Abstract
Super-reduced phases (SRPs), such as silicon carbide (SiC) and metal silicides, have increasingly been reported in various geological environments. However, their origin remains controversial. SRP inclusions (e.g., metal silicides and metallic silicon (Si0)) within SiC are commonly believed to indicate a natural origin. Here, we identified an unusual SRP assemblage (SiC, (Fe,Ni)Si2, and Si0) in situ in an H5-type Jingshan ordinary chondrite. Simultaneously, our analysis showed that the SiC abrasives contain (Fe,Ni)Si2 and Si0 inclusions. Other inclusions in the artificial SiC were similar to those in natural SiC (moissanite) reported in reference data, including diverse metal silicides (e.g., FeSi, FeSi2, Fe3Si7, and Fe5Si3), as well as a light rare earth element-enriched SiO phase and Fe-Mn-Cr alloys. These inclusions were produced by the in situ reduction of silica and the interaction between Si-containing coke and hot metals during the synthesis of the SiC abrasives. The results demonstrate that the SRP assemblage in the Jingshan chondrite originates from abrasive contamination and that the SRP inclusions (with a low content of Ca, Al, Ti, and Zr) cannot be used as a conclusive indicator for natural SiC. Additionally, the morphologies, biaxiality, and polytypes (determined by Raman spectroscopy) of SiC abrasives bear resemblance to those reported for natural SiC, and caution must be exercised when identifying the origin of SRP in samples processed by conventional methods using SiC abrasives. At the end of this paper, we propose more direct and reliable methods for distinguishing between natural and synthetic SiC.
1. Introduction
Natural SiC (moissanite), metal silicides, and metallic silicon (Si0) are super-reduced phases (SRPs), and are rare in nature. Thermodynamic calculations and experimental results indicate that SRPs are only stable in extremely reducing environments, for example, moissanite only occurs at an oxygen fugacity (fO2) of 6.5–7.5 log units below the iron–wüstite (IW) buffer, whereas metallic Si formation requires an environment with an fO2 of 3–5 log units below that of the SiC-forming reaction [1,2]. Thus, the occurrence of SRPs may be more important than their rare abundance in nature would indicate, because they offer valuable information on the formation conditions of rocks/meteorites [1].
SRPs have been reported in widespread geological settings. For example, moissanite has been identified in mantle-derived magmatic rocks, such as kimberlites [1,3,4,5]; volcanic breccias [6,7]; and as inclusions in kimberlite diamonds [8,9,10]. More enigmatic occurrences include those in metamorphic rocks, limestones, peralkaline syenite, pegmatites, and chromitite pods within ophiolites [11,12,13,14,15,16,17]. Various silicides (e.g., FeSi, FeSi2, and Fe3Si7) and native metals (e.g., Si0 and Fe0) have been reported as inclusions in some of the aforementioned SiC [1,4,5,7,11,12,14,15,17,18,19,20]. Moreover, diverse silicides have been successively identified from the heavy mineral separation of chromitites from the Luobusa ophiolite in Tibet, which generally hosts native Si0 inclusions [20,21,22,23,24]. Additionally, NiFe-silicides, Si0, and interstellar SiC have been found in meteorites and cosmic dust [25,26,27,28,29] while SiC has also been reported from rocks impacted by meteorites [30]. The widespread presence of SRPs in natural rocks raises several questions, such as, how do SRPs crystallise in such a wide range of environments, the majority of which do not provide extremely reducing conditions reported by experimental studies as crucial for SRP stability [5]? A comprehensive survey of the SRP literature revealed mineralogical evidence that definitively indicates the natural origin of SRPs in various terrestrial rocks limited to moissanite inclusions enclosed in diamonds and other encapsulating minerals and rock matrix [10,18,19,31,32,33,34,35].
The assumptions of the natural occurrence of SRPs have been controversial [1]. It is worth noting that most SRP grains in terrestrial rocks were obtained from heavy mineral concentrates through mineral separation techniques, and the risk of contamination during sample processing is high [36,37,38]. The common sources of contamination include the shedding of SiC grains from various rock-cutting/-drilling tools during mining, crushing, and separation processes. In addition, SiC abrasives and metal silicide impurities may be introduced during the grinding and polishing of thin rock sections or targets [1,36,39,40]. Menneken et al. [41] verified that abrasives or particles used for polishing can enter cavities in host minerals through openings only 2–3 µm wide. Methods related to the morphology, grain size, trace element chemistry, SRP inclusions, polytypes, biaxiality, and isotopic signatures have been proposed for distinguishing natural moissanite from synthetic SiC [1,5,13,16,17,18,42,43,44,45], where there is no doubt that the pre-solar origin of interstellar SiC in meteorites can be supported by the anomalous isotopic composition of C, Ne, Xe, Si, and N [42,43,44,45]. In contrast, the reliability of the above methods requires further validation when SiC grains possess a normal isotopic composition, i.e., isotopically similar to that of terrestrial contaminants. Notably, the presence of SRP inclusions such as metal silicides (e.g., FeSi, FeSi2, and Fe3Si7) and native metals (e.g., Si0 and Fe0) is often mentioned as one of the important evidence for ruling out contamination issues during presumably SiC-bearing samples and has been widely adopted as a consensus [1,5,7,14,17,19,46]. However, the evidence presented in this study challenges this prevailing viewpoint. It should be additionally noted that although some studies explicitly stated that their samples had not been drilled, cut, polished or otherwise prepared, or even claimed that any possibility of contamination has been ruled out during pre-processing, we should not neglect the discussion of the above-mentioned discrimination methods themselves. Any SRP particles in geological samples should be thoroughly reviewed and compared with potential contaminants to further rule it out, even when contamination appears unlikely.
In this study, we focus on an unusual SRP assemblage (SiC, (Fe,Ni)Si2, and Si0) identified in the Jingshan ordinary chondrite (hereafter referred to as the ‘Jingshan-SRP assemblage’). Further, we investigated the abrasive contamination through a comparative analysis using industrial green SiC abrasives and assessed the feasibility of the previously proposed methods for distinguishing between natural and artificial SiC, and explored more direct and reliable methods for the identification of natural SiC.
2. Materials and Methods
2.1. Sample Description and Preparation
Samples of the Jingshan chondrite and artificial green SiC abrasives were analysed in this study. Jingshan H5-type ordinary chondrite was discovered in 2006 in Jingshan County, Hubei Province, China. It has a total mass of 2245 g and is composed mainly of olivine (Fa15–17.5) and low-Ca pyroxene (Fs14.6–17Wo0–1.2) [47]. The accessory phases in the meteorite include monoclinic pyroxene, plagioclase, kamacite, troilite, chromite, and merrillite. The specimen examined in this study was cut from the bulk meteorite provided by Zuokai Ke. Prior to analysis, the Jingshan chondrite was cut using a micro-SiC (commercially known as ‘carborundum’) saw to expose a fresh surface. The clean slice was then glued onto a thin section (J-1) using epoxy resin. Different sizes of the green SiC abrasives (brand: GC) were used during the sample grinding process: 120# grit (approximately 50–200 μm) for preliminary grinding and 320# grit (approximately 20–45 μm) for fine grinding. Micro diamond powder was used for the final polishing. No additional industrial processes were performed during the sample preparation.
A supplementary polished thin section (J-2) of the Jingshan chondrite was prepared by diamond wire cutting and polished using an 800–1500# alumina sandpaper (SKY Lark; Suzhou, China) and a 0.05 μm alumina suspension (Qmaxis; Chicago, IL, USA). To prevent laboratory contamination, SiC abrasives were not used to prepare the thin section. Ultrasonic rinsing with distilled water was performed after each procedure to obtain a polished sample surface. This thin section was used for a comparative examination to determine the presence or absence of SRP within the Jingshan chondrite.
The industrial green SiC abrasive used in the Jingshan chondrite processing was also analysed to determine their constituents. Owing to the uniform production processes of the SiC abrasives of different sizes, we used the 120# SiC abrasive with a grain size range of 50–200 μm. The abrasive used in this study was manufactured by the Fuyang Qunchang Grinding Material Co., Ltd., Hangzhou, China, in accordance with the Chinese national standard [48]. Hundreds of SiC abrasive grains were affixed to a thin section using epoxy resin prior to analysis.
2.2. Optical Microscopy
The petrographic characterisation of the Jingshan chondrite and optical characterisation of the industrial SiC abrasives were performed using an Eclipse LV100POL optical microscope (Nikon; Tokyo, Japan) equipped with a digital camera system in both the transmitted and reflected light modes.
2.3. Scanning Electron Microscopy and Elemental Analysis
The micro morphology of the thin sections was recorded using a scanning electron microscope (SEM; FEI-FEG 650; Hillsboro, OR, USA) in the back-scattered electron (BSE) mode. The semi-quantitative analyses of the single-point elements of the Jingshan-SRP assemblage and inclusions in the SiC abrasives, coupled with multi-elemental mapping analysis of Si, Fe, Ni, Ti, Cr, Mg, and O in the region of the Jingshan-SRP assemblage, were performed using an energy-dispersive X-ray spectrometer (EDS; FEI-FEG 650). The non-negligible abundance of rare earth elements (REEs) in some inclusions of the SiC abrasives was qualitatively characterised using EDS. Both thin sections were coated with carbon before testing. The analyses were conducted in high-vacuum, all-element mode with an accelerating voltage range of 15–25 kV, a resolution of 2.5 nm, a working distance of approximately 10 mm, and an acquisition angle of 35°. The surface-scanning beam current spot range was set to 5.5–6.0 nm, and the scanning duration of the multi-elemental mapping analysis was 5 h.
