Laser-Induced Breakdown Spectroscopy in Mineral Exploration and Ore Processing
Abstract
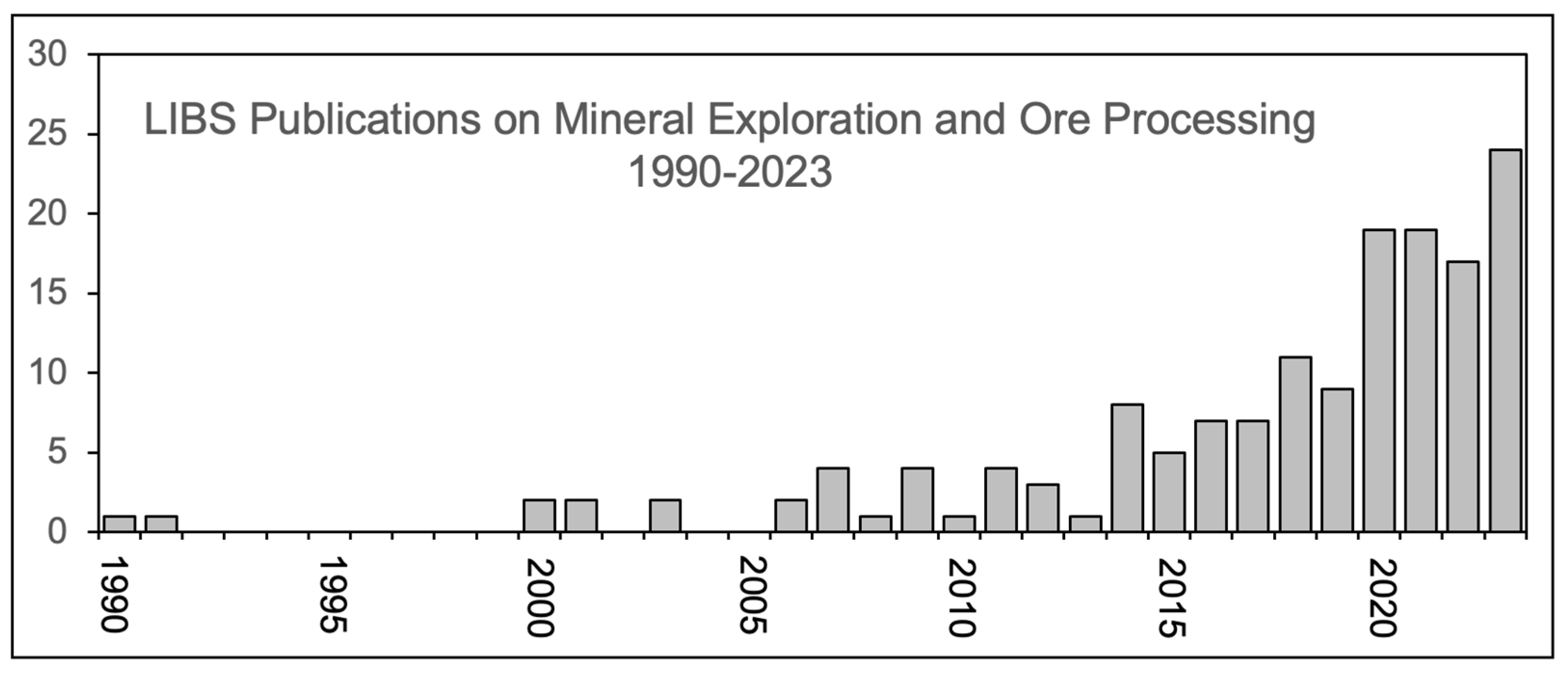
:1. Introduction
2. Laser-Induced Breakdown Spectroscopy
2.1. LIBS Instrumentation
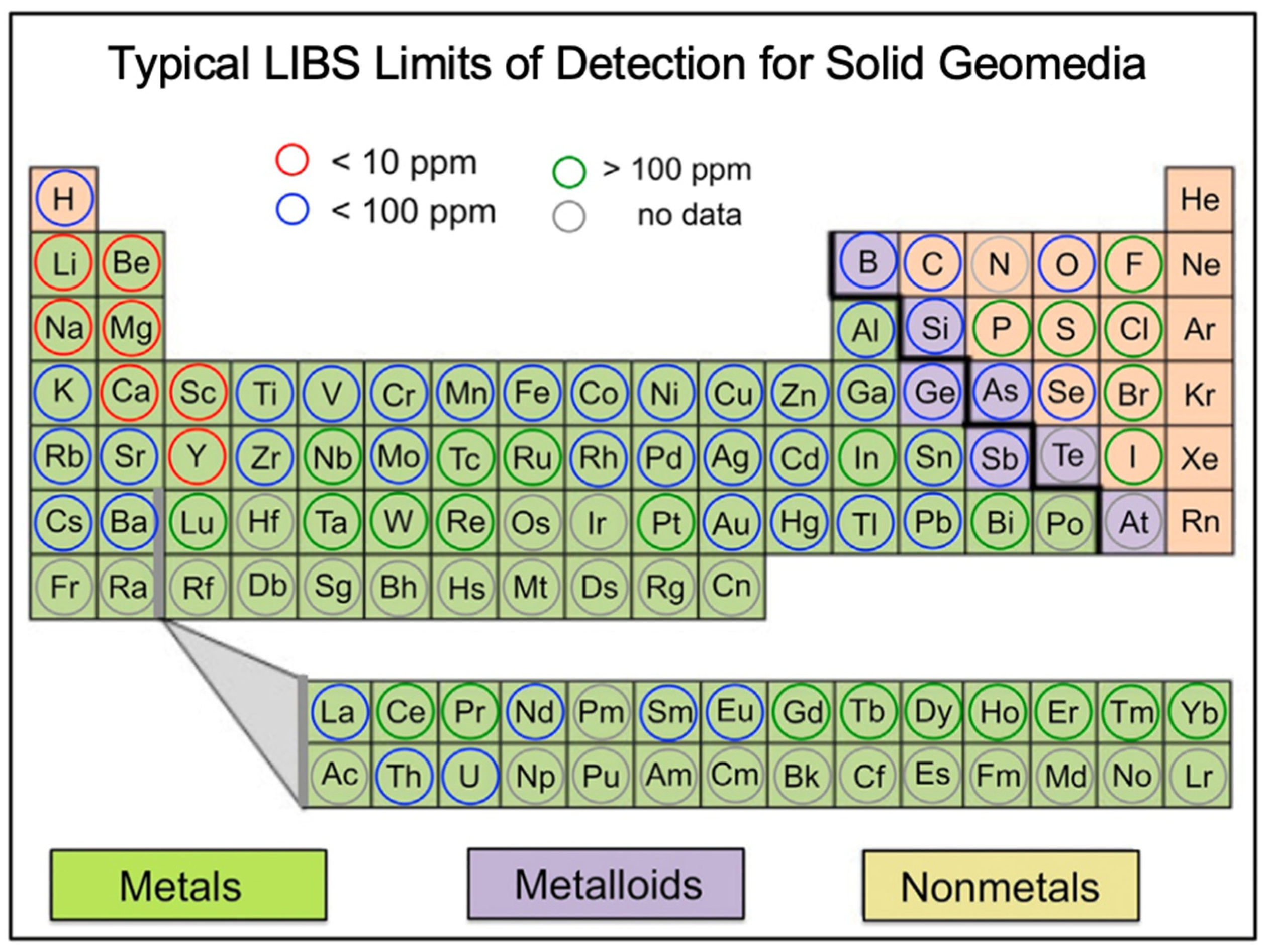
2.2. LIBS Attributes
2.3. Self-Absorption
2.4. Matrix Effects
2.5. Chemometrics in LIBS
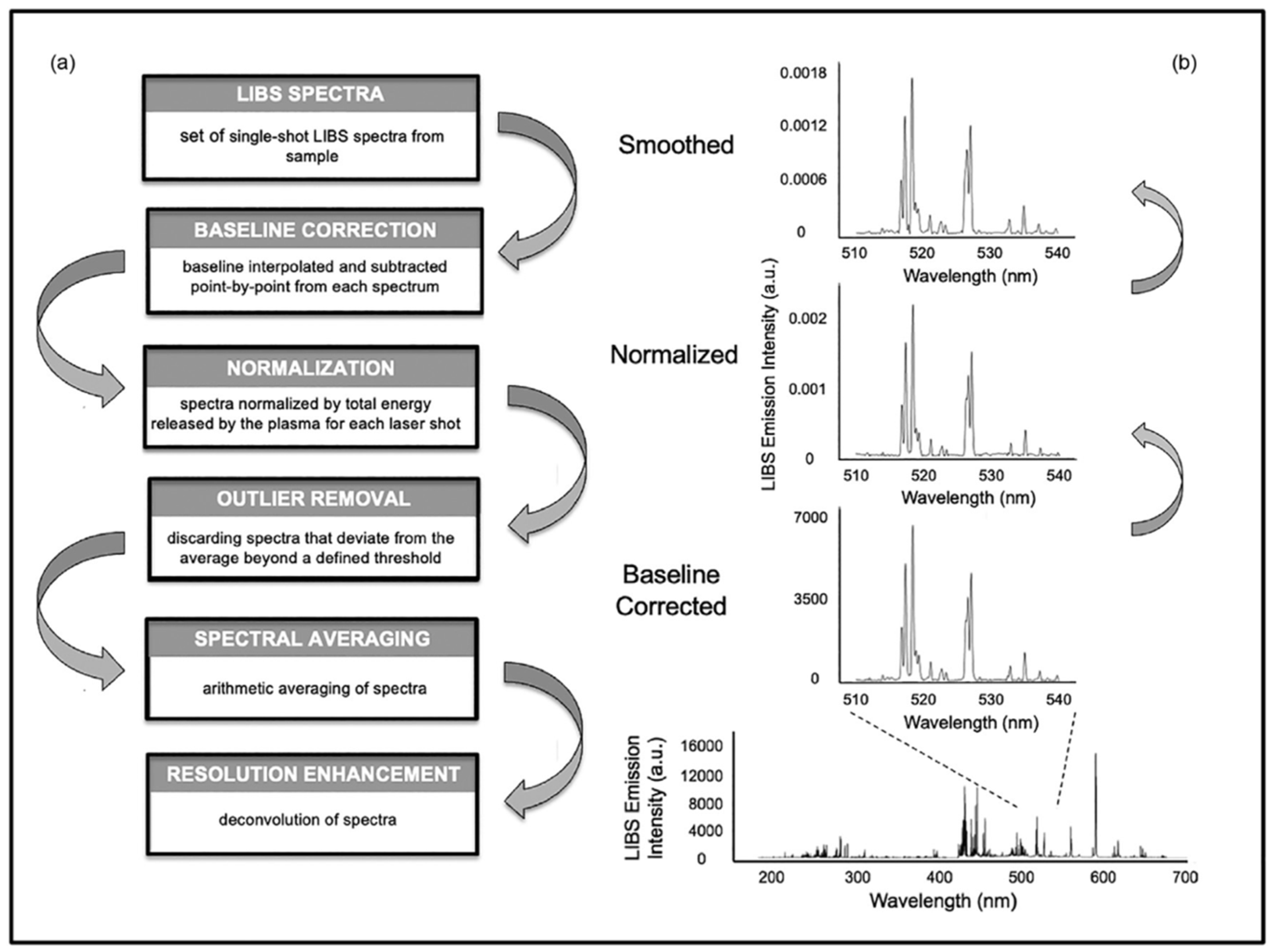
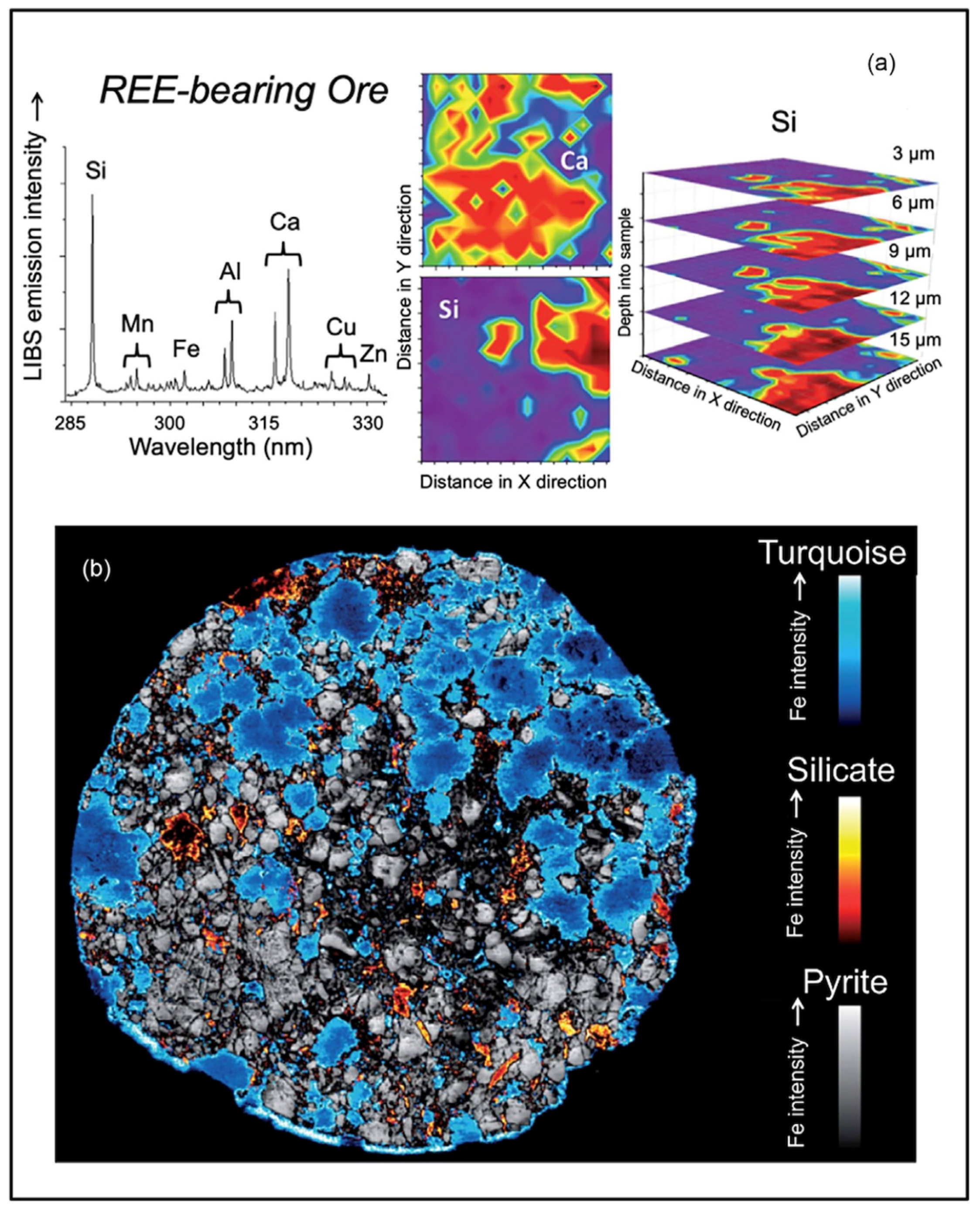
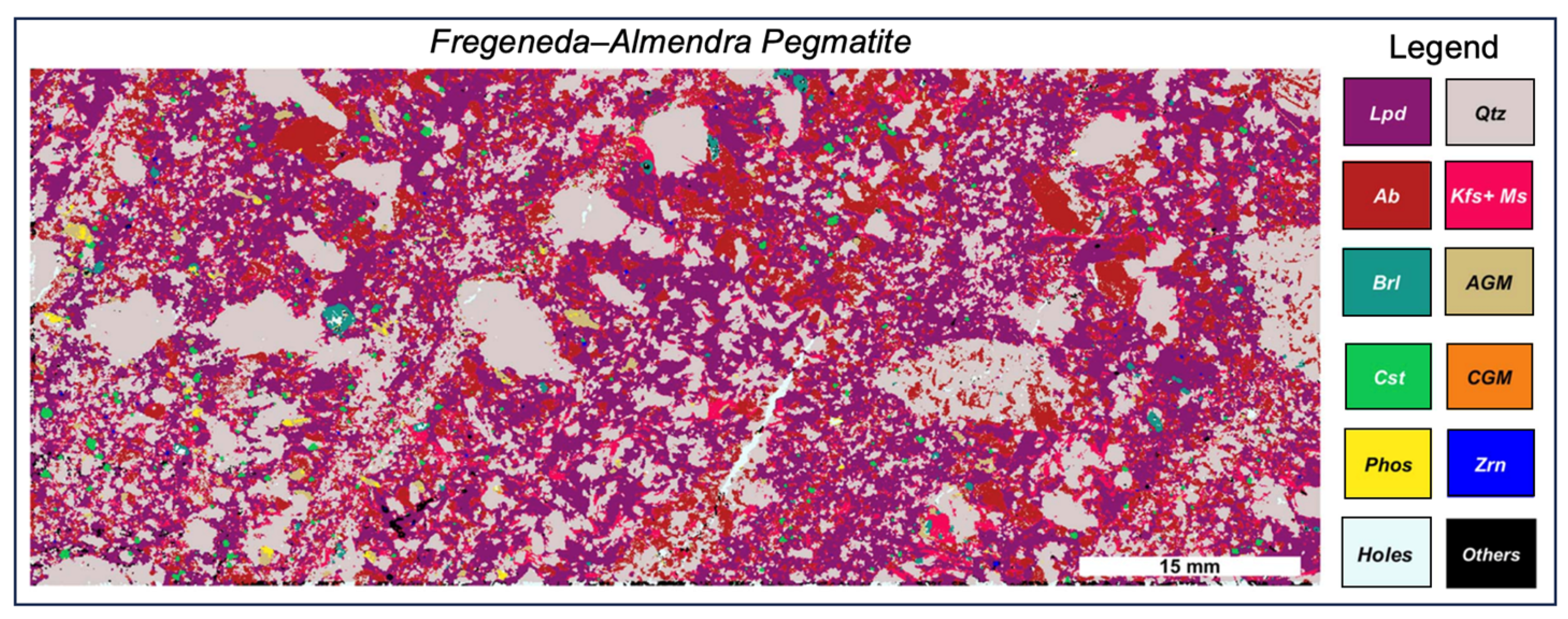
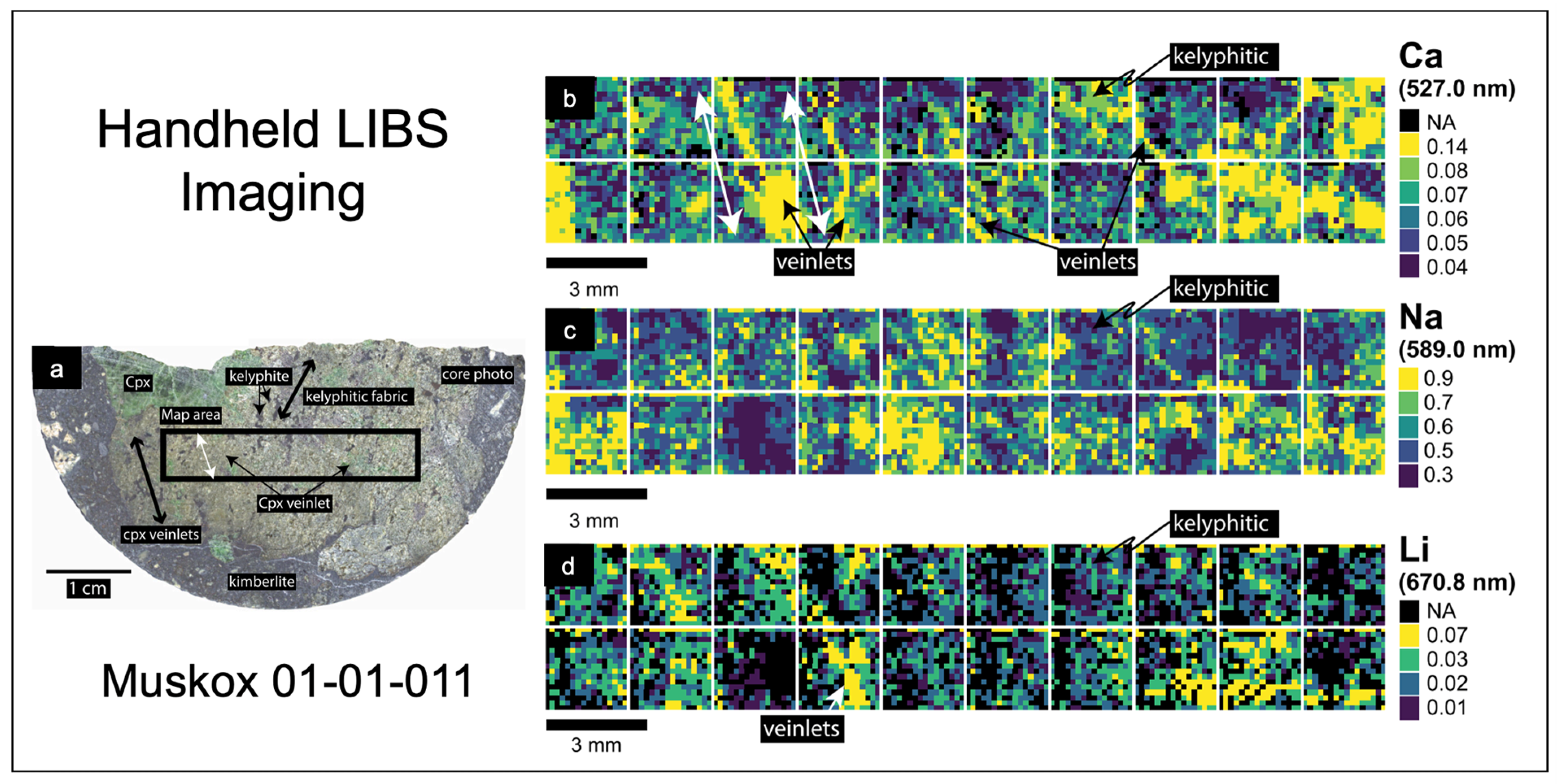
2.6. LIBS Imaging
2.7. Qualitative and Quantitative LIBS
3. LIBS in Mineral Exploration and Ore Processing
- (i)
- Detection—Is an element of interest present in this sample?
- (ii)
- Identification—What is this sample?
- (iii)
- Confirmation—Is this sample attribution correct?
- (iv)
- Classification—What assemblage does this sample belong to?
- (v)
- Quantification—What is the concentration an element in this sample?
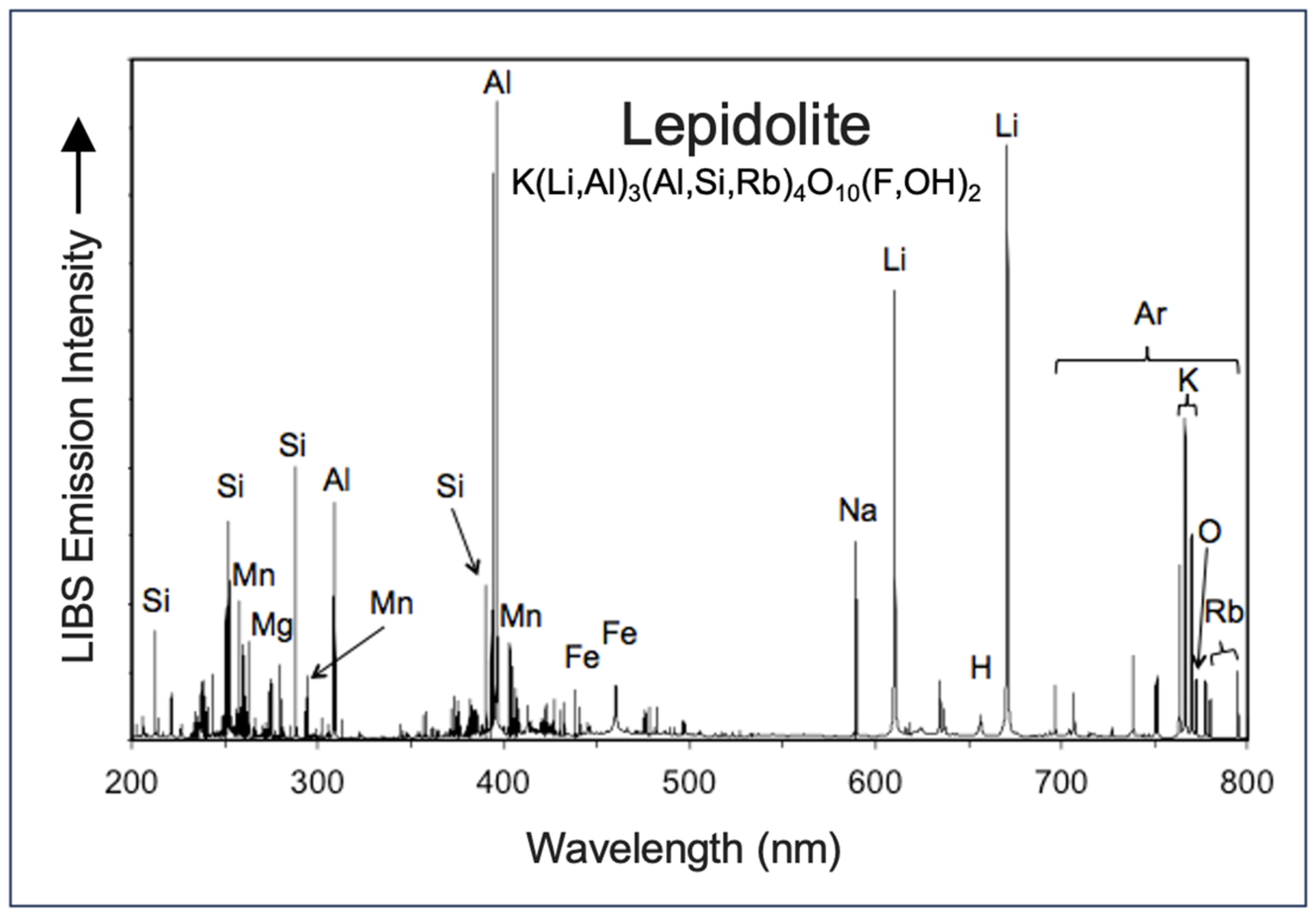
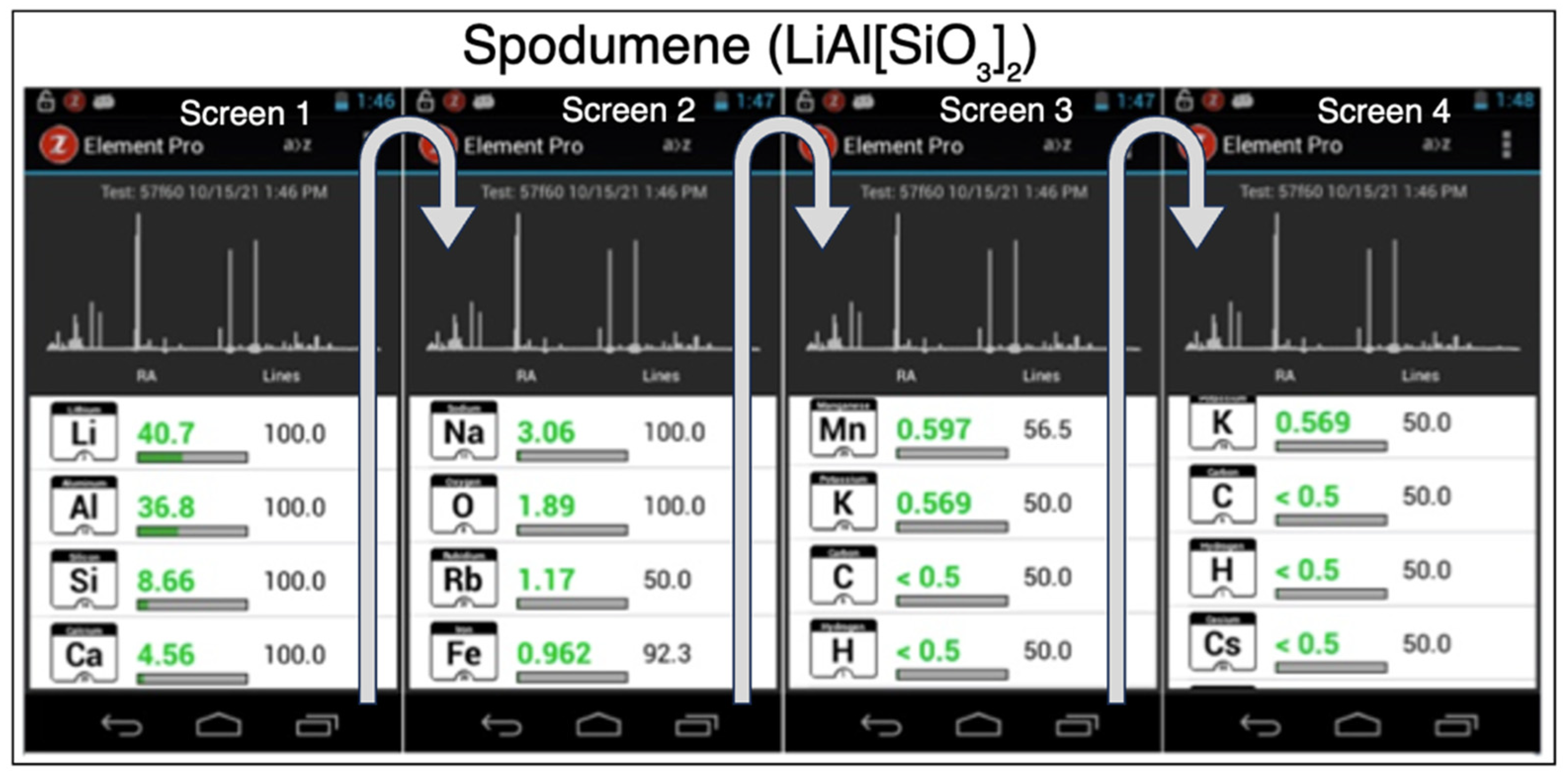
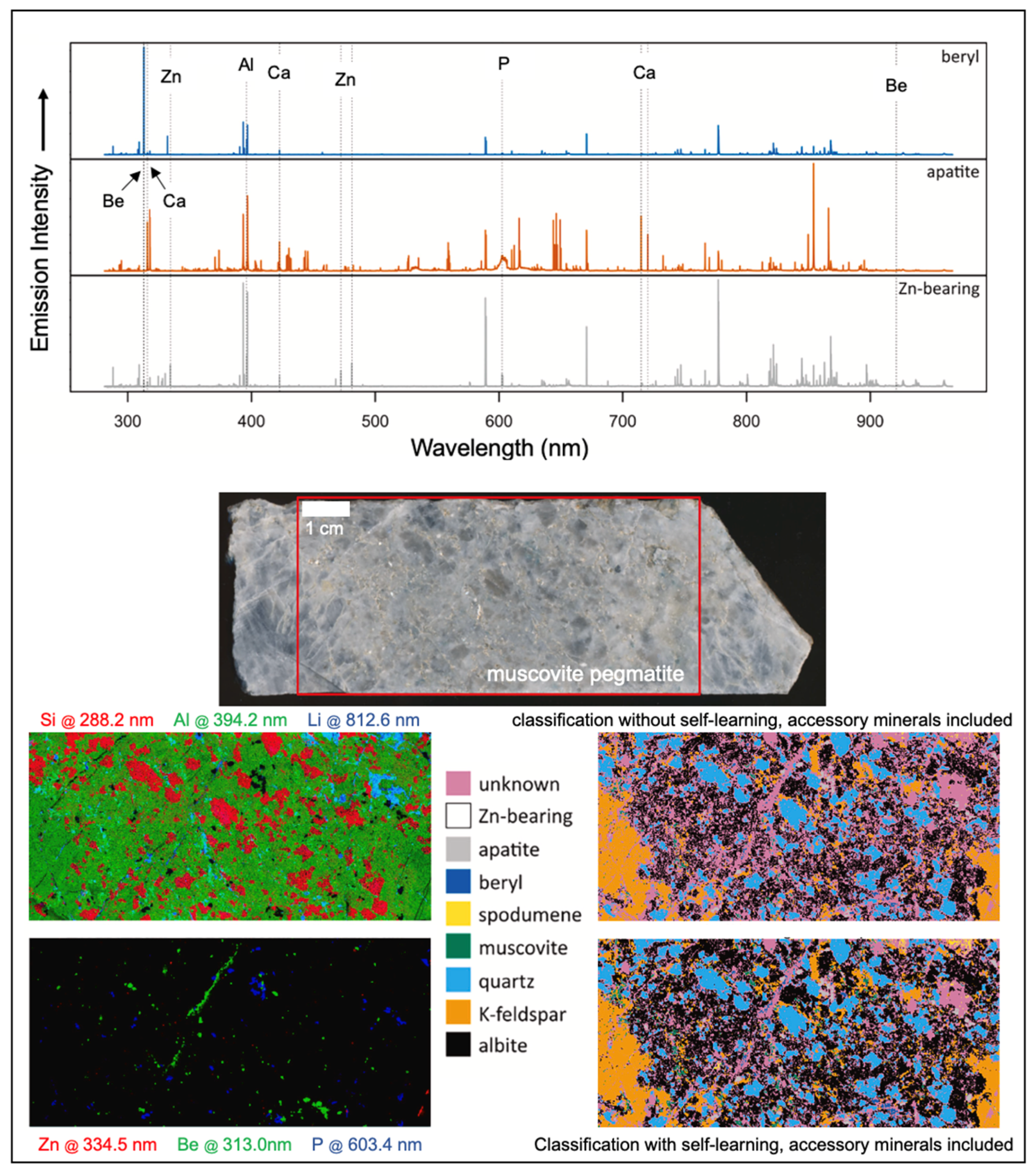
3.1. Lithium
3.2. Beryllium
3.3. Carbon
3.4. Fluorine
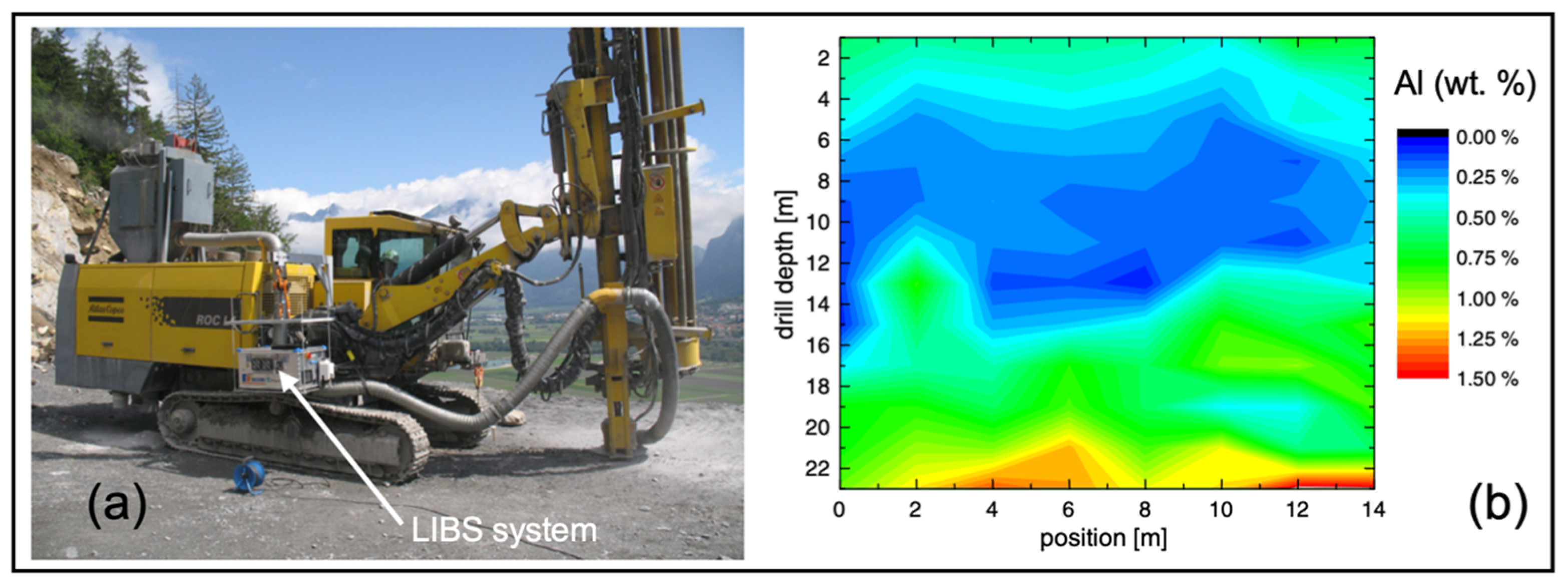
3.5. Aluminum, Phosphorus, Sulfur, and Calcium
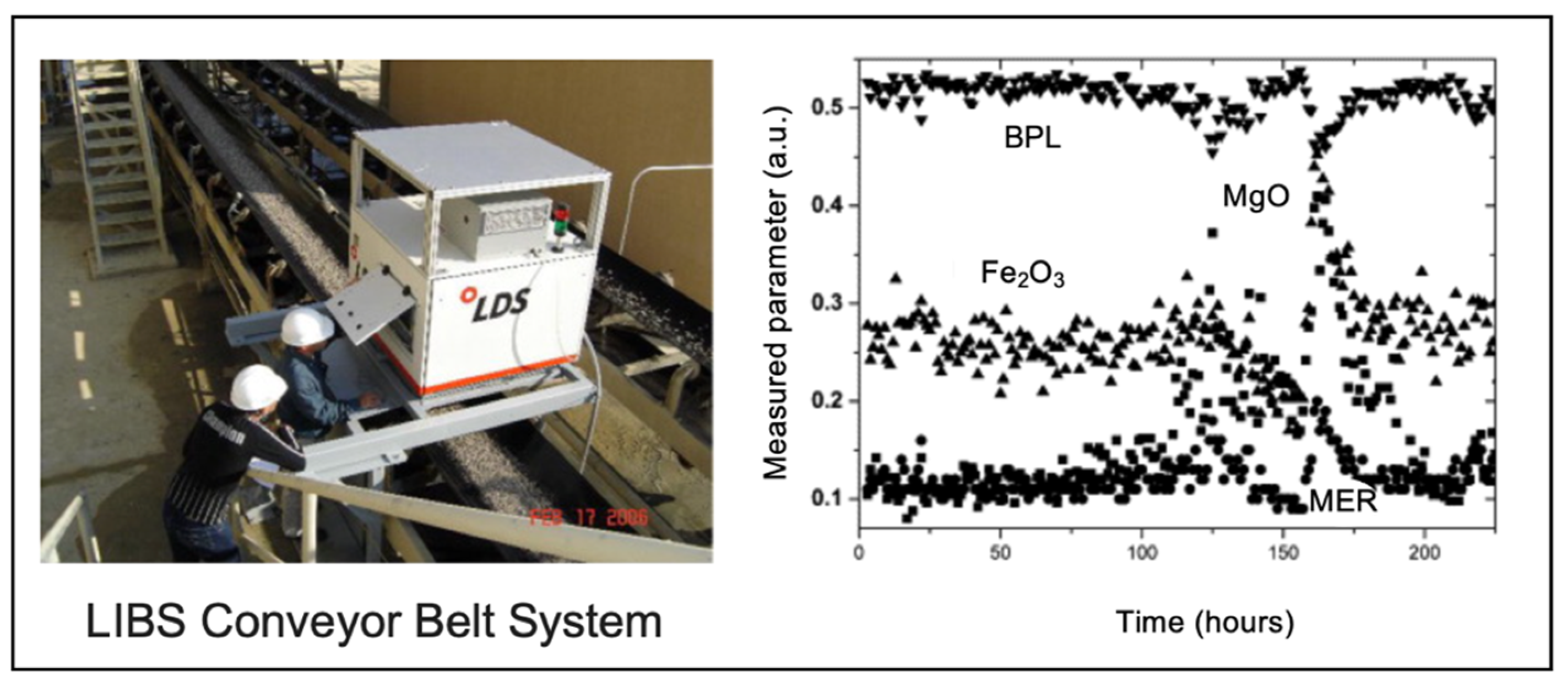
3.6. Transition Metals
3.7. Niobium and Tantalum
3.8. Platinum-Group Elements
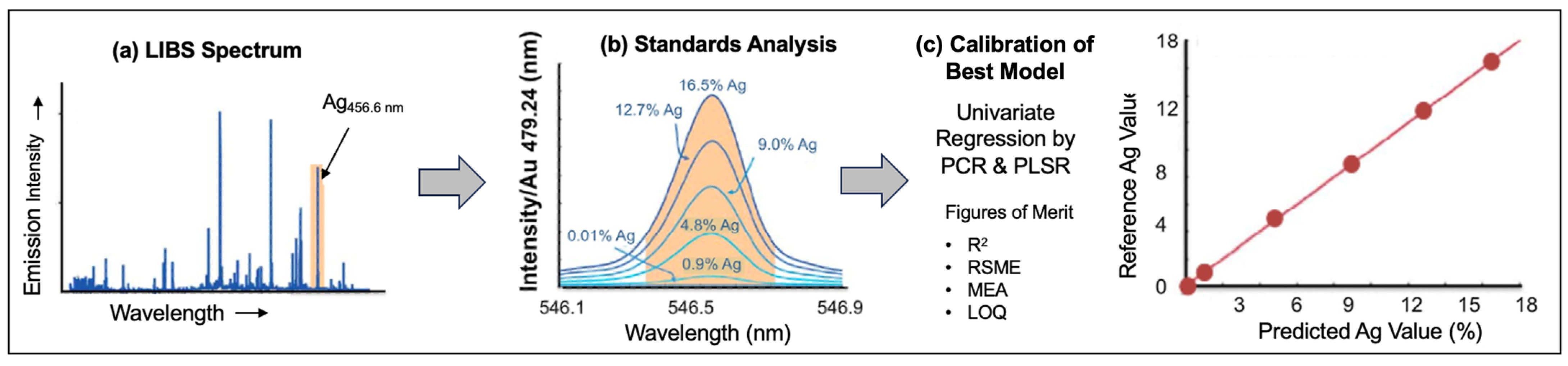
3.9. Precious Metals
3.10. Tin
3.11. Rare Earth Elements
3.12. Uranium
4. Tandem LIBS Methods
5. Summary and Future Prospects
Funding
Conflicts of Interest
References
- U.S. Geological Survey Releases 2022 List of Critical Minerals. Available online: https://www.usgs.gov/news/national-news-release/us-geological-survey-releases-2022-list-critical-minerals (accessed on 14 April 2024).
