Sulfidation of Smithsonite via Microwave Roasting under Low-Temperature Conditions
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
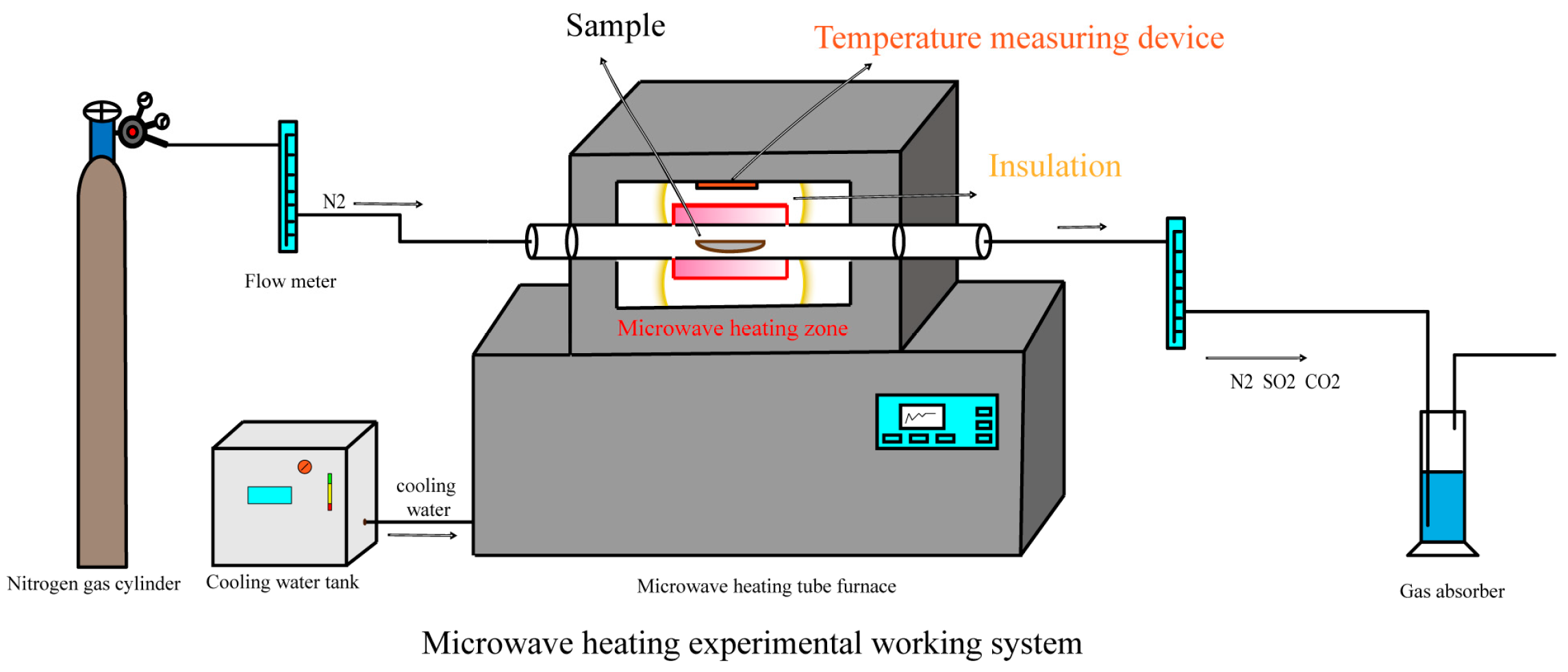
2.2. Experimental Device and Operation Method
2.3. Characterization Method
3. Results and Discussion
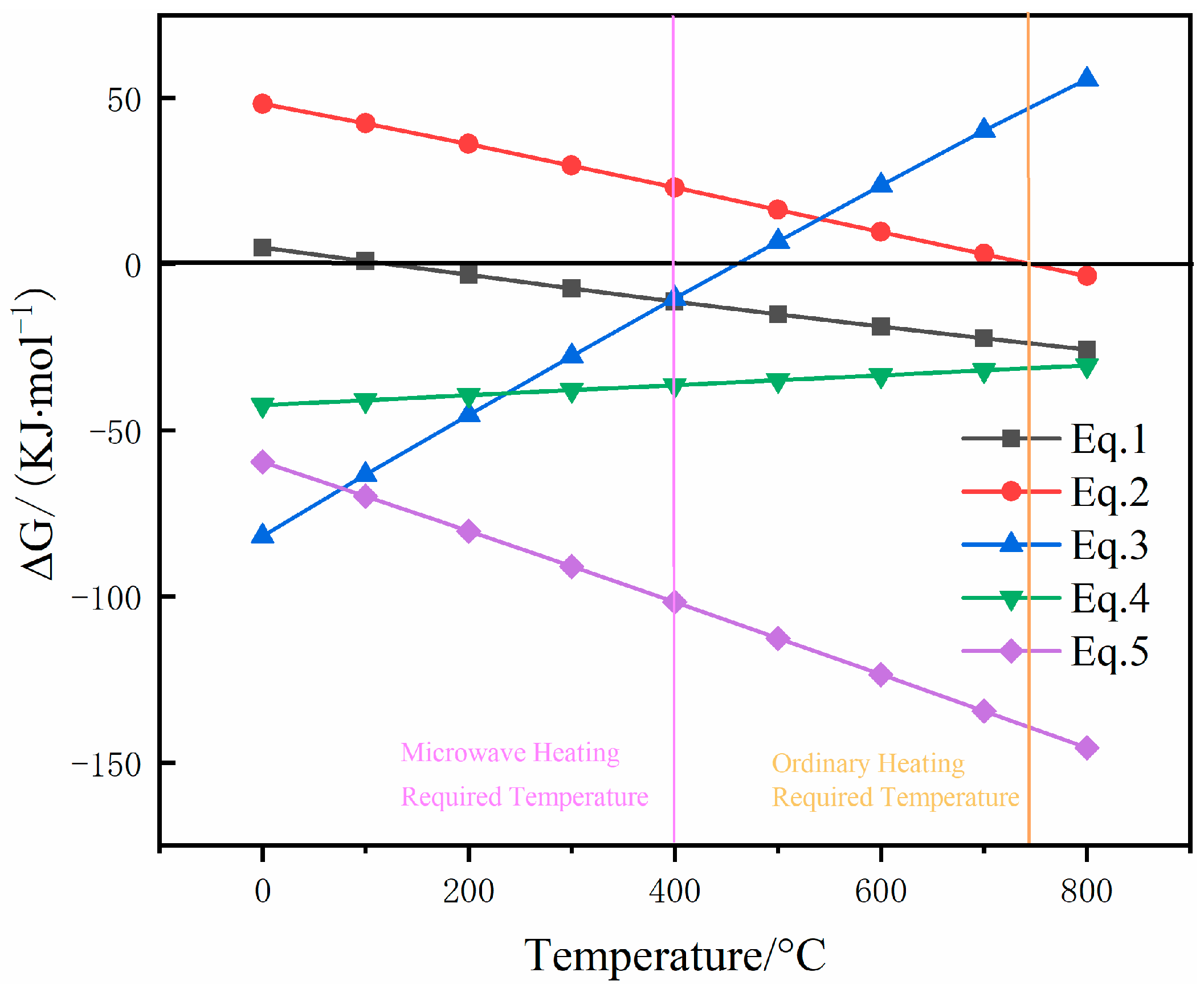