The elemental quantitative analysis of (Fe,Ni)Si2 in the Jingshan chondrite (hereafter referred to as ‘Jingshan-(Fe,Ni)Si2’) was conducted by an electron probe microanalyser (EPMA; JEOL JXA-8230; Tokyo, Japan) with four wavelength-dispersive spectrometers (WDS). The EPMA was operated based on the methods described by Li et al. [49] employing an accelerating voltage of 15 kV, beam current of 10 nA, diameter (‘spot mode’) range of 0–2 μm, and count time of 15–20 s. ZAF correction was applied to calibrate the analysis results.
2.4. Single-Crystal Electron Back-Scattered Diffraction
Prior to the electron back-scattered diffraction (EBSD) analysis, the thin section of the Jingshan chondrite (J-1) underwent vibrational polishing using the 0.05 μm alumina suspension (Buehler; Waukegan, IL, USA) to improve the sample surface flatness and increase the result’s accuracy. The EBSD analysis of Jingshan-(Fe,Ni)Si2 was performed using an SEM (FEI-FEG 650) equipped with an automated INCA-Synergy EBSD plug-in [50]. The analysis was performed at an accelerating voltage of 20 kV in the focused-beam mode, with the beam current spot set to six and the specimen stage tilted at 70°. A low-vacuum mode was employed after removing the carbon coating to enhance the EBSD analysis. The AZtecHKL software (version 3.1) was used to index the EBSD patterns; and the structure and cell parameters of Jingshan-(Fe,Ni)Si2 were determined by matching the experimental EBSD patterns with the known structures of the relevant Ni-Si and Fe-Si phases in the database.
2.5. Raman Spectroscopy
A confocal Raman micro-spectrometer (Renishaw inVia Reflex; London, UK) was used to characterise the Jingshan-SRP assemblage and various polytypes of the artificial SiC. The analyses were conducted with a laser wavelength of 532 nm and beam spot diameter of 1 μm. Laser intensity was 50%, with a spectral exposure time of 10 s and total spectra with four accumulations for each measurement. The scanning range was 100–1300 cm−1.
3. Results
3.1. Super-Reduced Phase Assemblage in Jingshan Chondrite
3.1.1. Appearance and Optical Properties
The SRP assemblage (SiC, (Fe,Ni)Si2, and Si0) in the Jingshan chondrite (J-1) was identified with SEM and optical microscopy (Figure 1a). SiC grains are about 2–20 μm, much larger than the size of interstellar SiC (<1 µm) reported in meteorites [42,44]. This significant difference in grain size may reduce the probability of its interstellar pre-solar origin to some extent. (Fe,Ni)Si2 occurs as an individual, rectangular grain approximately 5 × 10 μm in size and has a bright white, metallic lustre under reflected light (Figure 1b). The physical and chemical properties of (Fe,Ni)Si2, such as hardness, density, and refractive index, could not be determined because of the small grain size. The (Fe,Ni)Si2 grain was surrounded by SiC fragments, and the assemblage occurred in a crack between the pyroxene matrix and impact melt vein. Si-enriched zones (approximately 2–3 μm) were found within the (Fe,Ni)Si2 grain. The Si-enriched areas exhibited a darker contrast than the (Fe,Ni)Si2 grain in the BSE image (Figure 1c). No SRP assemblage was found in any area of the J-2 (Figure 1d,e). However, the alumina clusters (submicrons to microns in particle sizes) used for polishing were observed in a ~200 µm wide cavity between the matrix in J-2 (Figure 1f,g), but no diamond contamination was found.
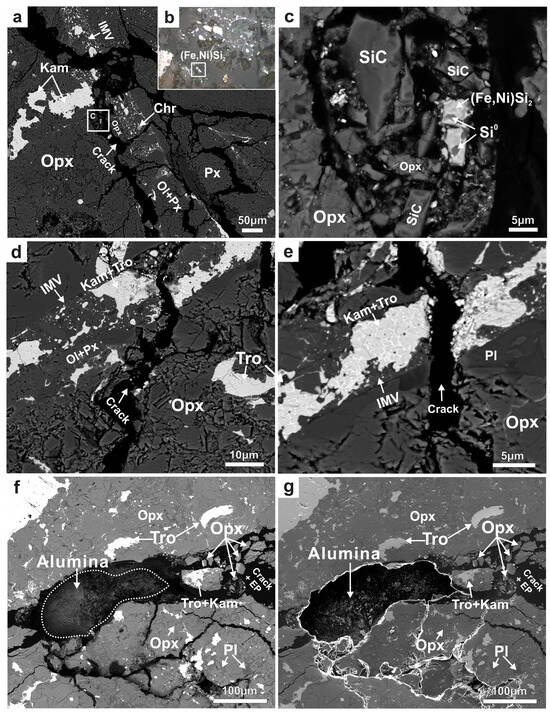
Figure 1.
(a) BSE image of the region containing the Jingshan-SRP assemblage in the thin section J-1. The (Fe,Ni)Si2 grain is located in a crack between the orthopyroxene (Opx) matrix and an impact melt vein (IMV); chromite (Chr), kamacite (Kam) blebs, olivine (Ol), and pyroxene (Px) are distributed within the vein; (b) optical microscope image under reflected light; (Fe,Ni)Si2 shows a bright white, metallic lustre; (c) the magnified BSE image shows the intergrowth of (Fe,Ni)Si2 and Si0, surrounded by the fragments of SiC and Opx; (d,e) the BSE images of the crack area between the Opx matrix and an IMV in the thin section J-2; no SRP assemblage was identified; (f) the BSE image and (g) SE image of the alumina contamination mixed into J-2. Tro—troilite; Pl—plagioclase; EP—epoxy resin.
3.1.2. Composition
X-ray elemental maps revealed the distribution of Si, Fe, Ni, Ti, Cr, Mg, and O in the Jingshan-SRP assemblage (Figure 2). The empirical formula of this Fe-Ni-Si phase (based on two Si atoms) was calculated from the average of the WDS analysis results (column ‘Jingshan’ in Table 1) as Ti0.04(Fe0.51Ni0.38)∑0.89Si2.00, simplified to (Fe,Ni)Si2, with the ideal compositions of Fe 24.61 wt %, Ni 25.87 wt %, and Si 49.52 wt %. Additionally, (Fe,Ni)Si2 contained trace amounts of Ti, Mg, Al, Cr, and F, as well as other impurity elements, which is similar to metal silicides such as (Ni,Fe)5(Si,P)2, Fe3Si, and Fe2Si, reported in previous studies from meteorites without SiC [25,26,28].
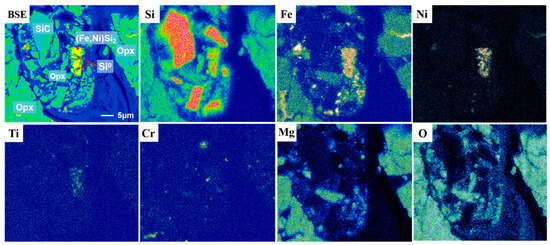
Figure 2.
BSE image and X-ray elemental maps for Si, Fe, Ni, Ti, Cr, Mg, and O in the region of the Jingshan-SRP assemblage.

Table 1.
EDS (WDS) analyses of the Jinshan-(Fe,Ni)Si2 and inclusions found in the green SiC abrasives.
3.1.3. Raman Spectroscopy
Figure 3a shows the Raman spectra of SiC, (Fe,Ni)Si2, and Si0 in the Jingshan chondrite sample. The SiC of the hexagonal 6H polytype (SiC-6H) was identified from the peaks of the folded transverse optic (FTO) phonon mode at 788 cm−1 and the folded longitudinal optic (FLO) phonon mode at 970 cm−1 (upper panel). The sharp peak at 514 cm−1 in the lower spectrum indicated the presence of Si0 (lower panel). The Raman spectrum of (Fe,Ni)Si2 is shown in Figure 3b. To accurately characterise the (Fe,Ni)Si2 phase and exclude potential analogues, the Raman spectrum of (Fe,Ni)Si2 was compared with the spectra of synthetic silicides NiSi2 and β-FeSi2 (Figure 3b). In the spectra of all three silicides, we observed sharp peaks at 514 cm−1 and approximately 521 cm−1 attributable to the first-order scattering line of transverse optic (TO) phonons from the Si substrate [51]. A notable distinction in the Raman spectra of (Fe,Ni)Si2 and β-FeSi2 suggested structural differences between the silicides, whereas (Fe,Ni)Si2 exhibited a close resemblance to the NiSi2 synthesised by Li et al. [52]. Only the peak of (Fe,Ni)Si2 was shifted towards a lower wavenumber (261 cm−1) than the broad peak in NiSi2 (286 cm−1). This peak shift may be associated with the substitution of Ni and Fe.