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Reck, B.K. On the materials basis of modern society. Proc. Natl. Acad. Sci. USA 2015, 112, 6295–6300. [Google Scholar] [CrossRef]
- Price, J.G.; Bickford, M.E. The challenges of mineral resources for society. In The Impact of the Geological Sciences on Society; Bickford, M.E., Ed.; Geological Society of America: Boulder, CO, USA, 2013; Special Paper 501; pp. 1–19. [Google Scholar]
- Ali, S.H.; Giurco, D.; Arndt, N.; Nickless, E.; Brown, G.; Demetriades, A.; Durrheim, R.; Enriquez, M.A.; Kinnaird, J.; Littleboy, A.; et al. Mineral supply for sustainable development requires resource governance. Nature 2017, 543, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Lusty, P.A.J.; Gunn, A.G. Challenges to global mineral resource security and options for future supply. In Ore Deposits in an Evolving Earth; Jenkin, G.R., Lusty, P.A., McDonald, I., Smith, M.P., Boyce, A.J., Wilkinson, J.J., Eds.; Geological Society of London: London, UK, 2016; Special Publication 393; pp. 65–276. [Google Scholar]
- Arndt, N.; Fontboté, L.; Hedenquist, J.; Kesler, S.; Thompson, J.; Wood, D. Future global mineral resources. Geochem. Perspect. 2017, 6, 171. [Google Scholar] [CrossRef]
- Harris, J.R.; Wilkinson, L.; Grunsky, E.C. Effective use and interpretation of lithogeochemical data in regional mineral exploration programs: Application of Geographic Information Systems (GIS) technology. Ore Geol. Rev. 2000, 16, 107–143. [Google Scholar] [CrossRef]
