Investigation of the Flotation of an Ore Containing Bastnaesite and Monazite: Kinetic Study and Process Flowsheet Simulation
Abstract
:1. Introduction
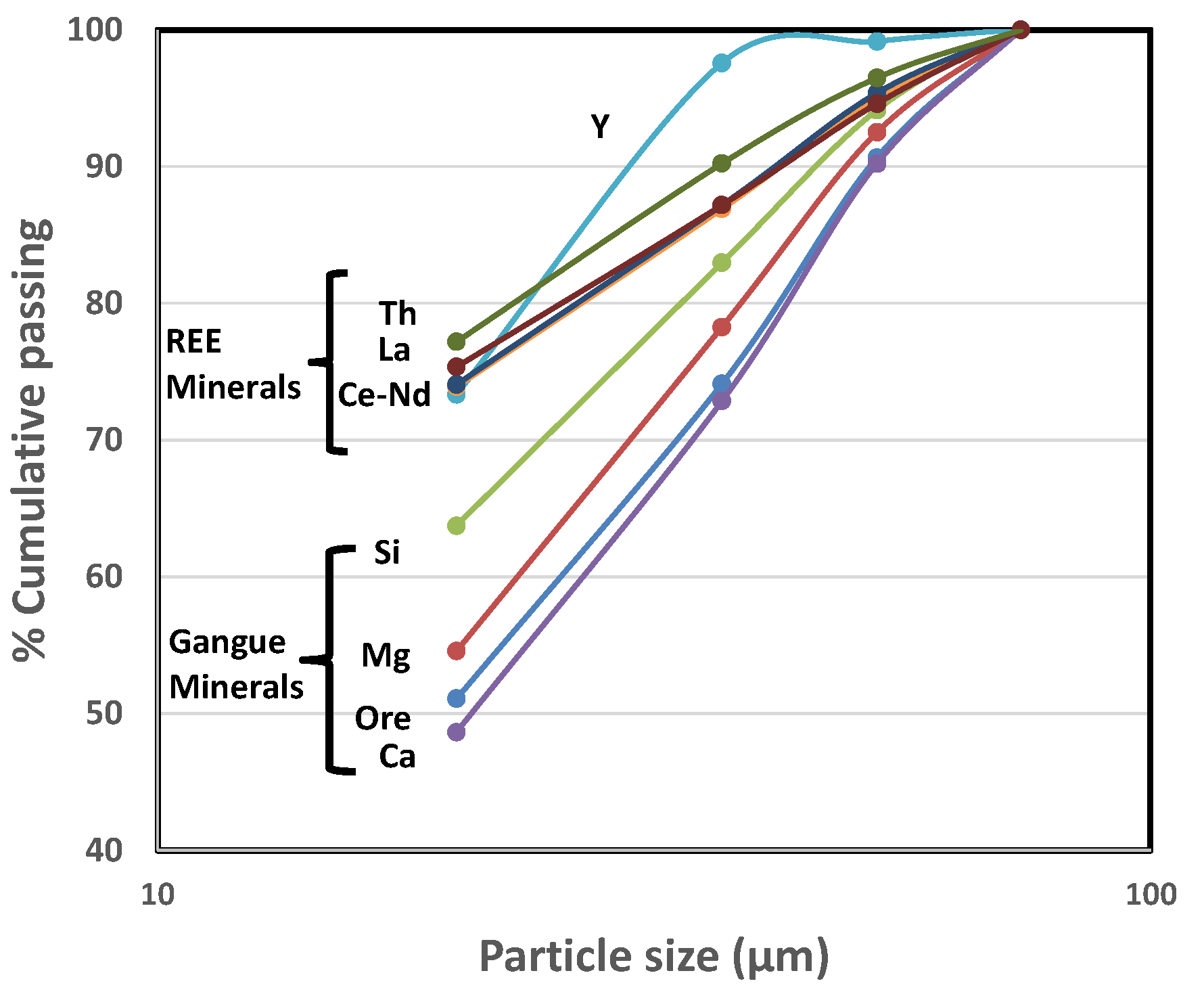
2. Ore Sample
3. Experimental Methodology
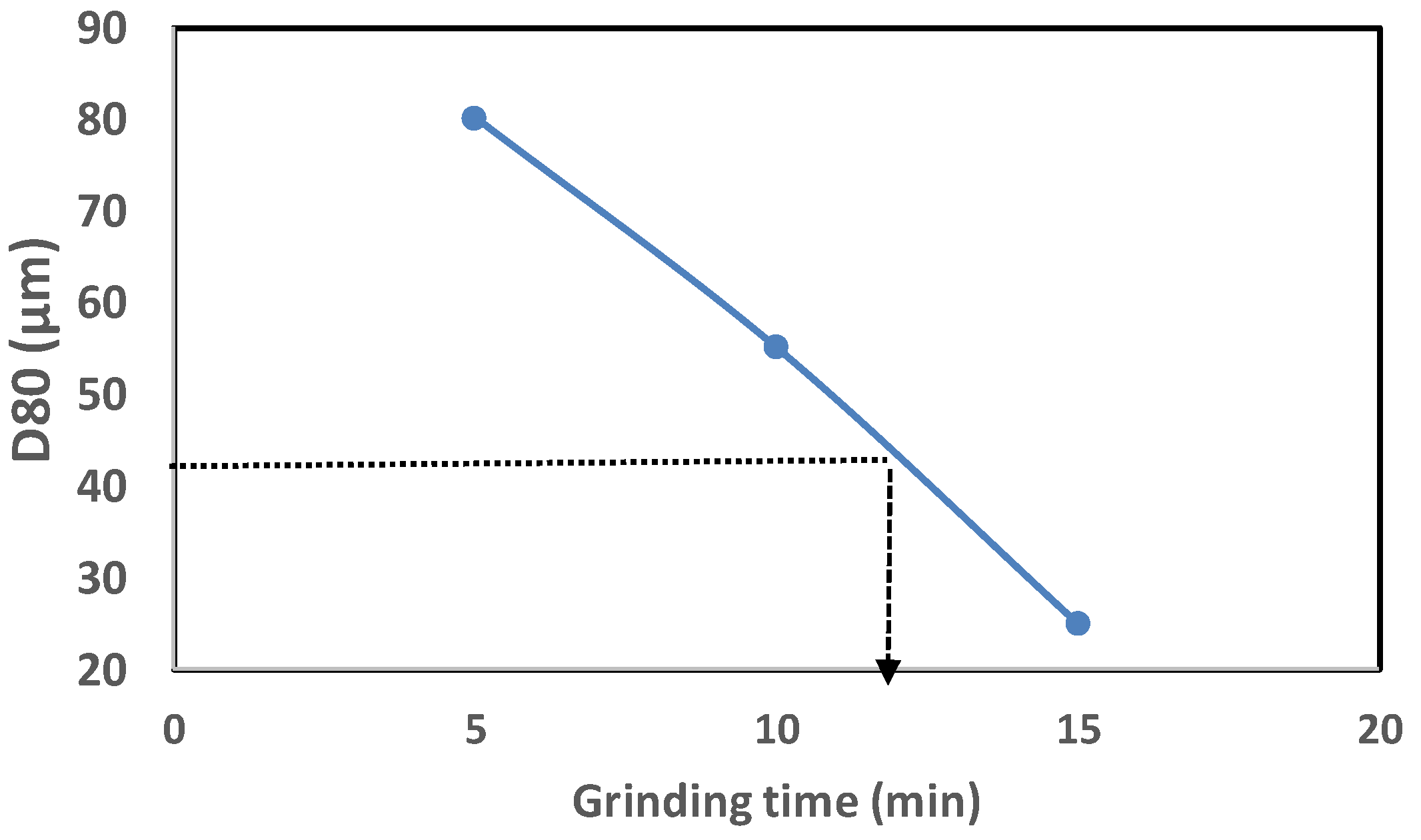
3.1. Size Reduction
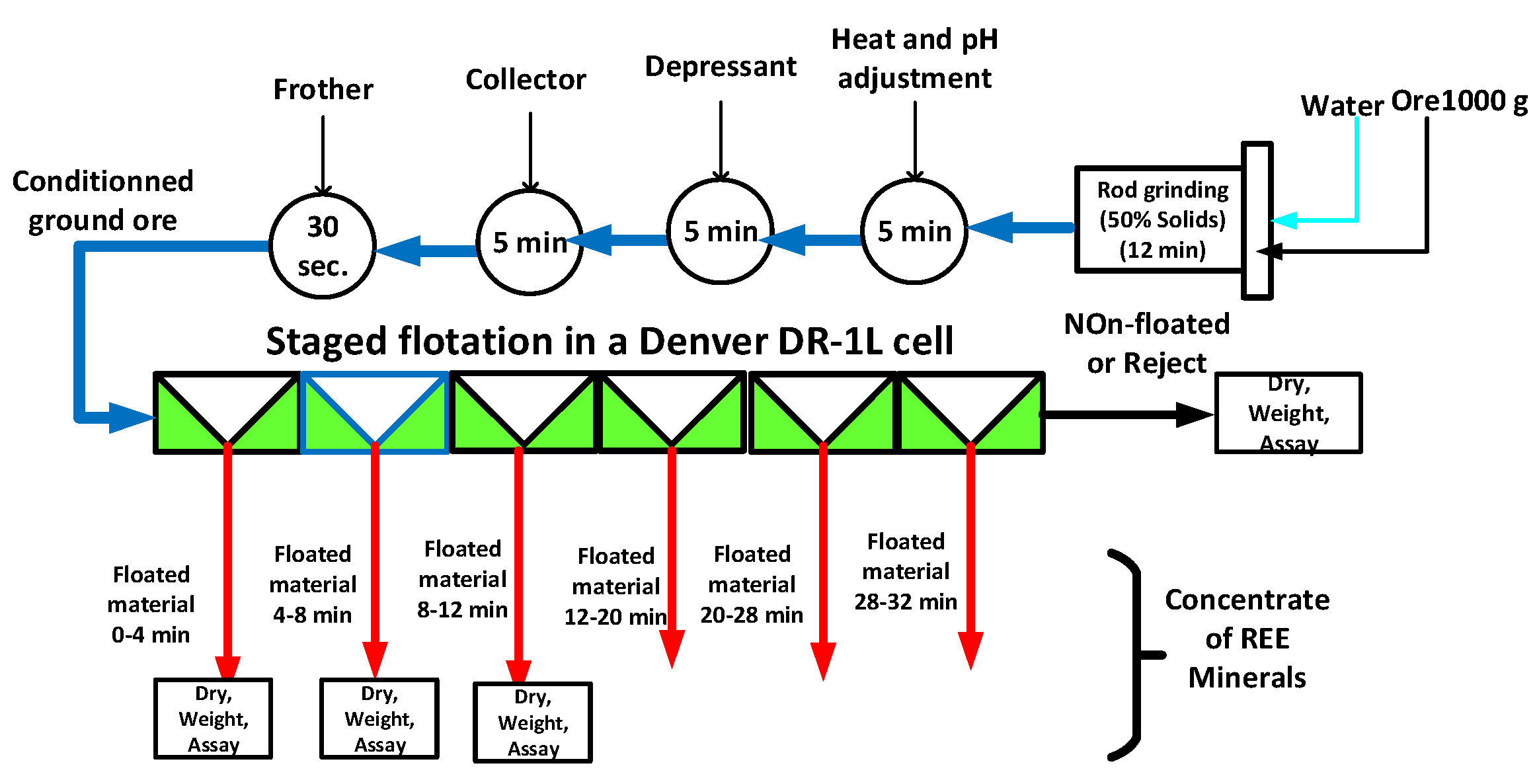
3.2. Flotation Tests
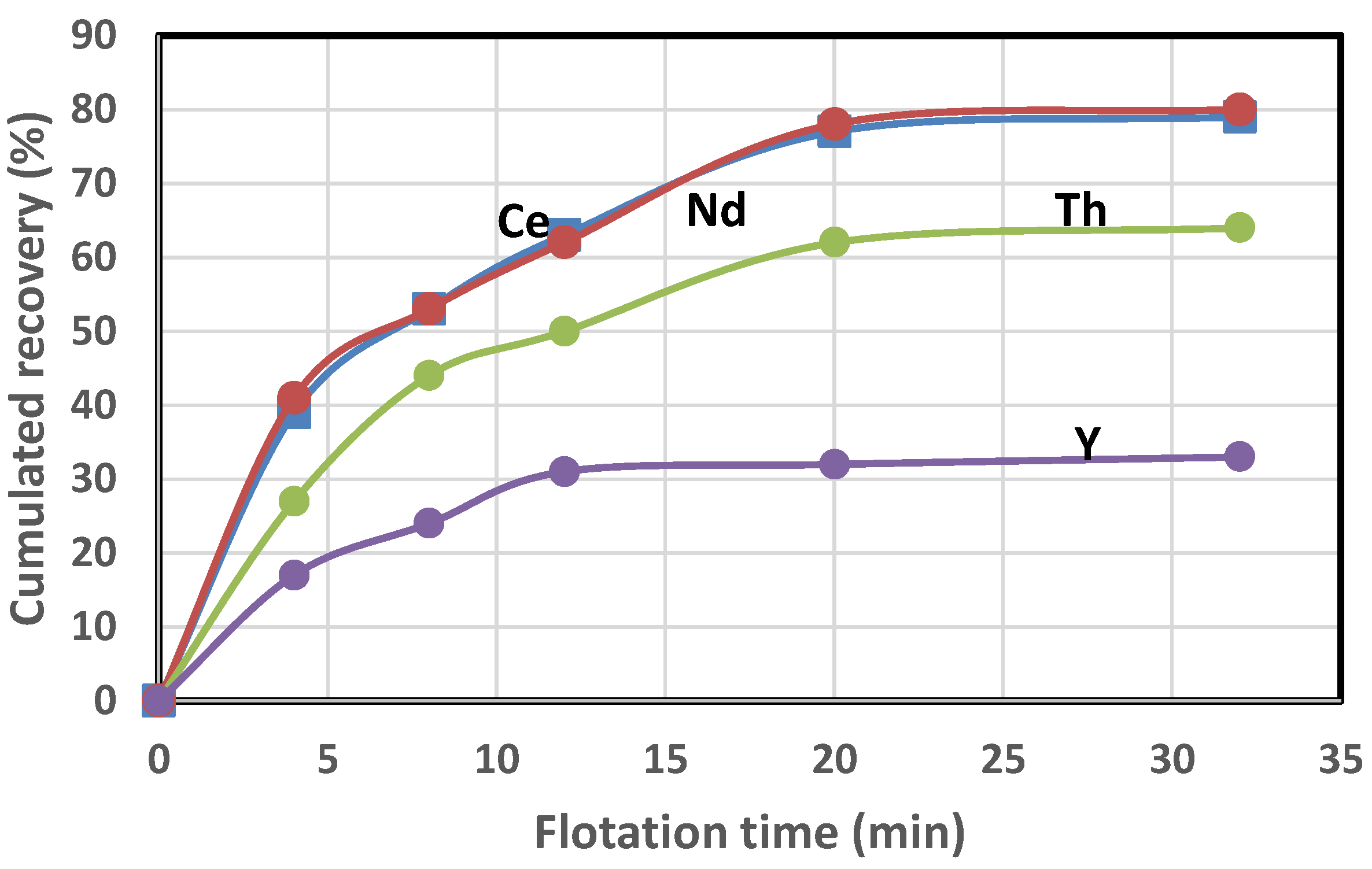
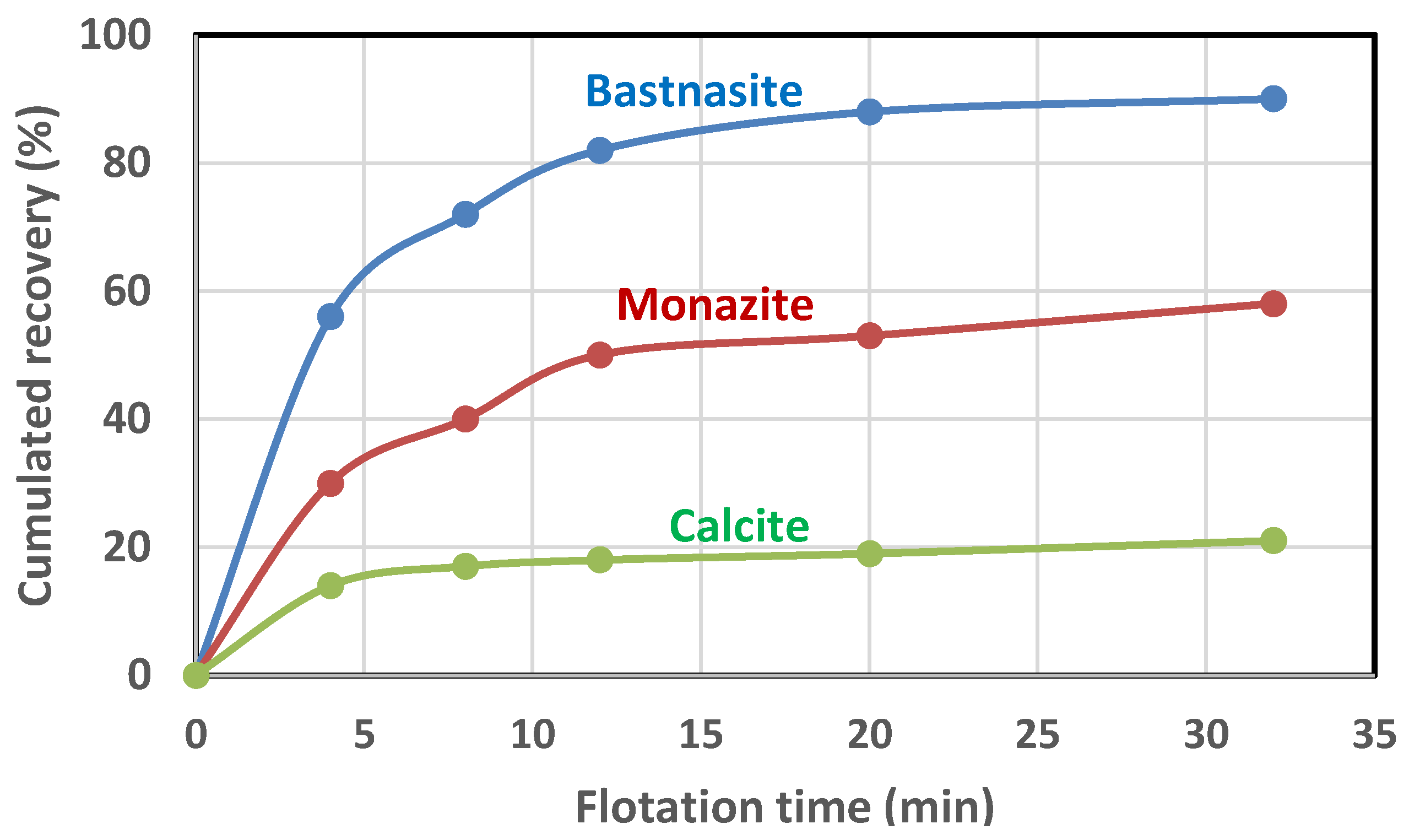
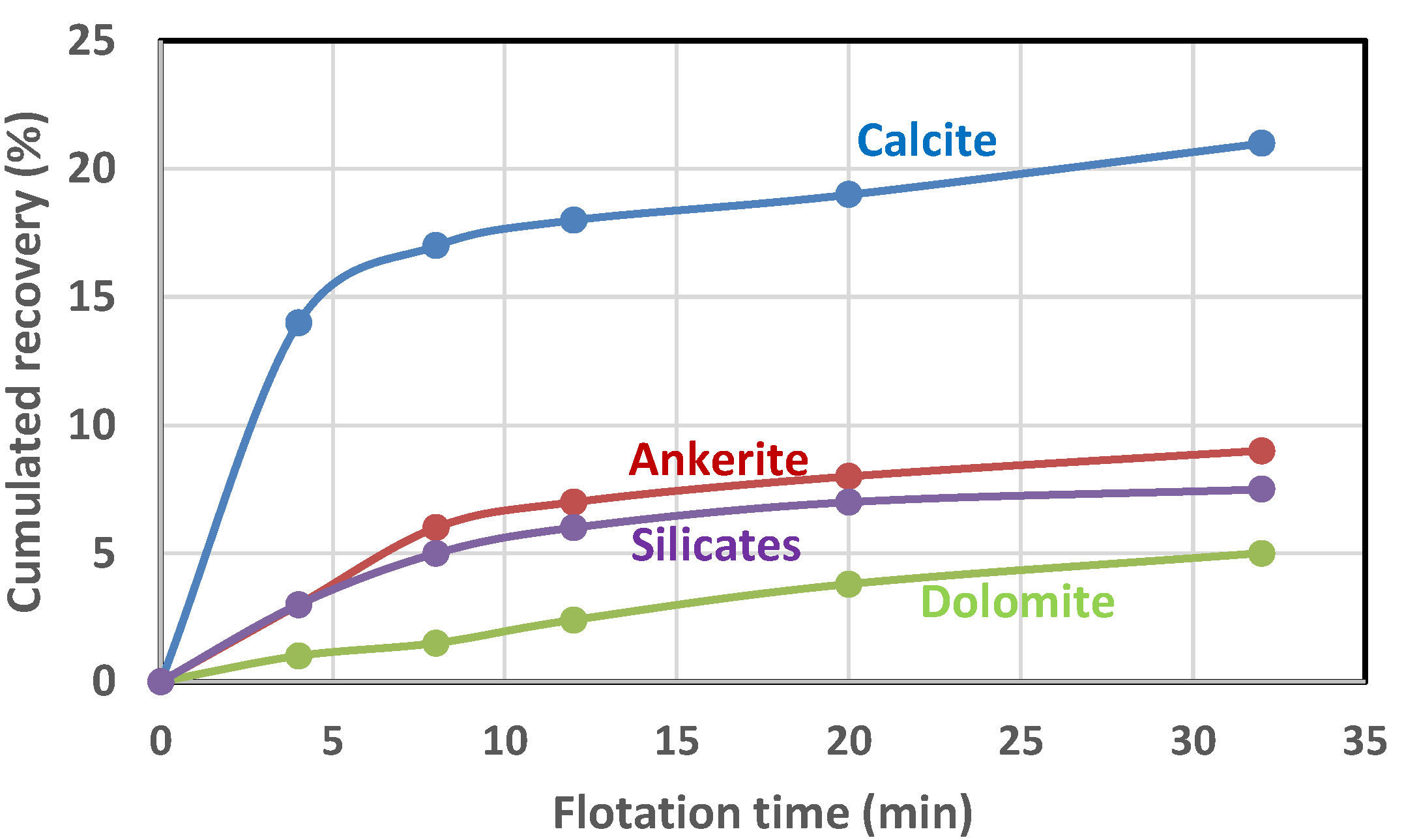
4. Analysis of the Flotation Test Results
5. Process Simulation
5.1. Model for the Flotation Process
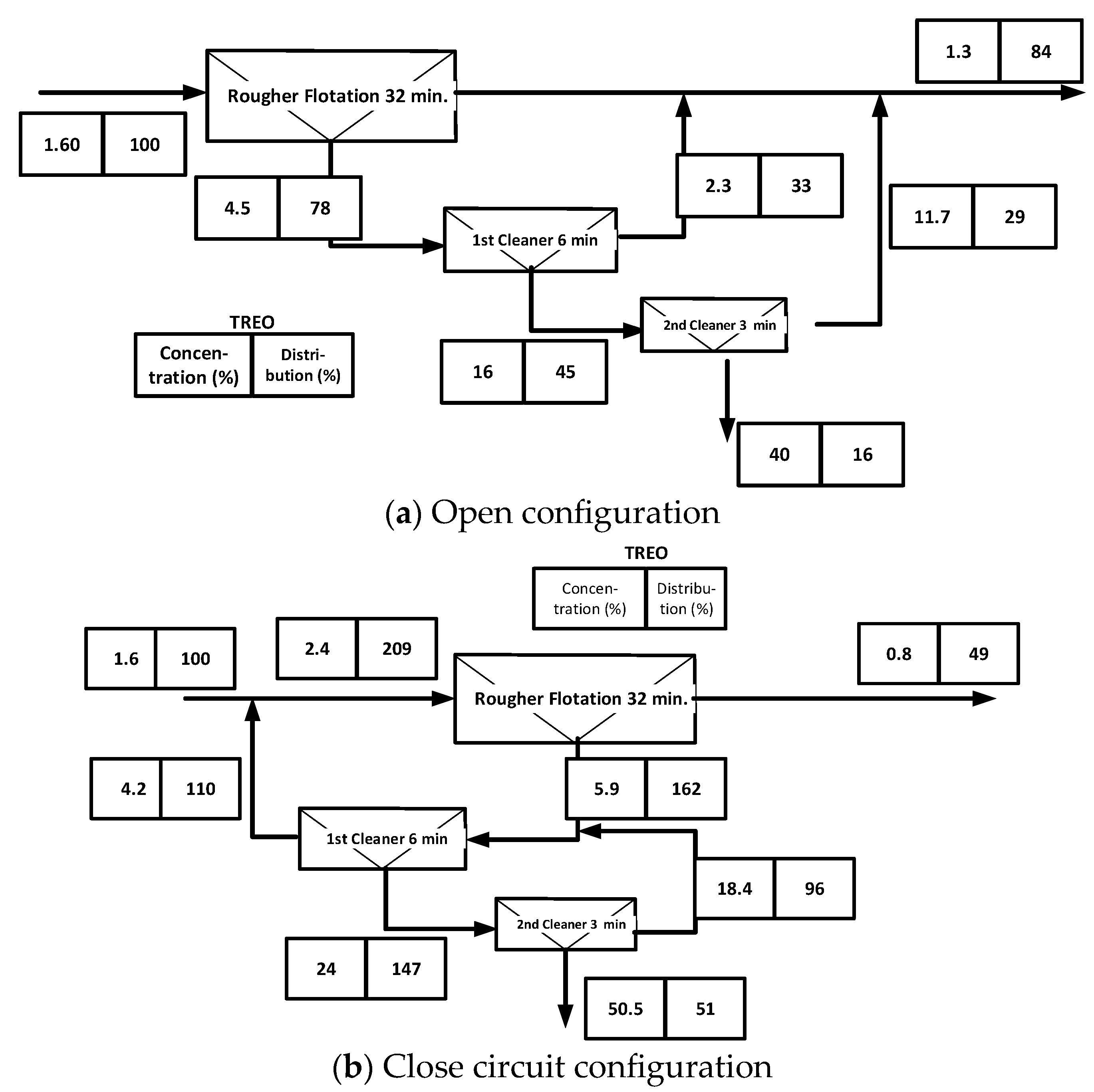
5.2. Simulation of the Production of a Bulk Bastnaesite–Monazite Concentrate of Hydrometallurgical Grade
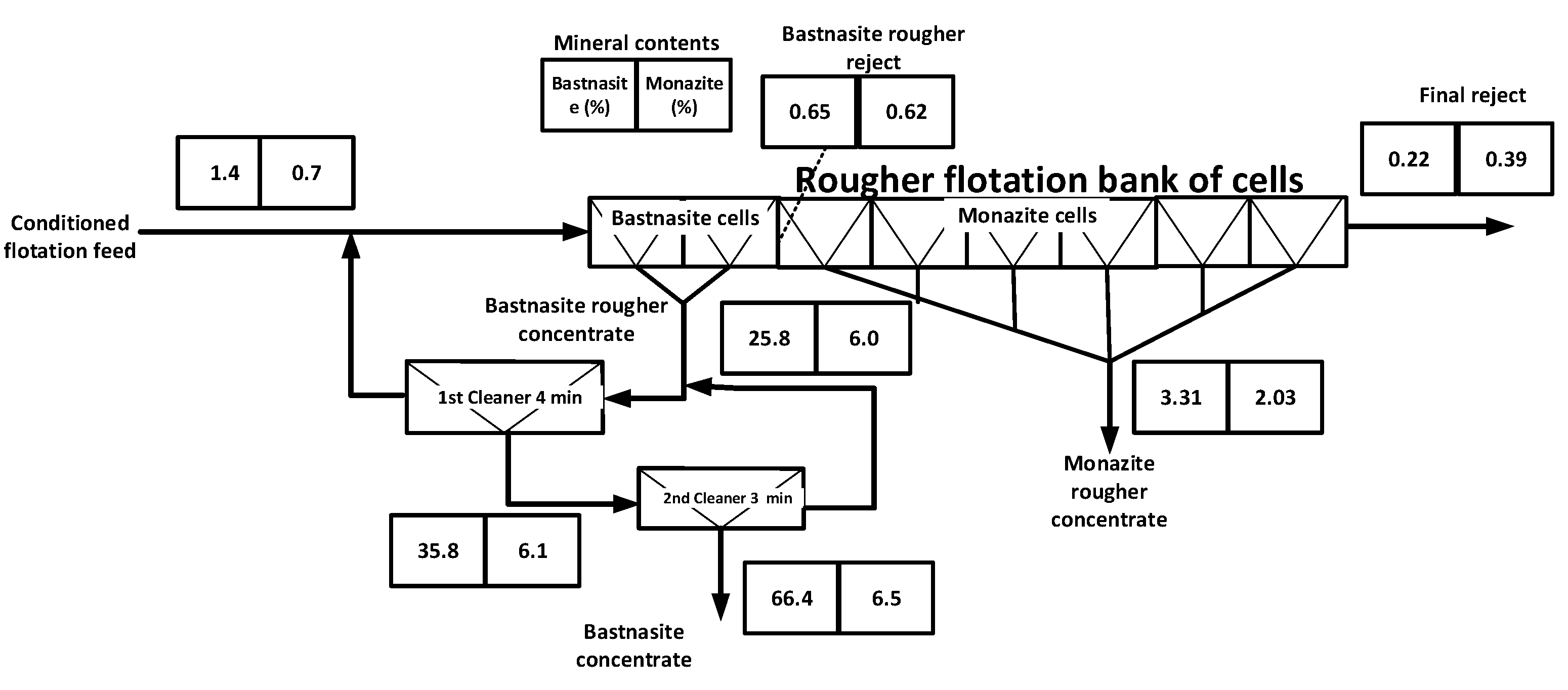
5.3. Separation of Bastnaesite and Monazite
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earth; CRC Press: Boca Raton, FL, USA, 2005; 484p. [Google Scholar]
- Sasai, R.; Shimamura, N.; Fujimura, T. Eco-Friendly Rare-Earth-Metal Recovering Process with High Versatility from Nd- 725 Fe-B Magnets. Acs Sustain. Chem. Eng. 2020, 8, 1507–1512. [Google Scholar] [CrossRef]
- Qi, D. Hydrometallurgy of Rare Earths: Extraction and Separation; Elseviser: Amsterdam, The Netherlands, 2018; 796p. [Google Scholar]
