New Insights into the Depressive Mechanism of Sodium Silicate on Bastnaesite, Parisite, and Fluorite: Experimental and DFT Study
Abstract
:1. Introduction
2. Experiment
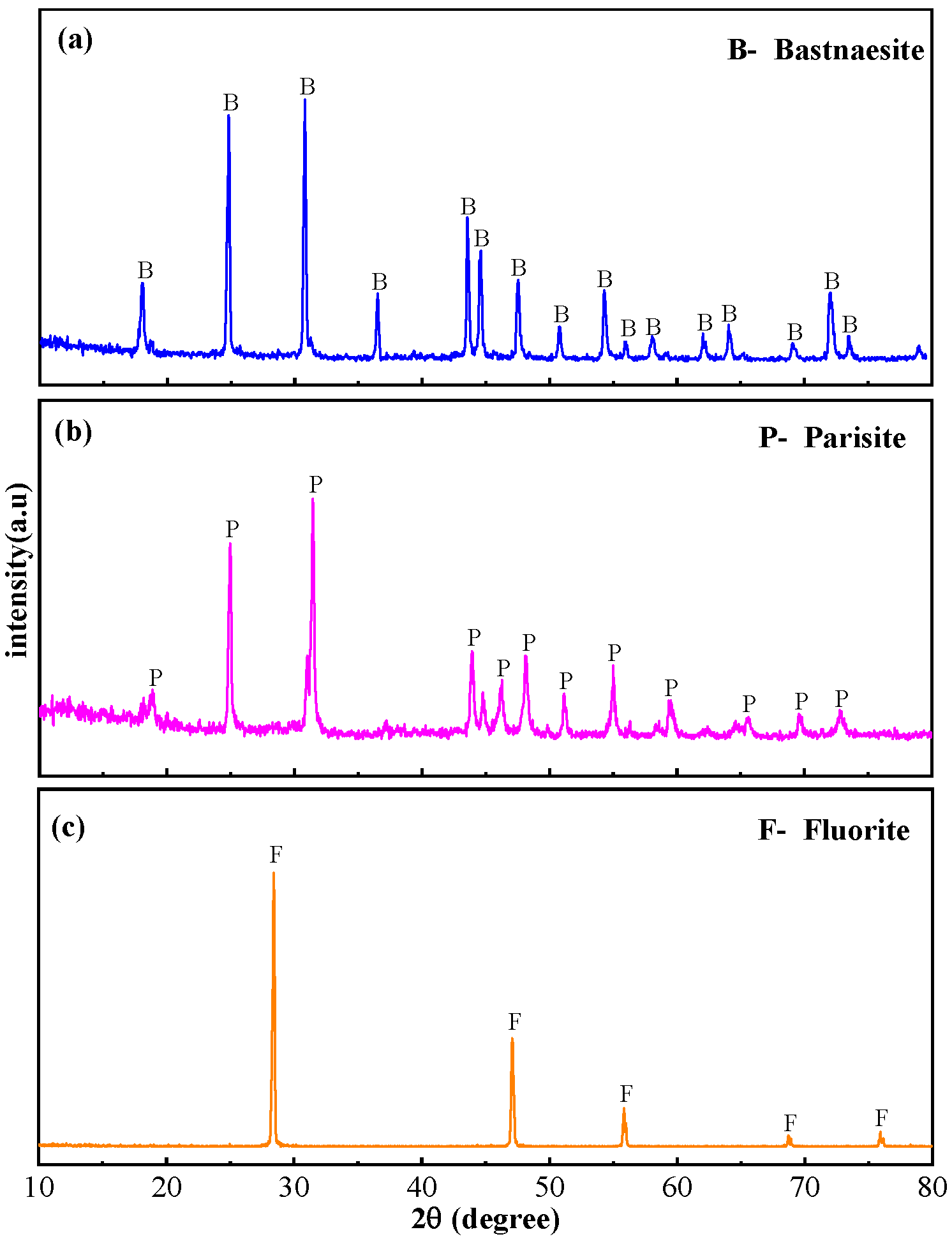
2.1. Materials and Reagents
2.2. Microflotation Experiments
2.3. Zeta Potential Measurements