3.1. Thermodynamic Analysis
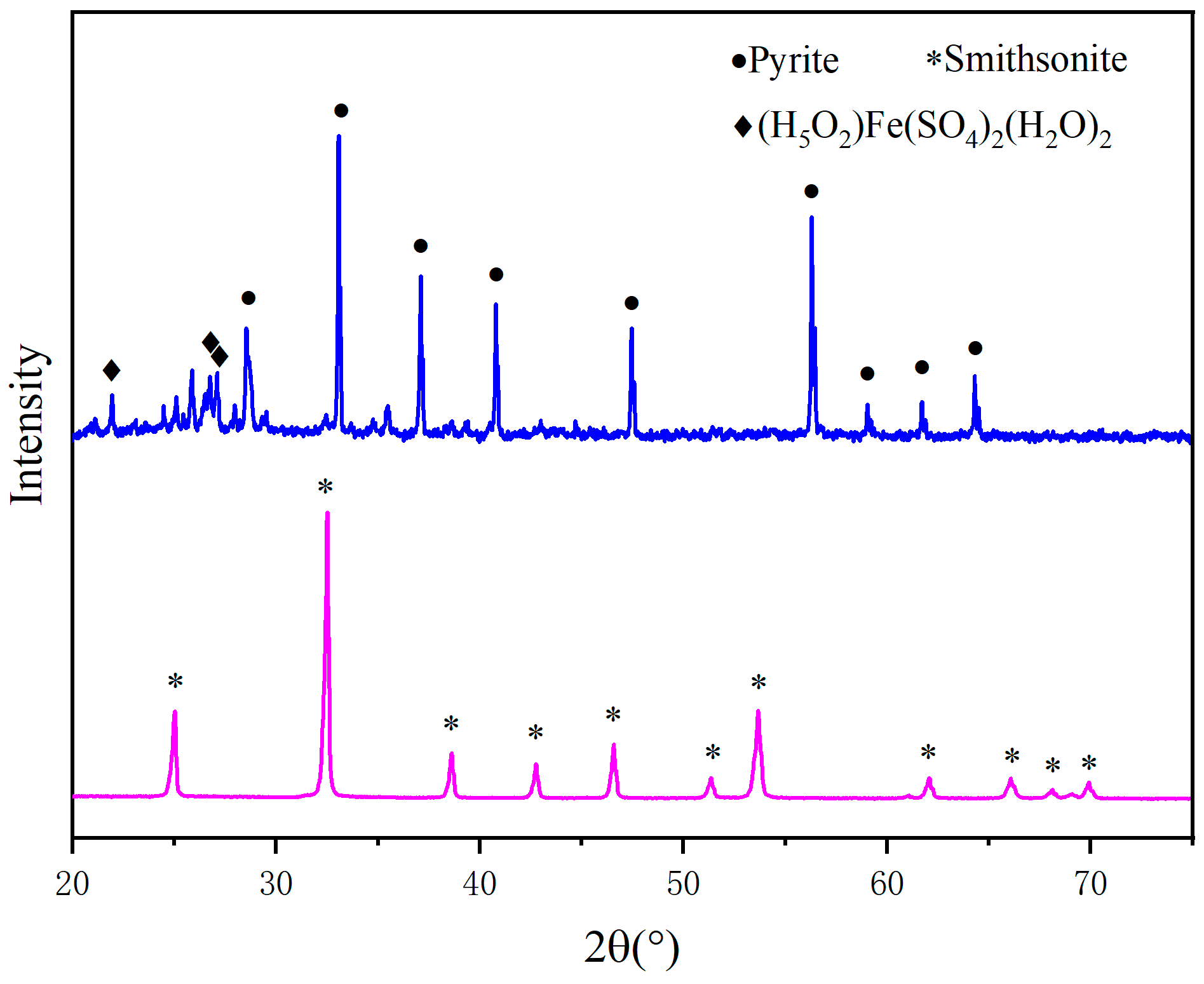
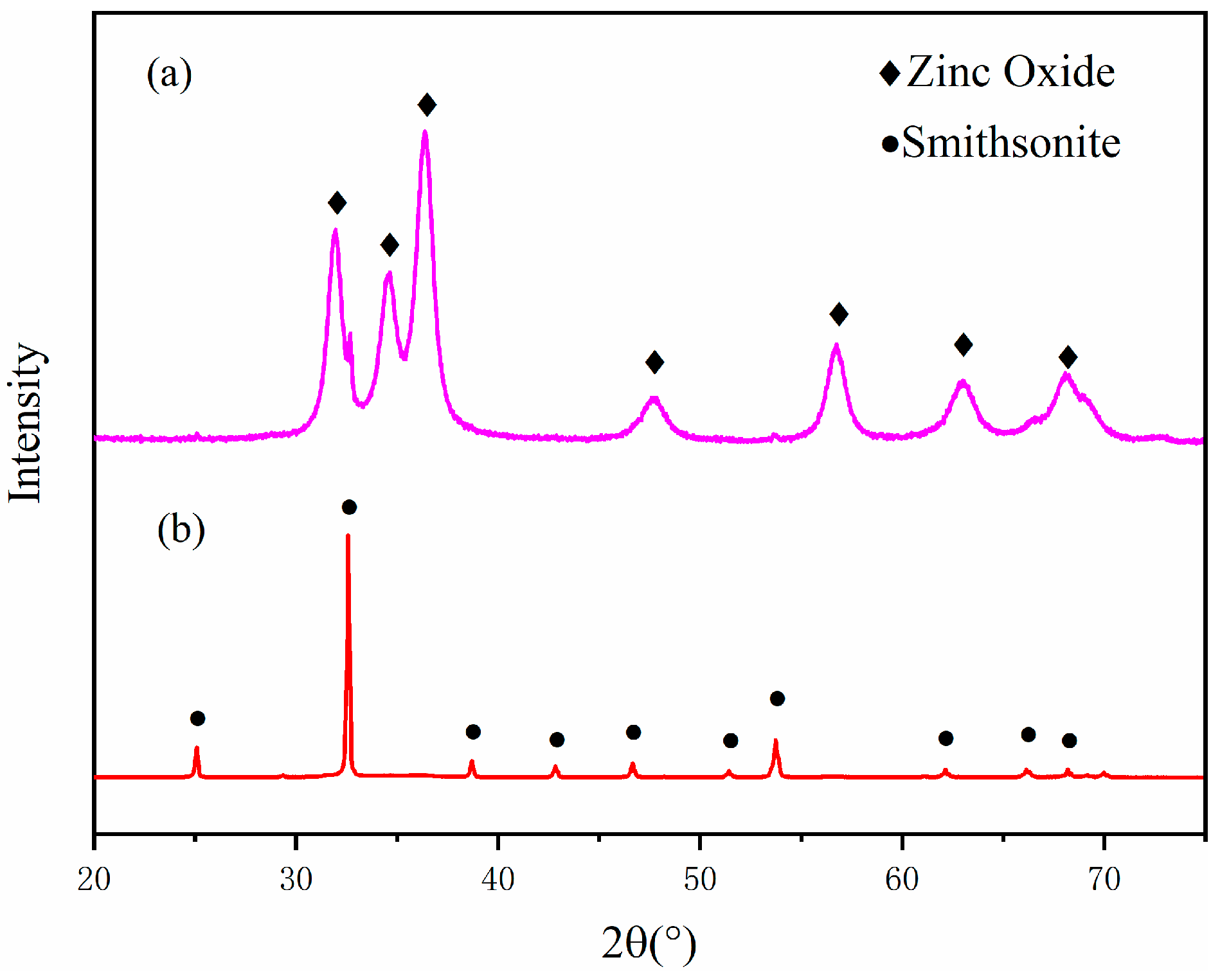
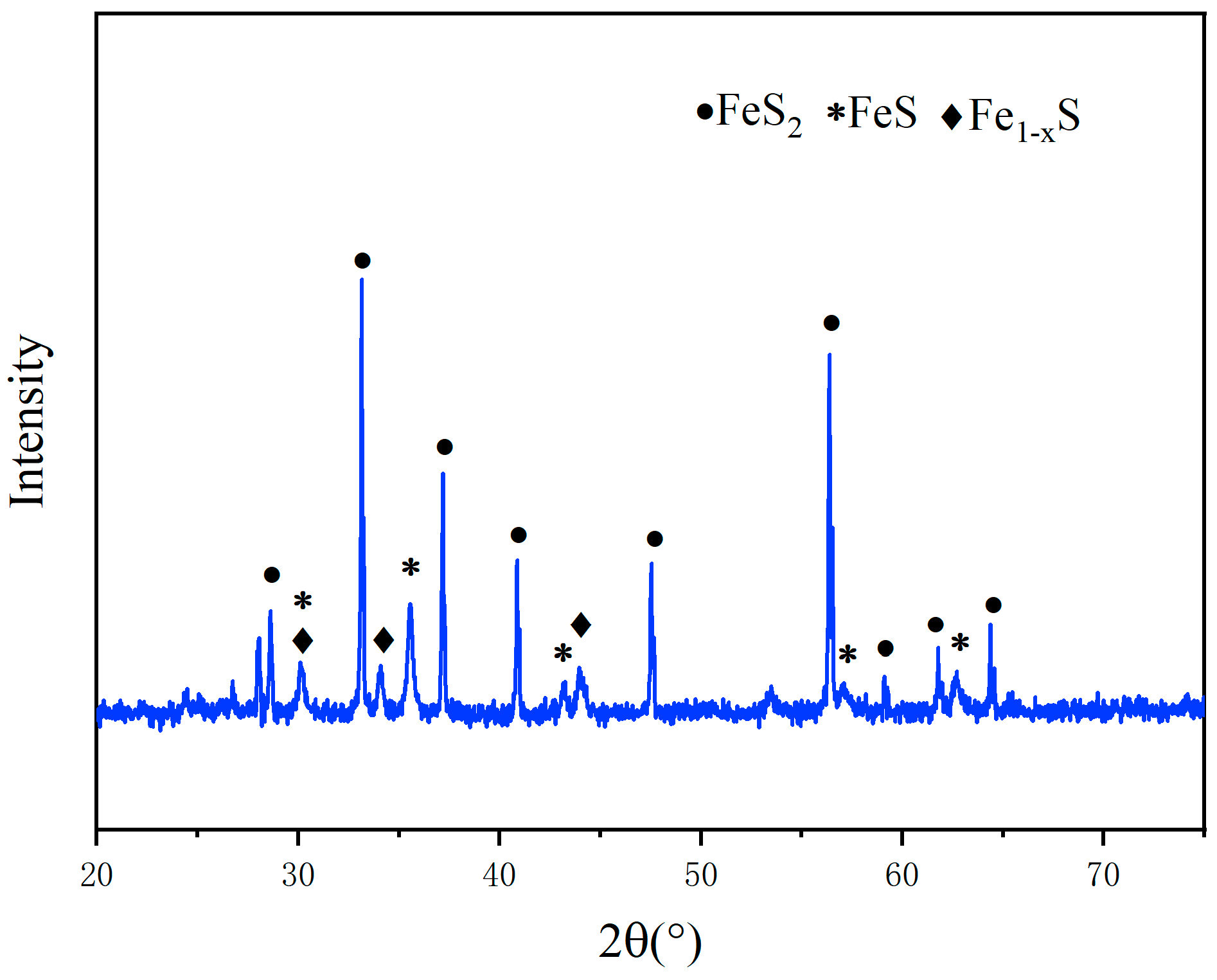
