Selective Inhibition Mechanisms of Fe(III) in the Flotation of Lepidolite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Micro-Flotation
2.3. Contact Angle
2.4. Zeta Potential
2.5. Fe(III) Adsorption Amount
2.6. Fourier-Transform Infrared Spectroscopy
2.7. X-ray Photoelectron Spectroscopy
3. Results and Discussion
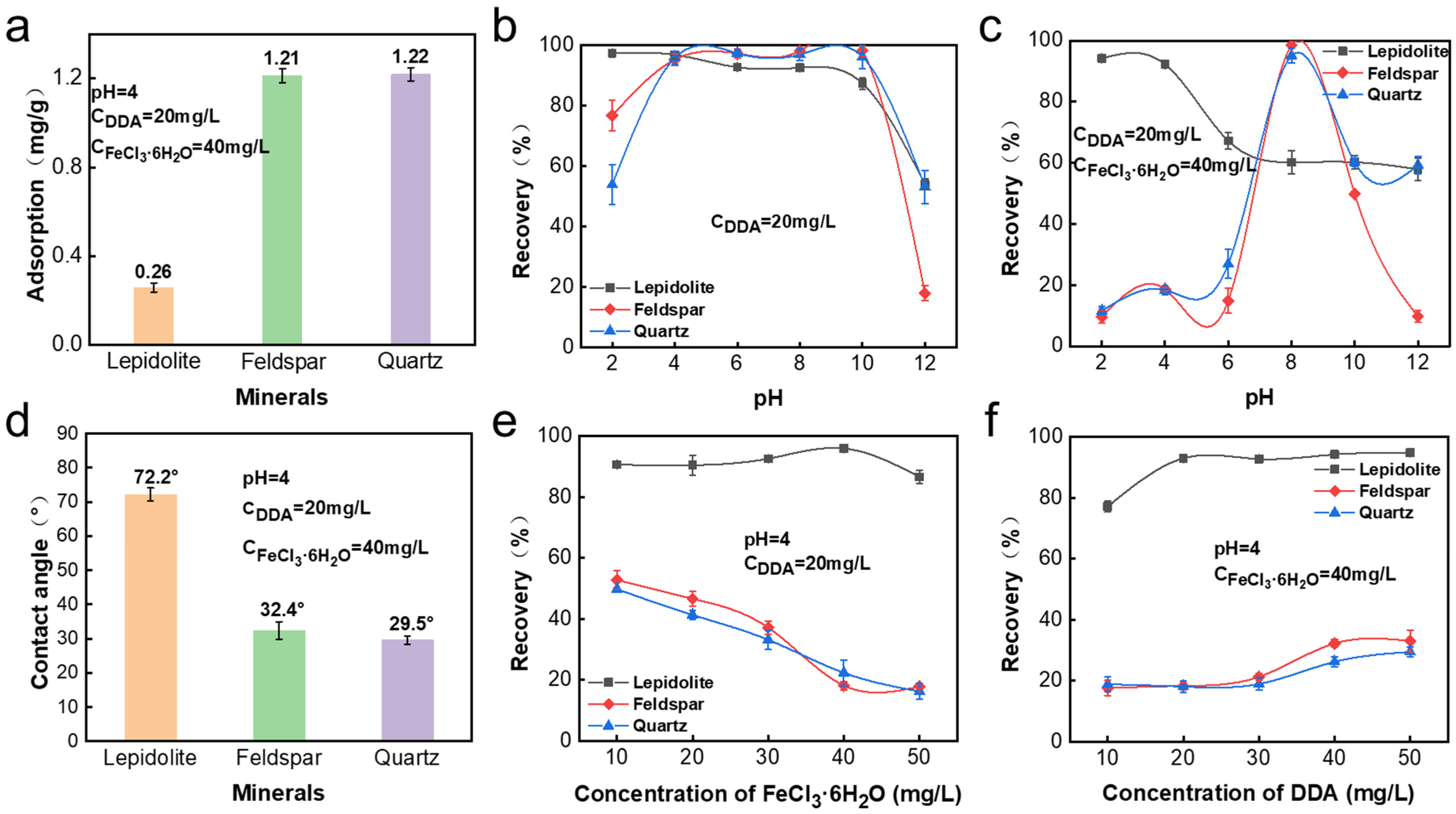
3.1. Adsorption Amounts of Fe(III)
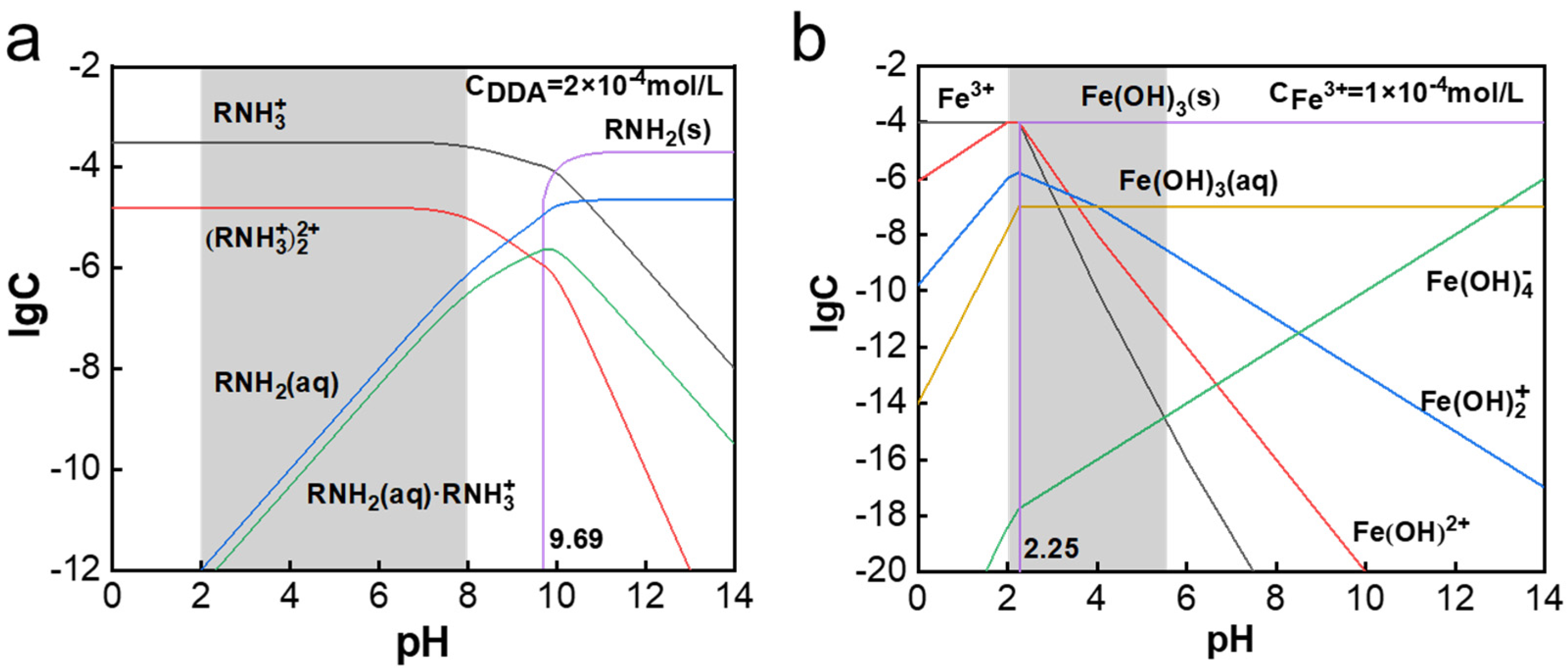
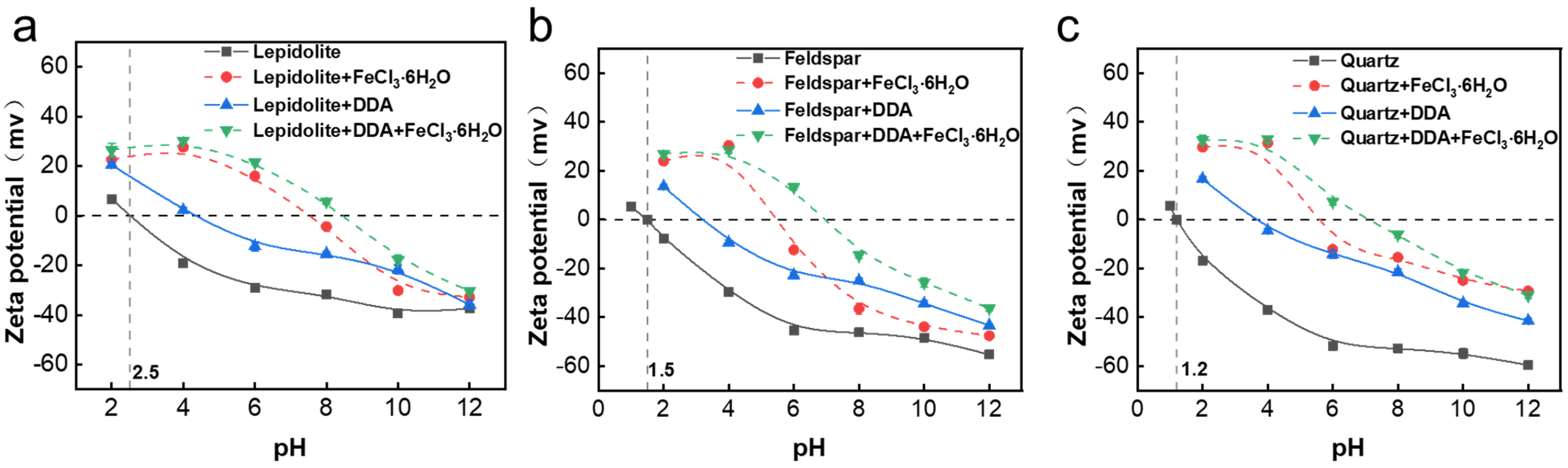
3.2. Solution Speciation and Zeta Potential Results
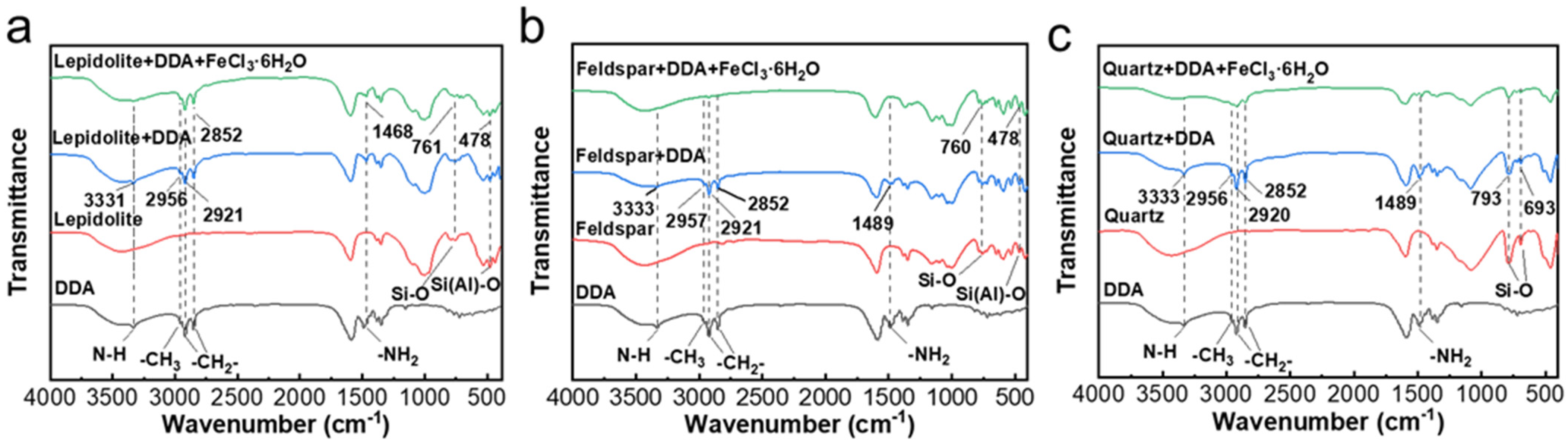
3.3. FTIR Results
3.4. XPS Results
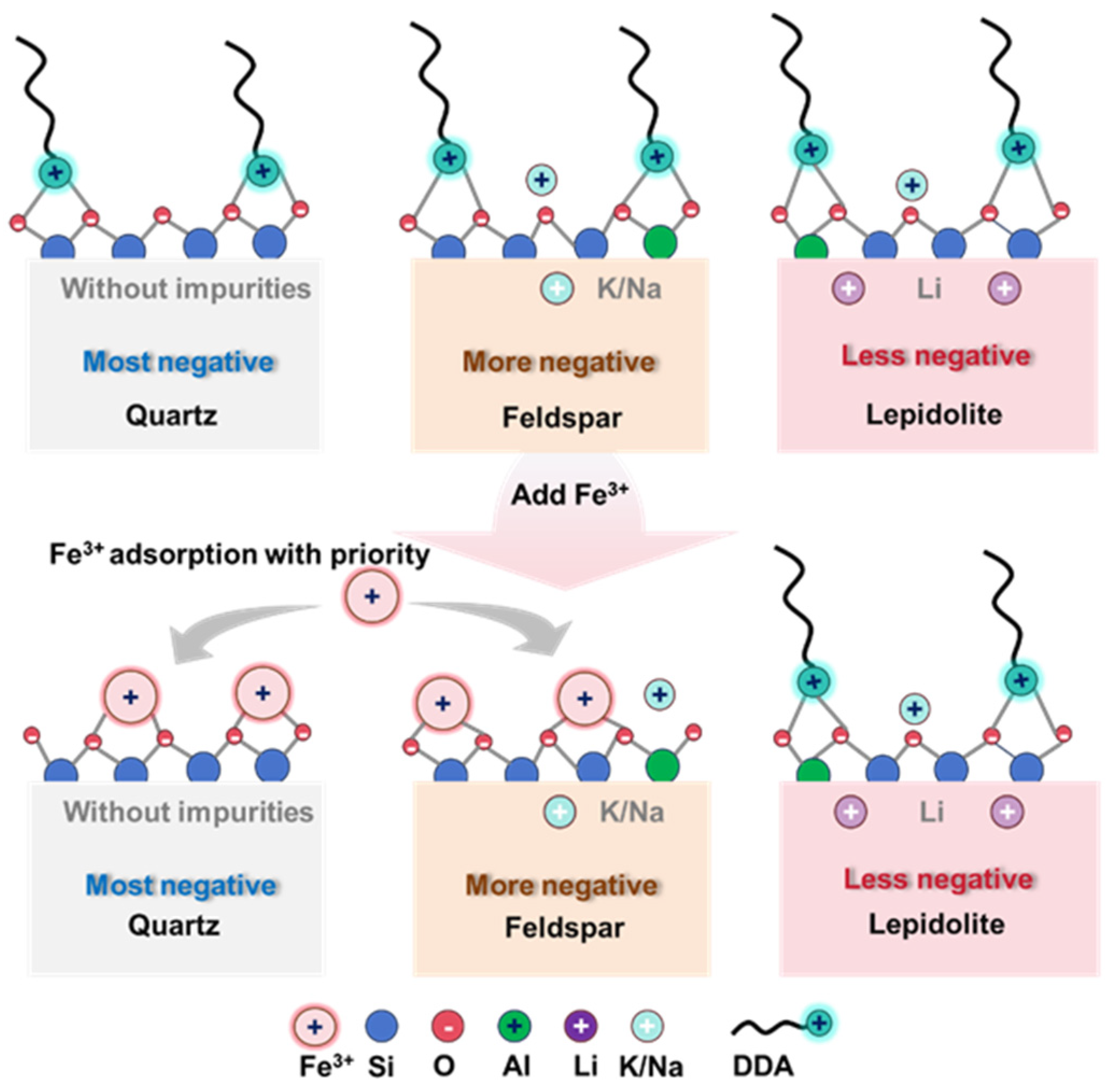
3.5. Selective Depressing Mechanism of Fe(III)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maisel, F.; Neef, C.; Marscheider-Weidemann, F.; Nissen, N.F. A Forecast on Future Raw Material Demand and Recycling Potential of Lithium-Ion Batteries in Electric Vehicles. Resour. Conserv. Recycl. 2023, 192, 106920. [Google Scholar] [CrossRef]
- Korbel, C.; Filippova, I.V.; Filippov, L.O. Froth Flotation of Lithium Micas—A Review. Miner. Eng. 2023, 192, 107986. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a Low-Carbon Society: A Review of Lithium Resource Availability, Challenges and Innovations in Mining, Extraction and Recycling, and Future Perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, Z.; Tong, X.; Xie, X.; Song, Q.; Fan, P. Review of the Research on the Development and Utilization of Clay-Type Lithium Resources. Particuology 2024, 87, 46–53. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Kuang, G. Recovery of Lithium from Mineral Resources: State-of-the-Art and Perspectives—A Review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y.; Wang, X.; Shumin, Z. Research Status of Spodumene Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2021, 42, 321–334. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The Beneficiation of Lithium Minerals from Hard Rock Ores: A Review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Z.; Wang, H.; Liu, R.; Cheng, C.; Shuai, S.; Hu, Y.; Guo, Z.; Yu, X.; He, G.; et al. Flotation Performance of a Novel Gemini Collector for Kaolinite at Low Temperature. Int. J. Min. Sci. Technol. 2021, 31, 1145–1152. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. Pradip A Century of Research Leading to Understanding the Scientific Basis of Selective Mineral Flotation and Design of Flotation Collectors. Min. Metall. Explor. 2019, 36, 3–20. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Adsorption of Trisiloxane Surfactant for Selective Flotation of Scheelite from Calcite at Room Temperature. Langmuir 2022, 38, 9010–9020. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Xu, L.; Wu, H.; Xu, Y.; Luo, L.; Yang, J.; Tang, Z.; Wang, Z. In Situ Adsorption of Mixed Anionic/Cationic Collectors in a Spodumene-Feldspar Flotation System: Implications for Collector Design. Langmuir 2020, 36, 8086–8099. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Yang, S.; Liu, C.; Xu, Y. Investigations on the Reverse Flotation of Quartz from Hematite Using Carboxymethyl Chitosan as a Depressant. Powder Technol. 2021, 393, 109–115. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Huang, G.; Li, C. Low-Temperature Performance of Cationic Collector Undecyl Propyl Ether Amine for Ilmenite Flotation. Miner. Eng. 2017, 114, 50–56. [Google Scholar] [CrossRef]
- Yang, B.; Fu, Y.F.; Yin, W.Z.; Sheng, Q.Y.; Zhu, Z.L.; Yin, X.M. Selective Collection Performance of an Efficient Quartz Collector and Its Response to Flotation Separation of Malachite from Quartz. Miner. Eng. 2021, 172, 107174. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice Flotation of Industrial Minerals(II). In Handbook of Flotation Reagents: Chemistry, Theory and Practice Flotation of Sulfide Ores; Elsevier Science & Technology Books: Peterborough, UK, 2015; p. 459. ISBN 9780444530295. [Google Scholar]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and Adsorption of Mixed Cationic/Anionic Collectors on Muscovite Mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Wei, Q.; Feng, L.; Dong, L.; Jiao, F.; Qin, W. Selective Co-Adsorption Mechanism of a New Mixed Collector on the Flotation Separation of Lepidolite from Quartz. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125973. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, S.; Cheng, C.; Wang, H.; Liu, R.; Hu, Y.; He, G.; Yu, X.; Fu, W. Recycling Lepidolite from Tantalum-Niobium Mine Tailings by a Combined Magnetic-Flotation Process Using a Novel Gemini Surfactant: From Tailings Dams to the “Bling” Raw Material of Lithium. ACS Sustain. Chem. Eng. 2020, 8, 18206–18214. [Google Scholar] [CrossRef]
- Marion, C.; Li, R.; Waters, K.E. A Review of Reagents Applied to Rare-Earth Mineral Flotation. Adv. Colloid. Interface Sci. 2020, 279, 102142. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Zheng, R.; Jin, Y.; Niu, C. Flotation Studies of Fluorite and Barite with Sodium Petroleum Sulfonate and Sodium Hexametaphosphate. J. Mater. Res. Technol. 2019, 8, 1267–1273. [Google Scholar] [CrossRef]
- Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J.S. Sodium Hexametaphosphate and Sodium Silicate as Dispersants to Reduce the Negative Effect of Kaolinite on the Flotation of Chalcopyrite in Seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Kang, J.; Sun, W.; Hu, Y.; Gao, Z.; Liu, R.; Zhang, Q.; Liu, H.; Meng, X. The Utilization of Waste By-Products for Removing Silicate from Mineral Processing Wastewater via Chemical Precipitation. Water Res. 2017, 125, 318–324. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sun, W.; Chen, D.; Gao, Z.; Zhang, C. Interface Interaction of Benzohydroxamic Acid with Lead Ions on Oxide Mineral Surfaces: A Coordination Mechanism Study. Langmuir 2021, 37, 3490–3499. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sun, W.; Zeng, H.; Fan, R.; Hu, W.; Gao, Z. Unraveling Roles of Lead Ions in Selective Flotation of Scheelite and Fluorite from Atomic Force Microscopy and First-Principles Calculations. Miner. Eng. 2022, 179, 107424. [Google Scholar] [CrossRef]
- Miao, Y.; He, J.; Zhu, X.; Zhu, G.; Cao, S.; Fan, G.; Li, G.; Cao, Y. Hardness of Surface Hydroxyls and Its Pivotal Role in the Flotation of Cassiterite from Quartz via Lead Ions Activation. Sep. Purif. Technol. 2024, 347, 127565. [Google Scholar] [CrossRef]
- Hu, Y.; He, J.; Zhang, C.C.; Zhang, C.C.; Sun, W.; Zhao, D.; Chen, P.; Han, H.; Gao, Z.; Liu, R.; et al. Insights into the Activation Mechanism of Calcium Ions on the Sericite Surface: A Combined Experimental and Computational Study. Appl. Surf. Sci. 2018, 427, 162–168. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, H.; Qin, W.; Zhang, M.; Li, Y.; Wei, P. Inhibition Mechanism of Ca2+, Mg2+ and Fe3+ in Fine Cassiterite Flotation Using Octanohydroxamic Acid. R. Soc. Open Sci. 2018, 5, 180158. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Hu, Y. Activation Mechanism of Fe (III) Ions in Cassiterite Flotation with Benzohydroxamic Acid Collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Miller, J.D.; Pray, R.E.; Perinne, B.F. Metal Ion Activation in Xanthate Flotation of Quartz. Min. Eng. 1966, 235, 359–365. [Google Scholar]
