Recycling of Au during Serpentinization of Ultramafic Rocks: A Case Study from Neoproterozoic Forearc Ophiolites, Egypt
Abstract
1. Introduction
1.1. Highly Siderophile Elements in Mantle Rocks
1.2. Sulfide Inclusions in Ophiolitic Peridotites: Primary vs. Hydrothermal Origins
2. The Gabal Abu Dahr Ophiolite
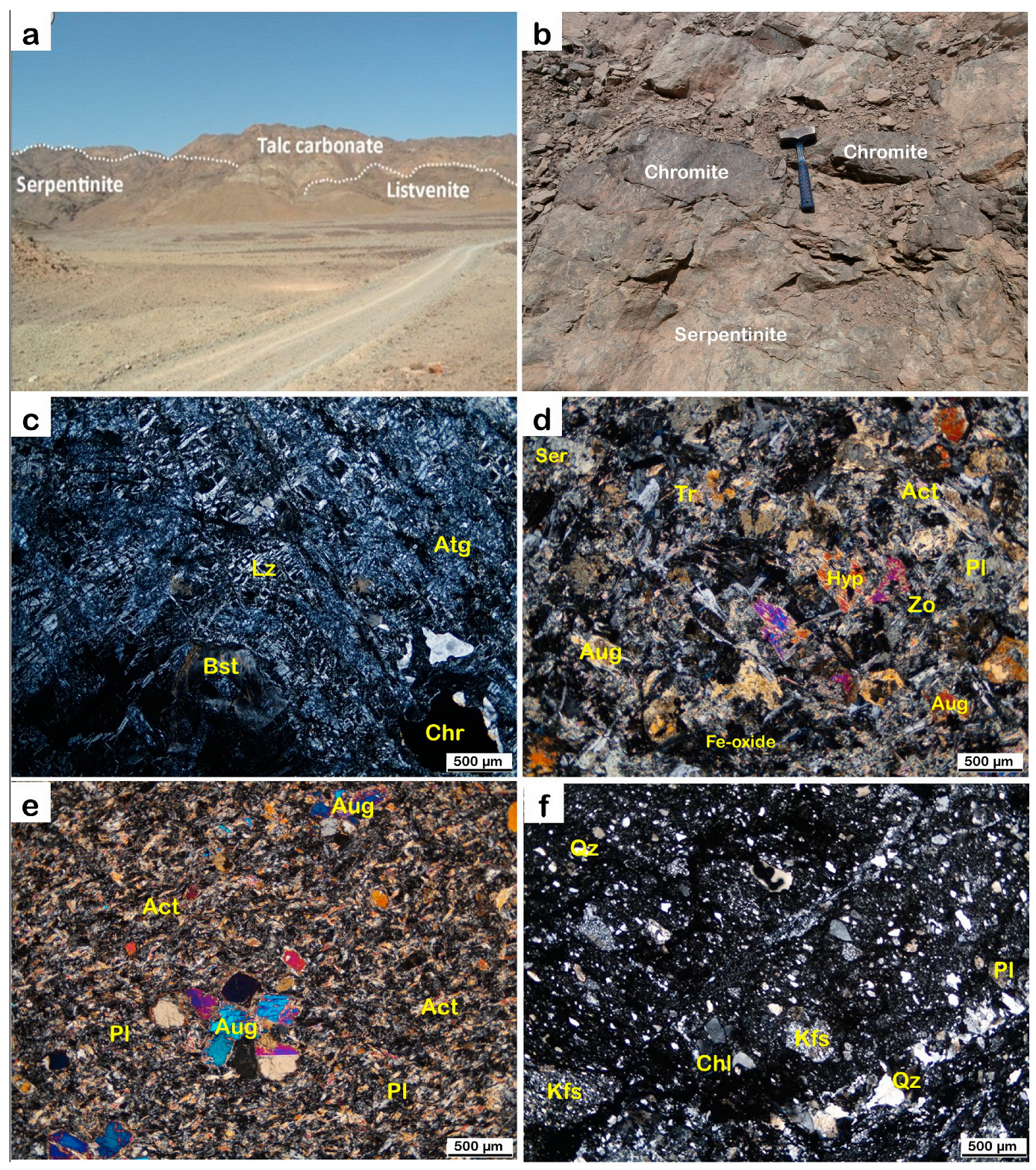
3. Field Work and Methods
4. Alteration and Sulfide Mineralogy
5. Results
5.1. Geochemical Characteristics of Abu Dahr Serpentinites and Alteration Products
5.2. EMPA Data of Sulfides
5.3. Trace Elements of Sulfide Minerals by LA-ICP-MS
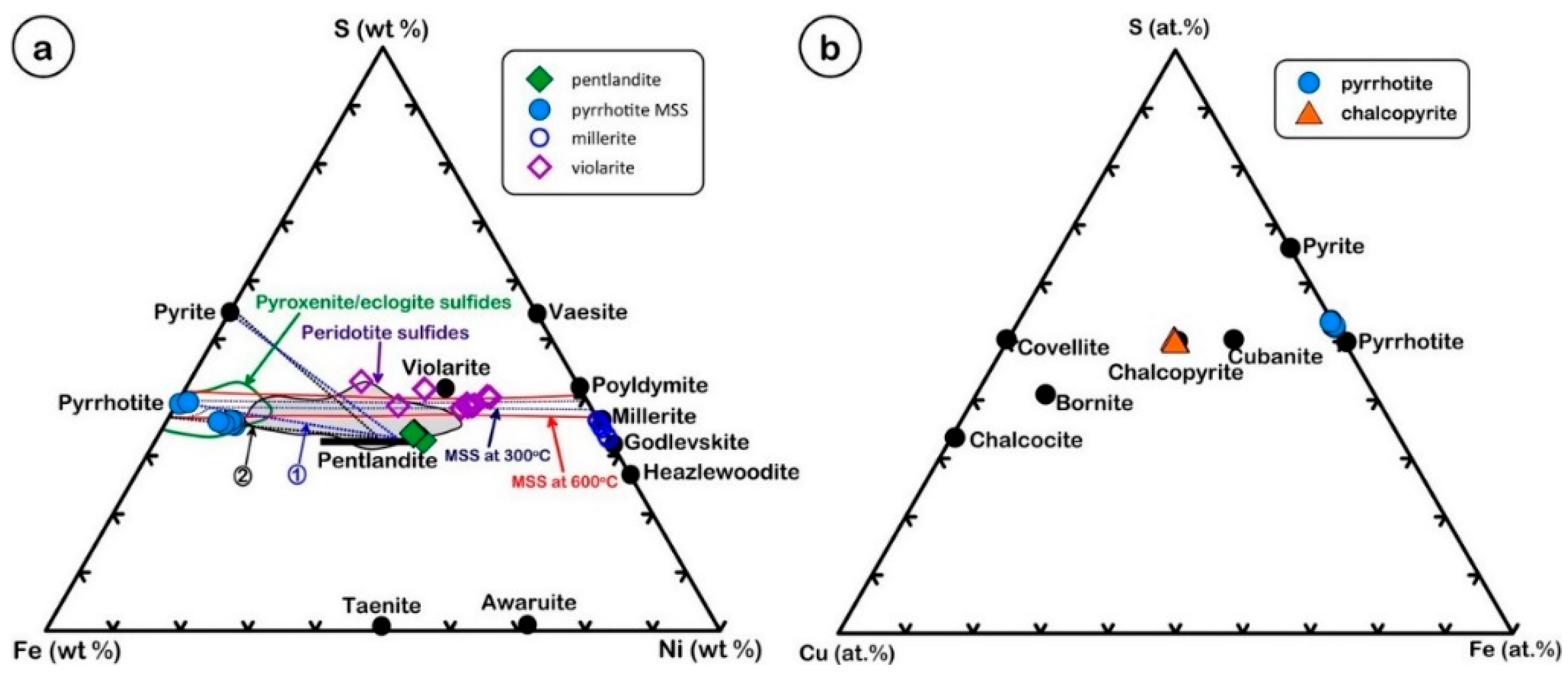
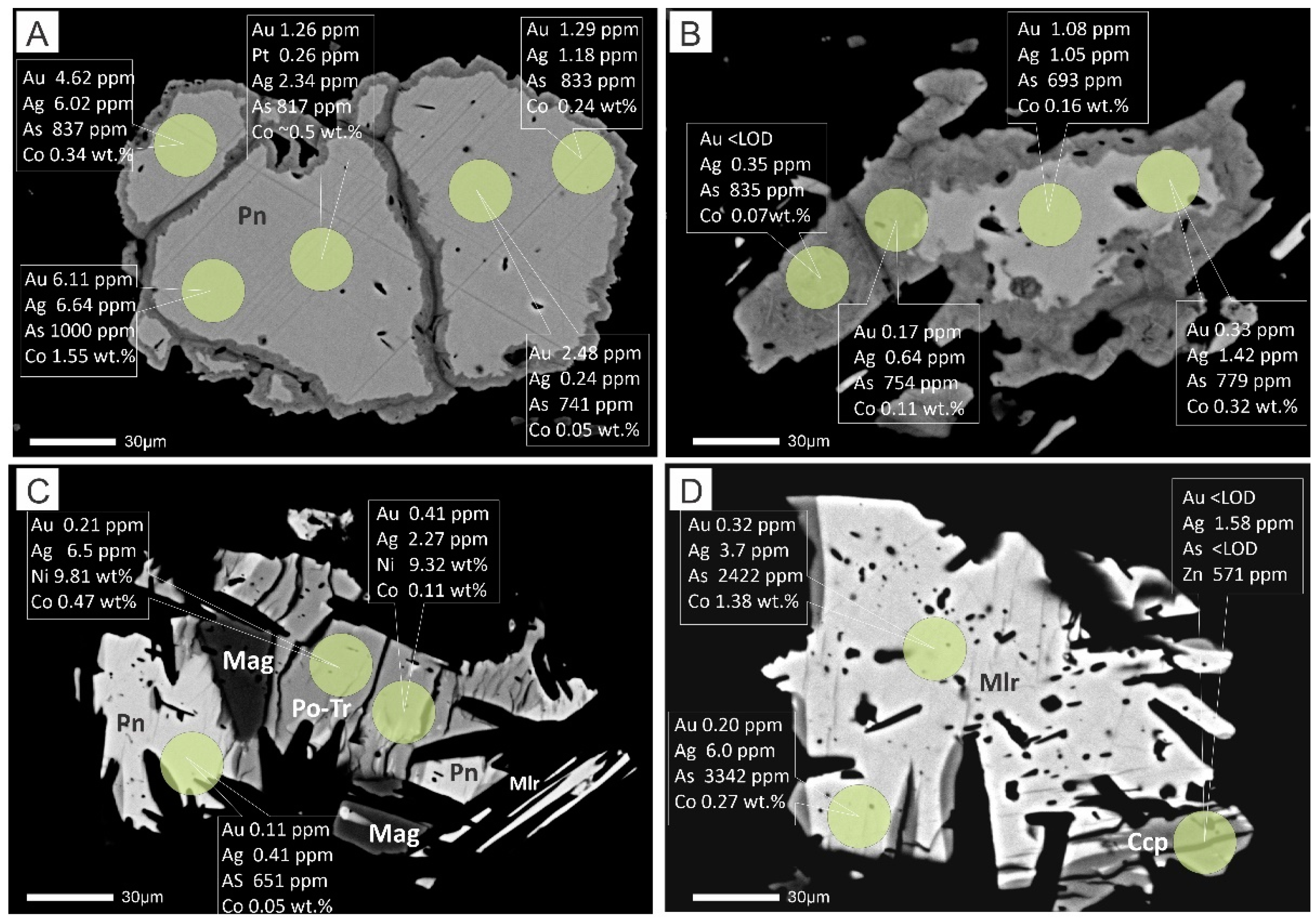
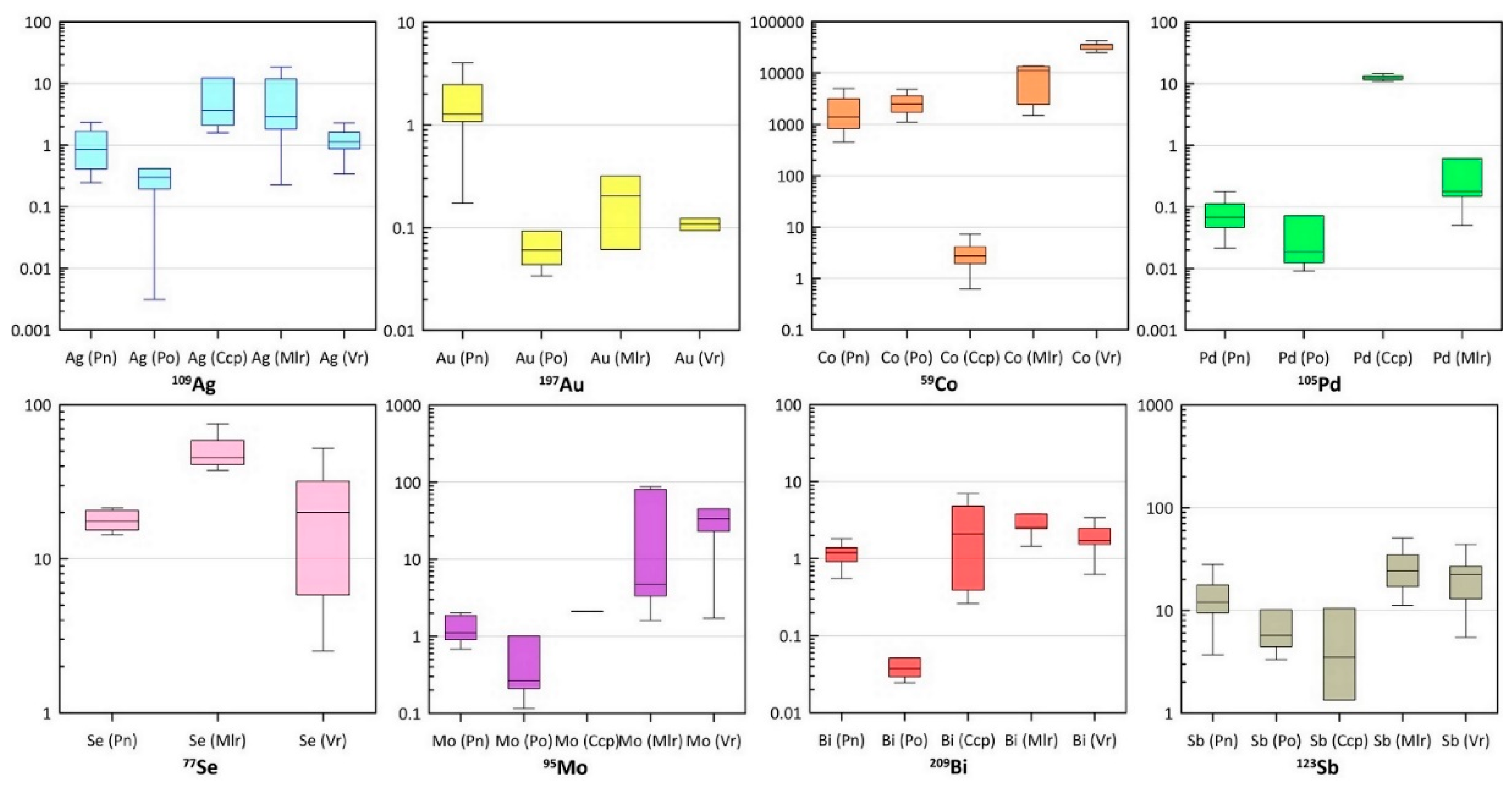
6. Discussion
6.1. The Significance of the S/Se Ratios in Ni-Sulfides
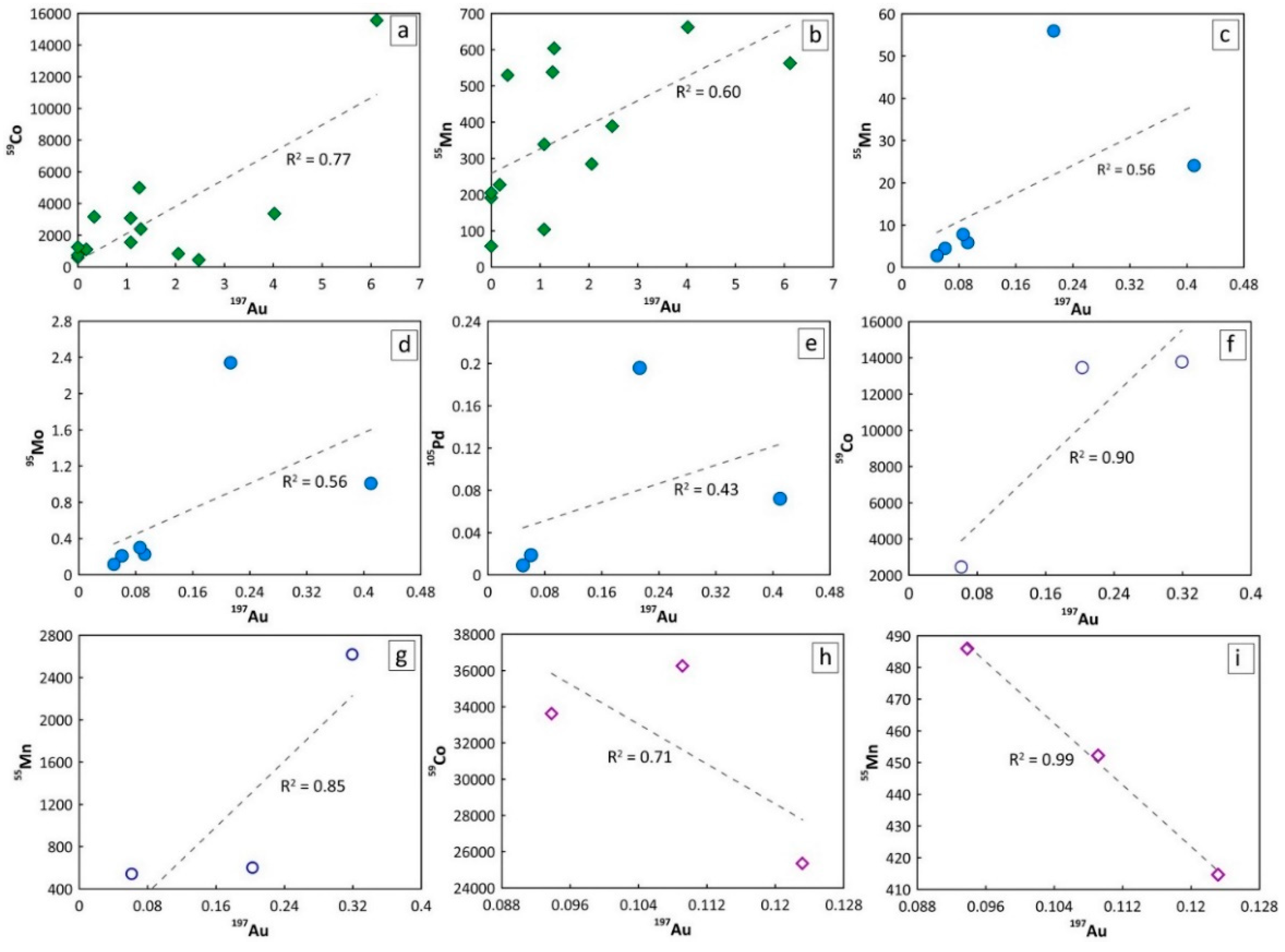
6.2. Implications of Pd Contents in the Examined Sulfides
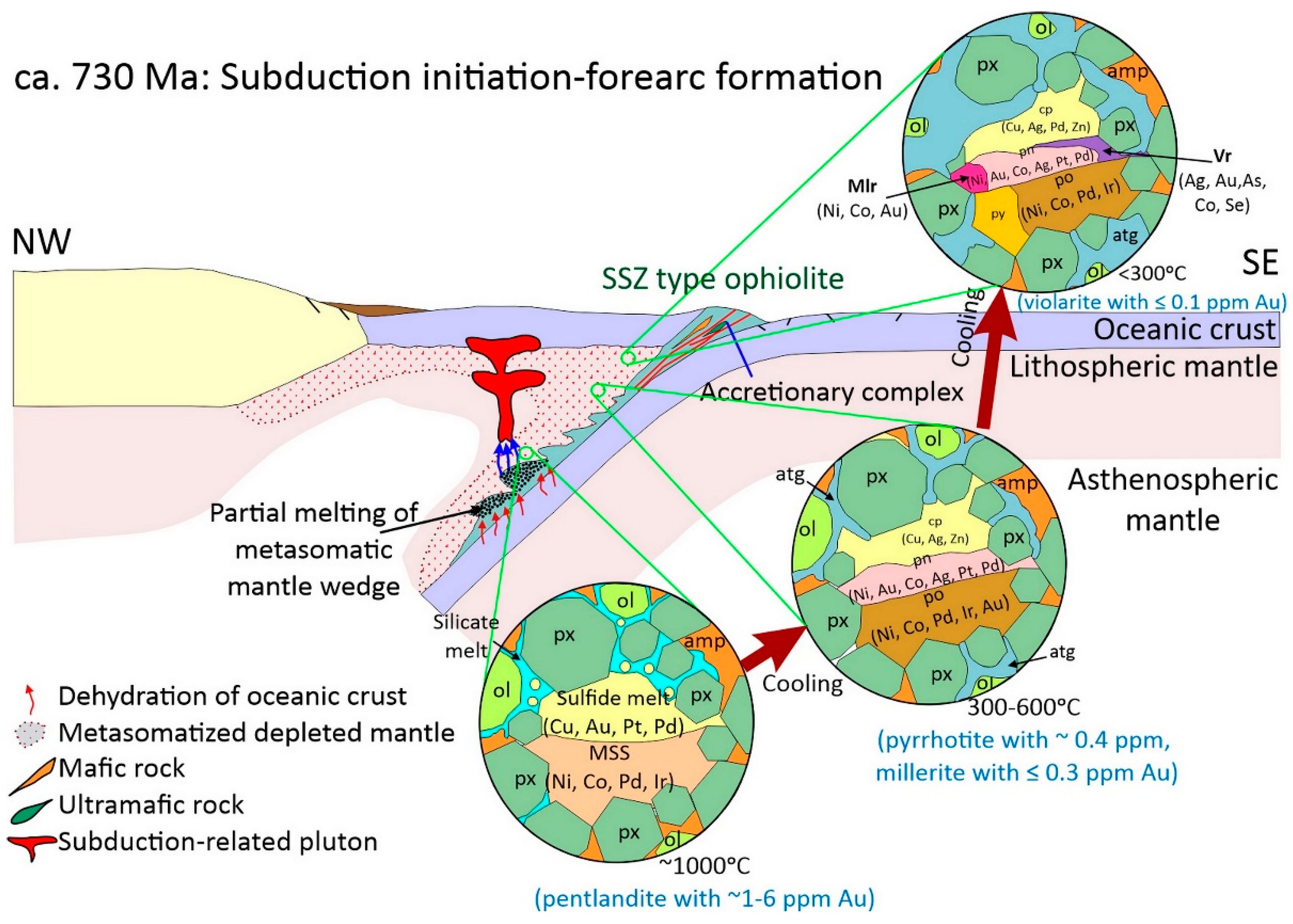
6.3. Refertilization of the Lithospheric Mantle and Au Mobilization
6.4. Geologic Evolution and Gold Recycling
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mungall, J.E. Roasting the mantle: Slab melting and the genesis of major Au and Au-rich Cu deposits. Geology 2002, 30, 915–918. [Google Scholar] [CrossRef]