- Dickerson, T. California minerals. Ind. Miner. 1985, 2, 21–33. [Google Scholar]
- Zhang, J.; Edwards, C. Mineral decomposition and leaching processes for treating rare earth ore concentrate. Can. Metall. Q. 2013, 52, 243–248. [Google Scholar] [CrossRef]
- Boulanger, J.-F.; Bazin, C.; Turgeon, K.; Verret, F.-O.; Downey, D. Production of partially separated rare Earth elements from a Quebec deposit. In Proceedings of the Extraction 2018 Metsoc Conference, Ottawa, ON, Canada, 19–23 August 2018; Boyd, D.R., Moats, M.S., Wang, S., Eds.; [Google Scholar]
- Grenier, L.; Tremblay, J.F.; Sirois, R. Ni 43-101 Technical Report, Updated Mineral Resource Estimate for Rare Earth Elements; 2012 Niobec Mine Property: Saint-Honore, QC, Canada, 2013; 166p. [Google Scholar]
- Jordens, A.; Marion, C.; Grammatikopoulos, T.; Hart, B.; Waters, K.E. Benefication of the Nechalacho rare earth deposit: Flotation response using benzohydroxamic acid. Miner. Eng. 2016, 99, 158–169. [Google Scholar] [CrossRef]
- Zhang, J.; Edwards, C. A Review of Rare Earth Mineral Processing Technology. In Proceedings of the 44th Annual Canadian Mineral Processors Operators Conference, Ottawa, ON, Canada, 17–19 January 2012. [Google Scholar]