- Govett, G.J.S. Rock Geochemistry in Mineral Exploration; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kyser, K.; Barr, J.; Ihlenfeld, C. Applied geochemistry in mineral exploration and mining. Elements 2015, 11, 241–246. [Google Scholar] [CrossRef]
- Zuo, R.; Wang, J.; Xiong, Y.; Wang, Z. The processing methods of geochemical exploration data: Past, present, and future. Appl. Geochem. 2021, 132, 105072. [Google Scholar] [CrossRef]
- Biqoqi Product List LIBS2500plus LIBS Systems. Available online: http://www.gzbiaoqi.com/ProductShowen.asp?ArticleID=12 (accessed on 5 April 2024).
- Applied Spectra J200 Laser-Induced Breakdown Spectroscopy (LIBS) Instrument. Available online: http://appliedspectra.com/products/j200-libs-instrument.html (accessed on 5 April 2024).
- DM6 M LIBS Material Analysis Microscope. Available online: http://Leica-microsystems.com/products/light-microscopes/p/libs-module/ (accessed on 5 April 2024).
- Connors, B.; Somers, A.; Day, D. Application of handheld laser-induced breakdown spectroscopy (LIBS) to geochemical analysis. Appl. Spectrosc. 2016, 70, 810–815. [Google Scholar] [CrossRef]
- Senesi, G.S.; Harmon, R.S.; Hark, R.R. Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status, and future prospects. Spectrochim. Acta Part B At. Spectrosc. 2021, 175, 106013. [Google Scholar] [CrossRef]
- Noll, R. Laser-Induced Breakdown Spectroscopy: Fundamentals and Applications; Springer: Berlin, Germany, 2012. [Google Scholar]
- Cremers, D.; Radziemski, L. Handbook of Laser-Induced Breakdown Spectroscopy; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- NIST Atomic Spectra Databases. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 5 April 2024).