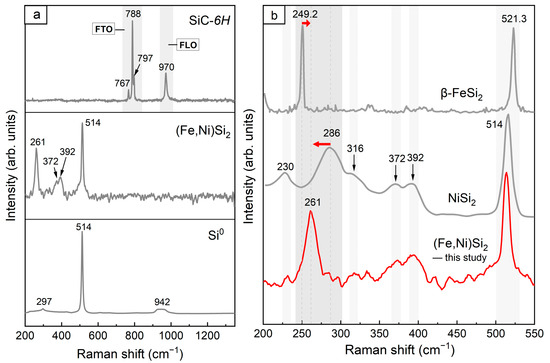
Figure 3.
(a) Raman spectra of SiC-6H, (Fe,Ni)Si2, and Si0 in the Jingshan chondrite; (b) the comparison of the Raman spectra of (Fe,Ni)Si2 with similar synthetic silicides β-FeSi2 [51] and NiSi2 [52]. The red arrows in (b) represent the shift of the corresponding peaks of (Fe,Ni)Si2 relative to β-FeSi2 and NiSi2, respectively.
3.1.4. EBSD Patterns
The Jingshan-(Fe,Ni)Si2 grain was too small (approximately 5 × 10 μm) to be tested using conventional single-crystal or powder XRD tests. Hence, the EBSD patterns (Figure 4) were used to determine the structure. The EBSD patterns of (Fe,Ni)Si2 were indexed by the Fmm fluorite-type structure and were most consistent with the synthetic NiSi2 cells from Wittmann et al. [53] (ICSD #76715, experimental PDF 43–989, calculated PDF 01–089–7166), with a mean angular deviation (MAD) of 0.73 (MAD < 1 was considered to indicate reliable data). The cell parameters of the synthetic NiSi2 phase [53] were a = 5.38 Å, V = 155.72 Å3, and Z = 4.
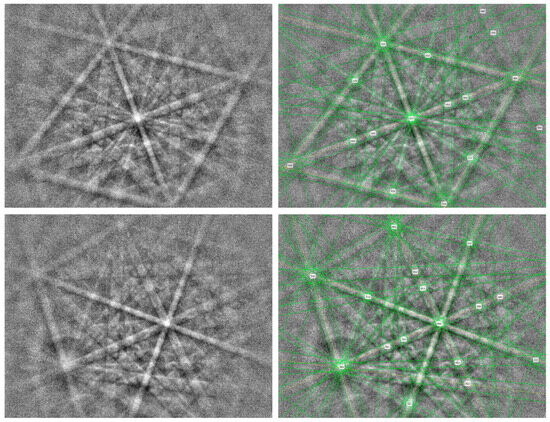
Figure 4.
EBSD patterns of (Fe,Ni)Si2 were recorded from two orientations (left) and their patterns indexed with the Fmm NiSi2 structure (right). Six diffraction bands were set.
(Fe,Ni)Si2 is a metal silicide that has not been discovered in nature. Some metal silicides approved by the International Mineralogical Association Committee on New Minerals, Nomenclature, and Classification (IMA-CNMNC) are presented in Table 2. Among these, FeSi2 (linzhiite) and Fe0.83Si2 (luobusaite) are similar in composition to (Fe,Ni)Si2. However, their crystal structures are entirely different. Linzhiite and luobusaite are tetragonal P4/mmm and orthorhombic Cmca, respectively, and their structures are similar to those of the α-FeSi2 and β-FeSi2 synthetics. Conversely, the (Fe,Ni)Si2 structure corresponds more with that of either γ-FeSi2 or γ-NiSi2. Therefore, (Fe,Ni)Si2 can be regarded as a solid solution of NiSi2 (fluorite-type structure, a = 5.38 Å) and γ-FeSi2 (fluorite-type structure, a = 5.39 Å) [54]; these silicides differ only in composition through the substitution of Fe and Ni. In (Fe,Ni)Si2, Fe and Ni are substituted at an approximate ratio of 5:4, based on which cell parameters of (Fe,Ni)Si2 are calculated as a = 5.3844 Å, V = 156.10 Å3, and Z = 4.

Table 2.
Some metal silicides that have been reported as natural minerals.
3.2. Analyses of Artificial Green SiC Abrasives
3.2.1. Morphology
The majority of the studied SiC abrasives were transparent fragments with different colours, ranging from bluish green to green and light green, or even colourless; green and bluish green SiC crystals were the most common (Figure 5a). Typically, the SiC abrasive grains exhibited irregular shapes and conchoidal fractures (Figure 5b), although some grains retained their regular shape (Figure 5c). A few black opaque coke residues were observed between the SiC grains (Figure 5d). These morphological features are highly similar to those reported for moissanite crystals in some natural rocks, e.g., [1,4,5,15,16,17].
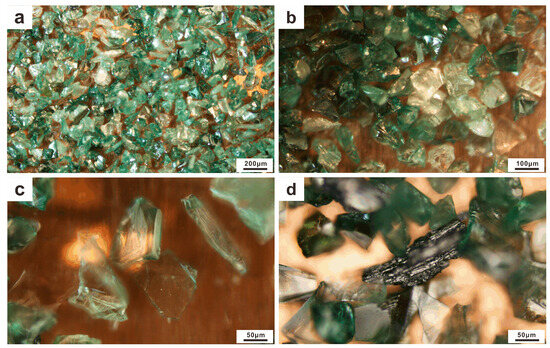
Figure 5.
Morphology of the artificial green SiC abrasives (120#) under the microscope: (a,b) colour varies from bluish green, green and light green to colourless, and the conchoidal fractures of the artificial SiC are visible; (c) an artificial SiC grain with a regular shape; (d) residual coke is visible between the SiC abrasives.
3.2.2. Inclusions
Figure 6 shows the presence of the various SRP inclusions within the SiC abrasive grains encompassing metal silicides, alloys, and native metals. The compositions of the host SiC and various inclusions are presented in Table 1. These inclusions showed a variety of irregular morphologies, including elongated triangular (Figure 6a,b), granular (Figure 6c,d), irregular planar (Figure 6e,f,j–l), ‘oil splash’ droplet-shaped (Figure 6g,k), lath-shaped (Figure 6h), lamellar (Figure 6m–o), and rounded (illustration in Figure 7a) with sizes ranging from submicrons to tens of microns. Fe-silicides were the most common inclusions in green artificial SiC, with Fe contents of 30.17–79.60 wt % (17.82–66.54 at %) and Si contents of 12.40–67.82 wt % (20.60–79.61 at %), reflecting the different proportions of Fe and Si. Their empirical formulae corresponded to Fe2Si9 (Figure 6f), Fe2Si7 (Figure 6b), Fe2Si5 (Figure 6d), FeSi3 (Figure 6g), Fe3Si7 (Figure 6h), FeSi2 (Figure 6c,k), and FeSi, Fe5Si3, Fe2Si, and Fe3Si (Figure 6j). These Fe-silicide inclusions generally contained minor amounts of Ni (0.82–5.38 wt %) and other impurity elements (e.g., Ti, Mn, Cr, and F). (Fe,Mn)Si3 with Mn contents up to 8.96 wt % was also identified within the SiC abrasive (Figure 6e).
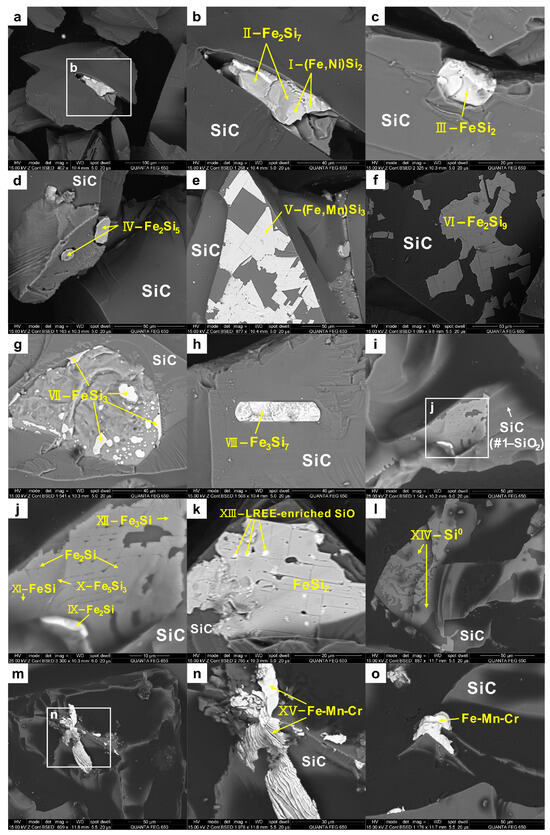
Figure 6.