2.4. Contact Angle Tests
2.5. XPS Measurements
2.6. First-Principles Calculations
3. Results and Discussion
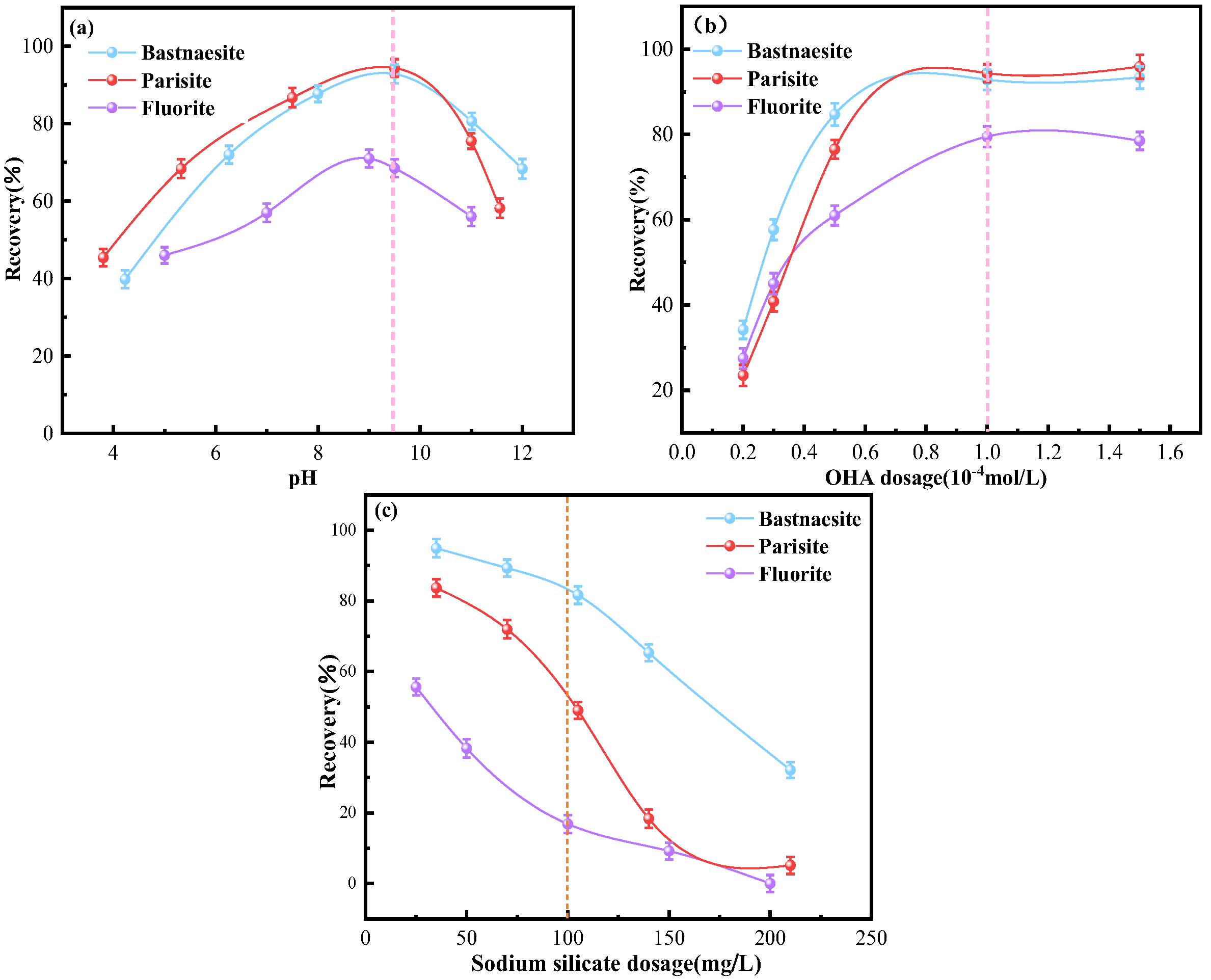
3.1. Microflotation Results
3.2. Zeta Potential Results
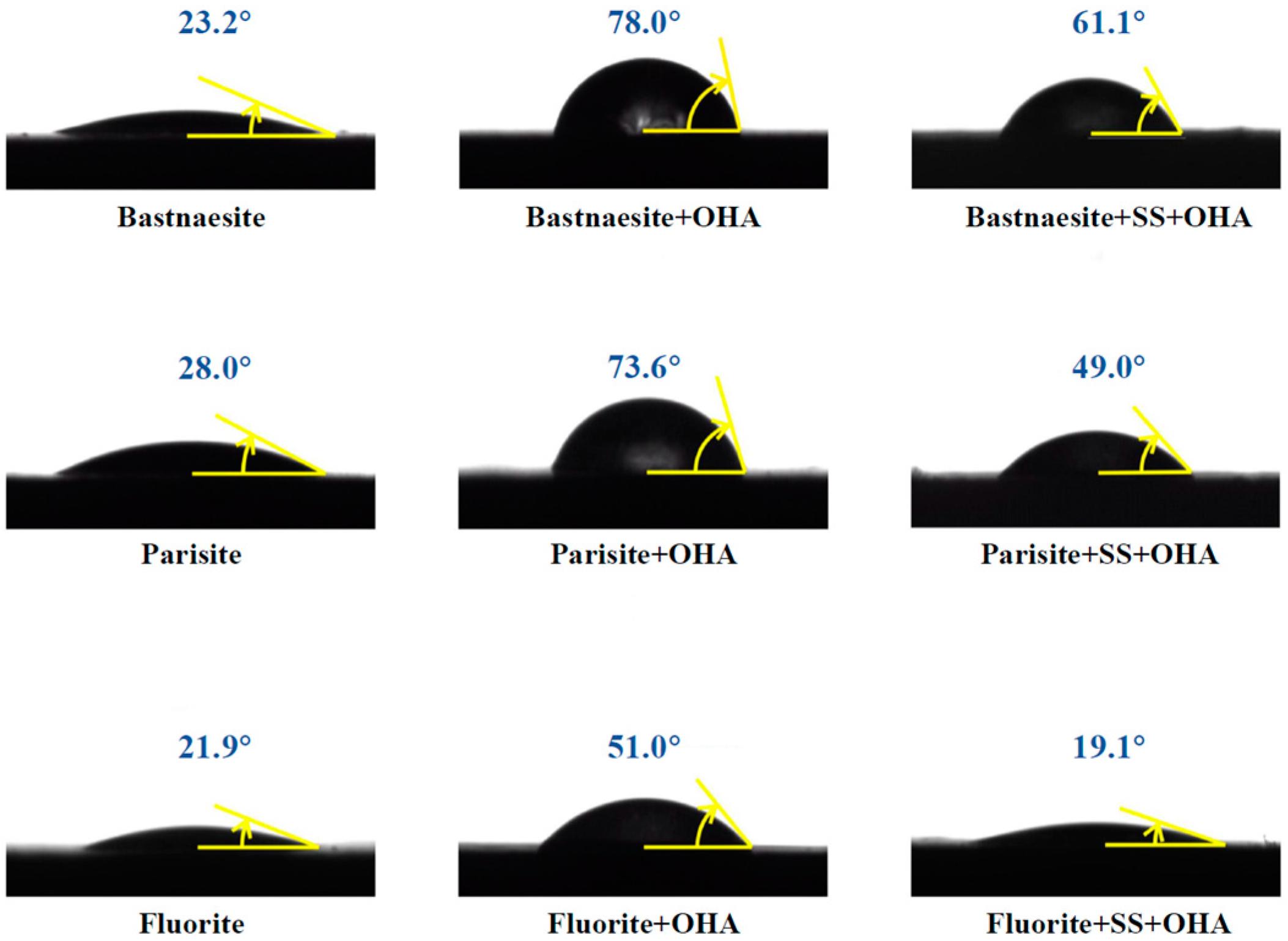
3.3. Contact Angle Measurements
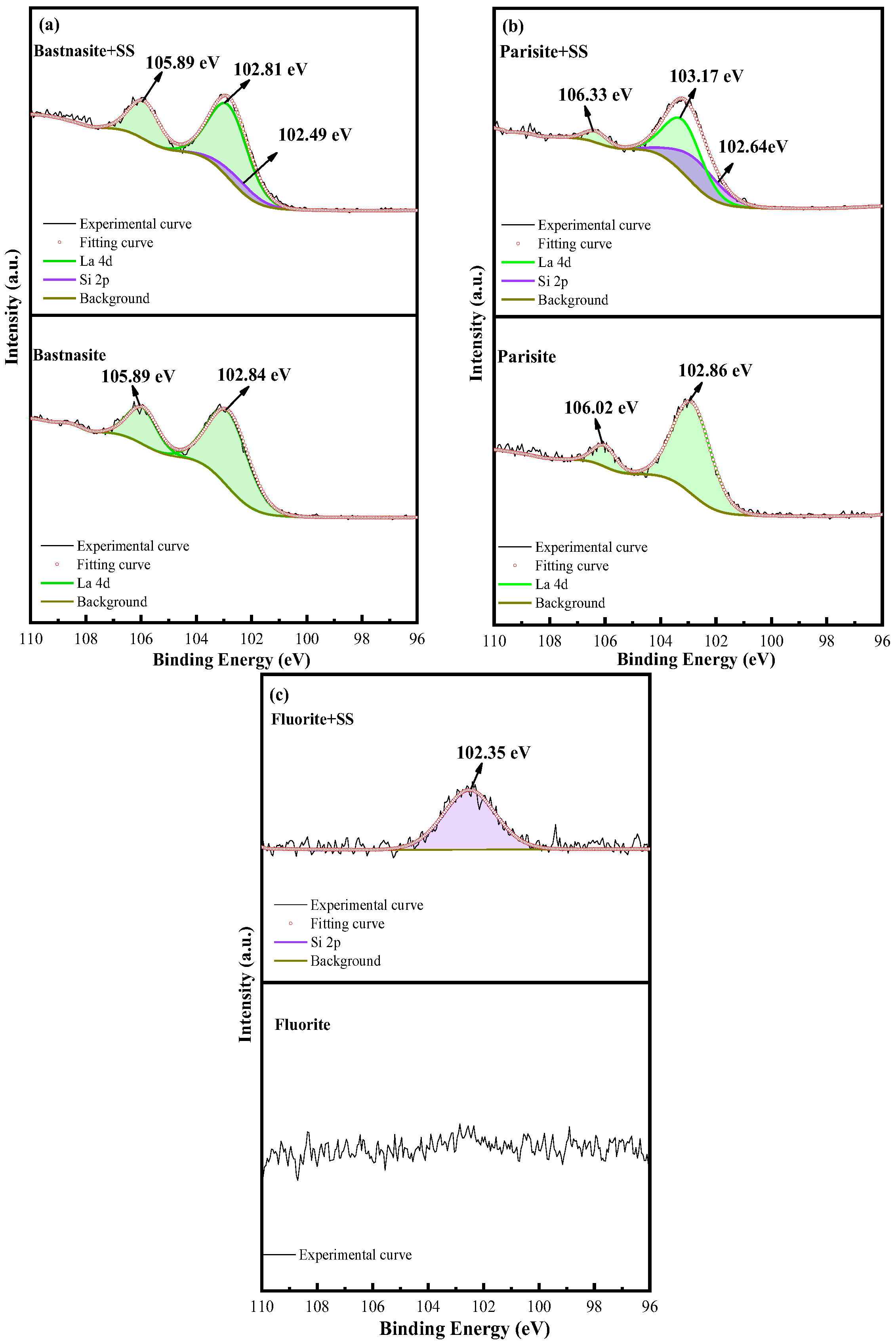
3.4. XPS Analysis
3.5. DFT Simulation
4. Conclusions
- OHA demonstrates a stronger collecting ability for bastnaesite compared to parisite, with the weakest collecting ability for fluorite. Conversely, SS exhibited the strongest depressing ability for fluorite, followed by parisite, and the weakest for bastnaesite.