- Wise, M.A.; Harmon, R.S.; Curry, A.C.; Jennings, M.; Grimac, Z.; Khashchevskaya, D. Handheld LIBS for Li exploration: An example from the Carolina Tin-Spodumene Belt, USA. Minerals 2022, 12, 77. [Google Scholar] [CrossRef]
- Harmon, R.S.; De Lucia, F.C.; Miziolek, A.W.; McNesby, K.L.; Walters, R.A.; French, P.D. Laser-induced breakdown spectroscopy (LIBS)—An emerging field-portable sensor technology for real-time, in-situ geochemical and environmental analysis. Geochem. Explor. Environ. Anal. 2005, 5, 21–28. [Google Scholar] [CrossRef]
- Harmon, R.S.; Russo, R.E. Laser-induced breakdown spectroscopy. In Treatise on Geochemistry, Analytical Geochemistry and Inorganic Instrumental Analysis; McDonough, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 15, pp. 245–272. [Google Scholar]
- Fabre, C. Advances in Laser-Induced Breakdown Spectroscopy analysis for geology: A critical review. Acta Part B At. Spectrosc. 2020, 166, 105799. [Google Scholar] [CrossRef]
- Harmon, R.S.; Senesi, G.S. Laser-induced breakdown Spectroscopy—A geochemical tool for the 21st century. Appl. Geochem. 2021, 128, 104929. [Google Scholar] [CrossRef]
- Lemière, B.; Harmon, R.S. XRF and LIBS for field geology. In Portable Spectroscopy and Spectrometry; Crocombe, R., Leary, P., Kammrath, B., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2021; pp. 455–497. [Google Scholar]
- Rifai, K.; Michaud Paradis, M.C.; Swierczek, Z.; Doucet, F.; Özcan, L.; Fayad, A.; Li, J.; Vidal, F. Emergence of new technology for ultrafast automated mineral phase identification and quantitative analysis using the CORIOSITY laser-induced breakdown spectroscopy (LIBS) system. Minerals 2020, 10, 918. [Google Scholar] [CrossRef]
- Rosenwasser, S.; Asimellis, G.; Bromley, B.; Hazlett, R.; Martin, J.; Pearce, T.; Zigler, A. Development of a method for automated quantitative analysis of ores using LIBS. Spectrochim. Acta Part B At. Spectrosc. 2001, 56, 707–714. [Google Scholar] [CrossRef]
- Fabre, C.; Ourti, N.E.; Ballouard, C.; Mercadier, J.; Cauzid, J. Handheld LIBS analysis for in situ quantification of Li and detection of the trace elements (Be, Rb and Cs). J. Geochem. Explor. 2022, 236, 106979. [Google Scholar] [CrossRef]
- Rifai, K.; Özcan, L.; Doucet, F.; Vidal, F. Quantification of copper, nickel and other elements in copper-nickel ore samples using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2020, 165, 105766. [Google Scholar] [CrossRef]
- Tognoni, E.; Cristoforetti, G.; Legnaiolia, S.; Palleschi, V. Calibration-free laser-induced breakdown spectroscopy: State of the art. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 1–14. [Google Scholar] [CrossRef]
- Praher, B.; Palleschi, V.; Viskup, P.; Heitz, J.; Pedarnig, J. Calibration free laser induced breakdown spectroscopy of oxide materials. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 671–679. [Google Scholar] [CrossRef]
- Thornton, B.; Takahashi, T.; Sato, T.; Sakka, T.; Tamura, A.; Matsumoto, A.; Nozaki, T.; Ohki, T.; Ohki, K. Development of a deep-sea laser-induced breakdown spectrometer for in situ multi-element chemical analysis. Deep Sea Res. 2015, 95, 20–36. [Google Scholar] [CrossRef]
- Guo, J.; Lu, Y.; Cheng, K.; Song, J.; Ye, W.; Li, N.; Zheng, R. Development of a compact underwater laser-induced breakdown spectroscopy (LIBS) system and preliminary results in sea trials. Appl. Opt. 2017, 56, 8196–8200. [Google Scholar] [CrossRef]
- Takahashi, T.; Yoshino, S.; Takaya, Y.; Nozaki, T.; Ohki, K.; Ohki, T.; Sakka, T.; Thornton, B. Quantitative in situ mapping of elements in deep-sea hydrothermal vents using laser-induced breakdown spectroscopy and multivariate analysis. Deep Sea Res. 2020, 158, 103232. [Google Scholar] [CrossRef]
- Wiens, R.C.; Wan, X.; Lasue, J.; Maurice, S. Laser-induced breakdown spectroscopy in planetary science. In Laser-Induced Breakdown Spectroscopy; Singh, J.P., Thakur, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 441–471. [Google Scholar]
- Wiens, R.C.; Maurice, S.; MSL Science Team. ChemCam: Chemostratigraphy by the first Mars microprobe. Elements 2015, 11, 33–38. [Google Scholar] [CrossRef]
- Xu, W.; Liu, X.; Yan, Z.; Li, L.; Zhang, Z.; Kuang, Y.; Jiang, H.; Yu, H.; Yang, F.; Liu, C.; et al. The MarSCoDe instrument suite on the Mars Rover of China’s Tianwen-1 mission. Space Sci. Rev. 2021, 217, 1–58. [Google Scholar] [CrossRef]
- Death, D.L.; Cunningham, A.P.; Pollard, L.J. Multi-element and mineralogical analysis of mineral ores using laser induced breakdown spectroscopy and chemometric analysis. Spectrochim. Acta Part B At. Spectrom. 2009, 64, 1048–1058. [Google Scholar] [CrossRef]
- Haavisto, O.; Kauppinen, T.; Häkkänen, H. Laser-induced breakdown spectroscopy for rapid elemental analysis of drillcore. IFAC-PapersOnLine 2013, 46, 87–91. [Google Scholar] [CrossRef]
- Pořízka, P.; Demidov, A.; Kaiser, J.; Keivanian, J.; Gornushkin, I.; Panne, U.; Riedel, J. Laser-induced breakdown spectroscopy for in situ qualitative and quantitative analysis of mineral ores. Spectrochim. Acta Part B At. Spectrom. 2014, 101, 155–163. [Google Scholar] [CrossRef]
- Pedarnig, J.D.; Trautner, S.; Grünberger, S.; Giannakaris, N.; Eschlböck-Fuchs, S.; Hofstadler, J. Review of element analysis of industrial materials by in-line laser-induced breakdown spectroscopy (LIBS). Appl. Sci. 2021, 11, 9274. [Google Scholar] [CrossRef]
- Barrette, L.; Turmel, S. On-line iron-ore slurry monitoring for real-time process control of pellet making processes using laser-induced breakdown spectroscopy: Graphitic vs. total carbon detection. Spectrochim. Acta Part B At. Spectrom. 2001, 56, 715–723. [Google Scholar] [CrossRef]
- Eseller, K.E.; Tripathi, M.M.; Yueh, F.Y.; Singh, J.P. Elemental analysis of slurry samples with laser induced breakdown spectroscopy. Appl. Opt. 2010, 49, C21–C26. [Google Scholar] [CrossRef]
- Khajehzadeh, N.; Haavisto, O.; Koresaar, L. On-stream and quantitative mineral identification of tailing slurries using LIBS technique. Miner. Eng. 2016, 98, 101–109. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, X.; Zhu, Z.; Guo, L.; Li, X.; Lu, Y.; Zeng, X. On-stream analysis of iron ore slurry using laser-induced breakdown spectroscopy. Appl. Opt. 2017, 56, 9144–9149. [Google Scholar] [CrossRef] [PubMed]
- Ytsma, C.R.; Knudson, C.A.; Dyar, M.D.; McAdam, A.C.; Michaud, D.D.; Rollosson, L.M. Accuracies and detection limits of major, minor, and trace element quantification in rocks by portable laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2020, 171, 105946. [Google Scholar] [CrossRef]
- Buckley, S. Geochemical analysis using laser-induced breakdown spectroscopy. Spectroscopy 2021, 36, 9–15. [Google Scholar] [CrossRef]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part II: Review of instrumental and methodological approaches to material analysis and applications to different fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef] [PubMed]
- Wisbrun, R.; Schechter, I.; Niessner, R.; Schroeder, H.; Kompa, K.L. Detector for trace elemental analysis of solid environmental samples by laser plasma spectroscopy. Anal. Chem. 1994, 66, 2964–2975. [Google Scholar] [CrossRef]
- Cáceres, J.O.; López, J.T.; Telle, H.H.; Ureña, A.G. Quantitative analysis of trace metal ions in ice using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2001, 56, 831–838. [Google Scholar] [CrossRef]
- Russo, R.E.; Mao, X.L.; Chan, W.T.; Bryant, M.F.; Kinard, W.F. Laser ablation sampling with inductively coupled plasma atomic emission spectrometry for the analysis of prototypical glasses. J. Anal. At. Spectrom. 1995, 10, 295–301. [Google Scholar] [CrossRef]
- Eppler, A.S.; Cremers, D.A.; Hickmott, D.D.; Ferris, M.J.; Koskelo, A.C. Matrix effects in the detection of Pb and Ba in soils using laser-induced breakdown spectroscopy. Appl. Spectrosc. 1996, 50, 1175–1181. [Google Scholar] [CrossRef]
- Gornushkin, S.I.; Gornushkin, I.B.; Anzano, J.M.; Smith, B.W.; Winefordner, J.D. Effective normalization technique for correction of matrix effects in laser-induced breakdown spectroscopy detection of magnesium in powdered samples. Appl. Spectrosc. 2002, 56, 433–436. [Google Scholar] [CrossRef]
- McMillan, N.J.; McManus, C.E.; Harmon, R.S.; De Lucia, F.C., Jr.; Miziolek, A.W. Laser-induced breakdown spectroscopy analysis of complex silicate minerals—beryl. Anal. Bioanal. Chem. 2006, 385, 263–271. [Google Scholar] [CrossRef]
- Radziemski, L.; Cremers, D. A A brief history of laser-induced breakdown spectroscopy: From the concept of atoms to LIBS 2012. Spectrochim. Acta Part B At. Spectrom. 2013, 87, 3–10. [Google Scholar] [CrossRef]
- Pořízka, P.; Klus, J.; Mašek, J.; Rajnoha, M.; Prochazka, D.; Modlitbová, P.; Novotný, J.; Burget, R.; Novotný, K.; Kaiser, J. Multivariate classification of echellograms: A new perspective in laser-induced breakdown spectroscopy analysis. Sci. Rep. 2017, 7, 3160. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.L. Chemometric analysis in LIBS. In Handbook of Laser-Induced Breakdown Spectroscopy; Cremers, D.A., Radziemski, L.J., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 223–255. [Google Scholar]
- Zhang, T.; Tang, H.; Li, H. Chemometrics in laser-induced breakdown spectroscopy. J. Chemom. 2018, 32, e2983. [Google Scholar] [CrossRef]
- Rifai, K.; Laflamme, M.; Constantin, M.; Vidal, F.; Sabsabi, M.; Blouin, A.; Bouchard, P.; Fytas, K.; Castello, M.; Kamwa, B.N. Analysis of gold in rock samples using laser-induced breakdown spectroscopy: Matrix and heterogeneity effects. Spectrochim. Acta Part B At. Spectrom. 2017, 134, 33–41. [Google Scholar] [CrossRef]
- Álvarez, J.; Velásquez, M.; Myakalwar, A.K.; Sandoval, C.; Fuentes, R.; Castillo, R.; Sbarbaro, D.; Yáñez, J. Determination of copper-based mineral species by laser induced breakdown spectroscopy and chemometric methods. J. Anal. At. Spectrom. 2019, 34, 2459–2468. [Google Scholar] [CrossRef]
- Nardecchia, A.; Fabre, C.; Cauzid, J.; Pelascini, F.; Motto-Ros, V.; Duponchel, L. Detection of minor compounds in complex mineral samples from millions of spectra: A new data analysis strategy in LIBS imaging. Anal. Chim. Acta 2020, 1114, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Meima, J.A. Mineral classification of lithium-bearing pegmatites based on laser-induced breakdown spectroscopy: Application of semi-supervised learning to detect known minerals and unknown material. Spectrochim. Acta Part B At. Spectrom. 2022, 189, 106370. [Google Scholar] [CrossRef]
- El Haddad, J.; Harhira, A.; Képeš, E.; Vrábel, J.; Kaiser, J.; Pořízka, P. Chemometric Processing of LIBS Data. In Laser Induced Breakdown Spectroscopy (LIBS) Concepts, Instrumentation, Data Analysis and Applications; Singh, V.K., Tripathi, D.K., Deguchi, Y., Wang, Z., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2023; Volume 1, pp. 241–275. [Google Scholar]
- Sobron, P.; Wang, A.; Sobron, F. Extraction of compositional and hydration information of sulfates from laser-induced plasma spectra recorded under Mars atmospheric conditions—Implications for ChemCam investigations on Curiosity rover. Spectrochim. Acta Part B At. Spectrom. 2012, 68, 1–6. [Google Scholar] [CrossRef]
- Fontana, F.F.; Tassios, S.; Stromberg, J.; Tiddy, C.; van der Hoek, B.; Uvarova, Y.A. Integrated laser-induced breakdown spectroscopy (LIBS) and multivariate wavelet tessellation: A new, rapid approach for lithogeochemical analysis and interpretation. Minerals 2021, 11, 312. [Google Scholar] [CrossRef]
- Yan, J.; Li, Q.; Qin, F.; Xiao, L.; Li, X. A polynomial interactive reconstruction method based on spectral morphological features for the classification of gem minerals using portable LIBS. J. Anal. At. Spectrom. 2022, 37, 1862–1868. [Google Scholar] [CrossRef]
- Boucher, T.F.; Ozanne, M.V.; Carmosino, M.L.; Dyar, M.D.; Mahadevan, S.; Breves, E.A.; Lepore, K.H.; Clegg, S.M. A study of machine learning regression methods for major elemental analysis of rocks using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2015, 107, 1–10. [Google Scholar] [CrossRef]
- Pagnotta, S.; Lezzerini, M.; Campanella, B.; Legnaioli, S.; Poggialini, F.; Palleschi, V. A new approach to non-linear multivariate calibration in laser-induced breakdown spectroscopy analysis of silicate rocks. Spectrochim. Acta Part B At. Spectrom. 2020, 166, 105804. [Google Scholar] [CrossRef]
- Limbeck, A.; Brunnbauer, L.; Lohninger, H.; Pořízka, P.; Modlitbová, P.; Kaiser, J.; Janovszky, P.; Kéri, A.; Galbács, G. Methodology and applications of elemental mapping by laser induced breakdown spectroscopy. Anal. Chim. Acta 2021, 1147, 72–98. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, L.; Leprince, M.; Moncayo, S.; Sorbier, L.; Lienemann, C.P.; Motto-Ros, V. Review of the recent advances and applications of LIBS-based imaging. Spectrochim. Acta Part B At. Spectrom. 2019, 151, 41–53. [Google Scholar] [CrossRef]
- Harmon, R.S.; Lawley, C.J.; Watts, J.; Harraden, C.L.; Somers, A.M.; Hark, R.R. Laser-induced breakdown spectroscopy—An emerging analytical tool for mineral exploration. Minerals 2019, 9, 718. [Google Scholar] [CrossRef]
- Lawley, C.J.; Somers, A.M.; Kjarsgaard, B.A. Rapid geochemical imaging of rocks and minerals with handheld laser induced breakdown spectroscopy (LIBS). J. Geochem Explor. 2021, 222, 106694. [Google Scholar] [CrossRef]
- Rifai, K.; Doucet, F.; Özcan, L.; Vidal, F. LIBS core imaging at kHz speed: Paving the way for real-time geochemical applications. Spectrochim. Acta Part B At. Spectrom. 2018, 150, 43–48. [Google Scholar] [CrossRef]
- Alvarez-Llamas, C.; Tercier, A.; Ballouard, C.; Fabre, C.; Hermelin, S.; Margueritat, J.; Duponchel, L.; Dujardin, C.; Motto-Ros, V. Ultrafast μLIBS imaging for the multiscale mineralogical characterization of pegmatite rocks. J. Anal. At. Spectrom. 2024, 39, 1077–1086. [Google Scholar] [CrossRef]