- Boulanger, J.-F.; Bazin, C.; Turgeon, K. Effect of depressant on bastnaesite and monazite flotation from a Canadian rare earth elements (REE) Ore. Minerals 2019, 9, 225–236. [Google Scholar] [CrossRef]
- LaDouceur, R.; Young, C.A.; Amelunxen, P.A. A Modeling and Optimization of Rare Earth Mineral Flotation using Salicylhydroxamic Acid. In International Mineral Processing Congress (IMPC) 2016; Canadian Institute of Mining, Metallurgy and Petroleum: Quebec City, QC, Canada, 2016. [Google Scholar]
- Zhang, W.; Honaker, R.Q.; Groppo, J.G. Flotation of monazite in the presence of calcite part 1: Calcium ion effects on the adsorption of hydroxamic acid. Miner. Eng. 2017, 100, 40–48. [Google Scholar] [CrossRef]
- Ren, J.; Song, S.; Lopez-Valdivieso, A.; Lu, S. Selective flotation of bastnaesite from monazite in rare earth concentrates using potassium alum as depressant. Int. J. Miner. Process. 2000, 59, 237–245. [Google Scholar] [CrossRef]
- Wang, W.; Li, E.; Peng, Z.; Guo, C.; Hou, S.; Li, Q. Flotation separation of bastnaesite from monazite using depressant dextrin hydrate and its depression mechanism. Miner. Eng. 2023, 200, 108151. [Google Scholar] [CrossRef]
- Lynch, A.J. Mineral and Coal Flotation Circuits: Their Simulation and Control; Elsevier: Amsterdam, The Netherlands, 1981; 291p. [Google Scholar]