- Griffin, W.; O’Reilly, S.Y.; Afonso, J.C.; Begg, G. The composition and evolution of lithospheric mantle: A re-evaluation and its tectonic implications. J. Petrol. 2009, 50, 1185–1204. [Google Scholar] [CrossRef]
- Luguet, A.; Reisberg, L. Highly siderophile element and 187Os signatures in non-cratonic basalt-hosted peridotite xenoliths: Unravelling the origin and evolution of the post-Archean lithospheric mantle. Rev. Mineral. Geochem. 2016, 81, 305–367. [Google Scholar] [CrossRef]
- Hamlyn, P.R.; Keays, R.R. Sulfur saturation and second-stage melts; application to the Bushveld platinum metal deposits. Econ. Geol. 1986, 81, 1431–1445. [Google Scholar] [CrossRef]
- Boyle, R. The Geochemistry of Gold and Its Deposits: Bulletin 280; Geological Survey of Canada: Ottawa, ON, Canada, 1979; pp. 1–584. [Google Scholar]
- Barnes, S.-J.; Maier, W.D. The fractionation of Ni, Cu and the noble metals in silicate and sulphide liquids. Short Course Notes-Geol. Assoc. Can. 1999, 13, 69–106. [Google Scholar]
- Barnes, S.-J.; Naldrett, A.J. Fractionation of the platinum-group elements and gold in some komatiites of the Abitibi greenstone belt, northern Ontario. Econ. Geol. 1987, 82, 165–183. [Google Scholar] [CrossRef]