- Harmon, R.S.; Khashchevskaya, D.; Morency, M.; Owen, L.A.; Jennings, M.; Knott, J.R.; Dortch, J.M. Analysis of rock varnish from the Mojave Desert by handheld laser-induced breakdown spectroscopy. Molecules 2021, 26, 5200. [Google Scholar] [CrossRef]
- Motto-Ros, V.; Moncayo, S.; Fabre, C.; Busser, B. LIBS imaging applications. In Laser-Induced Breakdown Spectroscopy; Singh, J.P., Thakur, S., Eds.; Elsevier: Amesterdam, The Netherlands, 2020; pp. 329–346. [Google Scholar]
- Chirinos, J.R.; Oropeza, D.D.; Gonzalez, J.J.; Hou, H.; Morey, M.; Zorba, V.; Russo, R.E. Simultaneous 3-dimensional elemental imaging with LIBS and LA-ICP-MS. J. Anal. At. Spectrom. 2014, 29, 1292–1298. [Google Scholar] [CrossRef]
- Moncayo, S.; Duponchel, L.; Mousavipak, N.; Panczer, G.; Trichard, F.; Bousquet, B.; Pelascini, F.; Motto-Ros, V. Exploration of megapixel hyperspectral LIBS images using principal component analysis. J. Anal. At. Spectrom. 2018, 33, 210–220. [Google Scholar] [CrossRef]
- Fabre, C.; Devismes, D.; Moncayo, S.; Pelascini, F.; Trichard, F.; Lecomte, A.; Bousquet, B.; Cauzid, J.; Motto-Ros, V. Elemental imaging by laser-induced breakdown spectroscopy for the geological characterization of minerals. J. Anal. At. Spectrom. 2018, 33, 1345–1353. [Google Scholar] [CrossRef]
- Harmon, R.S.; Remus, J.; McMillan, N.J.; McManus, C.; Collins, L.M.; Gottfried, J.L., Jr.; DeLucia, F.C., Jr.; Miziolek, A.W. LIBS analysis of geomaterials: Geochemical fingerprinting for the rapid analysis and discrimination of minerals. Appl. Geochem. 2009, 24, 1125–1141. [Google Scholar] [CrossRef]
- Hark, R.R.; Harmon, R.S. Geochemical fingerprinting using LIBS. In Laser Induced Breakdown Spectroscopy—Theory & Applications; Musazzi, S., Perini, U., Eds.; Springer: Heidelberg, Germany, 2014; pp. 309–348. [Google Scholar]
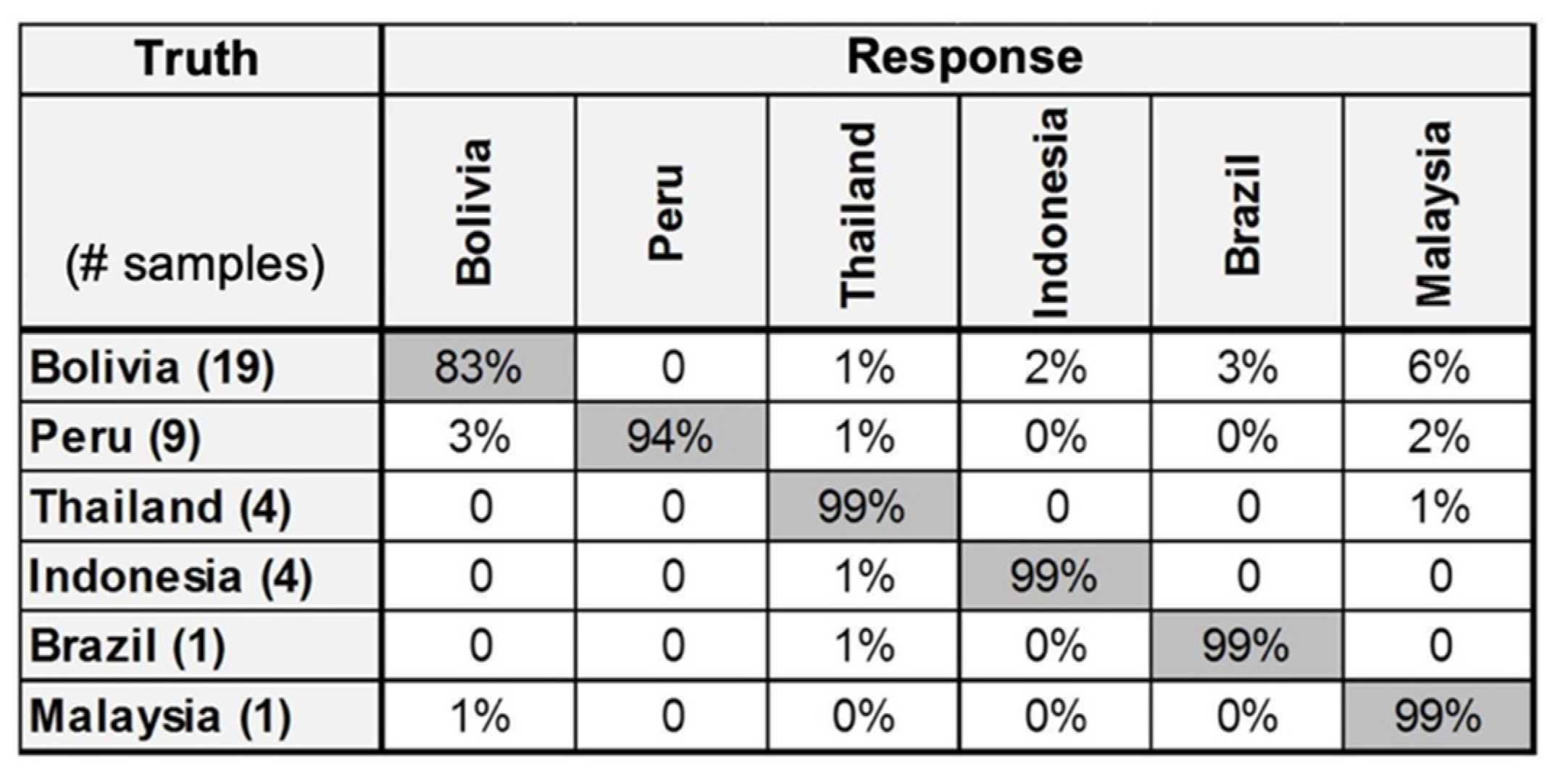
- Harmon, R.S.; Throckmorton, C.S.; Hark, R.R.; Gottfried, J.L.; Wörner, G.; Harpp, K.; Collins, L.M. Discriminating volcanic centers with handheld laser-induced breakdown spectroscopy (LIBS). J. Archaeol. Sci. 2018, 98, 112–127. [Google Scholar] [CrossRef]
- McMillan, N.J.; Montoya, C.; Chesner, W.H. Correlation of limestone beds using laser-induced breakdown spectroscopy and chemometric analysis. Appl. Opt. 2012, 51, B213–B222. [Google Scholar] [CrossRef] [PubMed]
- Harmon, R.S.; Hark, R.R.; Throckmorton, C.S.; Rankey, E.C.; Wise, M.A.; Somers, A.M.; Collins, L.M. Geochemical fingerprinting by handheld laser-induced breakdown spectroscopy. Geostand. Geoanal. Res. 2017, 41, 563–584. [Google Scholar] [CrossRef]
- Farnsworth-Pinkerton, S.; McMillan, N.J.; Dutrow, B.L.; Henry, D.J. Provenance of detrital tourmalines from Proterozoic metasedimentary rocks in the Picuris Mountains, New Mexico, using laser-induced breakdown spectroscopy. J. Geosci. 2018, 63, 193. [Google Scholar] [CrossRef]
- McMillan, N.J.; Curry, J.; Dutrow, B.L.; Henry, D.J. Identification of the host lithology of tourmaline using laser-induced breakdown spectroscopy for application in sediment provenance and mineral exploration. Can. Mineral. 2018, 56, 393–410. [Google Scholar] [CrossRef]
- Syvilay, D.; Bousquet, B.; Chapoulie, R.; Orange, M.; Le Bourdonnec, F.X. Advanced statistical analysis of LIBS spectra for the sourcing of obsidian samples. J. Anal. At. Spectrom. 2019, 34, 867–873. [Google Scholar] [CrossRef]
- Harmon, R.S.; Throckmorton, C.S.; Haverstock, G.; Baron, D.; Yohe, R.M.; Hark, R.R.; Knott, J.R. Connecting obsidian artifacts with their sources using multivariate statistical analysis of LIBS spectral signatures. Minerals 2023, 13, 1284. [Google Scholar] [CrossRef]
- Pochon, A.; Desaulty, A.M.; Bailly, L. Handheld laser-induced breakdown spectroscopy (LIBS) as a fast and easy method to trace gold. J. Anal. At. Spectrom. 2020, 35, 254–264. [Google Scholar] [CrossRef]
- Ciucci, A.; Corsi, M.; Palleschi, V.; Rastelli, S.; Salvetti, A.; Tognoni, E. New procedure for quantitative elemental analysis by laser-induced plasma spectroscopy. Appl. Spectros. 1999, 53, 960–964. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Wang, W.; Chen, F.; Xu, Y.; Nie, J.; Chu, Y.; Guo, L. A review of calibration-free laser-induced breakdown spectroscopy. TrAC Trends Anal. Chem. 2022, 152, 116618. [Google Scholar] [CrossRef]
- Kaski, S.; Häkkänen, H.; Korppi-Tommola, J. Sulfide mineral identification using laser-induced plasma spectroscopy. Miner. Eng. 2003, 16, 1239–1243. [Google Scholar] [CrossRef]
- Fabre, C.; Boiron, M.C.; Dubessy, J.; Moissette, A. Determination of ions in individual fluid inclusions by laser ablation optical emission spectroscopy: Development and applications to natural fluid inclusions. J. Anal. At. Spectrom. 1999, 14, 913–922. [Google Scholar] [CrossRef]
- Lanza, N.L.; Ollila, A.M.; Cousin, A.; Wiens, R.C.; Clegg, S.; Mangold, N.; Bridges, N.; Cooper, D.; Schmidt, M.; Berger, J.; et al. Understanding the signature of rock coatings in laser-induced breakdown spectroscopy data. Icarus 2015, 249, 62–73. [Google Scholar] [CrossRef]
- Khajehzadeh, N.; Kauppinen, T.K. Fast mineral identification using elemental LIBS technique. IFAC-PapersOnLine 2015, 48, 119–124. [Google Scholar] [CrossRef]
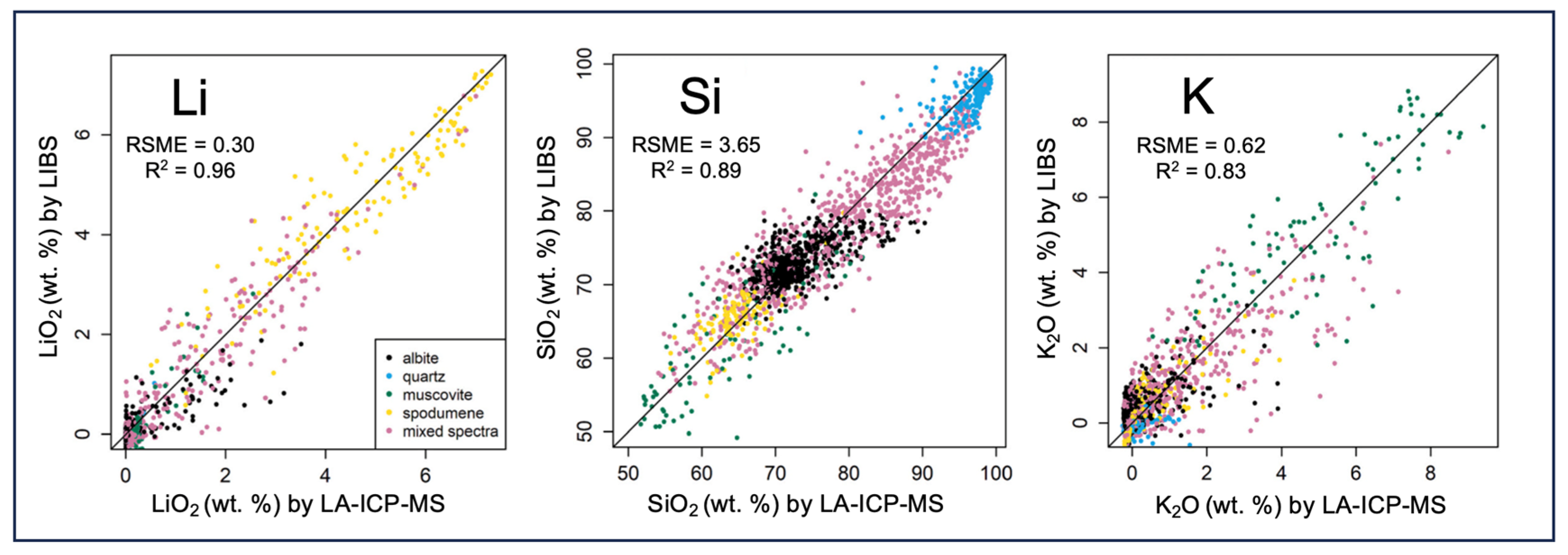
- Sweetapple, M.; Tassios, S. Laser-induced breakdown spectroscopy (LIBS) as a tool for in situ mapping and textural interpretation of lithium in pegmatite minerals. Am. Mineral. 2015, 100, 2141–2151. [Google Scholar] [CrossRef]
- Sandoval-Muñoz, C.; Velásquez, G.; Álvarez, J.; Pérez, F.; Velásquez, M.; Torres, S.; Sbarbaro-Hofer, D.; Motto-Ros, V.; Yáñez, J. Enhanced elemental and mineralogical imaging of Cu-mineralized rocks by coupling μ-LIBS and HSI. J. Anal. At. Spectrom. 2022, 37, 1981–1993. [Google Scholar] [CrossRef]
- Janovszky, P.; Jancsek, K.; Palásti, D.J.; Kopniczky, J.; Hopp, B.; Tóth, T.M.; Galbács, G. Classification of minerals and the assessment of lithium and beryllium content in granitoid rocks by laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2021, 36, 813–823. [Google Scholar] [CrossRef]
- Bolger, J.A. Semi-quantitative laser-induced breakdown spectroscopy for analysis of mineral drill core. Appl. Spectrosc. 2000, 54, 181–189. [Google Scholar] [CrossRef]
- Fontana, F.F.; van der Hoek, B.; Stromberg, J.; Tiddy, C.; Francis, N.; Tassios, S.; Uvarova, Y.A. On the use of laser-induced breakdown spectroscopy data for mineralogical investigations–constraints and application of a clustering method. Geochem. Explor. Environ. Anal. 2023, 23, geochem2023-003. [Google Scholar] [CrossRef]
- Noll, R.; Fricke-Begemann, C.; Brunk, M.; Connemann, S.; Meinhardt, C.; Scharun, M.; Sturm, V.; Makowe, J.; Gehlen, C. Laser-induced breakdown spectroscopy expands into industrial applications. Spectrochim. Acta Part B 2014, 93, 41–51. [Google Scholar] [CrossRef]
- Kim, T.; Lin, C.; Yoon, Y. Compositional mapping by laser-induced breakdown spectroscopy. J. Phys. Chem. B 1998, 102, 4284–4287. [Google Scholar] [CrossRef]
- Kuhn, K.; Meima, J.; Rammlmair, D.; Ohlendorf, C. Chemical mapping of mine waste drill cores with laser-induced breakdown spectroscopy (LIBS) and energy dispersive X-ray fluorescence (EDXRF) for mineral resource exploration. J. Geochem. Explor. 2016, 161, 72–84. [Google Scholar] [CrossRef]
- Streubel, L.; Jacobsen, L.; Merk, S.; Thees, M.; Rammlmair, D.; Meima, J.; Mory, D. Rapid analysis of geological drill-cores with LIBS. Opt. Photon. 2016, 11, 23–27. [Google Scholar] [CrossRef]
- Müller, S.; Meima, J.A.; Rammlmair, D. Detecting REE-rich areas in heterogeneous drill cores from Storkwitz using LIBS and a combination of k-means clustering and spatial raster analysis. J. Geochem. Explor. 2021, 221, 106697. [Google Scholar] [CrossRef]
- Müller, S.; Meima, J.A.; Gäbler, H.E. Improving spatially-resolved lithium quantification in drill core samples of spodumene pegmatite by using laser-induced breakdown spectroscopy and pixel-matched reference areas. J. Geochem. Explor. 2023, 250, 107235. [Google Scholar] [CrossRef]
- Meima, J.A.; Rammlmair, D. Investigation of compositional variations in chromitite ore with imaging laser induced breakdown spectroscopy and spectral angle mapper classification algorithm. Chem. Geol. 2020, 532, 119376. [Google Scholar] [CrossRef]
- Michaud, D.; Proulx, E.; Chartrand, J.G.; Barrette, L. Shooting slurries with laser-induced breakdown spectroscopy: Sampling is the name of the game. Appl. Opt. 2003, 42, 6179–6183. [Google Scholar] [CrossRef]
- Michaud, D.; Leclerc, R.; Proulx, É. Influence of particle size and mineral phase in the analysis of iron ore slurries by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2007, 62, 1575–1581. [Google Scholar] [CrossRef]
- Oh, S.Y.; Miller, T.; Yueh, F.Y.; Singh, J.P. Comparative study of laser-induced breakdown spectroscopy measurement using two slurry circulation systems. Appl. Opt. 2007, 46, 4020–4025. [Google Scholar] [CrossRef] [PubMed]
- Ayyalasomayajula, K.K.; Dikshit, V.; Yueh, F.Y.; Singh, J.P.; Smith, L.T. Quantitative analysis of slurry sample by laser-induced breakdown spectroscopy. Anal. Bioanal. Chem. 2011, 400, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Khajehzadeh, N.; Haavisto, O.; Koresaar, L. On-stream mineral identification of tailing slurries of an iron ore concentrator using data fusion of LIBS, reflectance spectroscopy and XRF measurement techniques. Miner. Eng. 2017, 113, 83–94. [Google Scholar] [CrossRef]
- Ayyalasomayajula, K.K.; Yueh, F.Y.; Singh, J.P. LIBS of slurry samples. In Laser-Induced Breakdown Spectroscopy; Singh, J.P., Thakur, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–274. [Google Scholar]
- Guo, L.B.; Cheng, X.; Tang, Y.; Tang, S.S.; Hao, Z.Q.; Li, X.Y.; Lu, Y.F.; Zeng, X.Y. Improvement of spectral intensity and resolution with fiber laser for on-stream slurry analysis in laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2019, 152, 38–43. [Google Scholar] [CrossRef]
- Chen, T.; Sun, L.; Yu, H.; Qi, L.; Shang, D.; Xie, Y. Efficient weakly supervised LIBS feature selection method in quantitative analysis of iron ore slurry. Appl. Opt. 2022, 61, D22–D29. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Sun, L.; Yu, H.; Zeng, P.; Qi, L. Online Fe grade monitoring of iron ore slurry by Morse wavelet transform and lightweight convolutional neural network based on LIBS. Spectrochim. Acta Part B At. Spectrom. 2023, 210, 106821. [Google Scholar] [CrossRef]
- Li, A.; Zhang, X.; Liu, X.; He, Y.; Shan, Y.; Sun, H.; Yi, W.; Liu, R. Real time and high-precision online determination of main components in iron ore using spectral refinement algorithm based LIBS. Opt. Exp. 2023, 31, 38728–38743. [Google Scholar] [CrossRef]
- Fabre, C.; Boiron, M.C.; Dubessy, J.; Cathelineau, M.; Banks, D.A. Palaeofluid chemistry of a single fluid event: A bulk and in-situ multi-technique analysis (LIBS, Raman spectroscopy) of an Alpine fluid (Mont-Blanc). Chem. Geol. 2002, 182, 249–264. [Google Scholar] [CrossRef]
- Derome, D.; Cuney, M.; Cathelineau, M.; Fabre, C.; Dubessy, J.; Bruneton, P.; Hubert, A. A detailed fluid inclusion study in silicified breccias from the Kombolgie sandstones (Northern Territory, Australia): Inferences for the genesis of middle-Proterozoic unconformity-type uranium deposits. J. Geochem. Explor. 2003, 80, 259–275. [Google Scholar] [CrossRef]
- Crocombe, R.A. Portable spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.J.; Paul, G.L.; O’Neill, J.A. Time-resolved laser-induced breakdown spectroscopy of iron ore. Appl. Spectrocsc. 1991, 44, 1711–1714. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global lithium sources—Industrial use and future in the electric vehicle industry: A review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global lithium availability: A constraint for electric vehicles? J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Fabre, C.; Boiron, M.C.; Dubessy, J.; Chabiron, A.; Charoy, B.; Crespo, T.M. Advances in lithium analysis in solids by means of laser-induced breakdown spectroscopy: An exploratory study. Geochim. Cosmochim Acta 2002, 66, 1401–1407. [Google Scholar] [CrossRef]
- Ribeiro, R.; Capela, D.; Ferreira, M.; Martins, R.; Jorge, P.; Guimarães, D.; Lima, A. X-ray fluorescence and laser-induced breakdown spectroscopy analysis of Li-rich minerals in veins from Argemela Tin Mine, Central Portugal. Minerals 2021, 11, 1169. [Google Scholar] [CrossRef]
- Rifai, K.; Constantin, M.; Yilmaz, A.; Özcan, L.Ç.; Doucet, F.R.; Azami, N. Quantification of lithium and mineralogical mapping in crushed ore samples using laser induced breakdown spectroscopy. Minerals 2022, 12, 253. [Google Scholar] [CrossRef]
- Días, F.; Ribeiro, R.; Gonçalves, F.; Lima, A.; Roda-Robles, E.; Martins, T. Calibrating a handheld LIBS for Li exploration in the Barroso–Alvão Aplite-Pegmatite Field, Northern Portugal: Textural precautions and procedures when analyzing spodumene and petalite. Minerals 2023, 13, 470. [Google Scholar] [CrossRef]
- Harmon, R.S.; Wise, M.A.; Curry, A.C.; Mistele, J.S.; Mason, M.S.; Grimac, Z. Rapid analysis of muscovites on a lithium pegmatite prospect by handheld LIBS. Minerals 2023, 13, 697. [Google Scholar] [CrossRef]
- Romppanen, S.; Pölönen, I.; Häkkänen, H.; Kaski, S. Optimization of spodumene identification by statistical approach for laser-induced breakdown spectroscopy data of lithium pegmatite ores. Appl. Spectrosc. Rev. 2023, 58, 297–317. [Google Scholar] [CrossRef]
- Wise, M.A.; Curry, A.C.; Harmon, R.S. Reevaluation of the K/Rb-Li Systematics in muscovite as a potential exploration tool for identifying Li mineralization in granitic pegmatites. Minerals 2024, 14, 117. [Google Scholar] [CrossRef]
- Beryllium: Economic Geology, Material Flow, and Global Importance of a Key Critical Mineral. Available online: https://www.usgs.gov/centers/geology-energy-and-minerals-science-center/science/beryllium-economic-geology-material-flow (accessed on 28 April 2024).