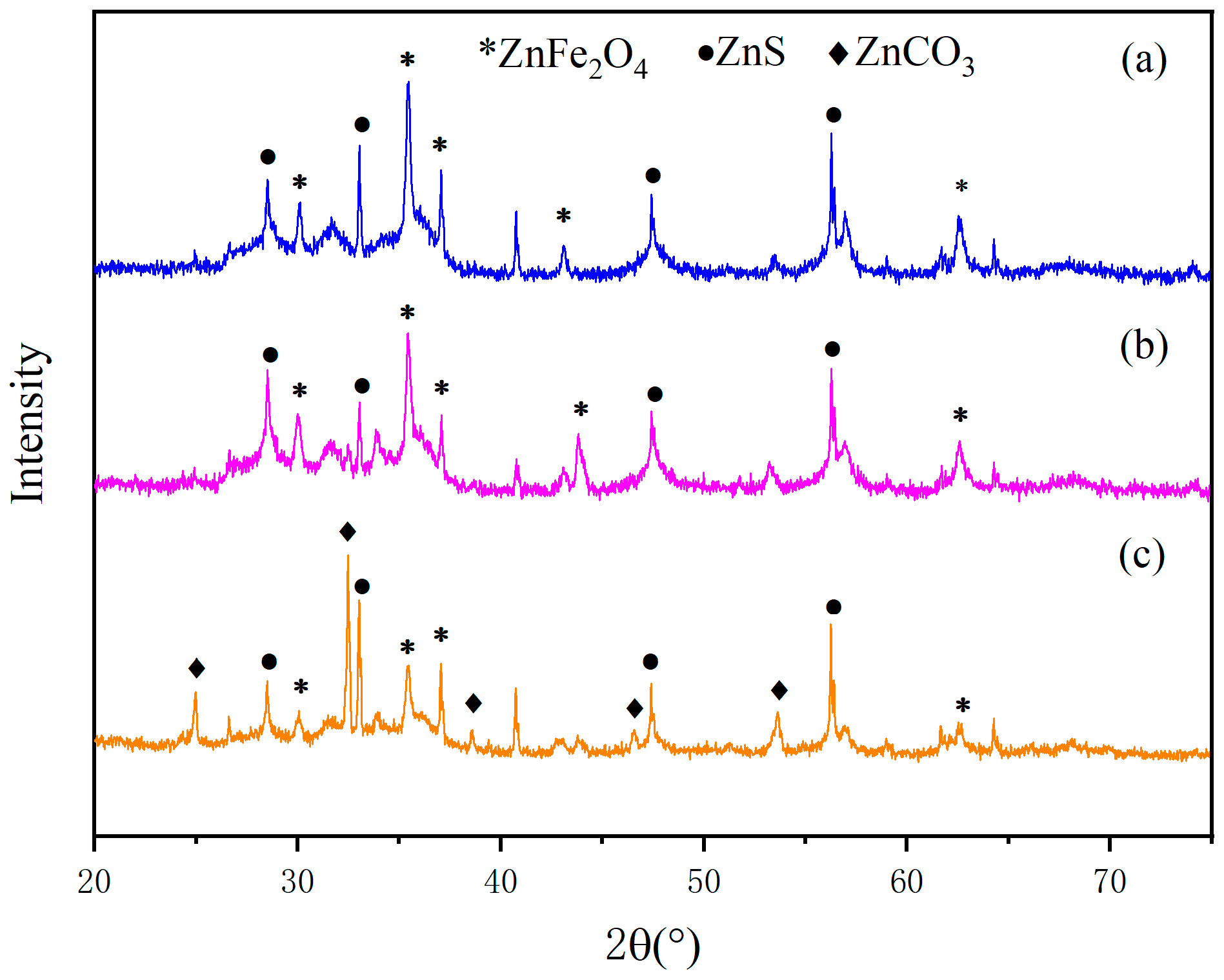
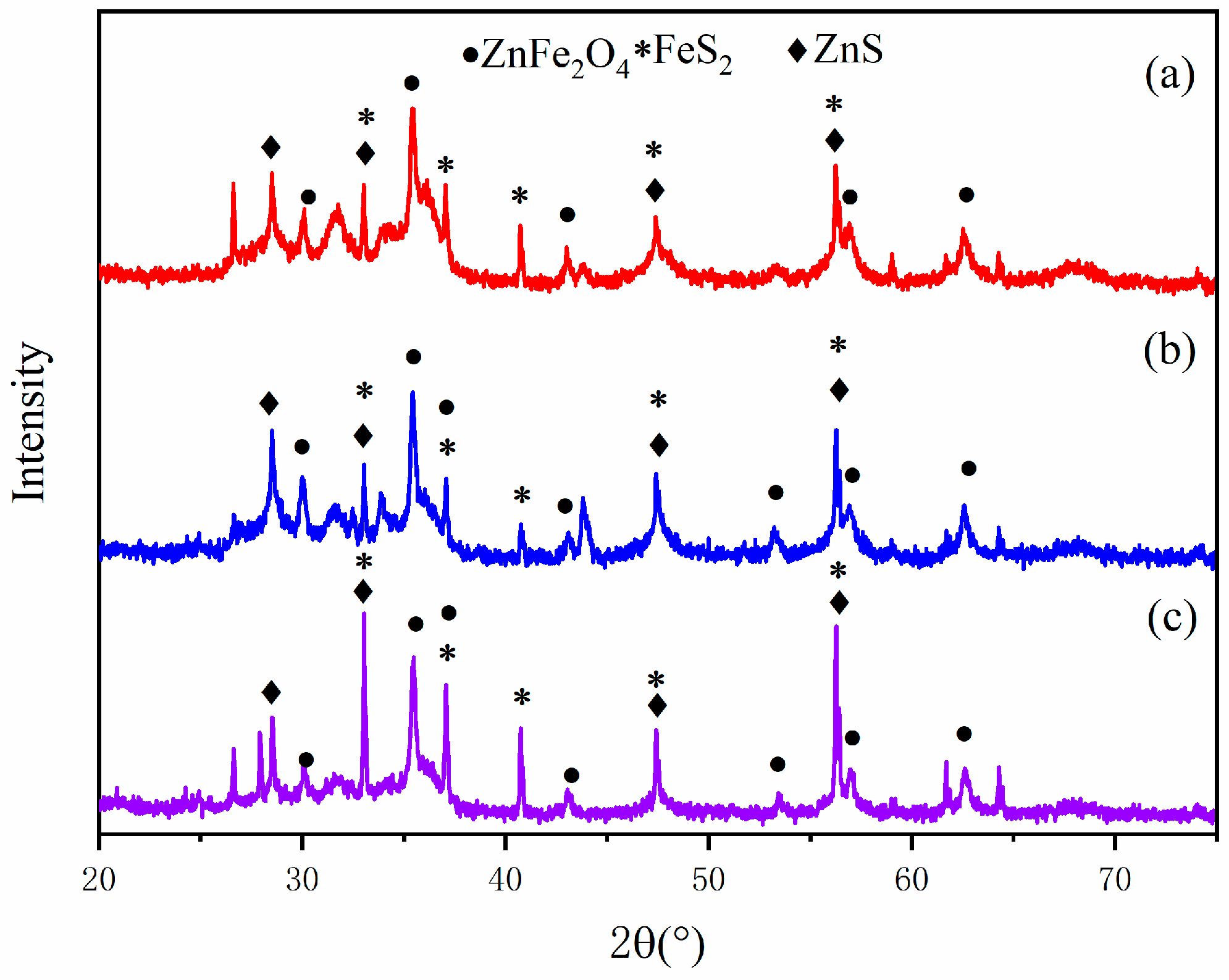
3.2. XRD Analysis
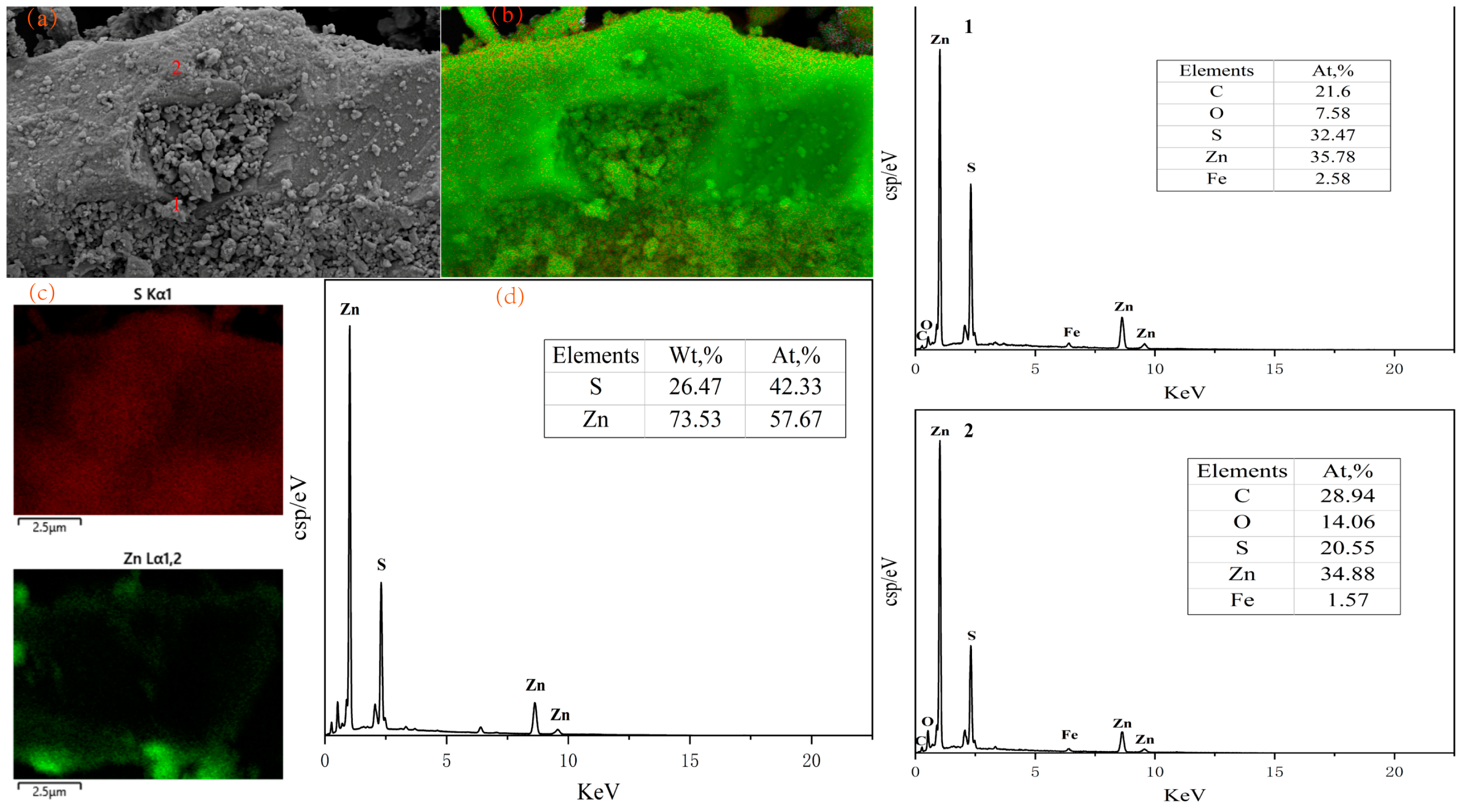
