Flotation Tailings from Cu-Au Mining (Bor, Serbia) as a Potential Secondary Raw Material for Valuable Metals Recovery
Abstract
:1. Introduction
2. Materials and Methods
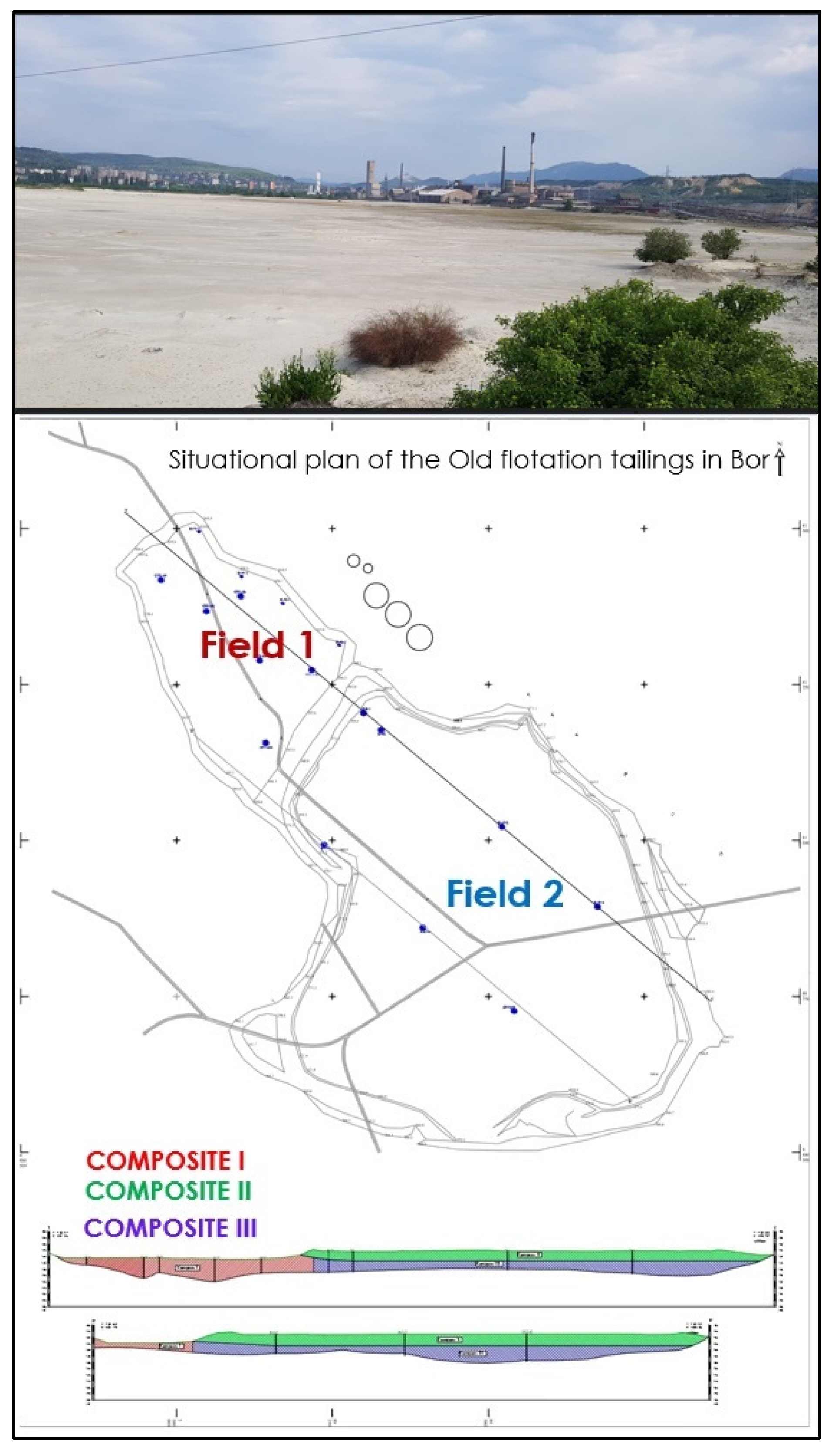
2.1. Sampling
2.2. Sample Preparation
3. Sample Characterization
3.1. Physico-Chemical Characterization of the Samples
3.2. Granulometric Composition of the Samples
3.3. Mineralogical Characterization of the Samples
4. Results and Discussion
4.1. Physico-Chemical Characterization of the Samples
4.2. Granulometric Composition of the Samples
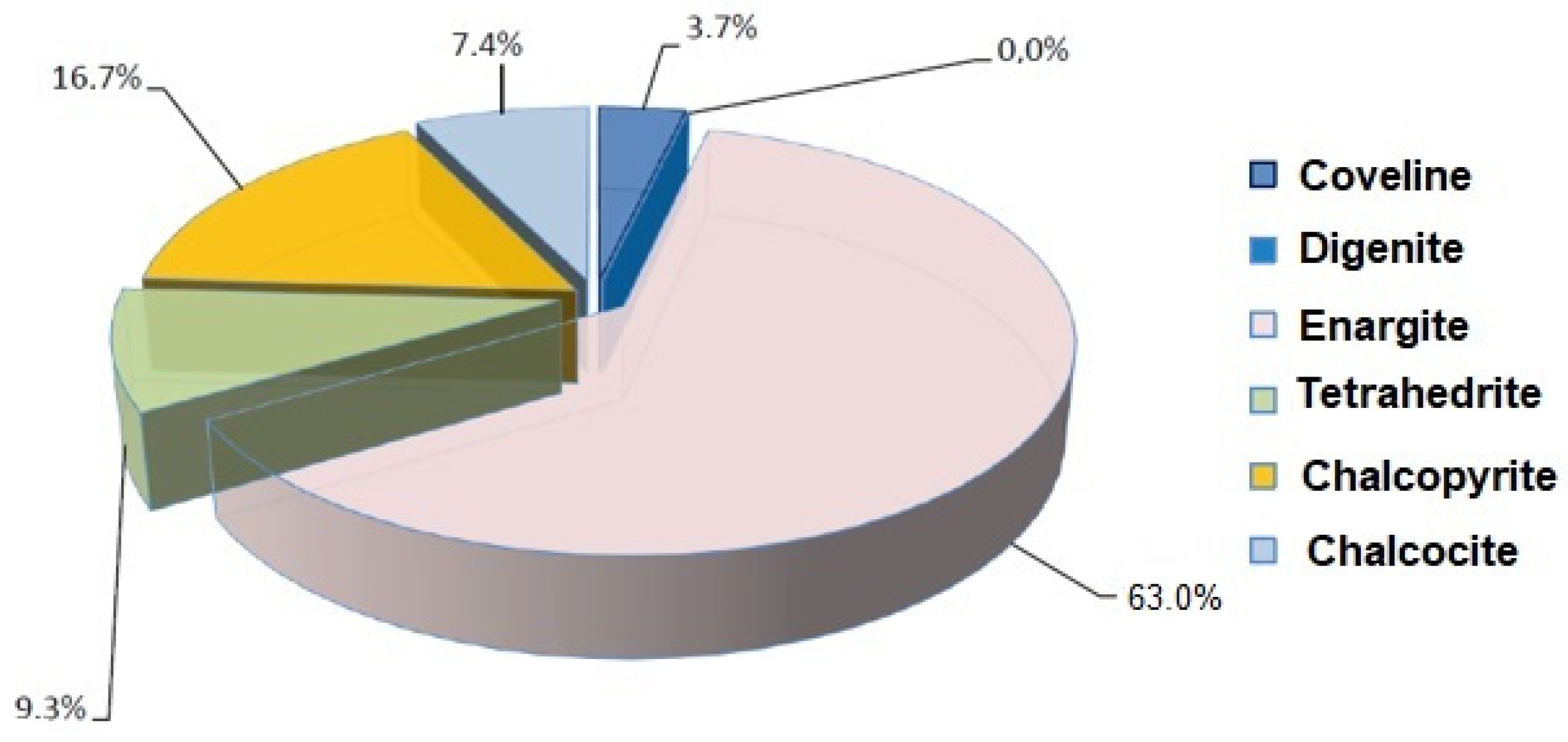
4.3. Mineralogical Characterization of the Samples
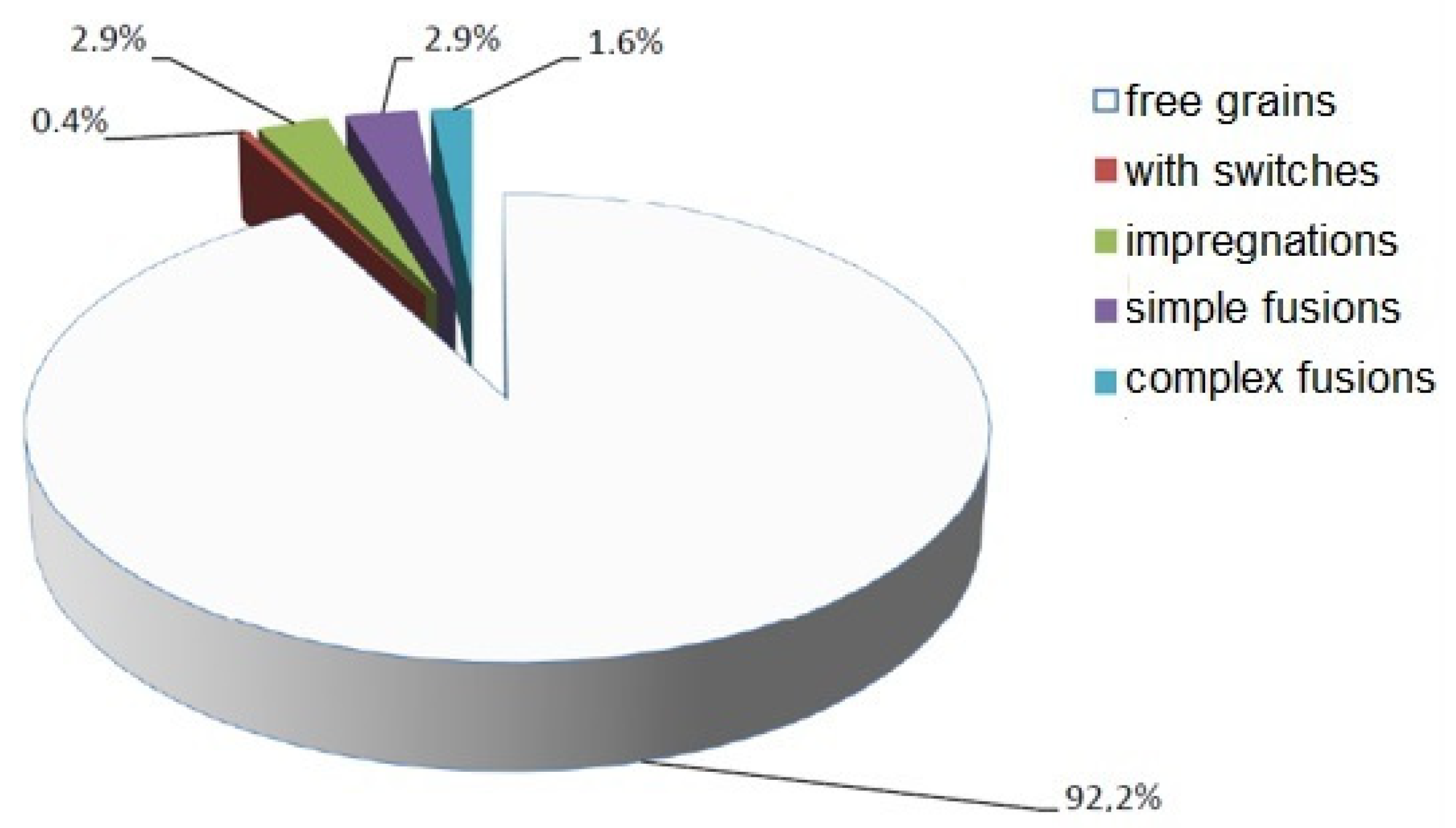
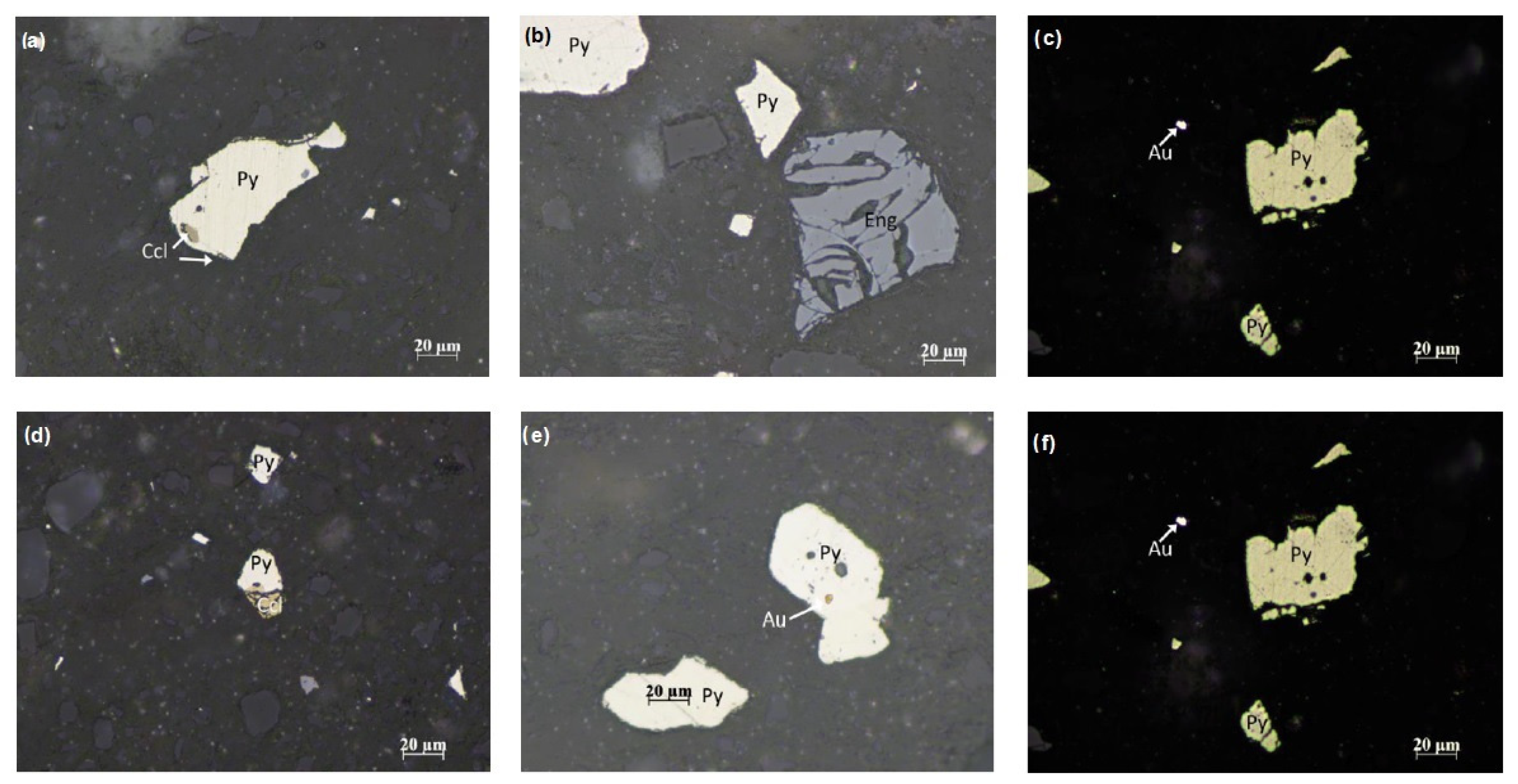
4.3.1. Polarization Microscope Analysis
4.3.2. XRD Analysis
4.3.3. SEM-EDS Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schueler, T.A.; Schippers, A.; Goldmann, D. Bioleaching for metals removal from mine tailings flotation fractions. Hydrometallurgy 2024, 225, 106286. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Bouzahzah, H.; Abdelmoula, M.; Dynes, J.J.; Jamieson, H.E. Role of secondary minerals in the acid generating potential of weathered mine tailings: Crystal-chemistry characterization and closed mine site management involvement. Sci. Total Environ. 2021, 784, 147105. [Google Scholar] [CrossRef]
- Abbadi, A.; Mucsi, G. A review on complex utilization of mine tailings: Recovery of rare earth elements and residue valorization. J. Environ. Chem. Eng. 2024, 12, 113118. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; Muñoz, J.Á. Leaching/Bioleaching and Recovery of Metals. Metals 2021, 11, 1732. [Google Scholar] [CrossRef]
- Santibáñez-Velásquez, L.E.; Guzmán, A.; Morel, M.J. Extraction of Iron and Other Metals from Copper Tailings through Leaching. Metals 2022, 12, 1924. [Google Scholar] [CrossRef]
- Opara, C.B.; Blannin, R.; Ebert, D.; Frenzel, M.; Pollmann, K.; Kutschke, S. Bioleaching of metal(loid)s from sulfidic mine tailings and waste rock from the Neves Corvo mine, Portugal, by an acidophilic consortium. Miner. Eng. 2022, 188, 107831. [Google Scholar] [CrossRef]