- SS has the strongest adsorption on fluorite, significantly hindering the adsorption of OHA. SS and OHA compete for adsorption on parisite surfaces. SS has weak adsorption on the surface of bastnaesite and has little effect on the adsorption of OHA.
- OHA ions form covalent bonds with metal ions on the surfaces of the three minerals. The five-membered hydroxamic-(O-O)-Ce/Ca chelate coordination formed between OHA and metal atoms on the three mineral surfaces is the most stable. Compared with parisite and fluorite, OHA exhibits a greater affinity for bastnaesite. SS interacts with metal ions on the mineral surface primarily through the SiO(OH)3− component, and SiO(OH)3− has the strongest affinity for fluorite, followed by parisite and bastnaesite.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Cheng, Z.; Hu, Y.; Cao, Y.; Wang, P.; Cao, Z. Depression behavior and mechanism of sodium silicate on bastnaesite and parisite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127691. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Xu, Y.; Shu, K.; Yang, J.; Luo, L.; Xu, L. Effect of dissolved fluorite and barite species on the flotation and adsorption behavior of bastnaesite. Sep. Purif. Technol. 2020, 237, 116387. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, Y.; Qu, Q.; Zhang, J.; Mu, Y. Separation of bastnäsite from fluorite using ethylenediamine tetraacetic acid as depressant. Min. Eng. 2019, 134, 134–141. [Google Scholar] [CrossRef]
- Liu, C.; Xu, L.; Deng, J.; Tian, J.; Wang, D.; Xue, K.; Zhang, X.; Wang, Y.; Fang, J.; Liu, J. A review of flotation reagents for bastnäsite-(Ce) rare earth ore. Adv. Colloid. Interface Sci. 2023, 321, 103029. [Google Scholar] [CrossRef] [PubMed]
- Chapleski, R.C., Jr.; Chowdhury, A.U.; Wanhala, A.K.; Gibson, L.D.; Stamberga, D.N.; Jansone-Popova, S.; Sacci, R.L.; Meyer, H.M., III; Stack, A.G.; Bocharova, V. Improving Rare-Earth Mineral Separation with Insights from Molecular Recognition: Functionalized Hydroxamic Acid Adsorption onto Bastnasite and Calcite. Langmuir 2022, 38, 5439–5453. [Google Scholar]
- Geneyton, A.; Filippov, L.; Heinig, T.; Buaron, N.; Menad, N.-E. Towards the efficient flotation of monazite from silicate-rich tailings with fatty acids collectors using a lanthanum salt as a selective phosphate activator. Min. Eng. 2021, 160, 106704. [Google Scholar] [CrossRef]
- Suslavich, B.; LaDouceur, R.; Mamudu, A.; Young, C. Synthesis and characterization of hydroxamic acid N, 3-dihydroxy-2-naphthamide and its copper (II) complex per keto/enol forms and rare earth flotation. Min. Min. Mater 2024, 3, 1. [Google Scholar] [CrossRef]