- McManus, C.E.; McMillan, N.J.; Harmon, R.S.; Whitmore, R.C.; De Lucia, F.C., Jr.; Miziolek, A.W. Use of laser induced breakdown spectroscopy in the determination of gem provenance: Beryls. Appl. Opt. 2008, 47, G72–G79. [Google Scholar] [CrossRef] [PubMed]
- Industrial Diamond Statistics and Information. Available online: http://www.usgs.gov/centers/national-minerals-information-center/industrial-diamond-statistics-and-information (accessed on 28 April 2024).
- Gurney, J.J.; Switzer, G.S. The discovery of garnets closely related to diamonds in thee Finsch pipe, South Africa. Contrib. Mineral. Petrol. 1973, 39, 103–116. [Google Scholar] [CrossRef]
- Bieri, F. From Blood Diamonds to the Kimberley Process How NGOs Cleaned up the Global Diamond Industry; Routledge: New York, NY, USA, 2016. [Google Scholar]
- McManus, C.E.; Dowe, J.; McMillan, N.J. Use of CC and CN molecular emissions in laser-induced breakdown spectroscopy data to determine diamond provenance. Microsc. Microanal. 2017, 23, 2282–2283. [Google Scholar] [CrossRef]
- McManus, C.E.; McMillan, N.J.; Dowe, J.; Bell, J. Diamonds certify themselves: Multivariate statistical provenance analysis. Minerals 2020, 10, 916. [Google Scholar] [CrossRef]
- Krzemnicki, M.S.; Hänni, H.A.; Walters, R.A. A new method for detecting Be diffusion-treated sapphires: Laser-induced breakdown spectroscopy (LIBS). Gems Gemol. 2004, 40, 314–322. [Google Scholar] [CrossRef]
- Agrosì, G.; Tempesta, G.I.; Scandale, E.; Legnaioli, S.; Lorenzetti, G.; Pagnotta, S.; Palleschi, V.; Mangone, A.; Lezzerini, M. Application of laser induced breakdown spectroscopy to the identification of emeralds from different synthetic processes. Spectrochim. Acta Part B At. Spectrom. 2014, 102, 48–51. [Google Scholar] [CrossRef]
- Fluorine. Available online: https://www.rsc.org/periodic-table/element/9/fluorine (accessed on 28 April 2024).
- Fluorine. Available online: https://www.usgs.gov/publications/fluorine (accessed on 28 April 2024).
- Quarles, C.D.; Gonzalez, J.J.; East, L.J.; Yoo, J.H.; Morey, M.; Russo, R.E. Fluorine analysis using laser induced breakdown spectroscopy (LIBS). J. Anal. At. Spectrom. 2014, 29, 1238–1242. [Google Scholar] [CrossRef]
- Álvarez, C.; Pisonero, J.; Bordel, N. Quantification of fluorite mass-content in powdered ores using a laser-induced breakdown spectroscopy method based on the detection of minor elements and CaF molecular bands. Spectrochim. Acta Part B At. Spectrom. 2014, 100, 123–128. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, R.; Hao, Z.; Zhang, W.; Li, Q.; Zeng, Q.; Li, X.; Zeng, X.; Lu, Y. Determination of fluorine in copper ore using laser-induced breakdown spectroscopy assisted by the SrF molecular emission band. J. Anal. At. Spectrom. 2020, 35, 754–761. [Google Scholar] [CrossRef]
- Foucaud, Y.; Fabre, C.; Demeusy, B.; Filippova, I.V.; Filippov, L.O. Optimisation of fast quantification of fluorine content using handheld laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2019, 158, 105628. [Google Scholar] [CrossRef]
- Gow, N.N.; Lozej, G.P. Bauxite. Geosci. Can. 1993, 20, 9–16. [Google Scholar]
- Fahad, M.; Ali, S.; Shah, K.H.; Shahzad, A.; Abrar, M. Quantitative elemental analysis of high silica bauxite using calibration-free laser-induced breakdown spectroscopy. Appl. Opt. 2019, 58, 7588–7596. [Google Scholar] [CrossRef] [PubMed]
- Meima, J.A.; Orberger, B.; Piña, C.G.; Prudhomme, A.; Dittrich, C. Increasing resource efficiency of bauxite using LIBS. Mater. Proc. 2021, 5, 81. [Google Scholar] [CrossRef]
- Phosphorus. Available online: https://www.rsc.org/periodic-table/element/15/phosphorus (accessed on 4 July 2024).
- Asimellis, G.; Giannoudakos, A.; Kompitsas, M. Phosphate ore beneficiation via determination of phosphorus-to-silica ratios by Laser Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2006, 61, 1253–1259. [Google Scholar] [CrossRef]
- Gaft, M.; Sapir-Sofer, I.; Modiano, H.; Stana, R. Laser induced breakdown spectroscopy for bulk minerals online analyses. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 1496–1503. [Google Scholar] [CrossRef]
- Gaft, M.; Nagli, L.; Groisman, Y.; Barishnikov, A. Industrial online raw materials analyzer based on laser-induced breakdown spectroscopy. Appl. Spectrosc. 2014, 68, 1004–1015. [Google Scholar] [CrossRef]
- Rifai, K.; Özcan, L.; Doucet, F.; Azami, N.; Deshays, L.; Lebbardi, A.; Vidal, F. Rapid analysis of phosphate slurries and pressed pellets using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2020, 163, 105735. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.; Qi, L.; Yu, H.; Zeng, P. A lightweight convolutional neural network model for quantitative analysis of phosphate ore slurry based on laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2021, 36, 2528–2535. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, L.; Qi, L.; Yu, H. On-line quantitative analysis of major elements in phosphate slurry using LIBS assisted by plasma information from orthogonal directions imaging. Measurement 2024, 234, 114827. [Google Scholar] [CrossRef]
- About Sulfur. Available online: https://www.sulphurinstitute.org/about-sulphur/introduction-to-sulphur/ (accessed on 3 May 2024).
- Gaft, M.; Nagli, L.; Fasaki, I.; Kompitsas, M.; Wilsch, G. Laser-induced breakdown spectroscopy for on-line sulfur analyses of minerals in ambient conditions. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 1098–1104. [Google Scholar] [CrossRef]
- Lime/Limestone. Available online: https://www.spglobal.com/commodityinsights/en/ci/products/lime-chemical-economics-handbook.html (accessed on 3 May 2024).
- Oates, J.A.H. Lime and Limestone: Chemistry and Technology, Production and Uses; John Wiley and Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Fahad, M.; Farooq, Z.; Abrar, M. Comparative study of calibration-free laser-induced breakdown spectroscopy methods for quantitative elemental analysis of quartz-bearing limestone. Appl. Opt. 2019, 58, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Agresti, J.; Indelicato, C.; Perotti, M.; Moreschi, R.; Osticioli, I.; Cacciari, I.; Mencaglia, A.A.; Siano, S. Quantitative compositional analyses of calcareous rocks for lime industry using LIBS. Molecules 2022, 27, 1813. [Google Scholar] [CrossRef] [PubMed]
- Transition Metals: Definition, Properties, Use, and Types. 2024. Available online: https://www.xometry.com/resources/materials/transition-metals/ (accessed on 6 May 2024).
- Grant, K.J.; Paul, G.L.; O’Neill, J.A. Quantitative elemental analysis of iron ore by laser-induced breakdown spectroscopy. Appl. Spectrosc. 1991, 45, 701–705. [Google Scholar] [CrossRef]
- Sun, Q.; Tran, M.; Smith, B.W.; Winefordner, J.D. Determination of Mn and Si in iron ore by laser-induced plasma spectroscopy. Anal. Chim. Acta 2000, 413, 187–195. [Google Scholar] [CrossRef]
- Death, D.L.; Cunningham, A.P.; Pollard, L.J. Multi-element analysis of iron ore pellets by laser-induced breakdown spectroscopy and principal components regression. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 763–769. [Google Scholar] [CrossRef]
- Yaroshchyk, P.; Death, D.L.; Spencer, S.J. Comparison of principal components regression, partial least squares regression, multi-block partial least squares regression, and serial partial least squares regression algorithms for the analysis of Fe in iron ore using LIBS. J. Anal. At. Spectrom. 2012, 27, 92–98. [Google Scholar] [CrossRef]
- Hao, Z.Q.; Li, C.M.; Shen, M.; Yang, X.Y.; Li, K.H.; Guo, L.B.; Li, X.Y.; Lu, Y.F.; Zeng, X.Y. Acidity measurement of iron ore powders using laser-induced breakdown spectroscopy with partial least squares regression. Opt. Exp. 2015, 23, 7795–7801. [Google Scholar] [CrossRef]
- Guo, Y.M.; Guo, L.B.; Hao, Z.Q.; Tang, Y.; Ma, S.X.; Zeng, Q.D.; Tang, S.S.; Li, X.Y.; Lu, Y.F.; Zeng, X.Y. Accuracy improvement of iron ore analysis using laser-induced breakdown spectroscopy with a hybrid sparse partial least squares and least-squares support vector machine model. J. Anal. At. Spectrom. 2018, 33, 1330–1335. [Google Scholar] [CrossRef]
- Wang, P.; Li, N.; Yan, C.; Feng, Y.; Ding, Y.; Zhang, T.; Li, H. Rapid quantitative analysis of the acidity of iron ore by the laser-induced breakdown spectroscopy (LIBS) technique coupled with variable importance measures-random forests (VIM-RF). Anal. Methods 2019, 11, 3419–3428. [Google Scholar] [CrossRef]
- Su, P.; Wu, X.; Li, C.; Yan, C.; An, Y.; Liu, S. A versatile method for quantitative analysis of total iron content in iron ore using laser-induced breakdown spectroscopy. Appl. Spectrosc. 2023, 77, 140–150. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, T.; Niu, G.; Wang, K.; Tang, H.; Duan, Y.; Li, H. Classification of iron ores by laser-induced breakdown spectroscopy (LIBS) combined with random forest (RF). J. Anal. At. Spectrom. 2015, 30, 453–458. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, X.; Zhang, L.; Ren, L. Application of scikit and keras libraries for the classification of iron ore data acquired by laser-induced breakdown spectroscopy (LIBS). Sensors 2020, 20, 1393. [Google Scholar] [CrossRef]
- Zhao, W.; Li, C.; Yan, C.; Min, H.; An, Y.; Liu, S. Interpretable deep learning-assisted laser-induced breakdown spectroscopy for brand classification of iron ores. Anal. Chim. Acta 2021, 1166, 338574. [Google Scholar] [CrossRef] [PubMed]
- Meima, J.A.; Rammlmair, D.; Junge, M. The use of laser induced breakdown spectroscopy for the mineral chemistry of chromite, orthopyroxene and plagioclase from Merensky Reef and UG-2 chromitite, Bushveld Complex, South Africa. Chem. Geol. 2021, 589, 120686. [Google Scholar] [CrossRef]
- Soulamidis, G.; Jatteau, M.; Stouraiti, C.; Voudouris, P.; Mavrogonatos, C.; Soukis, K.; Fabre, C.; Caumon, M.C.; Cauzid, J.; Tarantola, A. Application of portable spectroscopic tools in the exploration of manganese oxide minerals: Preliminary results from the case study of Drama Mn-oxide deposits, northern Greece. Mater. Proc. 2023, 15, 54. [Google Scholar] [CrossRef]
- Sangster, D.F. Geology of base metal deposits. In Encyclopedia of Life Support Systems; Cholakov, G.S., Nath, B., Eds.; UNESCO-EOLLS Publishers: Oxford, UK, 2009; Volume 6, pp. 91–116. [Google Scholar]
- Ahmad, N.; Ahmed, R.; Umar, Z.A.; Liaqat, U.; Manzoor, U.; Baig, M.A. Qualitative and quantitative analyses of copper ores collected from Baluchistan, Pakistan using LIBS and LA-TOF-MS. Appl. Phys. B 2018, 124, 160–170. [Google Scholar] [CrossRef]
- Ahmad, A.; Hafeez, M.; Abbasi, S.A.; Khan, T.M.; Faruque, M.R.I.; Khandaker, M.U.; Ahmad, P.; Rafique, M.; Haleem, N. Compositional analysis of chalcopyrite using calibration-free laser-induced breakdown spectroscopy. Appl. Sci. 2020, 10, 6848. [Google Scholar] [CrossRef]
- Hafeez, M.; Abbasi, S.A.; Rafique, M.; Hayder, R.; Sajid, M.; Iqbal, J.; Ahmad, N.; Shahida, S. Calibration-free laser-induced breakdown spectroscopic analysis of copper-rich mineral collected from the Gilgit-Baltistan region of Pakistan. Appl. Opt. 2020, 59, 68–76. [Google Scholar] [CrossRef]
- Baele, J.M.; Bouzahzah, H.; Papier, S.; Decrée, S.; Verheyden, S.; Burlet, C.; Pirard, E.; Franceschi, G.; Dejonghe, L. Trace-element imaging at macroscopic scale in a Belgian sphalerite-galena ore using laser-induced breakdown spectroscopy (LIBS). Geol. Belg. 2021, 24, 125–137. [Google Scholar] [CrossRef]
- Álvarez, J.; Velásquez, M.; Sandoval-Muñoz, C.; Castillo, R.D.P.; Bastidas, C.Y.; Luarte, D.; Sbárbaro, D.; Rammlmair, D.; Yáñez, J. Improved mineralogical analysis in copper ores by laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2022, 37, 1994–2004. [Google Scholar] [CrossRef]
- Varotsis, C.; Tselios, C.; Yiannakkos, K.A.; Andreou, C.; Papageorgiou, M.; Nicolaides, A. Application of double-pulse laser-induced breakdown spectroscopy (DP-LIBS), Fourier transform infrared micro-spectroscopy and Raman microscopy for the characterization of copper-sulfides. RSC Adv. 2022, 12, 631–639. [Google Scholar] [CrossRef]
- Velásquez, M.; Álvarez, J.; Sandoval, C.; Ramírez, E.; Bravo, M.; Fuentes, R.; Myakalwar, A.K.; Castillo, R.; Luarte, D.; Sbarbaro, D.; et al. Improved elemental quantification in copper ores by laser-induced breakdown spectroscopy with judicious data processing. Spectrochim. Acta Part B At. Spectrom. 2022, 188, 106343. [Google Scholar] [CrossRef]
- Velásquez, M.; Myakalwar, A.K.; Manzoor, S.; Vadillo, J.M.; Laserna, J.; Yanez, J. Progress in arsenic determination at low levels in copper ores by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2022, 195, 106501. [Google Scholar] [CrossRef]
- Brinkmann, P.; Köllner, N.; Merk, S.; Beitz, T.; Altenberger, U.; Löhmannsröben, H.G. Comparison of handheld and echelle spectrometer to assess copper in ores by means of laser-induced breakdown spectroscopy (LIBS). Minerals 2023, 13, 113. [Google Scholar] [CrossRef]
- Łazarek, Ł.; Antończak, A.J.; Wójcik, M.R.; Drzymała, J.; Abramski, K.M. Evaluation of the laser-induced breakdown spectroscopy technique for determination of the chemical composition of copper concentrates. Spectrochim. Acta Part B At. Spectrom. 2014, 97, 74–78. [Google Scholar] [CrossRef]
- Ormachea, O.; Villazón, A.; Terceros, I. Analysis of mining ore concentrates with a low cost portable LIBS system. Investig. Desarrollo 2018, 18, 81–90. [Google Scholar] [CrossRef]
- Fuentes, R.; Luarte, D.; Sandoval, C.; Myakalwar, A.K.; Yáñez, J.; Sbarbaro, D. Data fusion of laser induced breakdown spectroscopy and diffuse reflectance for improved analysis of mineral species in copper concentrates. Miner. Eng. 2021, 173, 107193. [Google Scholar] [CrossRef]
- Fuentes, R.; Luarte, D.; Sandoval, C.; Myakalwar, A.K.; Alvarez, J.; Yáñez, J.; Sbarbaro, D. Laser-induced breakdown spectroscopy and hyperspectral imaging data fusion for improved mineralogical analysis of copper concentrates. IFAC-PapersOnLine 2022, 55, 85–90. [Google Scholar] [CrossRef]
- Koh, E.J.; Amini, E.; Spier, C.A.; McLachlan, G.J.; Xie, W.; Beaton, N. A mineralogy characterisation technique for copper ore in flotation pulp using deep learning machine vision with optical microscopy. Miner. Eng. 2024, 205, 108481. [Google Scholar] [CrossRef]
- Niobium and Tantalum Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/niobium-and-tantalum-statistics-and-information (accessed on 8 May 2024).