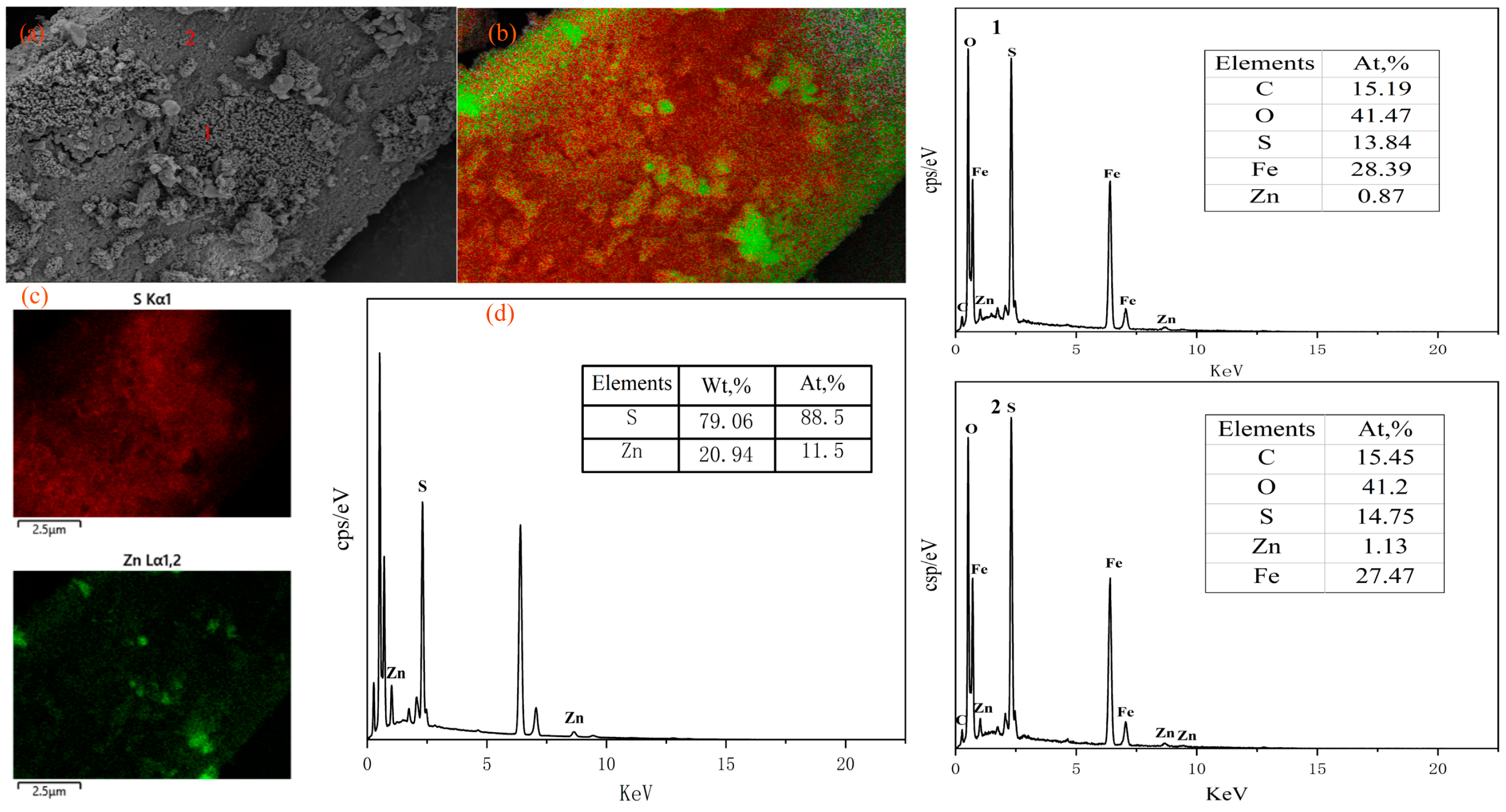
3.3. Sulfidation Surface Analysis
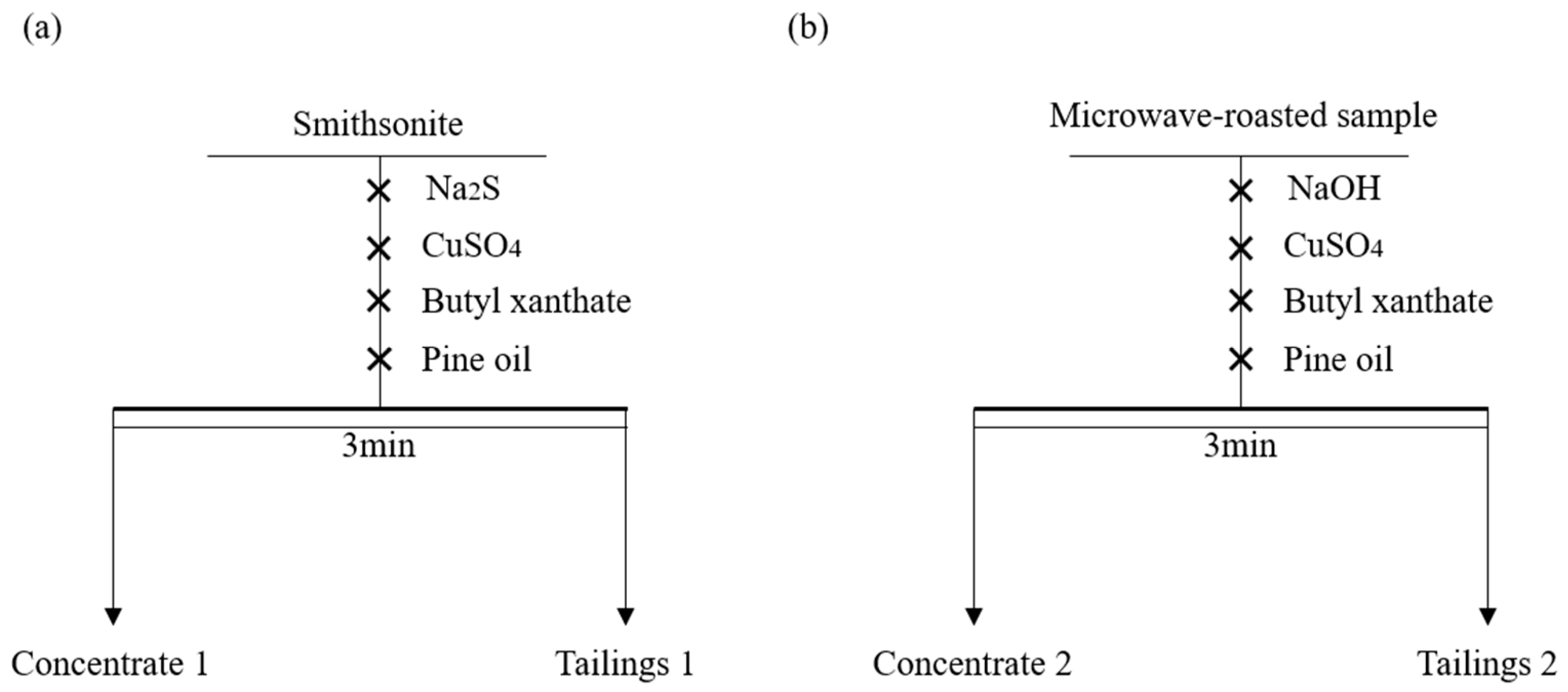
3.4. Flotation Experiment Verification
4. Conclusions
- Thermodynamic analysis indicates that the sulfidation of zinc carbonate is thermodynamically feasible, with an increase in temperature favoring ZnS formation.