- He, J.; Zhang, Y.; Wang, F.; Wu, Y.; Cao, Y.; Li, G.; Gao, Z. Interaction Mechanisms of Typical Collectors with Zircon Surfaces and Their Influence on Surface Morphology and Hydrophobicity for Selective Flotation. Appl. Surf. Sci. 2024, 648, 158941. [Google Scholar] [CrossRef]
- Christmann, P.; Gloaguen, E.; Labbé, J.F.; Melleton, J.; Piantone, P. Global Lithium Resources and Sustainability Issues. In Lithium Process Chemistry: Resources, Extraction, Batteries, and Recycling; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Fuerstenau, D.W. Pradip Zeta Potentials in the Flotation of Oxide and Silicate Minerals. Adv. Colloid. Interface Sci. 2005, 114–115, 9–26. [Google Scholar] [CrossRef]
- Berger, O.; Ortial, S.; Wein, S.; Denoyelle, S.; Bressolle, F.; Durand, T.; Escale, R.; Vial, H.J.; Vo-Hoang, Y. Evaluation of Amidoxime Derivatives as Prodrug Candidates of Potent Bis-Cationic Antimalarials. Bioorg Med. Chem. Lett. 2019, 29, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wen, S.; Feng, Q.; Zhang, Q.; Wang, Y.; Zhou, Y.; Nie, W. Mechanism of Depression by Fe3+ During Hemimorphite Flotation. Minerals 2020, 10, 790. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, Z.; Luo, H.; Xiao, C.; Zhou, F.; Chi, R. Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz. Minerals 2018, 8, 141. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y. New Insights into the Dodecylamine Adsorption on Scheelite and Calcite: An Adsorption Model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Lopez-Valdivieso, A.; Fuerstenau, D.W. Role of Hydrolyzed Cations in the Natural Hydrophobicity of Talc. Int. J. Miner. Process 1988, 23, 161–170. [Google Scholar] [CrossRef]