BSE images of different inclusions in the SiC abrasives (the serial numbers I–XV and #1 of the inclusions in this figure correspond to those in Table 1). (a,b) elongated triangular (Fe,Ni)Si2 and Fe2Si7; (c) granular FeSi2; (d) granular Fe2Si5; (e) irregular planar (Fe,Mn)Si3; (f) irregular planar Fe2Si9; (g) ‘oil splash’ droplet-shaped FeSi3; (h) lath-shaped Fe3Si7; (i) SiO2 was detected in SiC, which contains an irregular planar inclusion; (j) shows a magnified image of (i)—the distribution of Fe-silicides with different compositions in an irregular plane; (k) ‘oil splash’ droplet-shaped LREE-enriched SiO were dispersed in the planar FeSi2; (l) irregular planar Si0; (m–o) lamellar Fe-Mn-Cr.
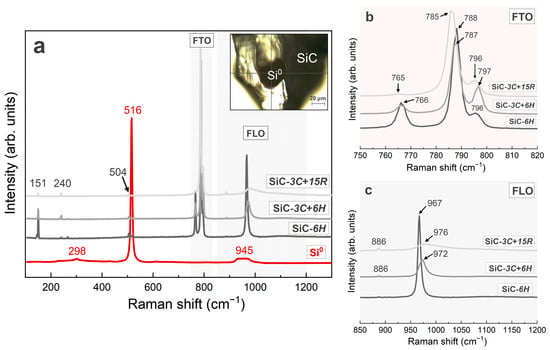
Figure 7.
(a) Several representative Raman spectra of the SiC abrasives (three grey lines) and Si0 inclusions (red line). The upper right illustration shows the test location of the Si0 inclusion; (b) the high-magnification detail of the SiC-FTO mode from the 750 to 820 cm−1 band; (c) the high-magnification detail of the SiC-FLO mode from the 850 to 1200 cm−1 band. FTA = folded transverse acoustic phonon; FLA = folded longitudinal acoustic phonon; FTO = folded transverse optic phonon; FLO = folded longitudinal optic phonon.
Some of the silicide inclusions contained high Ni contents (>30 wt %), thereby forming Fe-Ni-Si ternary phases such as (Fe,Ni)Si2, which principally comprised Fe 14.48 wt % (9.04 at %), Ni 30.43 wt % (18.09 at %), and Si 49.29 wt % (61.21 at %), as well as trace impurities of F and O. The composition of this phase is similar to that of the Jingshan-(Fe,Ni)Si2 (column ‘Jingshan’ in Table 1). Si0 also commonly occurred inside the SiC abrasives (Figure 6l; column XIV in Table 1) and often intergrew with metallic silicides. Raman spectroscopy showed the presence of Si0 inclusions in the artificial SiC (see Section 3.2.3). In the Jingshan chondrite, coexisting (Fe,Ni)Si2 and Si0 were surrounded by SiC fragments. The similarities between this SRP assemblage in the Jingshan chondrite and the inclusions in the SiC abrasives suggest a similar origin. Moreover, the examination of the thin section J-2 from the same meteorite that was not processed with the SiC abrasives did not reveal the presence of any SRPs. Hence, we inferred that the Jingshan-SRPs originated from the SiC abrasives during the grinding process and were subsequently embedded within the cracks of the thin section.
In addition, some micron-sized O-bearing inclusions enriched in light rare earth elements (LREE-enriched SiO) were dispersed in the FeSi2 inclusions of the SiC abrasives (Figure 6k). The SiO compound maintained an atomic ratio of approximately 1:1 between Si and O and contained various impurities, including Fe, Ni, Na, Ca, Cu, P, F, and Cl. The total amount of LREE reached 37.47 wt % and comprised 9.48 wt % La, 20.89 wt % Ce, and 8.43 wt % Nd. The LREE concentrations in this phase were sufficient for reliable identification using EDS. The detected enrichment of LREE was not a result of instrumental inaccuracies because the LREE-enriched SiO phases exhibited a sharp bright contrast in the BSE image, whereas REEs were undetectable in other phases during testing based on a similar procedure. The surface of the sample was covered with carbon (C), which was shielded during the test. Hence, it is unclear whether the SiO phase contained C. The exact composition of the Si-(C)-O phase depends on the physicochemical conditions of the oxidation reaction. LREE-enriched SiO has not been previously reported in synthetic SiC [46]; however, similar phases have been found in supposedly natural SiC, such as the O-bearing inclusions reported by Mathez et al. [1] and the Si-C-O phase reported by Shiryaev et al. [5].
3.2.3. Raman Spectroscopy of Artificial SiC Polytypes and Si0 Inclusions
According to the Raman analysis of numerous green SiC abrasives, three different representative Raman spectra of SiC were obtained (Figure 7a), which corresponded to three different SiC polytypes: 6H, 3C+6H, and 3C+15R. The SiC polytypes were identified using the FTO mode at 788 cm−1 (6H and 3C), 796–798 cm−1 (6H, 15R, and 3C), and 785 cm−1 (15R) (Figure 7b), and the FLO mode at 967 cm−1 (6H and 15R) and 972–978 cm−1 (3C) (Figure 7c) [19,55,56]. Among them, the uppermost Raman spectrum of the synthetic SiC (SiC-3C+15R) shows a very weak peak at ~970 cm−1, which is very similar to the biaxial moissanite [18]. The other two spectra have sharp peaks at ~970 cm−1, similar to the uniaxial moissanite (Figure 7a,c). Further, we also identified a dark rounded Si0 inclusion enclosed in a SiC abrasive. Si0 exhibited a sharp peak at approximately 515 cm−1, which did not belong to the host SiC, and weak peaks at 945 cm−1 and 298 cm−1 (Figure 7a).
4. Discussion
4.1. Comparison of SRP Inclusions in Artificial and Natural SiC
Numerous characteristic inclusions previously reported in natural SiC were identified in the synthetic SiC abrasives analysed in this study. Here, we discuss similarities between the inclusions present in artificial SiC abrasives and those in supposedly natural SiC from diverse geological environments.
According to Shiryaev et al. [4,5], SiC grains from the Mir, Aikhal, and Udachnaya kimberlite pipes (Yakutia) and Triassic limestones in Bulgaria contain the inclusions of Si0, Fe-silicides, and a Si-C-O phase. Si0 has been observed in the form of round or negative-crystal inclusions, which occasionally form networks between SiC grains. Many Si0 phases contain globules or rims of FeSi2, and SiC and SiO2 complexly coexist around FeSi2 grains. A similar assemblage of inclusions also exists in the studied SiC abrasives (Figure 6i,j). The presence of the Si-C-O phase and SiO2 suggests that the moissanite grew at high temperatures and pressures and was subsequently partially oxidised at high temperatures [5]. This process was not supposed to occur commonly in nature. However, the Si-(C)-O, SiO2, and Fe-silicide inclusions observed in this study are the by-products of artificial SiC, and their formation is common during industrial synthesis. The Fe-silicide phase described by Shiryaev et al. [4,5] showed considerable compositional variability, with an approximate chemical formula of FeSi2, and contained trace elements of Mn, Ni, and Ti. The FeSi2 component identified in our SiC abrasive (column III in Table 1) closely corresponds with the composition described in their study. Moreover, a second Fe-Si phase with a stoichiometry close to that of (Fe,Ti)Si3 was included in a SiC grain from Udachnaya [5], which is similar to the (Fe,Mn)Si3 and FeSi3 detected in our SiC abrasives (columns V and VII in Table 1). But we did not detect Ti in these two silicides. It is worth noting that in the study of Shiryaev et al. [4,5], they reported the exsolution of Ti0 in the FeSi2 grain attached to SiC. Ti0 was also not found in the inclusions of the synthetic SiC.
Nazzareni et al. [17] used Raman spectroscopy to identify dark, rounded Si0 inclusions (<40 μm) in deep blue to bluish green SiC from peralkaline syenite in the Azores, and stated that “the presence of Si0 inclusions supports the natural origin of these grains”. In this study, we also detected dark, rounded Si0 inclusions (~30 × 40 µm) in multiple synthetic SiC abrasives. The Raman spectra of the Si0 inclusions corresponded with those reported by Nazzareni et al. [17], and their morphologies were similar (Figure 7a).
Di Pierro et al. [7] reported the presence of Si0 and Fe3Si7 inclusions in moissanite grains discovered in kimberlite beach pebbles in the Aegean Sea region. Fe3Si7 has a composition of 37.14 wt % Fe and 52.72 wt % Si, coupled with trace amounts of Ni, Mn, Ti, Cr, and Al, which are comparable to the composition of Fe3Si7 found in the SiC abrasives in this study, as presented in column VIII of Table 1. Ca went undetected all through the analyses of the silicides in our SiC abrasive, whereas Di Pierro et al. [7] found Si2Ca (with 40.52 wt % Ca) and other Ca-rich silicide inclusions in moissanite, with Ca content ranging from 6.85 wt % to 8.01 wt %, e.g., Si-Fe-Al-Ca and Si2(Fe,Al,Ca)3. Al-rich inclusions were also not detected in the SiC abrasives, and the maximum content of Al in our analyses was 0.07 wt %, whereas Di Pierro et al. [7] reported that the Al content of the above inclusions in their moissanite was 20.99 wt % and up to 24.55 wt %. In addition to Al- and Ca-rich inclusions, Ti-rich inclusions were also not found in our analyses. The maximum amount of Ti was 1.7 wt % in the silicides and 3.29 wt % in Si0 (Table 1), whereas Si-Fe-Ti inclusions with up to 16.27 wt % Ti were reported in their moissanite [7]. Nevertheless, it cannot be ruled out that the presence of the above inclusions in the SiC abrasives was just not detected due to the limited number of particles we analysed.