- According to the analysis by XRD and XPS, when the pyrite content is low, the crystallinity degree of the generated sulfidized zinc is low. Conversely, when pyrite is in excess, most of the smithsonite completely reacts, leaving behind a large amount of pyrite. Additionally, as the FeS2 dosage increases, the proportions of zinc sulfate and iron sulfate also increase.
- SEM–EDS analysis reveals that the smithsonite surface appears relatively smooth and flat at 300 °C. However, when the temperature reaches 400 °C, the mineral surface becomes loose and porous, owing to the occurrence of gas pores generated by the decomposition of smithsonite into CO2. With the increase in the FeS2 dosage, the concentration of sulfur atoms on the smithsonite surface gradually increases. However, once a certain mass ratio is reached, the concentration of sulfur elements on the mineral surface remains roughly unchanged, indicating that the theoretical mass ratio for the complete conversion of smithsonite to zinc sulfide has been achieved.
- According to the results of the three analytical methods, the optimal microwave roasting conditions are determined as follows: a microwave roasting temperature of 400 °C and a ZnCO3:FeS2 mass ratio of 1:1.5.
- Comparative flotation experiments demonstrated that microwave-roasted smithsonite exhibited significantly higher floatability than the conventionally sulfidized method.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, J.; Liu, D.; Shen, P.; Zhang, X.; Song, K.; Jia, X.; Su, C. Effects of Heating-Sulfidation on the Formation of Zinc Sulfide Species on Smithsonite Surfaces and Its Response to Flotation. Min. Eng. 2021, 169, 106956. [Google Scholar] [CrossRef]
- Liao, R.; Wen, S.; Liu, J.; Bai, S.; Feng, Q. Synergetic Adsorption of Dodecylamine and Octyl Hydroxamic Acid on Sulfidized Smithsonite: Insights from Experiments and Molecular Dynamics Simulation. Sep. Purif. Technol. 2024, 329, 125106. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, G.; Zhang, G.; Zhao, W.; Han, G. Degradation Mechanism of Surface Hydrophobicity by Ferrous Ions in the Sulfidization Flotation System of Smithsonite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129119. [Google Scholar] [CrossRef]
- Lv, J.; Tong, X.; Zheng, Y.; Xie, X.; Wang, C. Study on the Surface Sulfidization Behavior of Smithsonite at High Temperature. Appl. Surf. Sci. 2018, 437, 13–18. [Google Scholar] [CrossRef]
- Chen, H.; Xie, H.; Chen, J.; Jin, Y.; Zeng, P.; Song, Z.; Zhang, Q.; Liu, D. Research Progress on Flotation Collectors for Lead-Zinc Oxide Ore. Conserv. Util. Miner. Resour. 2023, 43, 42–53. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wei, C.; Liu, C.-X.; Jiang, J.-B.; Wang, F. Sulfidation Roasting of Low Grade Lead–Zinc Oxide Ore with Elemental Sulfur. Min. Eng. 2010, 23, 563–566. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Zhu, G.; Xu, H.; Tang, X.; Luo, X. Flotation Behavior and Mechanism of Smithsonite under the System of Bidentate Ligand Sulfide Sodium Thiocyanate. Sep. Purif. Technol. 2024, 334, 126086. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, G.; Li, C.; Li, B.; Ye, G. The Surface Dissolution Process of Smithsonite and Its Effect on Flotation Behaviour. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132118. [Google Scholar] [CrossRef]
- Han, J.; Liu, W.; Zhang, T.; Xue, K.; Li, W.; Jiao, F.; Qin, W. Mechanism Study on the Sulfidation of ZnO with Sulfur and Iron Oxide at High Temperature. Sci. Rep. 2017, 7, 42536. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S. Formation of Zinc Sulfide Species on Smithsonite Surfaces and Its Response to Flotation Performance. J. Alloys Compd. 2017, 709, 602–608. [Google Scholar] [CrossRef]
- Yin, Z.; Cagnetta, G.; Huang, J. Mechanochemically Sulfidated Zero-Valent Iron as Persulfate Activation Catalyst in Permeable Reactive Barriers for Groundwater Remediation—A Feasibility Study. Chemosphere 2023, 311, 137081. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Min, X.-B.; Chai, L.-Y.; Zhou, B.-S.; Xue, K. Sulfidation Behavior of Zn and ZnS Crystal Growth Kinetics for Zn(OH)2–S–NaOH Hydrothermal System. Hydrometallurgy 2016, 161, 166–173. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, G.; Chen, J.; Peng, J.; Srinivasakannan, C.; Ruan, R. Preparation of Synthetic Rutile from High Titanium Slag Using Microwave Heating. Phase Transit. 2018, 91, 308–315. [Google Scholar] [CrossRef]
- Chen, G.; Ling, Y.; Li, Q.; Zheng, H.; Qi, J.; Li, K.; Chen, J.; Peng, J.; Gao, L.; Omran, M.; et al. Investigation on Microwave Carbothermal Reduction Behavior of Low-Grade Pyrolusite. J. Mater. Res. Technol. 2020, 9, 7862–7869. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Peng, J.; Ruan, R.; Srinivasakannan, C.; Chen, G. Pilot-Scale Study on Enhanced Carbothermal Reduction of Low-Grade Pyrolusite Using Microwave Heating. Powder Technol. 2020, 360, 846–854. [Google Scholar] [CrossRef]