- Araya, N.; Kraslawski, A.; Cisternas, L.A. Towards mine tailings valorization: Recovery of critical materials from Chilean mine tailings. J. Clean. Prod. 2020, 263, 121555. [Google Scholar] [CrossRef]
- Marín, O.A.; Kraslawski, A.; Cisternas, L.A. Estimating processing cost for the recovery of valuable elements from mine tailings using dimensional analysis. Miner. Eng. 2022, 184, 107629. [Google Scholar] [CrossRef]
- Hassan, S.; Bhadwal, S.S.; Khan, M.; Sabreena; Nissa, K.; Shah, R.A.; Bhat, H.M.; Bhat, S.A.; Lone, I.M.; Ganai, B.A. Revitalizing contaminated lands: A state-of-the-art review on the remediation of mine-tailings using phytoremediation and genomic approaches. Chemosphere 2024, 356, 141889. [Google Scholar] [CrossRef]
- Gümüşsoy, A.; Başyiğit, M.; Kart, E.U. Economic potential and environmental impact of metal recovery from copper slag flotation tailings. Resour. Policy 2023, 80, 103232. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.; Xu, H.; Cui, X.; Li, J.; Chen, J.; Zheng, B. Characterizations of heavy metal contamination, microbial community, and resistance genes in a tailing of the largest copper mine in China. Environ. Pollut. 2021, 280, 116947. [Google Scholar] [CrossRef] [PubMed]
- Eksteen, J.J.; Oraby, E.A.; Tanda, B.C. A conceptual process for copper extraction from chalcopyrite in alkaline glycinate solutions. Miner. Eng. 2017, 108, 53–66. [Google Scholar] [CrossRef]
- Arunachalam, K.P.; Avudaiappan, S.; Maureira, N.; Da Costa Garcia Filho, F.; Monteiro, S.N.; Batista, I.D.; de Azevedo, A.R.G. Innovative use of copper mine tailing as an additive in cement mortar. J. Mater. Res. Technol. 2023, 25, 2261–2274. [Google Scholar] [CrossRef]
- Gitari, M.W.; Akinyemi, S.A.; Thobakgale, R.; Ngoejana, P.C.; Ramugondo, L.; Matidza, M.; Mhlongo, S.E.; Dacosta, F.A.; Nemapate, N. Physicochemical and mineralogical characterization of Musina mine copper and New Union gold mine tailings: Implications for fabrication of beneficial geopolymeric construction materials. J. Afr. Earth Sci. 2018, 137, 218–228. [Google Scholar] [CrossRef]