- Palme, H.; O’Neill, H.S.C. Mantle Composition. In Treatise on Geochemistry; Carlson, R.W., Ed.; Elsevier-Pergamon: Oxford, UK, 2005; Volume 2, pp. 1–38. [Google Scholar]
- Mitchell, R.H.; Keays, R.R. Abundance and distribution of gold, palladium and iridium in some spinel and garnet lherzolites: Implications for the nature and origin of precious metal-rich intergranular components in the upper mantle. Geochim. Cosmochim. Acta 1981, 45, 2425–2442. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikov, V.V.; Robert, F.O.; Danyushevsky, L.V.; Chang, Z. Multistage Sedimentary and Metamorphic Origin of Pyrite and Gold in the Giant Sukhoi Log Deposit, Lena Gold Province, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Morey, A.A.; Tomkins, A.G.; Bierlein, F.P.; Weinberg, R.F.; Davidson, G.J. Bimodal distribution of gold in pyrite and arsenopyrite: Examples from the Archean Boorara and Bardoc shear systems, Yilgarn craton, Western Australia. Econ. Geol. 2008, 103, 599–614. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Meria, D.; Silcock, D.; Wade, B. Arsenopyrite-pyrite association in an orogenic gold ore: Tracing mineralization history from textures and trace elements. Econ. Geol. 2013, 108, 1273–1283. [Google Scholar] [CrossRef]