- Magdalinović, N. A procedure for rapid determination of the Bond Work Index. Int. J. Miner. Process. 1989, 27, 125–132. [Google Scholar] [CrossRef]
- Whitty-Leveille, L.; Turgeon, K.; Bazin, C.; Larivière, D. A Comparative Study of Sample Dissolution Techniques and Plasma-Based Instruments for the Precise and Accurate Quantification of REEs in Mineral Matrices. Anal. Chim. Acta 2017, 961, 33–41. [Google Scholar] [CrossRef]
- Boulanger, J.-F. Valorisation Des Terres Rares—Traitement de Minerais, Mise en Solution et Purification. Master’s Thesis, Université Laval, Québec, QC, Canada, 2017; 141p. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation methods for rare earth elements bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Yang, X.S.; Heino, N.; Pakkanen, L. Beneficiation Studies of a Rare Earth Ore from the Olserum Deposit. Nat. Resour. 2019, 10, 346–357. [Google Scholar] [CrossRef]
- Li, M.; Gao, K.; Zhang, D. The influence of temperature on rare earth flotation with Naphthyl Hydroxamic acid. J. Rare Earths 2018, 36, 99–107. [Google Scholar] [CrossRef]
- Pradip; Fuerstenau, D.W. Adsorption of Hydroxamate Collectors on Semisoluble Minerals Part II: Effect of Temperature on Adsorption. Colloids Surf. 1985, 15, 137–146. [Google Scholar] [CrossRef]