- Sun, R.; Liu, D.; Tian, X.; Zuo, Q.; Wang, D.; Wen, S. The role of copper ion and soluble starch used as a combined depressant in the flotation separation of fluorite from calcite: New insights on the application of modified starch in mineral processing. Min. Eng. 2022, 181, 107550. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, F.; Gao, H.; Chen, Z.; Peng, Y.; Liu, L. Selective separation of fluorite, barite and calcite with valonea extract and sodium fluosilicate as depressants. Minerals 2017, 7, 24. [Google Scholar] [CrossRef]
- Tao, L.; Sun, W.; Wang, J. Selective Separation of Fluorite from Calcite Using Saponified Tridecanoic Acid as a Novel Collector. Min. Process. Extr. Metall. Rev. 2023, 45, 509–521. [Google Scholar] [CrossRef]
- Penaloza, I.; Tita, A.; McNew, E.; Chu, P. Barite resources, production and recovery using froth flotation: A review. Min. Eng. 2023, 203, 108327. [Google Scholar] [CrossRef]
- Chen, X.; Gu, G.; Liu, D.; Zhu, R. The flotation separation of barite-calcite using sodium silicate as depressant in the presence of sodium dodecyl sulfate. Physicochem. Probl. Min. Process. 2019, 55, 346–355. [Google Scholar]
- Deng, J.; Liu, C.; Yang, S.; Li, H.; Liu, Y. Flotation separation of barite from calcite using acidified water glass as the depressant. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123605. [Google Scholar] [CrossRef]
- Qin, W.; Hu, J.; Zhu, H.; Jiao, F.; Jia, W.; Han, J.; Chen, C. Effect of depressants on flotation separation of magnesite from dolomite and calcite. Int. J. Min. Sci. Technol. 2023, 3, 83–91. [Google Scholar] [CrossRef]
- Ding, K.; Laskowski, J. Application of a modified water glass in a cationic flotation of calcite and dolomite. Can. Metall. Q. 2006, 45, 199–206. [Google Scholar] [CrossRef]
- Huang, H.; Gao, W.; Li, X. Effect of SO42-and PO43-on flotation and surface adsorption of dolomite: Experimental and molecular dynamics simulation studies. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132699. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, J.; Zhao, W. Utilization of citric acid to improve the depressive efficiency of sodium silicate on the flotation of calcite and fluorite. Min. Metall. Explor. 2022, 39, 855–862. [Google Scholar] [CrossRef]
- Jin, S.; Ou, L.; Ma, X.; Zhou, H.; Zhang, Z. Activation mechanisms of sodium silicate-inhibited fluorite in flotation under neutral and slightly alkaline conditions. Min. Eng. 2021, 161, 106738. [Google Scholar] [CrossRef]
- Xue, J.; Tu, H.; Shi, J.; An, Y.; Wan, H.; Bu, X. Enhanced inhibition of talc flotation using acidified sodium silicate and sodium carboxymethyl cellulose as the combined inhibitor. Int. J. Min. Metall. Mater. 2023, 30, 1310–1319. [Google Scholar] [CrossRef]