- Pearce, J.A.; Lippard, S.; Roberts, S. Characteristics and tectonic significance of supra-subduction zone ophiolites. Geol. Soc. Lond. Spec. Publ. 1984, 16, 77–94. [Google Scholar] [CrossRef]
- Boudier, F.; Nicolas, A. Harzburgite and lherzolite subtypes in ophiolitic and oceanic environments. Earth Planet. Sci. Lett. 1985, 76, 84–92. [Google Scholar] [CrossRef]
- Lorand, J. Mineralogy and chemistry of Cu-Fe-Ni sulfides in orogenic-type spinel peridotite bodies from Ariege (Northeastern Pyrenees, France). Contrib. Mineral. Petrol. 1989, 103, 335–345. [Google Scholar] [CrossRef]
- Frost, B.R. On the stability of sulfides, oxides, and native metals in serpentinite. J. Petrol. 1985, 26, 31–63. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W. Fe–Ni–Co–O–S phase relations in peridotite–seawater interactions. J. Petrol. 2009, 50, 37–59. [Google Scholar] [CrossRef]
- Frost, B.R.; Beard, J.S. On silica activity and serpentinization. J. Petrol. 2007, 48, 1351–1368. [Google Scholar] [CrossRef]
- Bach, W.; Paulick, H.; Garrido, C.J.; Ildefonse, B.; Meurer, W.P.; Humphris, S.E. Unraveling the sequence of serpentinization reactions: Petrography, mineral chemistry, and petrophysics of serpentinites from MAR 15 N (ODP Leg 209, Site 1274). Geophys. Res. Lett. 2006, 33, 4–7. [Google Scholar] [CrossRef]
- Alt, J.C.; Shanks, W.C., III. Sulfur in serpentinized oceanic peridotites: Serpentinization processes and microbial sulfate reduction. J. Geophys. Res. Solid Earth 1998, 103, 9917–9929. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; McCollom, T.M. Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 2013, 178, 55–69. [Google Scholar] [CrossRef]
- Eckstrand, O. The Dumont serpentinite; a model for control of nickeliferous opaque mineral assemblages by alteration reactions in ultramafic rocks. Econ. Geol. 1975, 70, 183–201. [Google Scholar] [CrossRef]
- Delacour, A.; Früh-Green, G.L.; Bernasconi, S.M. Sulfur mineralogy and geochemistry of serpentinites and gabbros of the Atlantis Massif (IODP Site U1309). Geochim. Cosmochim. Acta 2008, 72, 5111–5127. [Google Scholar] [CrossRef]