- Boisvert, L.; Bazin, C.; Caron, J.; Lavoie, F. Development and Testing of a Method to Estimate the Mineral Composition of Ore from Chemical Assays with a View toward Geometallurgy: Application to an Iron Ore Concentrator. Geomaterials 2022, 12, 70–92. [Google Scholar] [CrossRef]
- Lévesque, S.; Couet, F.; Pérez-Barnuevo, L.; Hennessey, C. Mineralogical tools for ore characterization-Key data at all steps of iron ore concentration. In Proceedings of the XXVIII International Mineral Processing Congress (IMPC), Quebec, QC, Canada, 11–15 September 2016; pp. 831–843. [Google Scholar]
- Dowling, E.C.; Klimpel, R.R.; Aplan, F.F. Model discrimination in the flotation of a porphyry copper ore. Miner. Metall. Process. 1985, 2, 87–101. [Google Scholar] [CrossRef]
- Agar, G.E. Calculation of locked cycle flotation test results. Miner. Eng. 2000, 13, 1533–1542. [Google Scholar] [CrossRef]
- Michaud, S.; Roberge, M.; Dumais, A. Canadian Royalties Inc.—Nunavik Nickel. In Canadian Milling Practice 2020; Bobicki, E., Gibson, C., Boulanger, J.-F., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum (CIM): Ottawa, ON, Canada, 2020. [Google Scholar]