Mathez et al. [1] observed the diverse micron-sized inclusions of moissanite in kimberlites from Yakutia, including Si0, Fe3Si7, Fe-Ti-Zr silicides (exhibit a variable major element composition but one subset is characterised by an approximately constant stoichiometry of Fe(Zr, Ti, Si)3, with Si/(Zr + Ti) ≈ 2.5), Mg-Fe silicate, LREE-enriched Fe-Ti silicides (up to approximately 16 wt % Ce and 25 wt % total LREEs), two O-bearing inclusions (Si2N2O and a silicate containing approximately 75 wt % LREE oxide) associated with the Si metal, and ferrosilicite inclusions. The authors state that only a few types of precipitates (e.g., B-compounds, solid carbon, and several silicides), all of which are submicroscopic or nano-sized, have been reported in synthetic SiC, e.g., [1,7,8,57]. Our investigation of the green SiC abrasives revealed the presence of the aforementioned metal silicide and Si0 inclusions, as well as an LREE-enriched O-bearing inclusion (Table 1, although the composition is not exactly the same as that found by Mathez et al. [1]), which contrasts with the reports finding “only a few types of precipitates”. All of these inclusions exist on a micron scale in our SiC abrasives, despite Mathez et al. [1] and Di Pierro et al. [7] reporting that the inclusions in synthetic SiC are “always nano-sized”. However, contrary to Yakutia moissanite [1], no Zr-/Ti-rich inclusions were found in our SiC abrasives, nor were any silicate inclusions found (see Section 4.3.2 for the detailed discussion).
Some silicides have been found in natural rocks rather than as inclusions in SiC (Table 2). These compounds are associated with Si0 or other silicides. For example, multiple mineral assemblages, including FeO, Fe, FexSiy, SiO2, and Si0, have been discovered in podiform chromitite from the Luobusa ophiolite in Tibet, China. The Si0 was intergrown with Fe3Si7 or formed as individual granular crystals [58]. FeSi2, TiFeSi2, and Si0 have been found in similar rocks [22,23]. With the exception of TiFeSi2, these mineral assemblages are very similar to the silicide inclusions in the aforementioned SiC abrasives and the Jingshan-(Fe,Ni)Si2 with the Si0 assemblage described in this study. Moreover, none of these studies demonstrated the contact relationship between the SRPs and their host rocks, including adjacent minerals; instead, individual grains were directly mounted in epoxy, thereby hindering a more effective discussion of their natural origins. The above suggests that the conclusion of their natural origins should be reassessed.
4.2. Synthesis Process of SiC Abrasives
Numerous methods, including carbothermal reduction, physical and chemical vapour deposition, high-temperature self-spreading, and sol–gel and plasma methods, have been used for SiC synthesis [19,59]. Carbothermal reduction is the most common and economical industrial method for producing green and black SiC abrasives, in which SiC is formed by high-temperature smelting (>2000 °C) in a highly reducing resistance furnace. In this process, coke (C) and high-quality silica (SiO2) are used as carbon and silicon sources, respectively, salt (NaCl) as a nucleating agent, and aluminium (Al) as an additive. The SiC formation reaction of the process is given by Equation (1) and involves two consecutive intermediate reaction processes (Equations (2) and (3)). First, coke (C) reacts with silica (SiO2) in a solid–solid or solid–liquid reaction, during which two intermediate products, gaseous silicon monoxide (SiO) and carbon monoxide (CO), are formed (Equation (2)). SiO undergoes a further gas–solid reaction with C to form SiC (Equation (3)). The equilibrium conditions for these processes depend on the temperature and partial pressure of the intermediate gas products, i.e., SiO and CO [60].
Moreover, the reactions expressed by Equations (4) and (5) [60] may also occur during SiC synthesis, producing metallic silicon (Si0) and SiO2.
Synthetic silicon carbides described in the literature are typically pure crystals grown under carefully controlled conditions. However, the synthesis of hundreds of SiC grains for industrial use does not require the meticulous control of the experimental conditions or material purity [61]. As such, industrial SiC abrasives typically contain a wide range of impurities; Si, C, Fe, and SiO2 are major impurities [59], whereas Ti, Al, Ni, V, Cr, Mn, N, and even certain REEs generally exist in trace amounts. These impurities are prone to form SRP inclusions, such as Fe(Ni)-Si compounds, Si0, or alloys, under the reducing conditions of SiC synthesis. Such SRP inclusions are very similar to those observed in natural SiC. The following explanations have been provided for the formation of certain impurity phases:
- (1)
- Different coke materials (as carbonaceous reducing agents) contain a variety of impurities, the compositions of which have been described in detail in several studies, e.g., [61,62].
- (2)
- During carbothermal reduction, fluctuations in the process conditions and the incomplete reactions of the raw materials may lead to the formation of free Si, C, SiO, and residual SiO2 impurities. After smelting, the inherent impurities in the raw materials and additional reaction processes result in the formation of new impurity phases in the SiC crystals. For example, Si0 can be generated according to the reactions in Equations (4) and (5). Additionally, Si powder may be introduced during conventional carbothermal reduction to increase the reaction yield at low temperatures, resulting in residual Si0 inclusions in synthetic SiC abrasives [59,63].
- (3)
- Silicide inclusions observed in SiC abrasives, such as FeSi, Fe3Si, and Fe5Si3, are believed to result from the in situ reduction of silica and the interaction between Si-containing coke and hot metals [64]. Fe-silicides generally contain minor amounts of Ni; when Ni is substituted for Fe at a given ratio, Fe-Ni-Si ternary-phase inclusions are formed. Significant amounts of Ni might be derived from the impurities in coke extensively used as a carbon source in the Acheson process.
- (4)
- Numerous inclusions in SiC abrasives contain trace amounts of fluorine because, as with corundum abrasives [61], small amounts of CaF2 or other fluorides are introduced as flux materials during the synthesis of SiC abrasives. Hence, fluorine-containing residues may be present in SiC abrasives.
In industries, the impurity phases in the SiC particles mainly exist in three forms: (1) the impurity phases exist around the SiC; (2) the impurity phases are embedded in the edges of the SiC particles; and (3) the impurity phases are completely wrapped in the SiC particles. The brittleness of the impurity phases, e.g., Fe-silicides and free-Si0, is poor, and the SiC crystals often undergo intergranular fracture during crushing. Thus, after the SiC crystals are crushed, some impurity phases exist around the SiC particles, some impurity phases are embedded in the edges of the SiC particles, and a small portion of impurity ions is doped into the crystal lattice of the SiC particles [65].
4.3. Distinguishing between Natural and Artificial SiC
Bauer et al. [6] and Xu et al. [18] reported that natural and synthetic SiC have similar morphological, physical, and chemical properties. Several of the most commonly used methods have been proposed to differentiate the two. The feasibility of each of these methods is discussed below.
4.3.1. Trace Element Chemistry
Shiryaev et al. [4,5] proposed that trace element chemistry and the nature of inclusions can provide a reliable basis for distinguishing between natural and synthetic SiC. They argued that the most plausible explanation for chemical differences between natural and synthetic SiC lies in the composition and abundance of submicroscopic accessory phases. Owing to the limited capacity of the SiC lattice to accommodate atoms substituting Si (or C), such as Al, N, and B, most other trace elements tend to be preferentially allocated to the inclusions rather than within the crystalline lattice of the SiC. Hence, most natural SiC grains exhibit low trace element contents (1–200 ppm), whereas the majority of trace elements analysed in the natural SiC probably reside in the inclusions of the SiC [4,5,17]. However, our results reveal that most of the synthetic host SiC was relatively pure. Diverse trace elements, including Mn, Cr, Ni, Ti, Al, Ca, and F, as well as a number of potential trace elements that we did not detect in this work, are predominantly located within the inclusions, as with those found in the ‘natural’ SiC inclusions in terrestrial rocks. Consequently, distinguishing between natural and synthetic SiC using only trace elements is challenging.
4.3.2. Inclusions
Numerous studies have pointed out that the most indisputable evidence for distinguishing synthetic from natural SiC is the presence of SRP inclusions, such as Si0 and multicomponent silicides in natural SiC, which have not previously been reported in industrial/synthetic SiC [1,5,7,14,17,19,31]. However, this study provides sufficient experimental results to show that the characteristics of SRP inclusions (especially the most commonly reported Fe(Ni)-silicides and Si0) in natural and synthetic SiC are extremely similar. Thus, it may not be feasible to identify natural SiC using these common SRP inclusions described above.