- Schwarzenbach, E.M.; Früh-Green, G.L.; Bernasconi, S.M.; Alt, J.C.; Shanks, W.C., III; Gaggero, L.; Crispini, L. Sulfur geochemistry of peridotite-hosted hydrothermal systems: Comparing the Ligurian ophiolites with oceanic serpentinites. Geochim. Cosmochim. Acta 2012, 91, 283–305. [Google Scholar] [CrossRef]
- Schwarzenbach, E.M.; Gazel, E.; Caddick, M.J. Hydrothermal processes in partially serpentinized peridotites from Costa Rica: Evidence from native copper and complex sulfide assemblages. Contrib. Mineral. Petrol. 2014, 168, 1079. [Google Scholar] [CrossRef]
- Abrajano, T.A.; Pasteris, J.D. Zambales ophiolite, Philippines. Contrib. Mineral. Petrol. 1989, 103, 64–77. [Google Scholar] [CrossRef]
- Tsushima, N.; Matsueda, H.; Ishihara, S. Polymetallic mineralization at the Nakakoshi copper deposits, central Hokkaido, Japan. Resour. Geol. 1999, 49, 89–97. [Google Scholar] [CrossRef]
- Zappettini, E.; Picot, P.; Sabourdy, G. Nouvelles données sur le gisement aurifère du Châtelet (Massif Central français), liaison génétique probable entre l’or et les roches ultrabasiques. C.-R. Séances L’académie Sci. Série 2 Mécanique-Phys. Chim. Sci. L’univers Sci. Terre 1983, 297, 351–354. [Google Scholar]