- Li, W.; Shi, D.; Han, Y. A selective flotation of fluorite from dolomite using caustic cassava starch and its adsorption mechanism: An experimental and DFT Study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127876. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, Z.; Wang, H.; He, G.; Yu, X.; Burov, V.E.; Poilov, V.Z.; Li, F.; Liu, R.; Li, W. Adsorption study of 3-tetradecylamine propyl amidoxime onto rhodochrosite surface: Implications for rhodochrosite-calcite flotation separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132469. [Google Scholar] [CrossRef]
- Lv, L.; Duan, H.; Liu, W.; Yue, T. Flotation separation of fluorite from calcite using bis hydroxamic acid collector. Min. Eng. 2022, 187, 107803. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Shuai, W.; Cao, Z.-F.; Xin, M.; Zhong, H. Structure-activity relationships of oxime compounds as flotation collectors by DFT calculations. Trans. Nonferrous Met. Soc. China 2022, 32, 4076–4087. [Google Scholar] [CrossRef]
- Guo, Z.; Khoso, S.A.; Wang, J.; Zhang, C.; Gao, Z.; Sun, W.; Tian, M.; Liu, Y. Interaction mechanism of 2- hydroxy-3-naphthyl hydroxamic acid and 1-hydroxy-2-naphthyl hydroxamic acid in the flotation separation of bastnaesite/fluorite: Experiments and first-principles calculations. Sep. Purif. Technol. 2022, 285, 120307. [Google Scholar] [CrossRef]
- Guo, Z.; Tian, M.; Qian, G.; Zhou, Y.; Gao, Z.; Sun, W. Flotation separation of bastnaesite and fluorite using styrylphosphonic acid and cinnamohydroxamic acid as collectors. J. Mol. Liq. 2022, 362, 119766. [Google Scholar] [CrossRef]
- Cao, Y.-Y.; Zhang, Y. Adsorption of H4SiO4 as a hydrolysate of sodium silicate on surfaces of fluorite (111), calcite (104), and scheelite (112): A density functional theory approach. Int. J. Electrochem. Sci. 2019, 14, 10807–10818. [Google Scholar] [CrossRef]
- Xu, L.; Liu, C.; Deng, J.; Wang, D.; Xue, K.; Wang, Y.; Meng, J.; Liu, J. Flotation and adsorption of novel Gemini decyl-bishydroxamic acid on bastnaesite: Experiments and density functional theory calculations. Int. J. Min. Sci. Technol. 2023, 33, 1193–1202. [Google Scholar] [CrossRef]
- Liu, P.; Guo, Z.; Zhang, W.; Tian, M.; Sun, W. N-[6-(hydroxyamino)-6-oxohexyl] octanamide: A collector derived from the structure of octyl hydroxamic acid and its application in bastnaesite flotation. Sep. Purif. Technol. 2024, 331, 125562. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williamsjones, A.E. The thermodynamic properties of bastnäsite-(Ce) and parisite-(Ce). Chem. Geol. 2015, 392, 87–101. [Google Scholar] [CrossRef]
- Guo, C.; Hou, S.; Wang, W.; Li, Q. Effect of an Environment-Friendly Depressant on the Flotation of Bastnaesite and Fluorite. Minerals 2024, 14, 165. [Google Scholar] [CrossRef]