- Ghazi, A.B.; Jamshidi-Zanjani, A.; Nejati, H. Utilization of copper mine tailings as a partial substitute for cement in concrete construction. Constr. Build. Mater. 2022, 317, 125921. [Google Scholar] [CrossRef]
- Nikvar-Hassani, A.; Hodges, R.; Zhang, L. Production of green bricks from low-reactive copper mine tailings: Durability and environmental aspects. Constr. Build. Mater. 2022, 337, 127571. [Google Scholar] [CrossRef]
- Lu, T.; Wei, Z.; Hesham El Naggar, M.; Wang, W.; Yang, Z.; Tian, X.; Guo, H. Effect of chemical environment on copper tailings reinforced by microbially induced carbonate precipitation. Constr. Build. Mater. 2023, 400, 132894. [Google Scholar] [CrossRef]
- Adewuyi, S.O.; Anani, A.; Luxbacher, K. Advancing sustainable and circular mining through solid-liquid recovery of mine tailings. Process Saf. Environ. Prot. 2024, 189, 31–46. [Google Scholar] [CrossRef]
- Kurniati, E.A.; Zeng, H.; Latypov, M.I.; Kim, H.J. Machine learning for predicting compressive strength of sustainable cement paste incorporating copper mine tailings as supplementary cementitious materials. Case Stud. Constr. Mater. 2024, 21, e03373. [Google Scholar] [CrossRef]
- Hansen, H.K.; Yianatos, J.B.; Ottosen, L.M. Speciation and leachability of copper in mine tailings from porphyry copper mining: Influence of particle size. Chemosphere 2005, 60, 1497–1503. [Google Scholar] [CrossRef]
- Munir, M.A.M.; Irshad, S.; Yousaf, B.; Ali, M.U.; Dan, C.; Abbas, Q.; Liu, G.; Yang, X. Interactive assessment of lignite and bamboo-biochar for geochemical speciation, modulation and uptake of Cu and other heavy metals in the copper mine tailing. Sci. Total Environ. 2021, 779, 146536. [Google Scholar] [CrossRef] [PubMed]
- García, S.; Camus, L.; Gonzalez-Diaz, E.; Collao, R.; Townley, B.; Parviainen, A.; Caraballo, M.A. The importance of geochemical and mineralogical characterization of fresh Cu-Porphyry mine tailings in mineral processing plants to optimize their revalorization potential. J. Geochem. Explor. 2024, 259, 107439. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, Y.; Zhao, Z. Utilization of limestone powder and water-reducing admixture in cemented paste backfill of coarse copper mine tailings. Constr. Build. Mater. 2016, 124, 31–36. [Google Scholar] [CrossRef]