- Mackinnnon, S.; Yan, D.; Dunne, R. The interaction of flash flotation with closed circuit grinding. Miner. Eng. 2003, 16, 1149–1160. [Google Scholar] [CrossRef]
- Li, L.Z.; Yang, X. China’s Rare Earth Ore Deposits and Beneficiation Techniques. In Proceedings of the ERES2014: 1st European Rare Earth Resources Conference, Milos Island, Greece, 4–7 September 2014. [Google Scholar]
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Y | Lu | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample #1 | 3208 | 6196 | 722 | 2479 | 278 | 60 | 196 | 14.9 | 29.9 | 4 | 12.5 | 1.2 | 3.9 | 82.5 | 0.99 | 13,289 |
| Sample #2 | 4597 | 8726 | 1066 | 1520 | 411 | 81 | 263 | 17.6 | 39.1 | 4.9 | 15.3 | 1.2 | 4.2 | 93.9 | 0.9 | 16,841 |
| Average | 3903 | 7461 | 894 | 2000 | 345 | 70.5 | 230 | 16.3 | 34.5 | 4.5 | 13.9 | 1.2 | 4.1 | 88.2 | 0.9 | 15,065 |
| Test Conditions | Metallurgical Results | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | Temp. | Depressant | Collector | REE recov. | Conc. Grade | Enrichment | ||
| °C | Name | g/t | Name | g/t | % | %TREE | TREE Con/TREE Feed | |
| 1 | 80 | FA-1 | 200 | 77% | 1.25% | 0.9 | ||
| 2 | 80 | FA-1 | 100 | 79% | 1.25% | 0.9 | ||
| 3 | 80 | FA-1 | 25 | 37% | 1.25% | 0.9 | ||
| 4 | 50 | FA-1 | 25 | 15% | 0.87% | 0.7 | ||
| 5 | 25 | FA-1 | 25 | 14% | 1.22% | 0.9 | ||