- Buisson, G.; Leblanc, M. Gold in mantle peridotites from Upper Proterozoic ophiolites in Arabia, Mali, and Morocco. Econ. Geol. 1987, 82, 2091–2097. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Corkhill, C.L. Mineralogy of sulfides. Elements 2017, 13, 81–87. [Google Scholar] [CrossRef]
- Guo, J.; Griffin, W.L.; O’Reilly, S.Y. Geochemistry and Origin of Sulphide Minerals in Mantle Xenoliths: Qilin, Southeastern China. J. Petrol. 1999, 40, 1125–1149. [Google Scholar] [CrossRef]
- Alard, O.; Griffin, W.L.; Lorand, J.P.; Jackson, S.E.; O’Reilly, S.Y. Non-chondritic distribution of the highly siderophile elements in mantle sulphides. Nature 2000, 407, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.; Graham, S.; O’Reilly, S.Y.; Pearson, N. Lithosphere evolution beneath the Kaapvaal Craton: Re–Os systematics of sulfides in mantle-derived peridotites. Chem. Geol. 2004, 208, 89–118. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Fonseca, R.O.; Smythe, D.J. Chalcophile elements and sulfides in the upper mantle. Elements 2017, 13, 111–116. [Google Scholar] [CrossRef]
- Andronikov, A.V.; Andronikova, I.E.; Sidorinova, T. Trace-Element Geochemistry of Sulfides in Upper Mantle Lherzolite Xenoliths from East Antarctica. Minerals 2021, 11, 773. [Google Scholar] [CrossRef]