- Ince, C.; Derogar, S.; Gurkaya, K.; Ball, R.J. Properties, durability and cost efficiency of cement and hydrated lime mortars reusing copper mine tailings of Lefke-Xeros in Cyprus. Constr. Build. Mater. 2021, 268, 121070. [Google Scholar] [CrossRef]
- Pan, W.; Kang, Z.; Liu, H.; Liao, Y.; Wang, S. Study on the bio-passivation of copper sulfide tailings combined with solid waste to inhibit acidic mine drainage. J. Water Process Eng. 2024, 61, 105253. [Google Scholar] [CrossRef]
- Petronijević, N.; Stanković, S.; Radovanović, D.; Sokić, M.; Marković, B.; Stopić, S.R.; Kamberović, Ž. Application of the Flotation Tailings as an Alternative Material for an Acid Mine Drainage Remediation: A Case Study of the Extremely Acidic Lake Robule (Serbia). Metals 2020, 10, 16. [Google Scholar] [CrossRef]
- Sarker, S.K.; Haque, N.; Bhuiyan, M.; Bruckard, M.; Pramanik, B.K. Recovery of strategically important critical minerals from mine tailings. J. Environ. Chem. Eng. 2022, 10, 107622. [Google Scholar] [CrossRef]
- Godirilwe, L.L.; Haga, K.; Altansukh, B.; Takasaki, Y.; Ishiyama, D.; Trifunovic, V.; Avramovic, L.; Jonovic, R.; Stevanovic, Z.; Shibayama, A. Copper Recovery and Reduction of Environmental Loading from Mine Tailings by High-Pressure Leaching and SX-EW Process. Metals 2021, 11, 1335. [Google Scholar] [CrossRef]
- Oliveira, D.; Horn, E.J.; Randall, D.G. Copper mine tailings valorization using microbial induced calcium carbonate precipitation. J. Environ. Manag. 2021, 298, 113440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Schippers, A. Stirred-tank bioleaching of copper and cobalt from mine tailings in Chile. Miner. Eng. 2022, 180, 107514. [Google Scholar] [CrossRef]
- Wong-Pinto, L.; Mercado, A.; Chong, G.; Salazar, P.; Ordóñez, J.I. Biosynthesis of copper nanoparticles from copper tailings ore—An approach to the ‘Bionanomining’. J. Clean. Prod. 2021, 315, 128107. [Google Scholar] [CrossRef]