- Guo, C.; Hou, S.; Wang, W.; Jin, H. Surface chemistry of xanthan gum interactions with bastnaesite and fluorite during flotation. Min. Eng. 2022, 189, 107891. [Google Scholar] [CrossRef]
- Guo, Z.; Khoso, S.A.; Tian, M.; Sun, W. Utilizing N-hydroxy-9-octadecenamide as a collector in flotation separation of bastnaesite and fluorite. J. Rare Earths 2023, 42, 1620–1628. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Y.; Zhu, Y.; Sun, N.; Wang, W. Investigating the performance of oxalic acid for separating bastnaesite from calcium-bearing gangue minerals based on experiment and theoretical calculation. Min. Eng. 2021, 170, 107047. [Google Scholar] [CrossRef]
- Cao, S.; Cao, Y.; Ma, Z.; Liao, Y.; Zhang, X. Structural and electronic properties of bastnaesite and implications for surface reactions in flotation. J. Rare Earths 2020, 38, 332–338. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Portela, R.; Baeza, P.; Alcolea-Rodriguez, V.; Villarroel, M.; Ávila, P. Zeta potential as a tool for functional materials development. Catal. Today 2023, 423, 113862. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Zhang, X.; Du, H.; Wang, X.; Miller, J. Surface chemistry aspects of bastnaesite flotation with octyl hydroxamate. Int. J. Min. Process. 2014, 133, 29–38. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, S.; Yi, H.; Song, S.; Jia, F. Flotation of bastnaesite by mixed collectors and adsorption mechanism. Chem. Phys. Lett. 2023, 830, 140793. [Google Scholar] [CrossRef]
- Letzel, A.; Santoro, M.; Frohleiks, J.; Ziefuß, A.R.; Reich, S.; Plech, A.; Fazio, E.; Neri, F.; Barcikowski, S.; Gökce, B. How the re-irradiation of a single ablation spot affects cavitation bubble dynamics and nanoparticles properties in laser ablation in liquids. Appl. Surf. Sci. 2019, 473, 828–837. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Wang, Z.; Miller, J. Flotation chemistry features in bastnaesite flotation with potassium lauryl phosphate. Min. Eng. 2016, 85, 17–22. [Google Scholar] [CrossRef]
- Espiritu, E.R.L.; Naseri, S.; Waters, K.E. Surface chemistry and flotation behavior of dolomite, monazite and bastnäsite in the presence of benzohydroxamate, sodium oleate and phosphoric acid ester collectors. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 254–265. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Chai, Y.; Zhang, Q. Crystal properties and interaction with flotation reagent of fluorapatite and dolomite. Min. Eng. 2023, 201, 108204. [Google Scholar] [CrossRef]
- Shuai, S.; Huang, Z.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W. Flotation separation of wolframite from calcite using a new trisiloxane surfactant as collector. Int. J. Min. Sci. Technol. 2023, 33, 379–387. [Google Scholar] [CrossRef]