It should be additionally mentioned, however, that Ti0 and Ca-, Al-, Ti-, or Zr-rich SRP inclusions (e.g., Si-Fe-Al-Ca, Si-Fe-Ti, Si2(Fe,Al,Ca)3, Si3(Fe,Al)2, Si2Ca, and Fe-Ti-Zr silicides), which are currently reported in a few moissanites [1,4,5,7] could potentially serve as distinguishing factors, as inferred from the fO2 necessary for Ca, Al, Ti, and Zr to reduce to their metallic states. For Ti, fO2 > 5 orders of magnitude lower than SiC + O2 = C + SiO2 (i) and SiC + Mg2SiO4 + O2 = C + 2MgSiO3 (ii) reactions calculated at 1 bar is required, whereas three further orders of magnitude are necessary to reduce Zr to its metallic state. The fO2 of Al is close to that of Ti, while Ca is the most difficult to reduce [66]. The reactions (i) and (ii) are already 5–6 log units below the IW buffer. Therefore, it is theoretically challenging to produce these SRP inclusions rich in Ca, Al, Ti, and Zr. Additionally, various silicate inclusions (e.g., Mg-Fe silicate and Ca-Al-silicate) have been found in some moissanites [1,46]. As predicted by thermodynamics [67], these oxidised silicates would not be stable under Achison conditions for artificial SiC production, since the corresponding components would be reduced to their metallic states. Therefore, Ca-, Al-, Ti-, or Zr-rich SRP inclusions and some silicate inclusions could be potential evidence to differentiate natural from synthetic SiC. However, due to the limited number of the samples analysed, further validation of its reliability is required.
4.3.3. Raman Spectroscopy of Polytypes
According to previous studies, numerous polytypes of synthetic SiC exist, the most common polytypes being 4H, 6H (hexagonal), 15R (rhombohedral), and 3C (cubic) [5]. Limited polytypes have been identified in moissanite, principally 6H and 15R, but very few 3C and 2H (hexagonal) [3,5,10,68]. 3C and 2H moissanite have been found in meteorites but rarely in terrestrial rocks [68]. The 6H, 4H, and 15R polytypes of moissanite have been reported in kimberlites, ophiolites, and volcanic rocks [5,7,19]. The Raman spectroscopy results for the SiC abrasives indicate that the common polytypes of SiC in natural materials (e.g., 6H and 15R) are also prevalent in most SiC abrasives (Figure 7). This implies that its origin cannot be effectively distinguished by the Raman spectroscopy of polytypes.
4.3.4. Biaxiality, Structural Defects, and Degree of Order
According to Xu et al. [18], “The biaxiality can be used to distinguish moissanitefrom synthetic carbide as well, because the biaxiality of moissanite occurs only when it is plastically deformed”. However, our Raman spectroscopy results show that the Raman spectra of some synthetic SiC are very similar to those of biaxial moissanite (see Section 3.2.3), suggesting that the optical biaxiality may also not be effective enough in distinguishing between synthetic and natural SiC. Fortunately, according to previous studies, e.g., [69], it may be possible to differentiate natural from synthetic SiC through the structural defects and degree of order determined by TEM and XRDT. The natural moissanite shows no evidence of any disorder, while the synthetic sample is relatively more disordered; and the synthetic SiC shows a high density of (001) stacking faults, which is not observed in the natural sample. Perhaps this method needs further validation as well; at least for now, it is executable.
In summary, to the best of our knowledge, distinguishing between natural and synthetic SiC requires a comprehensive consideration of their characteristics, including occurrence; Ca-, Al-, Ti- or Zr-rich SRP inclusions or silicate inclusions; mineral contact relationships and the formation conditions of coexisting minerals; structural defects and degree of order; and anomalous isotopic composition (for interstellar SiC). In rock or meteorite thin sections, exotic SiC grains and other contaminants are irregularly distributed as scattered or densely packed fragments within the interstices of the rock or fill surface fractures/cavities of minerals without being intricately intergrown with the host rock or adjacent primary minerals [40,70]. Conversely, natural moissanite crystals are typically enclosed completely inside a rigid host mineral or in close contact with adjacent minerals or the matrix, and alter the host and surrounding coexisting minerals, thereby resulting in morphological changes at the rims of the adjacent minerals [40,61,70]. Additionally, considering whether a correlation exists between the formation conditions of SiC and those of coexisting minerals, including the possibility of extreme formation conditions, is essential. The coexistence of other ultra-high-temperature/high-pressure phases may also provide robust evidence. Stable isotope geochemistry may also provide some useful evidence of possible relations with the host rocks or extraterrestrial materials, such as the SiC of interstellar origin can supported by the anomalous isotopic composition of C, Ne, Xe, Si, and N [42,43,44,45]. Furthermore, it is necessary to consider whether reasonable clues indicate the sources of Si, C, and SRP inclusions in the samples. Notably, the artificial contamination of SiC and silicides is not only observed for SiC abrasives but also for alumina abrasives [61,71] and synthetic diamonds [61,72].
5. Conclusions
The presence of SRP inclusions within SiC, including metal silicides (e.g., FeSi, FeSi2, and Fe3Si7) and native metals (e.g., Si0 and Fe0), has been inferred as evidence for natural SiC. The aim of this study was to demonstrate the fallacy of this assumption. The unusual SRP assemblage of SiC, (Fe,Ni)Si2 and Si0 observed in the Jingshan chondrite (J-1) indicates that anthropogenic contaminants may have been produced by the conventional sample processing methods. The analysis of artificial green SiC abrasives further demonstrates the similarities between synthetic and natural SiC in terms of their morphology, inclusions, and polytypes. The inclusions in the SiC abrasives observed in this study include Si0, various Fe(Ni)-silicides, Fe-Mn-Cr alloys, and even the LREE-enriched SiO phase. The results provide compelling evidence for SRP inclusions in artificial SiC, previously believed to exist exclusively in natural SiC. Hence, neither SRP inclusions (with a low content of Ca, Al, Ti, and Zr) nor morphological, biaxial, or polymorphic (determined by Raman spectroscopy) features can be used to distinguish between natural and artificial SiC. Multiple types of SiC abrasives produced by different methods and other forms of abrasives (e.g., alumina and diamond abrasives), especially the diversity of inclusions within, may warrant further investigation to gain insights into the possible sources of SRP contamination.
Author Contributions
Conceptualization, S.Q. and Y.M.; methodology, Y.M., M.C. and S.Q.; investigation, data curation, and formal analysis, Y.M.; writing—original draft preparation, Y.M. and M.M.; writing—review and editing, M.C., S.Q., Y.M. and M.M.; project administration and funding acquisition, S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the National Natural Science Foundation of China, grant number 42072047.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Zuokai Ke for providing the Jingshan chondrite samples for the experiments, and three anonymous reviewers for their constructive comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mathez, E.A.; Fogel, R.A.; Hutcheon, I.D.; Marshintsev, V.K. Carbon isotopic composition and origin of SiC from kimberlites of Yakutia, Russia. Geochim. Cosmochim. Acta 1995, 59, 781–791. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Gao, C.G.; Golubkova, A.; Rohrbach, A.; Connolly, J.A. Natural moissanite (SiC)—A low temperature mineral formed from highly fractionated ultra-reducing COH-fluids. Prog. Earth Planet. Sci. 2014, 1, 27. [Google Scholar] [CrossRef]