- Chen, H.; Nikvar-Hassani, A.; Ormsby, S.; Ramey, D.; Zhang, L. Mechanical and microstructural investigations on the low-reactive copper mine tailing-based geopolymer activated by phosphoric acid. Constr. Build. Mater. 2023, 393, 132030. [Google Scholar] [CrossRef]
- Lei, B.; Li, X.; Guo, Y.; Qu, F.; Zhao, C.; Tam, V.W.Y.; Wu, V.; Li, W. Recycling of copper tailing as filler material in asphalt paving mastic: A sustainable solution for mining waste recovery. Case Stud. Constr. Mater. 2024, 20, e03237. [Google Scholar] [CrossRef]
- Alcalde, J.; Kelm, U.; Vergara, D. Historical assessment of metal recovery potential from old mine tailings: A study case for porphyry copper tailings, Chile. Miner. Eng. 2018, 127, 334–338. [Google Scholar] [CrossRef]
- Chen, W.; Yin, S.; Zhou, G.; Li, Z.; Song, Q. Copper recovery from tailings through bioleaching and further application of bioleached tailings residue: Comprehensive utilization of tailings. J. Clean. Prod. 2022, 332, 130129. [Google Scholar] [CrossRef]
- Adrianto, L.R.; Pfister, S. Prospective environmental assessment of reprocessing and valorization alternatives for sulfidic copper tailings. Resour. Conserv. Recycl. 2022, 186, 106567. [Google Scholar] [CrossRef]
- Li, Z.; Guo, L.; Zhao, Y.; Peng, X.; Kyegyenbai, K. A Particle Size Distribution Model for Tailings in Mine Backfill. Metals 2022, 12, 594. [Google Scholar] [CrossRef]
- Liu, J.; Peng, H.; Peng, H.; Zheng, J.; Zhang, Z.; Ye, H.; Wang, H.; Yu, Z.; Zhu, X.; Wang, Q. Production of glass-ceramics from MSW-fly ash and Dexing copper mine-tailing: Crystallization kinetics, structure, properties and immobilization of heavy metals. Ceram. Int. 2023, 49, 35428–35437. [Google Scholar] [CrossRef]
- Nikvar-Hassani, A.; Vashaghian, H.; Hodges, R.; Zhang, L. Production of green bricks from low-reactive copper mine tailings: Chemical and mechanical aspects. Constr. Build. Mater. 2022, 324, 126695. [Google Scholar] [CrossRef]
- Huynh, T.P.; Nguyen, T.M.; Lam, T.K. Utilization of high volumes of copper mine tailings in the production of fine-grained concrete. Mater. Today Proc. 2022, 65, 543–548. [Google Scholar] [CrossRef]