- Zou, S.; Wang, S.; Ma, X.; Yang, J.; Zhong, H. Synthesis of a novel dithiocarbamate collector and its selective adsorption mechanism in galena flotation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130649. [Google Scholar] [CrossRef]
- Cao, Z.; Cheng, Z.; Wang, J.; Cao, Y. Synergistic depression mechanism of Ca2+ ions and sodium silicate on bastnaesite flotation. J. Rare Earths 2022, 40, 988–995. [Google Scholar] [CrossRef]
- Wu, J.; Yang, B.; Song, S.; Quintana, M.; Jia, F.; Tian, X. The efficient recovery of molybdenite fines using a novel collector: Flotation performances, adsorption mechanism and DFT calculation. Min. Eng. 2022, 188, 107848. [Google Scholar] [CrossRef]
- Azizi, D.; Larachi, F. Surface interactions and flotation behavior of calcite, dolomite and ankerite with alkyl hydroxamic acid bearing collector and sodium silicate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 126–138. [Google Scholar] [CrossRef]
- Kumar, D.; Srinivasan, S.G.; Jain, V.; Rai, B. Understanding flotation processes at the atomic scale using density functional theory–A case study on adsorption of 2-Mercaptobenzothiazole on chalcopyrite and pyrite surfaces. Appl. Surf. Sci. 2022, 579, 152112. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, Y.; Huang, Y.; Zhu, Y.; Shi, C.; Wang, W. Adsorption of ferric ions on the surface of bastnaesite and its significance in flotation. Min. Eng. 2020, 158, 106588. [Google Scholar] [CrossRef]



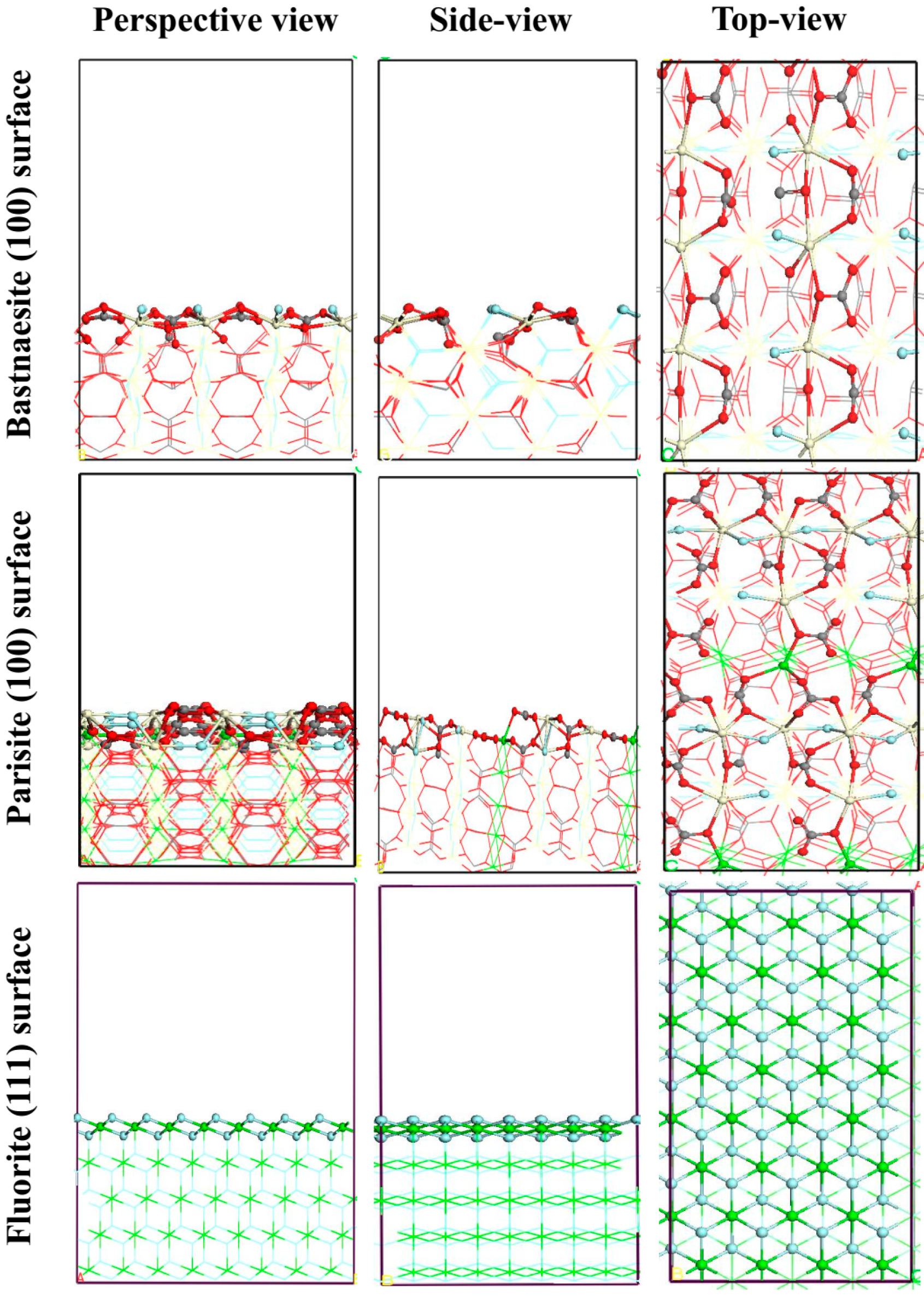

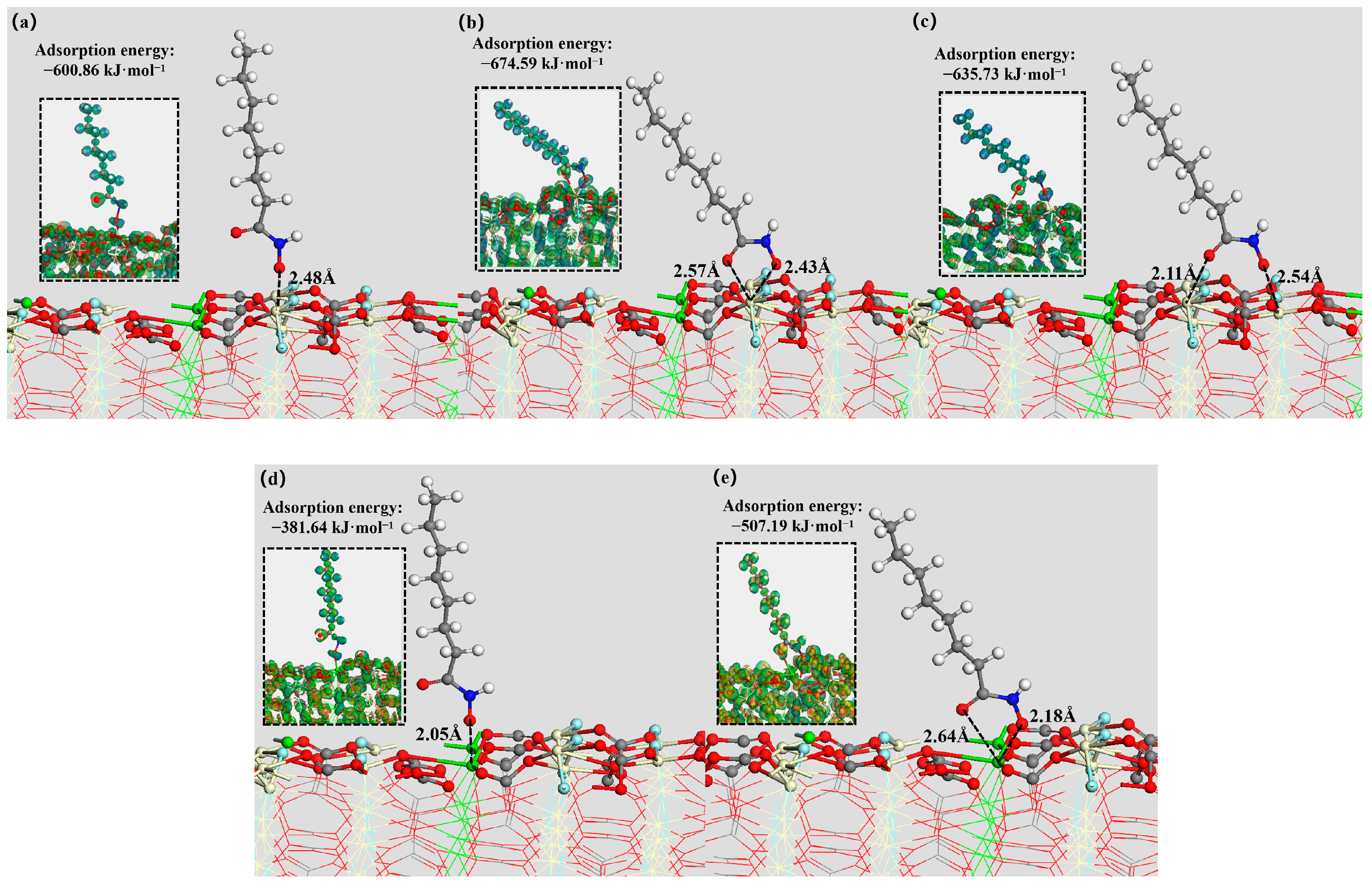
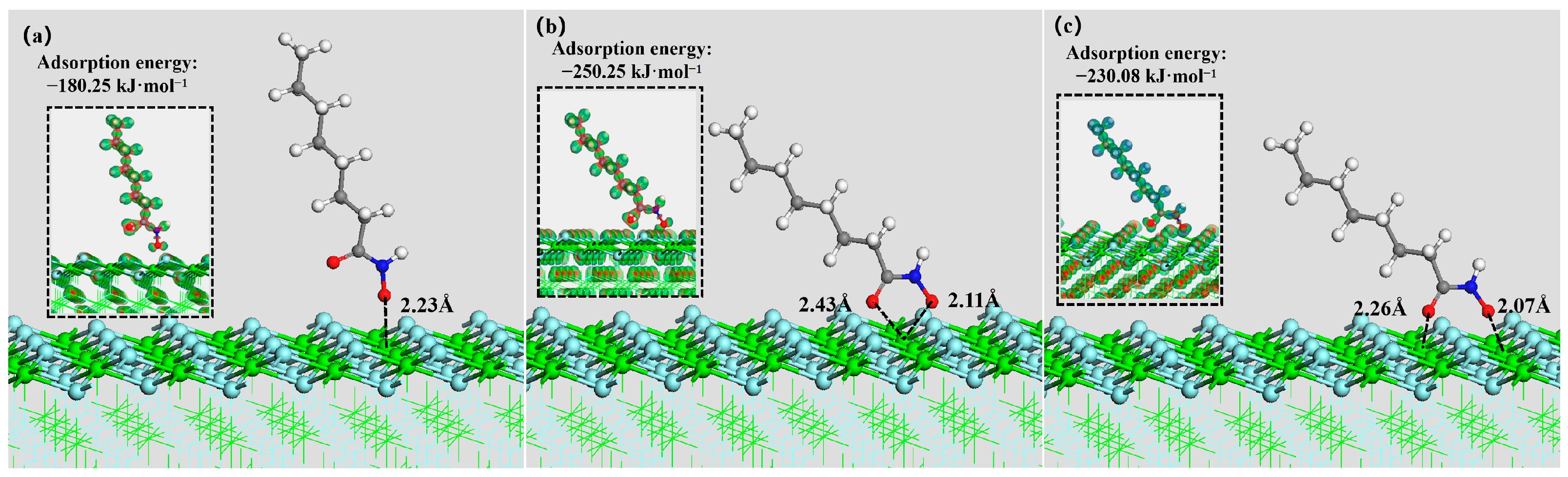


| Mineral Surface | Active Site | OHA Adsorption Energy (kJ mol−1) | SS Adsorption Energy (kJ mol−1) |
|---|---|---|---|
| Bastnaesite (100) surface | Ce | −767.68 | −624.77 |
| Parisite (100) surface | Ce | −674.59 | −678.53 |
| Ca | −507.19 | −480.79 | |
| Fluorite (100) surface | Ca | −250.25 | −678.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lu, W.; Cao, Z.; Wu, X.; Wang, P.; Wang, X.; Liu, W. New Insights into the Depressive Mechanism of Sodium Silicate on Bastnaesite, Parisite, and Fluorite: Experimental and DFT Study. Minerals 2024, 14, 870. https://doi.org/10.3390/min14090870
Wang J, Lu W, Cao Z, Wu X, Wang P, Wang X, Liu W. New Insights into the Depressive Mechanism of Sodium Silicate on Bastnaesite, Parisite, and Fluorite: Experimental and DFT Study. Minerals. 2024; 14(9):870. https://doi.org/10.3390/min14090870
Chicago/Turabian StyleWang, Jieliang, Wenda Lu, Zhao Cao, Xu Wu, Peng Wang, Xiaoping Wang, and Wenli Liu. 2024. "New Insights into the Depressive Mechanism of Sodium Silicate on Bastnaesite, Parisite, and Fluorite: Experimental and DFT Study" Minerals 14, no. 9: 870. https://doi.org/10.3390/min14090870