- Montague, D. Stolen goods: Coltan and conflict in the Democratic Republic of Congo. SAIS Rev. Intl. Aff. 2002, 22, 103. [Google Scholar] [CrossRef]
- Gäbler, H.E.; Melcher, F.; Graupner, T.; Bahr, A.; Sitnikova, M.A.; Henjes-Kunst, F.; Oberthür, T.; Brätz, H.; Gerdes, A. Speeding up the analytical workflow for Coltan fingerprinting by an integrated mineral liberation analysis/LA-ICP-MS approach. Geostand. Geoanal. Res. 2011, 35, 431–448. [Google Scholar] [CrossRef]
- Savu-Krohn, C.; Rantitsch, G.; Auer, P.; Melcher, F.; Graupner, T. Geochemical fingerprinting of coltan ores by machine learning on uneven datasets. Nat. Resour. Res. 2011, 20, 177–191. [Google Scholar] [CrossRef]
- Harmon, R.S.; Shughrue, K.M.; Remus, J.J.; Wise, M.A.; East, L.J.; Hark, R.R. Can the provenance of the conflict minerals columbite and tantalite be ascertained by laser-induced breakdown spectroscopy? Anal. Bioanal. Chem. 2011, 400, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Hark, R.R.; Remus, J.J.; East, L.J.; Harmon, R.S.; Wise, M.A.; Tansi, B.M.; Shughrue, K.M.; Dunsin, K.S.; Liu, C. Geographical analysis of “conflict minerals” utilizing laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2012, 74, 131–136. [Google Scholar] [CrossRef]
- Platinum-Group Elements. Available online: https://www.usgs.gov/publications/platinum-group-elements# (accessed on 8 May 2024).
- Maier, W.D. Platinum-group element (PGE) deposits and occurrences: Mineralization styles, genetic concepts, and exploration criteria. J. Afr. Earth Sci. 2005, 41, 165–191. [Google Scholar] [CrossRef]
- Rifai, K.; Özcan, L.Ç.; Doucet, F.R.; Rhoderick, K.; Vidal, F. Ultrafast elemental mapping of platinum group elements and mineral identification in platinum-palladium ore using laser induced breakdown spectroscopy. Minerals 2020, 10, 207. [Google Scholar] [CrossRef]
- Mohamed, N.; Rifai, K.; Selmani, S.; Constantin, M.; Doucet, F.R.; Özcan, L.Ç.; Sabsabi, M.; Vidal, F. Chemical and mineralogical mapping of platinum-group element ore samples using laser-induced breakdown spectroscopy and micro-X-ray fluorescence. Geostand. Geoanal. Res. 2021, 45, 539–550. [Google Scholar] [CrossRef]
- Selmani, S.; Mohamed, N.; Elhamdaoui, I.; Fernandes, J.; Bouchard, P.; Constantin, M.; Sabsabi, M.; Vidal, F. Laser-induced breakdown spectroscopy analysis of palladium in rock ore. Spectrochim. Acta Part B At. Spectrom. 2022, 196, 106523. [Google Scholar] [CrossRef]
- Elhamdaoui, I.; Selmani, S.; Sabsabi, M.; Constantin, M.; Bouchard, P.; Vidal, F. Quantifying platinum and palladium in solid ore using laser-induced breakdown spectroscopy assisted by the laser-induced fluorescence (LIBS-LIF) technique. Appl. Spectrosc. 2023, 77, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Selmani, S.; Elhamdaoui, I.; Mohamed, N.; Bouchard, P.; Constantin, M.; Sabsabi, M.; Vidal, F. Laser-produced craters in minerals of a palladium ore sample. Appl. Phys. A 2023, 129, 737. [Google Scholar] [CrossRef]
- Precious Metals: Definition, Properties, Uses, and Types. 2024. Available online: https://www.xometry.com/resources/materials/precious-metals/ (accessed on 4 July 2024).
- Díaz, D.; Hahn, D.W.; and Molina, A. Quantification of gold and silver in minerals by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2017, 136, 106–115. [Google Scholar] [CrossRef]
- Díaz, D.; Molina, A.; Hahn, D. Effect of laser irradiance and wavelength on the analysis of gold-and silver-bearing minerals with laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2018, 145, 86–95. [Google Scholar] [CrossRef]
- Fahad, M.; Ali, S.; Shahzad, A.; Shah, K.H. Determination of base metals and gold in Pb-Sb sulfosalt mineral boulangerite using laser-induced breakdown spectroscopy and inductively coupled plasma optical emission spectrometry. Laser Phys. 2020, 30, 095701. [Google Scholar] [CrossRef]
- Díaz, D.; Molina, A.; Hahn, D.W. Laser-induced breakdown spectroscopy and principal component analysis for the classification of spectra from gold-bearing ores. Appl. Spectrosc. 2020, 74, 42–54. [Google Scholar] [CrossRef]
- Elhamdaoui, I.; Rifai, K.; Iqbal, J.; Mohamed, N.; Selmani, S.; Fernandes, J.; Bouchard, P.; Constantin, M.; Laflamme, M.; Sabsabi, M.; et al. Measuring the concentration of gold in ore samples by laser-induced breakdown spectroscopy and comparison with the gravimetry/atomic absorption techniques. Spectrochim. Acta Part B At. Spectrom. 2021, 183, 106256. [Google Scholar] [CrossRef]
- Gervais, F.; Rifai, K.; Plamondon, P.; Özcan, L.; Doucet, F.; Vidal, F. Compositional tomography of a gold-bearing sample by laser-induced breakdown spectroscopy. Terra Nova 2019, 31, 479–484. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Hedenquist, J.W. Linkages between volcanotectonic settings, ore-fluid compositions, and epithermal precious metal deposits. Soc. Econ. Geol. Sp. Pub. 2003, 10, 315–343. [Google Scholar]
- Loen, J.S. Use of placer gold characteristics to locate bedrock gold mineralization. Explor. Mining Geol. 1995, 4, 335–339. [Google Scholar]
- Tin. Available online: https://www.rsc.org/periodic-table/element/50/tin (accessed on 4 July 2024).
- Rare Earth Elements. Available online: https://www.smenet.org/What-We-Do/Technical-Briefings/Rare-Earth-Elements (accessed on 10 May 2024).
- Abedin, K.M.; Haider, A.F.M.Y.; Rony, M.A.; Khan, Z.H. Identification of multiple rare earths and associated elements in natural monazite sands by laser induced breakdown spectroscopy. Opt. Laser Technol. 2011, 43, 45–49. [Google Scholar] [CrossRef]
- Romppanen, S.; Häkkänen, H.; Kaski, S. Singular value decomposition approach to the yttrium occurrence in mineral maps of rare earth element ores using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2017, 134, 69–74. [Google Scholar] [CrossRef]
- Bhatt, C.R.; Jain, J.C.; Goueguel, C.L.; McIntyre, D.L.; Singh, J.P. Determination of rare earth elements in geological samples using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2018, 72, 114–121. [Google Scholar] [CrossRef]
- Gaft, M.; Raichlin, Y.; Pelascini, F.; Panzer, G.; Ros, V.M. Imaging rare-earth elements in minerals by laser-induced plasma spectroscopy: Molecular emission and plasma-induced luminescence. Spectrochim. Acta Part B At. Spectrom. 2019, 151, 12–19. [Google Scholar] [CrossRef]
- Manard, B.T.; Wylie, E.M.; Willson, S.P. Analysis of rare earth elements in uranium using handheld laser-induced breakdown spectroscopy (HH LIBS). Appl. Spectrosc. 2018, 72, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, C.R.; Hartzler, D.; Jain, J.C.; McIntyre, D.L. Evaluation of analytical performance of double pulse laser-induced breakdown spectroscopy for the detection of rare earth elements. Opt. Laser Technol. 2020, 126, 106110. [Google Scholar] [CrossRef]
- Afgan, M.S.; Hou, Z.; Song, W.; Liu, J.; Song, Y.; Gu, W.; Wang, Z. On the spectral identification and wavelength dependence of rare-earth ore emission by laser-induced breakdown spectroscopy. Chemosensors 2022, 10, 350. [Google Scholar] [CrossRef]
- Akhmetzhanov, T.F.; Popov, A.M. Direct determination of lanthanides by LIBS in REE-rich ores: Comparison between univariate and DoE based multivariate calibrations with respect to spectral resolution. J. Anal. At. Spectrom. 2022, 37, 2330–2339. [Google Scholar] [CrossRef]
- Li, J.; Tang, Z.; Hao, Z.; Huang, Z.; Zhu, C.; Li, Q.; He, B.; Chen, J.; Li, X. Determination of La in ore using laser-induced breakdown spectroscopy combined with laser-induced fluorescence and standard addition method. J. Anal. At. Spectrom. 2022, 37, 2176–2181. [Google Scholar] [CrossRef]
- Akhmetzhanov, T.F.; Labutin, T.A.; Korshunov, D.M.; Samsonov, A.A.; Popov, A.M. Determination of Ce and La in REE-rich ores using handheld LIBS and PLS regression. J. Anal. At. Spectrom. 2023, 38, 2134–2143. [Google Scholar] [CrossRef]
- Deng, Z.; Hao, Z.; Liu, L.; Xu, Z.; Zhao, Z.; Lu, Y.; Shi, J.; He, X. Detection of Y, La, Yb, and Dy elements in rare earth ores by double-pulse laser-induced breakdown spectroscopy. J. Laser Appl. 2023, 35, 022003. [Google Scholar] [CrossRef]
- Fayyaz, A.; Asghar, H.; Alshehri, A.M.; Alrebdi, T.A. LIBS assisted PCA analysis of multiple rare-earth elements (La, Ce, Nd, Sm, and Yb) in phosphorite deposits. Heliyon 2023, 9, e13957. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, A.; Ali, R.; Waqas, M.; Liaqat, U.; Ahmad, R.; Umar, Z.A.; Baig, M.A. Analysis of rare earth ores using laser-induced breakdown spectroscopy and laser ablation time-of-flight mass spectrometry. Minerals 2023, 13, 787. [Google Scholar] [CrossRef]
- Liu, X.; Yan, C.; An, D.; Yue, C.; Zhang, T.; Tang, H.; Li, H. Rapid quantitative analysis of rare earth elements Lu and Y in rare earth ores by laser induced breakdown spectroscopy combined with iPLS-VIP and partial least squares. RSC Adv. 2023, 13, 15347–15355. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Yang, C.; Gao, J.; Zhu, Z.; Sun, S.; Liu, X.; Liu, Z. Determination of La in rare earth ores using laser-induced breakdown spectroscopy combined with bidirectional long short-term memory. Appl. Phys. B 2024, 130, 1–9. [Google Scholar] [CrossRef]
- Uranium Overview. Available online: https://www.cameco.com/uranium_101/uranium-overview (accessed on 28 April 2024).
- Uranium. Available online: https://www.rsc.org/periodic-table/element/92/uranium (accessed on 10 May 2024).