- Esmaeili, J.; Al-Mwanes, A.O. Production of eco-friendly UHPC with high durability and resistance to harsh environmental conditions using copper mine tailings. J. Build. Eng. 2023, 76, 107297. [Google Scholar] [CrossRef]
- Lutandula, M.S.; Maloba, B. Recovery of cobalt and copper through reprocessing of tailings from flotation of oxidised ores. J. Environ. Chem. Eng. 2013, 1, 1085–1090. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, A.; Tapia, J.C.J.; Lapidus, G.T. Evaluation of acid mine drainage (AMD) from tailings and their valorization by copper recovery. Miner. Eng. 2023, 191, 107979. [Google Scholar] [CrossRef]
- Maksimović, M.; Bugarin, M.; Marinković, V.; Jonović, R.; Avramović, L. Annual Report on Applied, Detailed Geological Investigations of Technogenic Mineral Raw Materials in the Area of the Old Flotation Tailings Bor (Field 1 and Field 2), in the Second Research Year (2019/20); Mining and Metallurgy Institute Bor: Bor, Serbia, 2020. [Google Scholar]
- Marinković, V.; Jovanović, M.; Avramović, L.; Jonović, R.; Pačkovski, G.; Kržanović, D. Project of Applied, Detailed Geological Research of Technogenic Mineral Raw Materials in the Area of Old Flotation Tailings Bor (Field 1 and Field 2), at 2021/23; Mining and Metallurgy Institute Bor: Bor, Serbia, 2021. [Google Scholar]
- Jovanović, M.; Maksimović, M.; Marinković, V.; Pačkovski, G.; Avramović, L. Final Report on Applied, Detailed Geological Investigations of Technogenic Mineral Raw Materials in the Area of the Old Flotation Tailings Bor (Field 1 and Field 2), in Period 2021 to 2023; Mining and Metallurgy Institute Bor: Bor, Serbia, 2023. [Google Scholar]
- Özdemir, E.; Lang, A.; Saari, J.; Liipo, J. Recovery of Sulfur, Copper, and Gold by Reprocessing Old Floitation Tailings at Bor, Serbia. Asp. Min. Miner. Sci. 2024, 12, 1366–1377. [Google Scholar]
- Velizar, S.; Milošević, V.; Milićević, D.; Gorgievski, M.; Bogdanović, G. Reprocessing of the Old Flotation Tailings Deposited on the RTB Bor Tailings Pond—A Case Study. Chem. Ind. Chem. Eng. Q. 2018, 24, 333–344. [Google Scholar]