- Marshintsev, V.K. Natural silicon carbide in Yakutian kimberlites. Mineral. Zhurnal 1990, 12, 17–26. [Google Scholar]
- Shiryaev, A.A.; Griffin, W.L.; Stoyanov, E.; Kagi, H. Natural silicon carbide from different geological settings: Polytypes, trace elements, inclusions. In Proceedings of the 9th International Kimberlite Conference: Extended Abstracts, Frankfurt, Germany, 15 October 2008; Volume 9. [Google Scholar]
- Shiryaev, A.A.; Griffin, W.L.; Stoyanov, E. Moissanite (SiC) from kimberlites: Polytypes, trace elements, inclusions and speculations on origin. Lithos 2011, 122, 152–164. [Google Scholar] [CrossRef]
- Bauer, J.; Fiala, J.; Hřichová, R. Natural α-silicon carbide. Am. Mineral. 1963, 48, 620–634. [Google Scholar]
- Di Pierro, S.; Gnos, E.; Grobety, B.H.; Armbruster, T.; Bernasconi, S.M.; Ulmer, P. Rock-forming moissanite (natural α-silicon carbide). Am. Mineral. 2003, 88, 1817–1821. [Google Scholar] [CrossRef]
- Moore, R.O.; Otter, M.L.; Rickard, R.S.; Harris, J.W.; Gurney, J.J. The occurrence of moissanite and ferro-periclase as inclusions in diamond. In Proceedings of the 4th International Kimberlite Conference: Extended Abstracts, Perth, Australia, 11–15 August 1986; pp. 409–411. [Google Scholar]
- Moore, R.O.; Gurney, J.J. Mineral inclusions in diamond from the Monastery kimberlite, South Africa. In Kimberlites and Related Rocks; Ross, J., Ed.; Blackwell Science Publication: Carlton, Australia, 1989; Volume 14, pp. 1029–1041. [Google Scholar]
- Leung, I.S. Silicon carbide cluster entrapped in a diamond from Fuxian, China. Am. Mineral. 1990, 75, 1110–1119. [Google Scholar]
- Lian, D.Y.; Yang, J.S.; Dilek, Y.; Wu, W.W.; Zhang, Z.M.; Xiong, F.H.; Liu, F.; Zhou, W.D. Deep mantle origin and ultra-reducing conditions in podiform chromitite: Diamond, moissanite, and other unusual minerals in podiform chromitites from the Pozanti-Karsanti ophiolite, southern Turkey. Am. Mineral. 2017, 102, 1101–1113. [Google Scholar] [CrossRef]
- Gnoevaja, N.; Grozdanov, L. Moissanite from Triassic rocks, NW Bulgaria. Proc. Bulg. Geol. Soc. 1965, 26, 89–95. [Google Scholar]
- Lyakhovich, V.V. Origin of accessory moissanite. Int. Geol. Rev. 1980, 22, 961–970. [Google Scholar] [CrossRef]
- Trumbull, R.B.; Yang, J.; Robinson, P.T.; Di Pierro, S.; Vennemann, T.; Wiedenbeck, M. The carbon isotope composition of natural SiC (moissanite) from the Earth’s mantle: New discoveries from ophiolites. Lithos 2009, 113, 612–620. [Google Scholar] [CrossRef]
- Yang, J.S.; Meng, F.C.; Xu, X.Z.; Robinson, P.T.; Dilek, Y.; Makeyev, A.B.; Wirth, R.; Wiedenbeck, M.; Cliff, J. Diamonds, native elements and metal alloys from chromitites of the Ray-Iz ophiolite of the Polar Urals. Gondwana Res. 2015, 27, 459–485. [Google Scholar] [CrossRef]
- Pujol-Solà, N.; Proenza, J.A.; Garcia-Casco, A.; González-Jiménez, J.M.; Andreazini, A.; Melgarejo, J.C.; Gervilla, F. An alternative scenario on the origin of ultra-high pressure (UHP) and super-reduced (SuR) minerals in ophiolitic chromitites: A case study from the Mercedita deposit (Eastern Cuba). Minerals 2018, 8, 433. [Google Scholar] [CrossRef]
- Nazzareni, S.; Nestola, F.; Zanon, V.; Bindi, L.; Scricciolo, E.; Petrelli, M.; Zanatta, M.; Mariotto, G.; Giuli, G. Discovery of moissanite in a peralkaline syenite from the Azores Islands. Lithos 2019, 324, 68–73. [Google Scholar] [CrossRef]
- Xu, S.T.; Wu, W.P.; Xiao, W.S.; Yang, J.S.; Chen, J.; Ji, S.Y.; Liu, Y.C. Moissanite in serpentinite from the Dabie Mountains in China. Mineral. Mag. 2008, 72, 899–908. [Google Scholar] [CrossRef]
- Dobrzhinetskaya, L.; Mukhin, P.; Wang, Q.; Wirth, R.; O’Bannon, E.; Zhao, W.; Eppelbaum, L.; Sokhonchuk, T. Moissanite (SiC) with metal-silicide and silicon inclusions from tuff of Israel: Raman spectroscopy and electron microscope studies. Lithos 2018, 310, 355–368. [Google Scholar] [CrossRef]
- Robinson, P.T.; Bai, W.J.; Malpas, J.; Yang, J.S.; Zhou, M.F.; Fang, Q.S.; Hu, X.F.; Cameron, S.; Staudigel, H. Ultra-high pressure minerals in the Loubusa Ophiolite, Tibet, and their tectonic implications. Geol. Soc. Lond. Spec. Publ. 2004, 226, 247–271. [Google Scholar] [CrossRef]
- Bai, W.J.; Shi, N.C.; Fang, Q.S.; Li, G.W.; Yang, J.S.; Xiong, M.; Rong, H. Ruobusaite—A new mineral. Acta Geol. Sin. 2006, 80, 1487–1490. (In Chinese) [Google Scholar]
- Li, G.W.; Fang, Q.S.; Shi, N.C.; Bai, W.J.; Yang, J.S.; Xiong, M.; Ma, Z.S.; Rong, H. Zangboite, TiFeSi2, a new mineral species from Luobusha, Tibet, China, and its crystal structure. Can. Mineral. 2009, 47, 1265–1274. [Google Scholar] [CrossRef]
- Li, G.W.; Bai, W.J.; Shi, N.C.; Fang, Q.S.; Xiong, M.; Yang, J.S.; Ma, Z.S.; Rong, H. Linzhiite, FeSi2, a redefined and revalidated new mineral species from Luobusha, Tibet, China. Eur. J. Mineral. 2012, 24, 1047–1052. [Google Scholar] [CrossRef]
- Shi, N.C.; Bai, W.J.; Li, G.W.; Xiong, M.; Yang, J.S.; Ma, Z.S.; Rong, H. Naquite, FeSi, a new mineral species from Luobusha, Tibet, western China. Acta Geol. Sin.-Engl. 2012, 86, 533–538. [Google Scholar] [CrossRef]
- Wai, C.M. The metal phase of Horse Creek, Mount Egerton, and Norton County enstatitic meteorites. Mineral. Mag. 1970, 37, 905–908. [Google Scholar] [CrossRef]
- Keil, K.; Berkley, J.L.; Fuchs, L.H. Suessite, Fe3Si: A new mineral in the North Haig ureilite. Am. Mineral. 1982, 67, 126–131. [Google Scholar]
- Yu, Z.X. Two new minerals gupeiite and xifengite in cosmic dusts from Yanshan. Acta Petrol. Mineral. Anal. 1984, 3, 231–237. (In Chinese) [Google Scholar]
- Anand, M.; Taylor, L.A.; Nazarov, M.A.; Shu, J.; Mao, H.; Hemley, R.J. Space weathering on airless planetary bodies: Clues from the lunar mineral hapkeite. Proc. Natl. Acad. Sci. USA 2004, 101, 6847–6851. [Google Scholar] [CrossRef]
- Garvie, L.A.; Ma, C.; Ray, S.; Domanik, K.; Wittmann, A.; Wadhwa, M. Carletonmooreite, Ni3Si, a new silicide from the Norton County aubrite meteorite. Am. Mineral. 2021, 106, 1828–1834. [Google Scholar] [CrossRef]
- Gromilov, S.A.; Afanasiev, V.P.; Pokhilenko, N.P. Moissanites of the Popigai astrobleme. Dokl. Earth Sci. 2018, 481, 997–999. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Yang, J.S.; Ernst, W.G.; Jahn, B.M.; Iizuka, Y.; Guo, G.L. Discovery of in situ super-reducing, ultrahigh-pressure phases in the Luobusa ophiolitic chromitites, Tibet: New insights into the deep upper mantle and mantle transition zone. Am. Mineral. 2016, 101, 1285–1294. [Google Scholar] [CrossRef]
- Qi, X.X.; Yang, J.S.; Xu, Z.Q.; Bai, W.J.; Zhang, Z.M.; Fang, Q.S. Discovery of moissanite in retrogressive eclogite from the Pre-pilot Hole of the Chinese Continental Scientific Drilling Project (CCSD-PP2) and its geological implication. Acta Petrol. Sin. 2007, 23, 3207–3214. [Google Scholar]
- Golovko, A.V.; Kaminsky, F.V. The shoshonite-absarokite-picrite Karashoho Pipe, Uzbekistan: An unusual diamond deposit in an atypical tectonic environment. Econ. Geol. 2010, 105, 825–840. [Google Scholar] [CrossRef]
- Perraki, M.; Faryad, S.W. First finding of microdiamond, coesite and other UHP phases in felsic granulites in the Moldanubian Zone: Implications for deep subduction and a revised geodynamic model for Variscan Orogeny in the Bohemian Massif. Lithos 2014, 202–203, 157–166. [Google Scholar] [CrossRef]
- Robinson, P.T.; Trumbull, R.B.; Schmitt, A.; Yang, J.S.; Li, W.J.; Zhou, M.F.; Erzinger, J.; Dare, S.; Xiong, F.H. The origin and significance of crustal minerals in ophiolitic chromitites and peridotites. Gondwana Res. 2015, 27, 486–506. [Google Scholar] [CrossRef]
