatable Gangue Depression. Minerals 2019, 9, 231. [Google Scholar] [CrossRef]
- Parolis, L.A.S.; van der Merwe, R.; Groenmeyer, G.V.; Harris, P.J. The influence of metal cations on the behaviour of carboxymethyl celluloses as talc depressants. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 109–115. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Nicholson, T.; Lauten, R.A. The different effects of bentonite and kaolin on copper flotation. Appl. Clay Sci. 2015, 114, 48–52. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Nicholson, T.; Lauten, R.A. The role of cations in copper flotation in the presence of bentonite. Miner. Eng. 2016, 96–97, 108–112. [Google Scholar] [CrossRef]
- Huang, L.; Song, S.; Gu, G.; Wang, Y. The Interaction between Cations in Saline Water and Calcium Bentonite in Copper Flotation. Min. Met. Explor. 2021, 38, 693–699. [Google Scholar] [CrossRef]
- Du, S.; Peng, T.; Song, S.; Gu, G.; Wang, Y. Effect of calcium ions on bentonite network structure. Miner. Eng. 2022, 186, 107724. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, N.; Peng, Y. The entrainment of kaolinite particles in copper and gold flotation using fresh water and sea water. Powder Technol. 2015, 286, 431–437. [Google Scholar] [CrossRef]
- Cruz, N.; Peng, Y.; Farrokhpay, S.; Bradshaw, D. Interactions of clay minerals in copper–gold flotation: Part 1—Rheological properties of clay mineral suspensions in the presence of flotation reagents. Miner. Eng. 2013, 50–51, 30–37. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Ndlovu, B.; Bradshaw, D. Behavior of talc and mica in copper ore flotation. Appl. Clay Sci. 2018, 160, 270–275. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Y.; Zhao, Y.; Chen, T.; Chen, L.; Jiao, X.; Qing, C.; Song, S. Effects of clay species on coal flotation under the cationic regulation. Chem. Phys. Lett. 2020, 753, 137626. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Peng, Y. The interaction between kaolinite and saline water in affecting the microstructure, rheology and settling of coal flotation products. Powder Technol. 2020, 372, 76–83. [Google Scholar] [CrossRef]
- Wang, L.; Li, C. A Brief Review of Pulp and Froth Rheology in Mineral Flotation. J. Chem. 2020, 2020, 3894542. [Google Scholar] [CrossRef]
- Shi, F.N.; Zheng, X.F. The rheology of flotation froths. Int. J. Miner. Process. 2003, 69, 115–128. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Nicholson, T.; Peng, Y. The effect of saline water on the settling of coal slurry and coal froth. Powder Technol. 2019, 344, 161–168. [Google Scholar] [CrossRef]
- Li, C.; Runge, K.; Shi, F.; Farrokhpay, S. Effect of froth rheology on froth and flotation performance. Miner. Eng. 2018, 115, 4–12. [Google Scholar] [CrossRef]
- Wang, Z.; Mu, Y.; Zhang, M.; Cao, Y.; Li, C. Effect of clay crystal structure on froth rheology in flotation. Powder Technol. 2024, 435, 119395. [Google Scholar] [CrossRef]
- Li, C.; Farrokhpay, S.; Shi, F.; Runge, K. A novel approach to measure froth rheology in flotation. Miner. Eng. 2015, 71, 89–96. [Google Scholar] [CrossRef]
- Bazar, J.A.; Rahimi, M.; Fathinia, S.; Jafari, M.; Chipakwe, V.; Chelgani, S.C. Talc flotation—An overview. Minerals 2021, 11, 662. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Y.; Runge, K.; Bradshaw, D. A review of entrainment: Mechanisms, contributing factors and modelling in flotation. Miner. Eng. 2015, 70, 77–91. [Google Scholar] [CrossRef]
- Li, C.; Cao, Y.; Peng, W.; Shi, F. On the correlation between froth stability and viscosity in flotation. Miner. Eng. 2020, 149, 106269. [Google Scholar] [CrossRef]
- Li, C.; Runge, K.; Shi, F.; Farrokhpay, S. Effect of flotation froth properties on froth rheology. Powder Technol. 2016, 294, 55–65. [Google Scholar] [CrossRef]
- Li, C.; Runge, K.; Shi, F.; Farrokhpay, S. Effect of flotation conditions on froth rheology. Powder Technol. 2018, 340, 537–542. [Google Scholar] [CrossRef]
- Dong, Y.; Li, H.; Fan, Y.; Ma, X.; Sun, D.; Wang, Y.; Gao, Z.; Dong, X. Tunable dewatering behavior of montmorillonite suspension by adjusting solution pH and electrolyte C-concentration. Minerals 2020, 10, 293. [Google Scholar] [CrossRef]
- Stickland, A.D.; Kumar, A.; Kusuma, T.E.; Scales, P.J.; Tindley, A.; Biggs, S.; Buscall, R. The effect of premature wall yield on creep testing of strongly flocculated suspensions. Rheol. Acta 2015, 54, 337–352. [Google Scholar] [CrossRef]
- Krieger, I.M.; Maron, S.H. Direct Determination of the Flow Curves of Non-Newtonian Fluids. J. Appl. Phys. 1952, 23, 147–149. [Google Scholar] [CrossRef]
- Dzuy, N.Q.; Boger, D.V. Yield stress measurement for concentrated suspensions. J. Rheol. 1983, 27, 321–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).