- Serafimovski, T.; Ristović, I.; Boev, B.; Tasev, G.; Boev, I.; Serafimovski, D.; Dolenec, M. Some Geochemical and Mineralogical Features of Samples from Old Bor’s Tailing Dam. Geol. Maced. 2021, 5, 157–164. [Google Scholar]
- Han, B.; Altansukh, B.; Haga, K.; Stevanovic, Z.; Jonovic, R.; Markovic, R.; Avramovic, L.; Obradovic, L.; Takasaki, Y.; Masuda, N.; et al. Copper Upgrading and Recovery Process from Mine Tailing of Bor Region, Serbia Using Flotation. Int. J. Soc. Mater. Eng. Resour. 2014, 20, 225–229. [Google Scholar] [CrossRef]

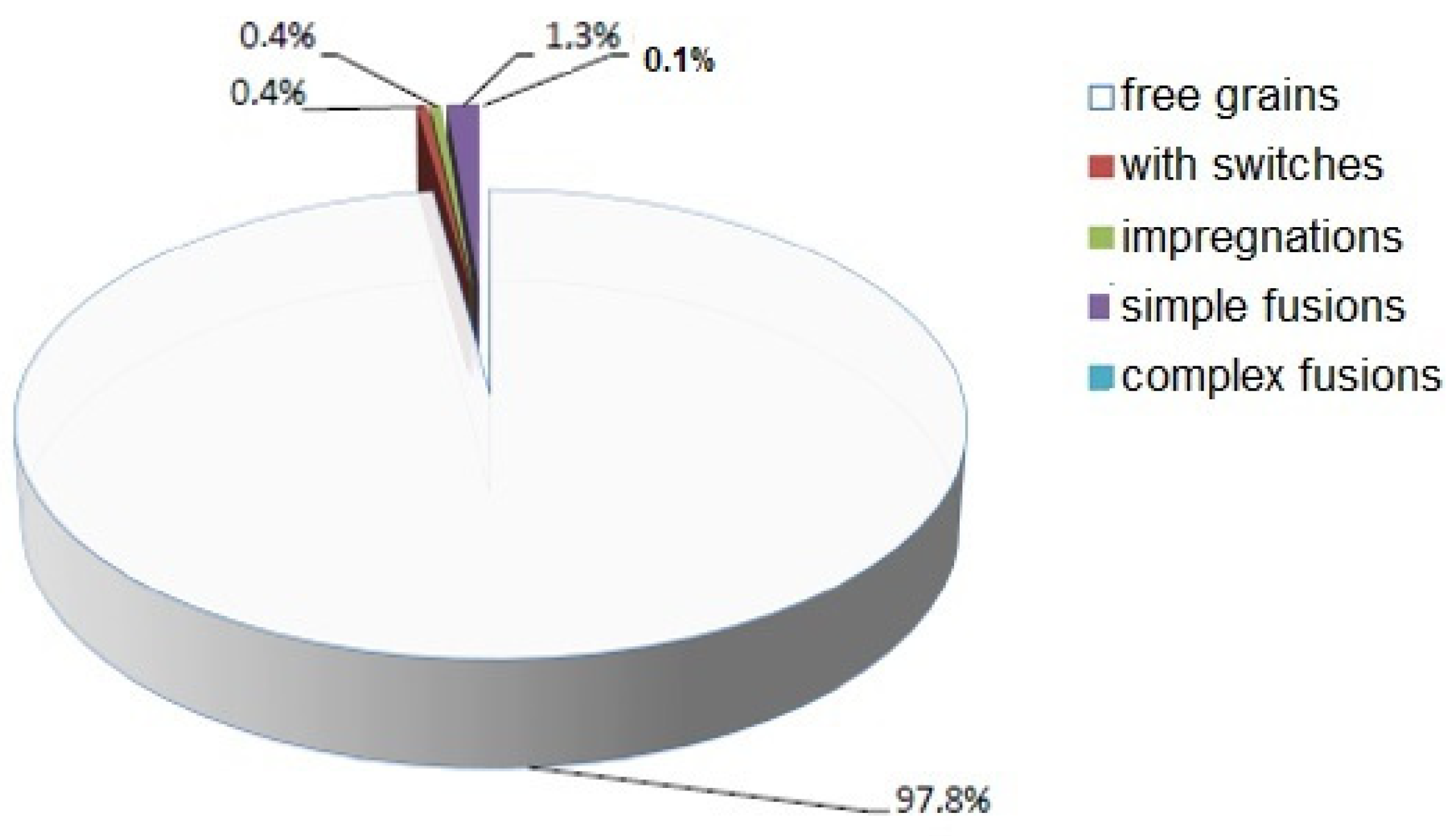




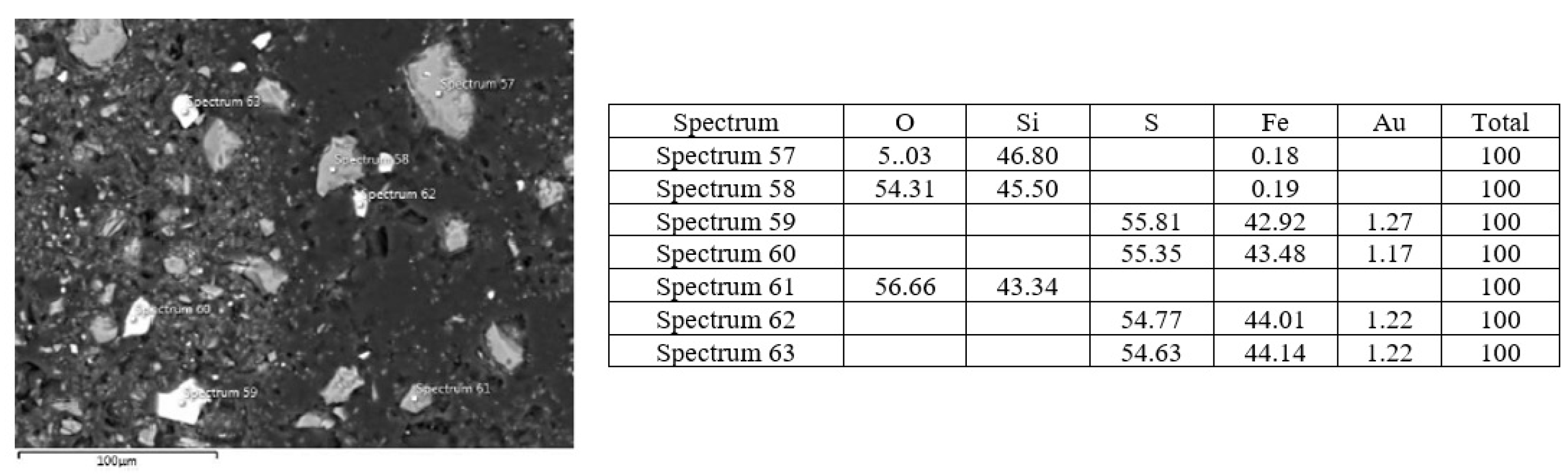
| Sample Name | Sample Density (g·cm−3) | Bulk Mass (kg·m−3) | pH Value |
|---|---|---|---|
| COMPOSITE I+III | 2.765 | 1.176 | 2.82 |
| COMPOSITE II | 2.690 | 0.976 | 2.77 |
| Element | Unit | Content | |
|---|---|---|---|
| COMPOSITE I+III | COMPOSITE II | ||
| Cutotal | % | 0.25 | 0.23 |
| Cuox | % | 0.18 | 0.12 |
| Fe | % | 7.62 | 8.90 |
| Ca | % | 0.78 | 0.74 |
| K | % | 0.54 | 0.59 |
| Na | % | 0.23 | 0.19 |
| S | % | 9.34 | 12.38 |
| SiO2 | % | 52.88 | 49.88 |
| Al2O3 | % | 10.76 | 11.00 |
| Au | ppm | 0.45 | 0.40 |
| Ag | ppm | 1.40 | 2.00 |
| Sr | ppm | 648 | 642 |
| As | ppm | 122 | 166 |
| Zn | ppm | 23.6 | 49.1 |
| Sieve Opening Size d (mm) | Mass Participation m (%) | Sieve Reflection R (%) | Sieve Sifting D (%) |
|---|---|---|---|
| −4+2.36 | 7.60 | 7.60 | 100.00 |
| −2.36+1.70 | 0.20 | 7.80 | 92.40 |
| −1.70+0.850 | 0.40 | 8.20 | 92.20 |
| −0.850+0.600 | 0.40 | 8.60 | 91.80 |
| −0.600+0.425 | 0.30 | 8.90 | 91.40 |
| −0.425+0.300 | 6.70 | 15.60 | 91.10 |
| −0.300+0.212 | 1.10 | 16.70 | 84.40 |
| −0.212+0.150 | 4.40 | 21.10 | 83.30 |
| −0.150+0.106 | 6.30 | 27.40 | 78.90 |
| −0.106+0.075 | 6.10 | 33.50 | 72.60 |
| −0.075+0.053 | 5.50 | 39.00 | 66.50 |
| −0.053+0.038 | 5.50 | 44.50 | 61.00 |
| −0.038+0.00 | 55.50 | 100.00 | 55.50 |
| Sieve Opening Size d (mm) | Mass Participation m (%) | Sieve Reflection R (%) | Sieve Sifting D (%) |
|---|---|---|---|
| −4+2.36 | 0.30 | 0.30 | 100.00 |
| −2.36+1.70 | 0.10 | 0.40 | 99.70 |
| −1.70+0.850 | 0.10 | 0.50 | 99.60 |
| −0.850+0.600 | 0.10 | 0.60 | 99.50 |