- Goldbaum, M.W.; Elliott, R.; Forster, J.; Maham, Y.; Bobicki, E.R. Investigating the Microwave Heating Behaviour of Pyrrhotite Tailings. Min. Eng. 2020, 146, 106152. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Y. Study on Microwave-Assisted Grinding and Liberation Characteristics for Ludwigite. J. Microw. Power Electromagn. Energy 2021, 55, 28–44. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Han, Y. Efficient Flotation Separation of Lead–Zinc Oxide Ores Using Mineral Sulfidation Reconstruction Technology: A Review. Green. Smart Min. Eng. 2024, 1, 175–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, J.; Chen, J.; Yu, R.; Xing, X. A Low-Cost and Large-Scale Synthesis of Nano-Zinc Oxide from Smithsonite. Inorg. Chem. Commun. 2014, 43, 138–141. [Google Scholar] [CrossRef]
- Lan, Z.; Lai, Z.; Zheng, Y.; Lv, J.; Pang, J.; Ning, J. Thermochemical Modification for the Surface of Smithsonite with Sulfur and Its Flotation Response. Min. Eng. 2020, 150, 106271. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Qin, W.; Jiao, F.; Han, J.; Yang, K.; Luo, H. Sulfidation Roasting of Lead and Zinc Carbonate with Sulphur by Temperature Gradient Method. J. Cent. South. Univ. 2015, 22, 1635–1642. [Google Scholar] [CrossRef]
- Zhang, X.; Kou, J.; Sun, C. A Comparative Study of the Thermal Decomposition of Pyrite under Microwave and Conventional Heating with Different Temperatures. J. Anal. Appl. Pyrolysis 2019, 138, 41–53. [Google Scholar] [CrossRef]
- Tian, C.; Rao, Y.; Su, G.; Huang, T.; Xiang, C. The Thermal Decomposition Behavior of Pyrite-Pyrrhotite Mixtures in Nitrogen Atmosphere. J. Chem. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Zhang, K.; Zhang, S.; Li, K.; Li, J.; Peng, G. Thermal Decomposition and Oxidation of Pyrite with Different Morphologies in the Coal Gangue of North China. J. Therm. Anal. Calorim. 2023, 148, 2023–2038. [Google Scholar] [CrossRef]
- He, C.L.; Ma, S.J.; Su, X.J.; Mo, Q.H.; Yang, J.L. Comparison of the Microwave Absorption Characteristics of Hematite, Magnetite and Pyrite. J. Microw. Power Electromagn. Energy 2015, 49, 131–146. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, L.; Han, J.; Jiao, F.; Qin, W. Sulfidation Mechanism of ZnO Roasted with Pyrite. Sci. Rep. 2018, 8, 9516. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Du, Y.; Wang, J. Sulfuric Acid Pretreatment of Oxidized Pyrrhotite in Flotation Desulphurization of Magnetite Concentrate. Min. Eng. 2023, 203, 108347. [Google Scholar] [CrossRef]
- Zheng, Y.; Bao, L.; Lv, J.; Pang, J.; Hu, P.; Huang, Y. Flotation Response of Cerussite after Hydrothermal Treatment with Sulfur and the Sulfidation Mechanism. J. Mater. Res. Technol. 2021, 15, 2933–2942. [Google Scholar] [CrossRef]
- Li, C.; Bai, S.; Ding, Z.; Yu, P.; Wen, S. Visual MINTEQ Model, ToF–SIMS, and XPS Study of Smithsonite Surface Sulfidation Behavior: Zinc Sulfide Precipitation Adsorption. J. Taiwan Inst. Chem. Eng. 2019, 96, 53–62. [Google Scholar] [CrossRef]
- Xu, L.; Liu, D.; Sun, R.; Wang, Y.; Liu, Z.; Shao, P.; Wang, C.; Wen, S. Flotation Separation of Smithsonite and Calcite in Sodium Oleate System Using Soluble Starch as Depressant. Min. Eng. 2024, 205, 108490. [Google Scholar] [CrossRef]
- Duchoslav, J.; Steinberger, R.; Arndt, M.; Stifter, D. XPS Study of Zinc Hydroxide as a Potential Corrosion Product of Zinc: Rapid X-ray Induced Conversion into Zinc Oxide. Corros. Sci. 2014, 82, 356–361. [Google Scholar] [CrossRef]


| ZnCO3/FeS2 Mass Ratio | Binding Energy (eV) | Relative Contents (%) | |||||
|---|---|---|---|---|---|---|---|
| FeSO4 ZnSO4 | Iron Sulfides | ZnS | Fe/ZnSO4 | Iron Sulfides | ZnS | ||
| 1:1 | 168.62 | 169.72 | 162.86 | 161.76 | 4.32 | 38.78 | 38.72 |
| 1:1.5 | 168.21 | 169.31 | 162.53 | 161.43 | 14.9 | 42.62 | 42.71 |
| 1:2 | 168.88 | 169.98 | 163.03 | 161.93 | 22.53 | 47.82 | 47.86 |
| ZnCO3/FeS2 Mass Ratio | Binding Energy (eV) | Relative Contents (%) | ||
|---|---|---|---|---|
| C | CO32− C-O-C | ZnCO3 | C | |
| 1:1 | 284.82 | 288.74 286.34 | 24.35 | 75.65 |
| 1:1.5 | 284.79 | 288.43 286.38 | 23.73 | 76.22 |
| 1:2 | 284.77 | 288.59 286.12 | 15.79 | 84.21 |
| ZnCO3/FeS2 Mass Ratio | Binding Energy (eV) | Relative Contents (%) | ||
|---|---|---|---|---|
| Metal Oxide | Metal Carbonates | ZnO | ZnCO3 | |
| 1:1 | 529.71 | 531.93 | 11.05 | 88.95 |
| 1:1.5 | 529.14 | 532.27 | 10.27 | 89.73 |
| 1:2 | 529.77 | 531.96 | 10.44 | 89.56 |
| Product Name | Yield/% | Grade/% | Recovery Rate/% |
|---|---|---|---|
| Concentrate 1 | 71.70 | 48.56 | 71.70 |
| Tailings 1 | 28.30 | 48.56 | 28.30 |
| Smithsonite | 100.00 | 48.56 | 100.00 |
| Product Name | Yield/% | Grade/% | Recovery Rate/% |
|---|---|---|---|
| Concentrate 2 | 34.93 | 50.21 | 90.32 |
| Tailings 2 | 65.07 | 2.89 | 9.68 |
| Microwave-roasted sample | 100.00 | 19.42 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Yin, S.; Li, M.; Zhang, X.; Wen, X.; Zhang, H.; Nie, Q.; Lei, T. Sulfidation of Smithsonite via Microwave Roasting under Low-Temperature Conditions. Minerals 2024, 14, 855. https://doi.org/10.3390/min14090855
Kang J, Yin S, Li M, Zhang X, Wen X, Zhang H, Nie Q, Lei T. Sulfidation of Smithsonite via Microwave Roasting under Low-Temperature Conditions. Minerals. 2024; 14(9):855. https://doi.org/10.3390/min14090855
Chicago/Turabian StyleKang, Jiawei, Shubiao Yin, Mingxiao Li, Xingzhi Zhang, Xujie Wen, Hanping Zhang, Qi Nie, and Ting Lei. 2024. "Sulfidation of Smithsonite via Microwave Roasting under Low-Temperature Conditions" Minerals 14, no. 9: 855. https://doi.org/10.3390/min14090855