| 6 | 80 | FA-1 | 25 | 46% | 1.51% | 1.1 | ||
| 7 | 80 | A6493 | 25 | 11% | 1.69% | 1.3 | ||
| 8 | 80 | A6493 | 150 | 36% | 2.45% | 1.8 | ||
| 9 | 25 | A6493 | 100 | 25% | 2.00% | 1.5 | ||
| 10 | 80 | FA-1 | 25 | 47% | 1.25% | 0.9 | ||
| 11 | 25 | FA-1 | 25 | 29% | 1.58% | 1.2 | ||
| 12 | 25 | A6493 | 100 | 40% | 1.64% | 1.2 | ||
| 13 | 25 | S-9849 | 25 | 31% | 2.14% | 1.6 | ||
| 14 | 25 | A704 | 25 | 19% | 2.23% | 1.7 | ||
| 15 | 80 | A704 | 25 | 44% | 1.60% | 1.2 | ||
| 16 | 80 | FA-1 | 25 | 35% | 1.62% | 1.2 | ||
| 17 | 25 | A6494 | 200 | 78% | 1.73% | 1.2 | ||
| 18 | 25 | A6493 | 200 | 71% | 1.81% | 1.3 | ||
| 19 | 25 | CS | 200 | A6493 | 200 | 85% | 1.48% | 1.1 |
| 20 | 25 | CS | 200 | FA-1 | 100 | 64% | 1.64% | 1.1 |
| 21 | 25 | SS | 1000 | A6493 | 200 | 43% | 1.31% | 1.1 |
| 22 | 25 | SS | 1000 | A6493 | 200 | 47% | 2.13% | 1.7 |
| 23 | 25 | SS | 1000 | FA-1 | 150 | 54% | 1.09% | 1.0 |
| 24 | 80 | SS | 1000 | FA-1 | 25 | 37% | 1.81% | 1.4 |
| 25 | 25 | SS | 1000 | DGA | 500 | 45% | 2.23% | 1.3 |
| 26 | 25 | SS | 1000 | SA | 200 | 70% | 1.91% | 1.6 |
| 27 | 25 | SS | 500 | BA | 500 | 62% | 1.90% | 1.4 |
| 28 | 25 | SS | 500 | BA | 250 | 59% | 2.03% | 1.6 |
| 29 | 25 | SS + GG | 500 + 500 | BA | 500 | 74% | 2.00% | 1.3 |