| −0.600+0.425 | 0.10 | 0.70 | 99.40 |
| −0.425+0.300 | 0.40 | 1.10 | 99.30 |
| −0.300+0.212 | 7.20 | 8.30 | 98.90 |
| −0.212+0.150 | 6.30 | 14.60 | 91.70 |
| −0.150+0.106 | 8.60 | 23.20 | 85.40 |
| −0.106+0.075 | 7.70 | 30.90 | 76.80 |
| −0.075+0.053 | 7.80 | 38.70 | 69.10 |
| −0.053+0.038 | 6.20 | 44.90 | 61.30 |
| −0.038+0.00 | 55.10 | 100.00 | 55.10 |
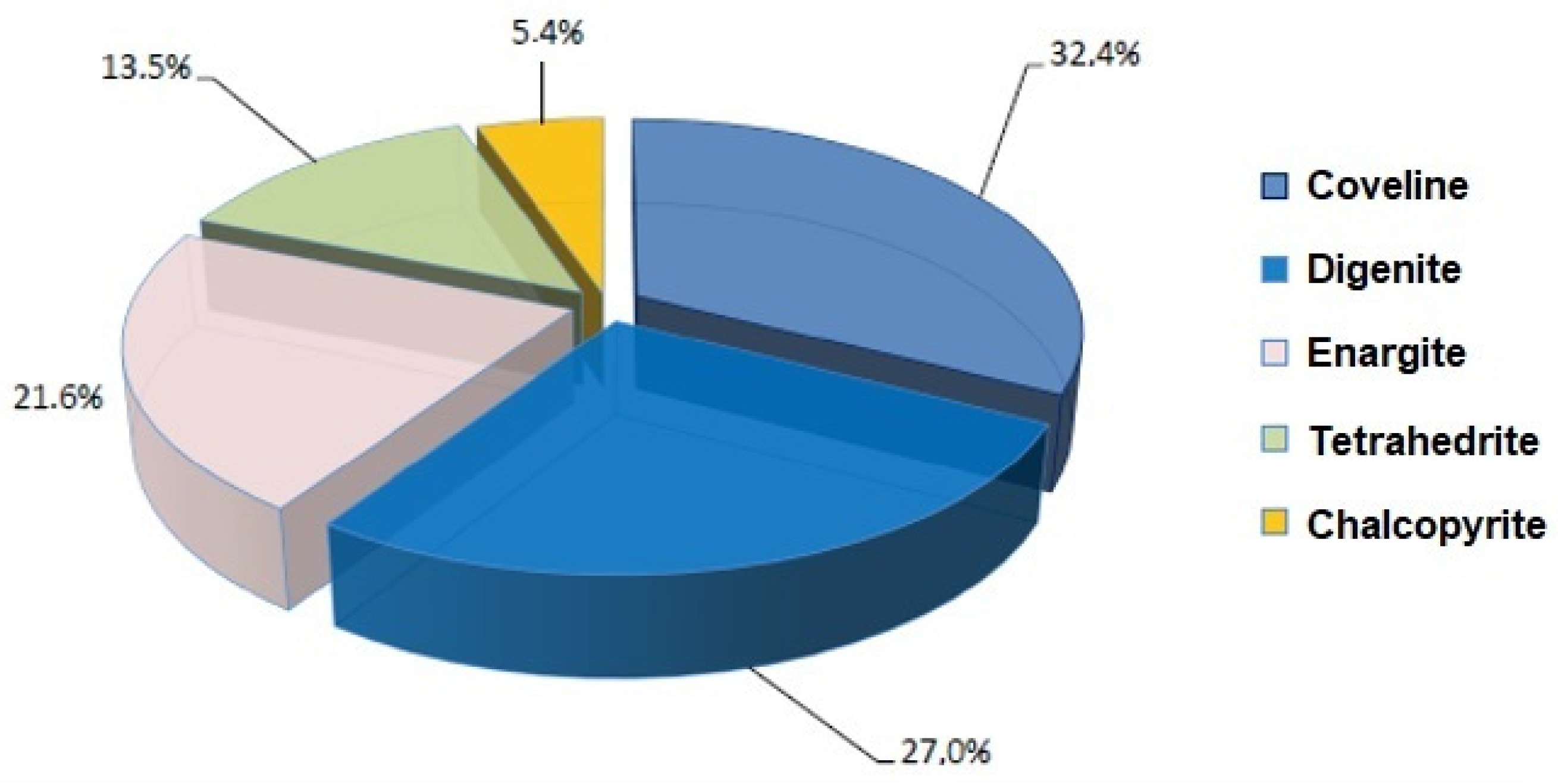
| Mineral | COMPOSITE I+III | COMPOSITE II |
|---|---|---|
| Pyrite (FeS2) | 17.15 | 22.96 |
| Coveline (CuS) | 0.02 | 0.12 |
| Digenite (Cu9S5) | - | 0.10 |
| Enargite (Cu3AsS4) | 0.34 | 0.08 |
| Tetrahedrite (CuFeSbS) | 0.05 | 0.05 |
| Chalcopyrite (CuFeS2) | 0.09 | 0.02 |
| Chalcocite (CuS2) | 0.04 | <0.01 |
| Native gold | <0.01 | - |
| Magnetite (Fe3O4) | 0.32 | 0.19 |
| Hematite (Fe2O3) | 0.11 | 0.04 |
| Rutile (TiO2) | 0.21 | 0.27 |
| Leucoxene (TiO2) | 0.31 | 0.61 |
| Cassiterite (SnO2) | - | 0.03 |
| Cu-limonite (CuFe2O3∙H2O) | 0.90 | 0.72 |
| Tailings minerals | 80.46 | 74.81 |
| In total: | 100.00 | 100.00 |
| Mineral | Assessment of Mineral Content (%) | |
|---|---|---|
| COMPOSITE I+III | COMPOSITE II | |
| Quartz (SiO2) | 46 | 43 |
| Pyrite (Fe2S) | 17 | 20 |
| Kaolinite (Al2Si2O5(OH)4) | 31 | 31 |
| Alunite (KAl3(SO4)2(OH)6) | 6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifunović, V.; Avramović, L.; Božić, D.; Jonović, M.; Šabaz, D.; Bugarin, D. Flotation Tailings from Cu-Au Mining (Bor, Serbia) as a Potential Secondary Raw Material for Valuable Metals Recovery. Minerals 2024, 14, 905. https://doi.org/10.3390/min14090905
Trifunović V, Avramović L, Božić D, Jonović M, Šabaz D, Bugarin D. Flotation Tailings from Cu-Au Mining (Bor, Serbia) as a Potential Secondary Raw Material for Valuable Metals Recovery. Minerals. 2024; 14(9):905. https://doi.org/10.3390/min14090905
Chicago/Turabian StyleTrifunović, Vanja, Ljiljana Avramović, Dragana Božić, Marija Jonović, Dragan Šabaz, and Dejan Bugarin. 2024. "Flotation Tailings from Cu-Au Mining (Bor, Serbia) as a Potential Secondary Raw Material for Valuable Metals Recovery" Minerals 14, no. 9: 905. https://doi.org/10.3390/min14090905