- Zhang, H.; Chai, W.; Cao, Y. Flotation Separation of Quartz from Gypsum Using Benzyl Quaternary Ammonium Salt as Collector. Appl. Surf. Sci. 2022, 576, 151834. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Hu, Y.; Yu, X.; He, G.; Fu, W. Froth Flotation Separation of Lepidolite Ore Using a New Gemini Surfactant as the Flotation Collector. Sep. Purif. Technol. 2022, 282, 119122. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. A Self-Assembly Mixed Collector System and the Mechanism for the Flotation Separation of Spodumene from Feldspar and Quartz. Miner. Eng. 2021, 171, 107082. [Google Scholar] [CrossRef]
- Keszthelyi, T.; Pászti, Z.; Rigó, T.; Hakkel, O.; Telegdi, J.; Guczi, L. Investigation of Solid Surfaces Modified by Langmuir-Blodgett Monolayers Using Sum-Frequency Vibrational Spectroscopy and X-Ray Photoelectron Spectroscopy. J. Phys. Chem. B 2006, 110, 8701–8714. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation Mechanism of Lead Ions in Cassiterite Flotation with Salicylhydroxamic Acid as Collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Pongpaiboonkul, S.; Phokharatkul, D.; Hodak, J.H.; Wisitsoraat, A.; Hodak, S.K. Enhancement of H2S-Sensing Performances with Fe-Doping in CaCu3Ti4O12 Thin Films Prepared by a Sol-Gel Method. Sens. Actuators B Chem. 2016, 224, 118–127. [Google Scholar] [CrossRef]