- Sirven, J.B.; Pailloux, A.; M’Baye, Y.; Coulon, N.; Alpettaz, T.; Gosse, S. Towards the determination of the geographical origin of yellow cake samples by laser-induced breakdown spectroscopy and chemometrics. J. Anal. At. Spectrom. 2009, 24, 451–459. [Google Scholar] [CrossRef]
- Kim, Y.S.; Han, B.Y.; Shin, H.S.; Kim, H.D.; Jung, E.C.; Jung, J.H.; Na, S.H. Determination of uranium concentration in an ore sample using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2012, 74, 190–193. [Google Scholar] [CrossRef]
- Barefield II, J.E.; Judge, E.J.; Campbell, K.R.; Colgan, J.P.; Kilcrease, D.P.; Johns, H.M.; Wiens, R.C.; McInroy, R.E.; Martinez, R.K.; Clegg, S.M. Analysis of geological materials containing uranium using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2016, 120, 1–8. [Google Scholar] [CrossRef]
- Ji, J.; Song, W.; Hou, Z.; Li, L.; Yu, X.; Wang, Z. Raw signal improvement using beam shaping plasma modulation for uranium detection in ore using laser-induced breakdown spectroscopy. Anal. Chim. Acta. 2022, 1235, 340551. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Tang, Z.; Zhou, R.; Yan, J.; Zhu, C.; Liu, K.; Li, X.; Zeng, X. Determination of uranium in ores using laser-induced breakdown spectroscopy combined with laser-induced fluorescence. J. Anal. At. Spectrom. 2020, 35, 626–631. [Google Scholar] [CrossRef]
- Klus, J.; Mikysek, P.; Prochazka, D.; Pořízka, P.; Prochazková, P.; Novotný, J.; Trojek, T.; Novotný, K.; Slobodník, M.; Kaiser, J. Multivariate approach to the chemical mapping of uranium in sandstone-hosted uranium ores analyzed using double pulse laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2016, 123, 143–149. [Google Scholar] [CrossRef]
- Holá, M.; Novotný, K.; Dobeš, J.; Krempl, I.; Wertich, V.; Mozola, J.; Kubeš, M.; Faltusová, V.; Leichmann, J.; and Kanický, V. Dual imaging of uranium ore by laser ablation inductively coupled plasma mass spectrometry and laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrom. 2021, 186, 106312. [Google Scholar] [CrossRef]
- Krempl, I.; Novotný, K.; Wertich, V.; Škoda, R.; Kanický, V.; Leichmann, J. Distinguishing secondary uranium mineralizations in uranium ore using LIBS imaging. Spectrochim. Acta Part B At. Spectrom. 2023, 206, 1067340. [Google Scholar] [CrossRef]
- Andrews, H.B.; Quarles, C.D., Jr.; Bradley, V.C.; Spano, T.L.; Petrus, J.A.; Paul, B.; Zirakparvar, N.A.; Dunlap, D.R.; Hexel, C.R.; Manard, B.T. Advancing elemental and isotopic analysis of uranium mineral inclusions: Rapid screening via laser-induced breakdown spectroscopy and high-resolution laser ablation-ICP-MS mapping. Microchem. J. 2024, 196, 109605. [Google Scholar] [CrossRef]
- Gibaga, C.R.L.; Montano, M.O.; Samaniego, J.O.; Tanciongco, A.M.; Quierrez, R.N.M.; Gervasio, J.H.C.; Reyes, R.C.G.; Peralta, M.J.V.; Catague, G.V., Jr. Comparative study on determination of critical minerals in Ni laterites using handheld LIBS, handheld XRF, and ICP-MS. Philipp J. Sci. 2023, 151, 1595–1600. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, Y.; Yan, J.; Wu, Z.; Li, Y. Classification and discrimination of minerals using laser induced breakdown spectroscopy and Raman spectroscopy. Plasma Sci. Technol. 2015, 17, 923. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, J.; Li, Y.; Lu, Y.; Guo, J.; Tian, Y.; Ren, L. Mid-level data fusion of Raman spectroscopy and laser-induced breakdown spectroscopy: Improving ores identification accuracy. Anal. Chim. Acta 2023, 1240, 340772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, F.; Jiang, X.; Wu, S.; Xu, M. On-stream mineral identification of tailing slurries of tungsten via NIR and XRF data fusion measurement techniques. Anal. Methods 2020, 12, 3296–3307. [Google Scholar] [CrossRef]
- Bol’shakov, A.A.; Mao, X.; González, J.J.; Russo, R.E. Laser ablation molecular isotopic spectrometry (LAMIS): Current state of the art. J. Anal. At. Spectrom. 2016, 31, 119–134. [Google Scholar] [CrossRef]




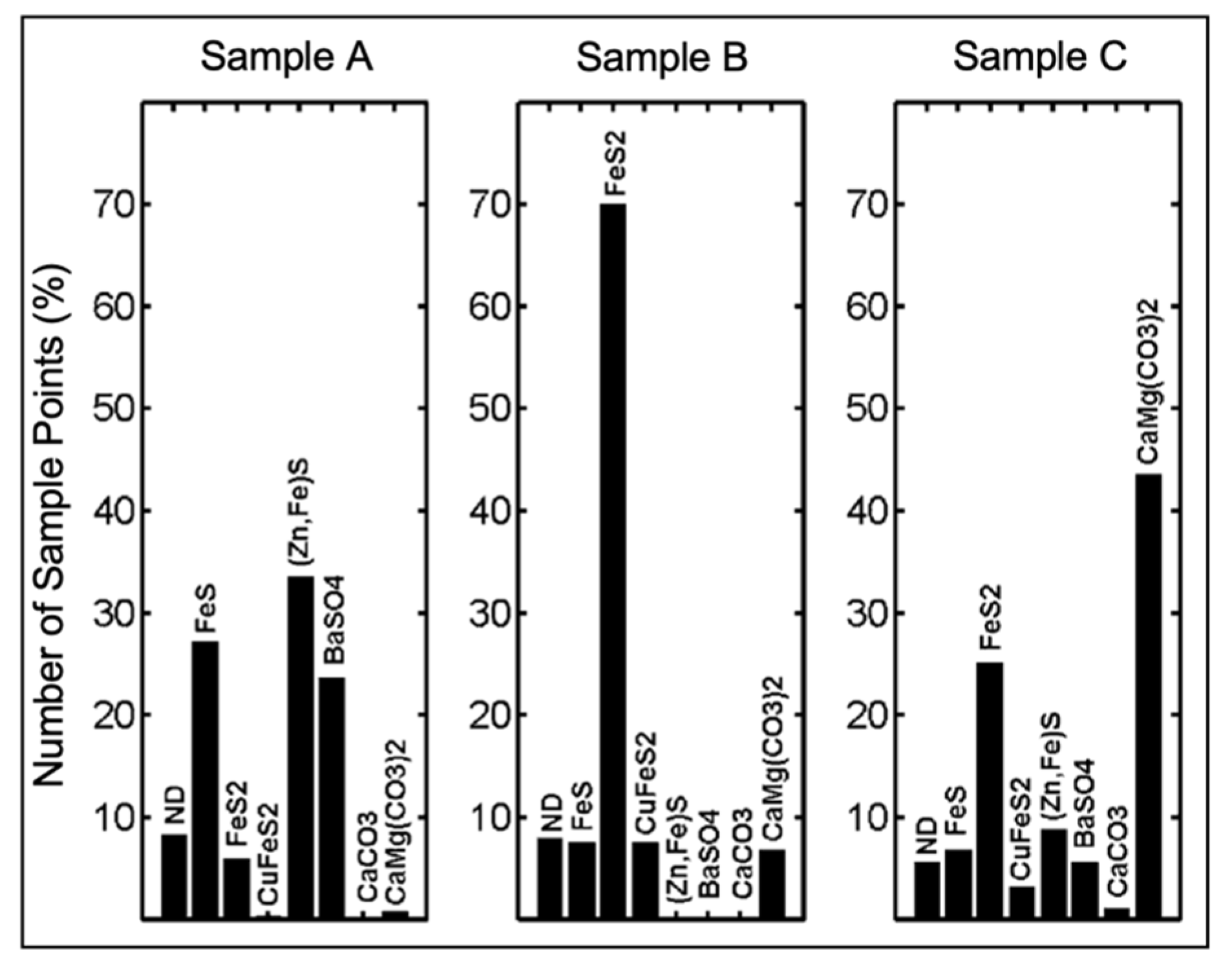
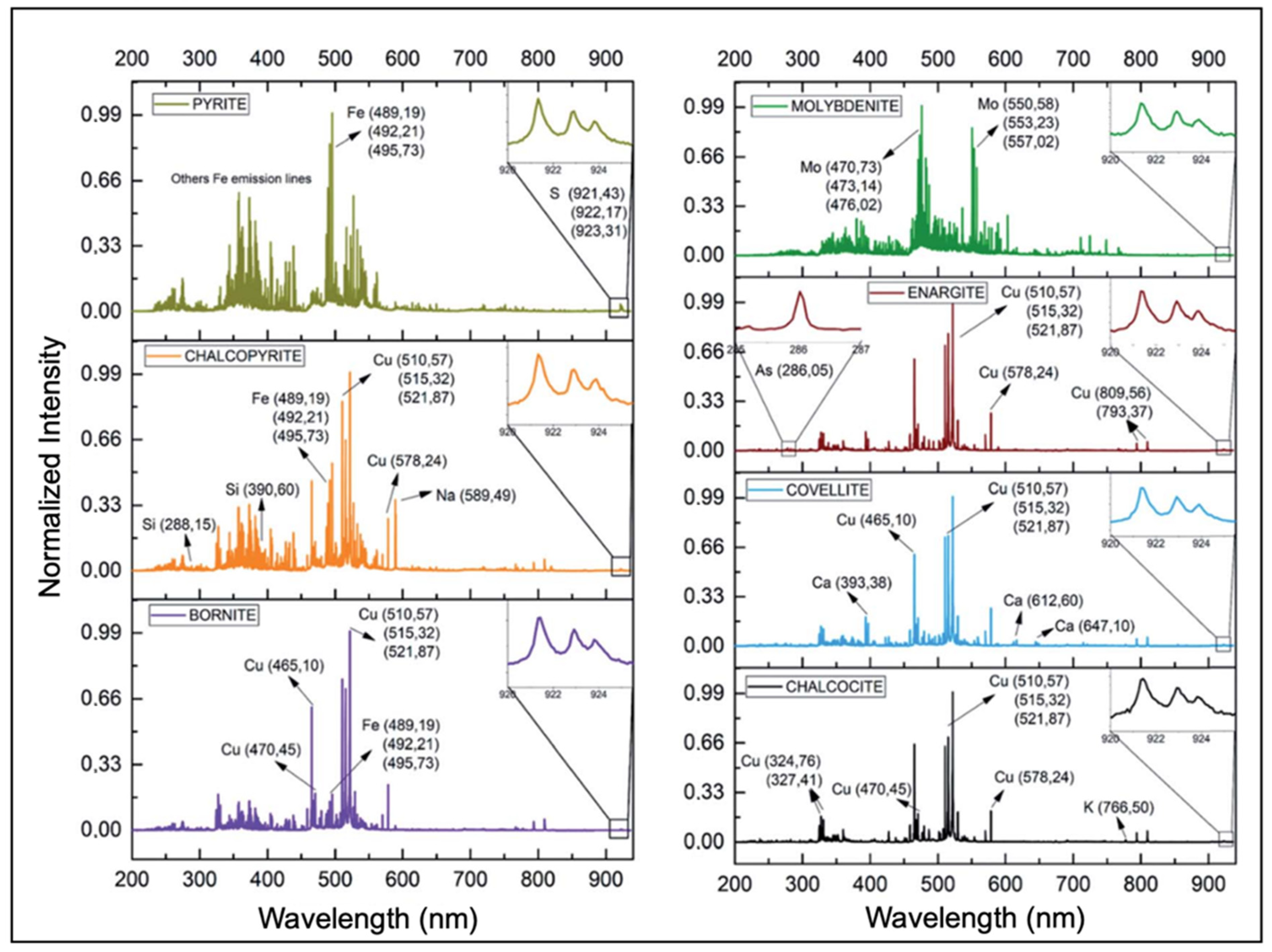
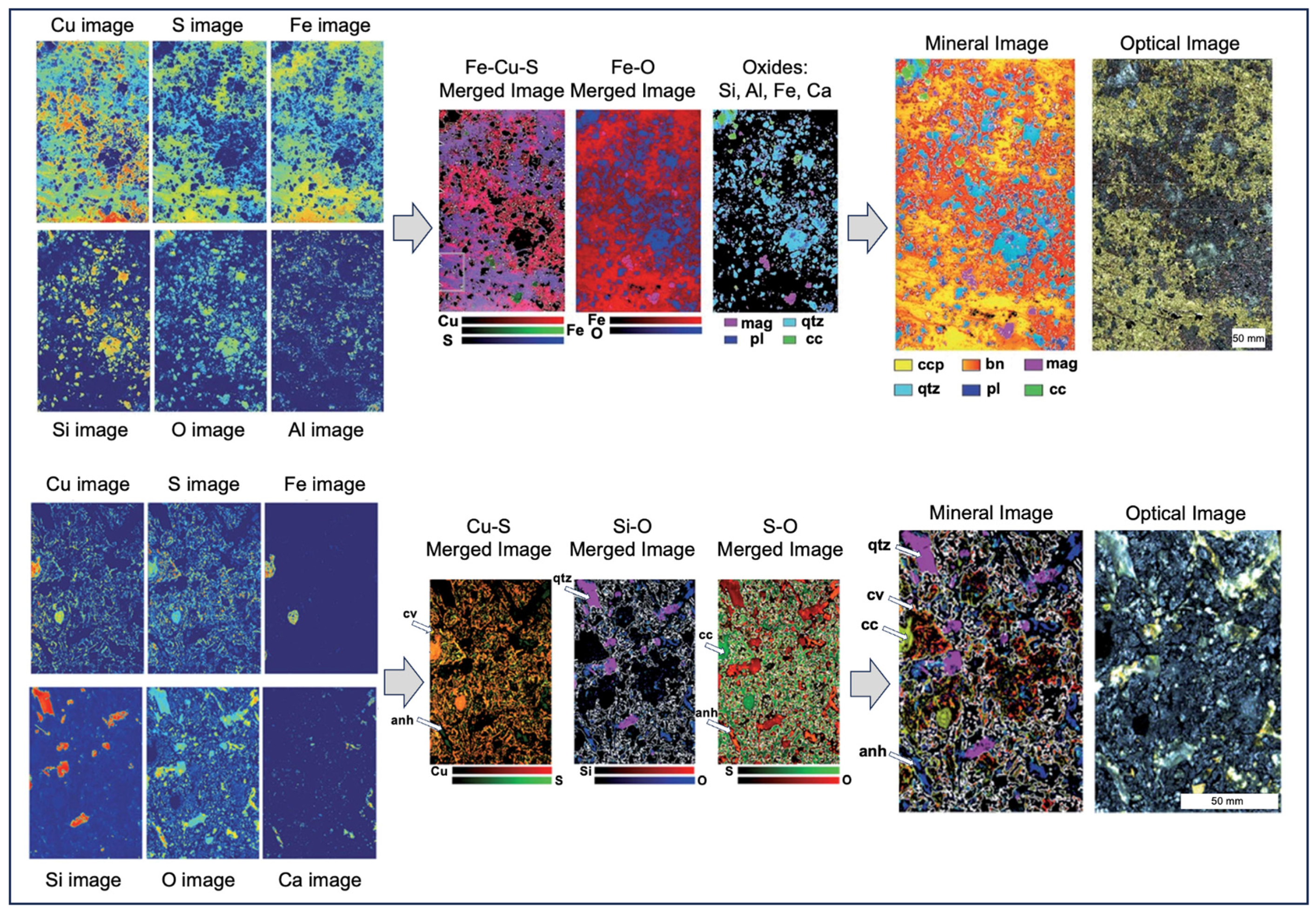


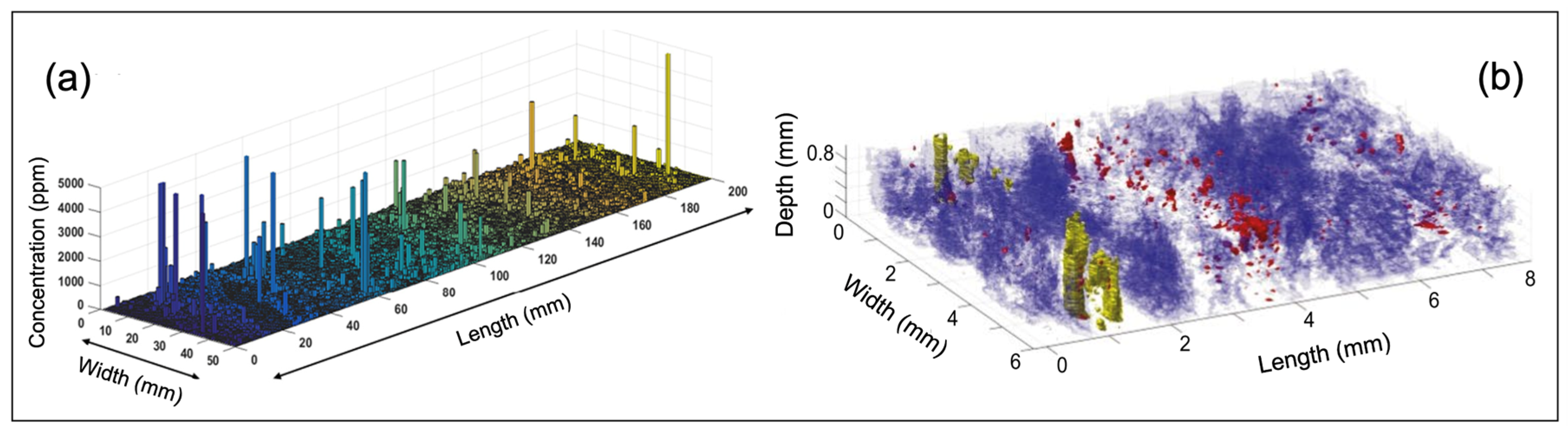



| System architecture | Simple, requiring only a laser, optics, detector/spectrograph, and computer, so is less expensive than many other analytical technologies |
| Analytical capability | Full periodic table, but particularly sensitive to the light elements H, Li, Be, B, and C and with isotopic analysis demonstrated for certain elements |
| Application | Solids, liquids, or gases in the laboratory or outside in the ambient environment for in situ field analysis |
| Analytical time | Rapid multi-element analysis (<1 s) |
| Surface analysis | In situ analysis of ultra-thin alteration layers, coatings, and contamination on a sample surface |
| Preparation | Little to no sample preparation needed. The LIBS plasma shock wave can be used to remove allochthonous surface materials and contamination by ‘cleaning’ shots prior to analysis |
| Depth analysis | Stratigraphic profiling to >100 mm depth and in situ analysis of individual particles and mineral grains as well as both liquid and solid inclusions |
| Imaging | Megapixel compositional imaging of rock and mineral surfaces at tens of μm spatial resolution |
| Deployment | As laboratory instrumentation, field-portable and handheld analyzers, and as bespoke systems for use in harsh industrial settings and geologically extreme environments |
| Integration | Easily combined with other analytical techniques such as LIF, RS, IR, µXRF, or LA-ICP-MS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmon, R.S. Laser-Induced Breakdown Spectroscopy in Mineral Exploration and Ore Processing. Minerals 2024, 14, 731. https://doi.org/10.3390/min14070731
Harmon RS. Laser-Induced Breakdown Spectroscopy in Mineral Exploration and Ore Processing. Minerals. 2024; 14(7):731. https://doi.org/10.3390/min14070731
Chicago/Turabian StyleHarmon, Russell S. 2024. "Laser-Induced Breakdown Spectroscopy in Mineral Exploration and Ore Processing" Minerals 14, no. 7: 731. https://doi.org/10.3390/min14070731