| 30 | 25 | SS + GG | 500 + 200 | F8920 | 1000 | 77% | 1.71% | 1.1 |
| 31 | 25 | SS | 500 | F8920+ | 1000 | 78% | 1.79% | 1.2 |
| 32 | 25 | SS | 500 | F8920+ | 1500 | 77% | 1.89% | 0.0 |
| 33 | 50 | SS | 500 | F8920 | 2100 | 92% | 4.36% | 2.7 |
| Parameter | Bastnaesite | Monazite | Calcite * | Ankerite * | Dolomite * | Silicates * | Total |
|---|---|---|---|---|---|---|---|
| (%) | 85.6 | 65.6 | 29.6 | 9.1 | 18.7 | 7.6 | |
| (min−1) | 0.26 | 0.17 | 0.29 | 0.12 | 0.07 | 0.1 | |
| Head (%) + | 0.6 | 1.7 | 17.4 | 41.9 | 28.6 | 10.3 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazin, C.; Boulanger, J.-F. Investigation of the Flotation of an Ore Containing Bastnaesite and Monazite: Kinetic Study and Process Flowsheet Simulation. Minerals 2024, 14, 906. https://doi.org/10.3390/min14090906
Bazin C, Boulanger J-F. Investigation of the Flotation of an Ore Containing Bastnaesite and Monazite: Kinetic Study and Process Flowsheet Simulation. Minerals. 2024; 14(9):906. https://doi.org/10.3390/min14090906
Chicago/Turabian StyleBazin, Claude, and Jean-François Boulanger. 2024. "Investigation of the Flotation of an Ore Containing Bastnaesite and Monazite: Kinetic Study and Process Flowsheet Simulation" Minerals 14, no. 9: 906. https://doi.org/10.3390/min14090906