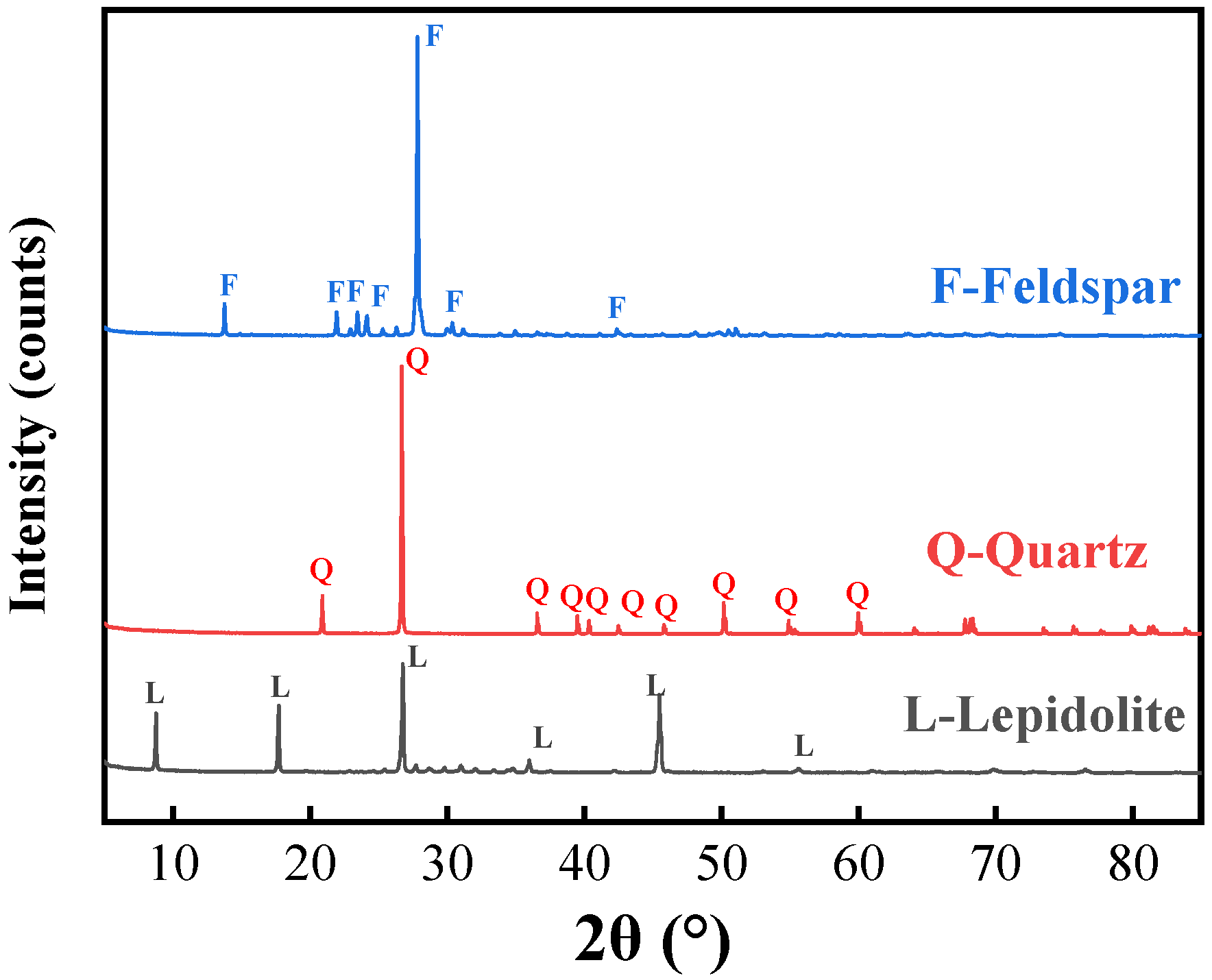

| Composition | Li2O | SiO2 | Al2O3 | K2O | Rb2O | MnO | Na2O | Cs2O | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|
| Weight(%) | 7.20 | 52.62 | 21.13 | 13.02 | 2.02 | 1.13 | 0.36 | 0.23 | 0.21 |
| Composition | SiO2 | Al2O3 | Na2O | CaO | K2O |
|---|---|---|---|---|---|
| Weight(%) | 68.69 | 18.16 | 12.33 | 0.42 | 0.11 |
| Composition | SiO2 | Al2O3 | P2O5 | ZnO | CaO |
|---|---|---|---|---|---|
| Weight(%) | 99.57 | 0.19 | 0.04 | 0.04 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, L.; Zhang, J.; Cao, Y.; He, J.; Li, G. Selective Inhibition Mechanisms of Fe(III) in the Flotation of Lepidolite. Minerals 2024, 14, 851. https://doi.org/10.3390/min14090851
Wang F, Liu L, Zhang J, Cao Y, He J, Li G. Selective Inhibition Mechanisms of Fe(III) in the Flotation of Lepidolite. Minerals. 2024; 14(9):851. https://doi.org/10.3390/min14090851
Chicago/Turabian StyleWang, Feifan, Lei Liu, Jingjing Zhang, Yijun Cao, Jianyong He, and Guosheng Li. 2024. "Selective Inhibition Mechanisms of Fe(III) in the Flotation of Lepidolite" Minerals 14, no. 9: 851. https://doi.org/10.3390/min14090851
APA StyleWang, F., Liu, L., Zhang, J., Cao, Y., He, J., & Li, G. (2024). Selective Inhibition Mechanisms of Fe(III) in the Flotation of Lepidolite. Minerals, 14(9), 851. https://doi.org/10.3390/min14090851