- Machev, P.; O’Bannon, E.F.; Bozhilov, K.N.; Wang, Q.; Dobrzhinetskaya, L. Not all moissanites are created equal: New constraints on moissanite from metamorphic rocks of Bulgaria. Earth Planet. Sci. Lett. 2018, 498, 387–396. [Google Scholar] [CrossRef]
- Litasov, K.D.; Kagi, H.; Bekker, T.B.; Hirata, T.; Makino, Y. Cuboctahedral type Ib diamonds in ophiolitic chromitites and peridotites: The evidence for anthropogenic contamination. High Press. Res. 2019, 39, 480–488. [Google Scholar] [CrossRef]
- Ballhaus, C.; Helmy, H.M.; Fonseca, R.O.C.; Wirth, R.; Schreiber, A.; Jöns, N. Ultra-reduced phases in ophiolites cannot come from Earth’s mantle. Am. Mineral. 2021, 106, 1053–1063. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Igarashi, M.; Tsai, C.; Iwasaki, I.; Liou, J.G. Possibility of the misidentification of contaminated microdiamonds in UHP metamorphic rocks: An example of diamond grains in Dabie garnet clinopyroxenite. In Proceedings of the American Geophysical Union Fall Meeting Abstracts, San Francisco, CA, USA, 5–9 December 2011; pp. V23E–2609. [Google Scholar]
- Nasdala, L.; Steger, S.; Reissner, C. Raman study of diamond-based abrasives, and possible artefacts in detecting UHP microdiamond. Lithos 2016, 265, 317–327. [Google Scholar] [CrossRef]
- Menneken, M.; Geisler, T.; Nemchin, A.A.; Pollok, K.; Whitehouse, M.; Pidgeon, R.; Wilde, S. Is there really carbon in the detrital zircons from Jack Hills, Western Australia? In Proceedings of the European Geosciences Union General Assembly Conference Abstracts, Vienna, Austria, 27 April–2 May 2014; p. 13489. [Google Scholar]
- Bernatowicz, T.; Fraundorf, G.; Ming, T.; Anders, E.; Wopenka, B.; Zinner, E.; Fraundorf, P. Evidence for interstellar SiC in the Murray carbonaceous meteorite. Nature 1987, 330, 728–730. [Google Scholar] [CrossRef]
- Zinner, E.; Ming, T.; Anders, E. Interstellar SiC in the Murchison and Murray meteorites: Isotopic composition of Ne, Xe, Si, C, and N. Geochim. Cosmochim. Acta 1989, 53, 3273–3290. [Google Scholar] [CrossRef]
- Alexander, C.M.O.’D.; Swan, P.; Walker, R.M. In situ measurement of interstellar silicon carbide in two CM chondrite meteorites. Nature 1990, 348, 715–717. [Google Scholar] [CrossRef]
- Stone, J.; Hutcheon, I.D.; Epstein, S.; Wasserburg, G.J. Correlated Si isotope anomalies and large13C enrichments in a family of exotic SiC grains. Earth Planet. Sci. Lett. 1991, 107, 570–581. [Google Scholar] [CrossRef]
- Di Pierro, S.; Gnos, E. Ca-Al-silicate inclusions in natural moissanite (SiC). Am. Mineral. 2016, 101, 71–81. [Google Scholar] [CrossRef]
- Bouvier, A.; Gattacceca, J.; Agee, C.; Grossman, J.; Metzler, K. The Meteoritical Bulletin, No. 104. Meteorit. Planet. Sci. 2017, 52, 2284. [Google Scholar] [CrossRef]
- GB/T 2480−2008; Conventional Abrasive—Silicon Carbide. National Standard of the People’s Republic of China: Beijing, China, 2008. (In Chinese)
- Li, X.L.; Zhang, L.F.; Wei, C.J.; Slabunov, A.I.; Bader, T. Quartz and orthopyroxene exsolution lamellae in clinopyroxene and the metamorphic P-T path of Belomorian eclogites. J. Metamorph. Geol. 2018, 36, 1–22. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, C.Y.; Zhang, J.J.; Wang, J.M.; Zhong, D.L.; Wang, Y.; Lai, Q.Z.; Yue, Y.H.; Zhou, Q.Y. Midcrustal shearing and doming in a Cenozoic compressive setting along the Ailao Shan-Red River shear zone. Geochem. Geophys. Geosyst. 2017, 18, 400–433. [Google Scholar] [CrossRef]
- Maeda, Y.; Umezawa, K.; Hayashi, Y.; Miyake, K. Raman spectroscopic study of ion-beam synthesized polycrystalline β-FeSi2 on Si (100). Thin Solid Films 2001, 381, 219–224. [Google Scholar] [CrossRef]
- Li, F.; Lustig, N.; Klosowski, P.; Lannin, J.S. Disorder-induced Raman scattering in NiSi2. Phys. Rev. B 1990, 41, 10210. [Google Scholar] [CrossRef]
- Wittmann, A.; Burger, K.O.; Nowotny, H. Untersuchungen im Dreistoff: Ni-Al-Si sowie von Mono-und Disilicidsystemen einiger Übergangsmetalle. Monatsh. Chem. Verwandte Teile And. Wiss. 1962, 93, 674–680. [Google Scholar] [CrossRef]
- Langkau, S.; Wagner, G.; Kloess, G.; Heuer, M. TEM analysis of (Ni,Fe)Si2 precipitates in Si. Phys. Status Solidi A 2010, 207, 1832–1844. [Google Scholar] [CrossRef]
- Nakashima, S.; Harima, H. Raman investigation of SiC polytypes. Phys. Status Solidi A 1997, 162, 39–64. [Google Scholar] [CrossRef]
- Chikvaidze, G.; Mironova-Ulmane, N.; Plaude, A.; Sergeev, O. Investigation of silicon carbide polytypes by Raman spectroscopy. Latv. J. Phys. Tech. Sci. 2014, 51, 51–57. [Google Scholar] [CrossRef]
- Backhaus-Ricoult, M.; Mozdzierz, N.; Eveno, P. Impurities in silicon carbide ceramics and their role during high temperature creep. J. Phys. III 1993, 3, 2189–2210. [Google Scholar] [CrossRef]
- Bai, W.J.; Yang, J.S.; Fang, Q.S.; Yan, B.G.; Zhang, Z.M. Ultra-high pressure minerals: FeO, Fe, FeSi, Si and SiO2 assemblage from ophiolite in Tibet and its earth dynamic significance. Acta Geosci. Sin. 2002, 23, 395–402. (In Chinese) [Google Scholar]
- Ye, J.; Yue, X.Y.; Ru, H.Q.; Zhang, C.P. Progress in synthesis of high-purity submicron SiC powders by SiO2. Ceramics 2023, 450, 21–28. (In Chinese) [Google Scholar]
- Vix-Guterl, C.; Ehrburger, P. Effect of the properties of a carbon substrate on its reaction with silica for silicon carbide formation. Carbon 1997, 35, 1587–1592. [Google Scholar] [CrossRef]
- Litasov, K.D.; Kagi, H.; Bekker, T.B. Enigmatic super-reduced phases in corundum from natural rocks: Possible contamination from artificial abrasive materials or metallurgical slags. Lithos 2019, 340, 181–190. [Google Scholar] [CrossRef]
- Narciso-Romero, F.J.; Rodríguez-Reinoso, F.; Díez, M.A. Influence of the carbon material on the synthesis of silicon carbide. Carbon 1999, 37, 1771–1778. [Google Scholar] [CrossRef]
- Li, K.Z.; Wei, J.; Li, H.J.; Wang, C.; Jiao, G.S. Silicon assistant carbothermal reduction for SiC powders. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2008, 15, 484–488. [Google Scholar] [CrossRef]
- Ye, Z.Z.; Gupta, S.; Kerkkonen, O.; Kanniala, R.; Sahajwalla, V. SiC and ferro-silicides formation in tuyere cokes. ISIJ Int. 2013, 53, 181–183. [Google Scholar] [CrossRef][Green Version]
- Feng, D.; Qin, Z.B.; Ren, Q.X.; Sun, S.H.; Xia, Q.; Ru, H.Q.; Wang, W.; Ren, S.Y.; Zhang, C.P. Occurrence forms of major impurity elements in silicon carbide. Ceram. Int. 2022, 48, 205–211. [Google Scholar] [CrossRef]
- Ulmer, G.C.; Grandstaff, D.E.; Woermann, E.; Göbbels, M.; Schönitz, M.; Woodland, A.B. The redox stability of moissanite (SiC) compared with metal-metal oxide buffers at 1773 K and at pressures up to 90 kbar. Neues Jahrb. Mineral. Abh. 1998, 172, 279–307. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Telle, R. Purifying mechanism in the Acheson process-a thermodynamic study. Mater. Sci. Forum 2010, 645, 41–44. [Google Scholar] [CrossRef]
- Daulton, T.L.; Bernatowicz, T.J.; Lewis, R.S.; Messenger, S.; Stadermann, F.J.; Amari, S. Polytype distribution in circumstellar silicon carbide. Science 2002, 296, 1852–1855. [Google Scholar] [CrossRef]
- Tempesta, G.; Agrosì, G.; Capitani, G.C.; Scandale, E. XRDT and TEM study of defects and polytypism in natural moissanite and synthetic SiC crystals. Adv. Sci. Technol. 2006, 48, 61–66. [Google Scholar] [CrossRef]
- Dobrzhinetskaya, L.; Wirth, R.; Green, H. Diamonds in Earth’s oldest zircons from Jack Hills conglomerate, Australia, are contamination. Earth Planet. Sci. Lett. 2014, 387, 212–218. [Google Scholar] [CrossRef]
- Galuskin, E.; Galuskina, I. Evidence of the anthropogenic origin of the ‘Carmel sapphire’ with enigmatic super-reduced minerals. Mineral. Mag. 2023, 87, 619–630. [Google Scholar] [CrossRef]
- Yin, L.W.; Zou, Z.D.; Li, M.S.; Liu, Y.X.; Cui, J.J.; Hao, Z.Y. Characteristics of some inclusions contained in synthetic diamond single crystals. Mater. Sci. Eng. A 2000, 293, 107–111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).