- Fontboté, L.; Kouzmanov, K.; Chiaradia, M.; Pokrovski, G.S. Sulfide minerals in hydrothermal deposits. Elements 2017, 13, 97–103. [Google Scholar] [CrossRef]
- Ashmawy, M.H. The Ophiolitic Mélange of the South Eastern Desert of Egypt: Remote Sensing, Field Work and Petrographic Investigations; D. Reimer: Winnipeg, MB, Canada, 1987; Volume 84. [Google Scholar]
- Zoheir, B.A.; Mehanna, A.M.; Qaoud, N.N. Geochemistry and geothermobarometry of the Um Eleiga Neoproterozoic island arc intrusive complex, SE Egypt: Genesis of a potential gold-hosting intrusion. Appl. Earth Sci. 2008, 117, 89–111. [Google Scholar] [CrossRef]
- Gahlan, H.A.; Azer, M.K.; Khalil, A.E. The neoproterozoic Abu Dahr ophiolite, South Eastern Desert, Egypt: Petrological characteristics and tectonomagmatic evolution. Mineral. Petrol. 2015, 109, 611–630. [Google Scholar] [CrossRef]
- Seleem, T.; Hamimi, Z.; Zaky, K.; Zoheir, B.A. ASTER mapping and geochemical analysis of chromitite bodies in the Abu Dahr ophiolites, South Eastern Desert, Egypt. Arab. J. Geosci. 2020, 13, 731. [Google Scholar] [CrossRef]
- Zoheir, B.; Abd El-Rahman, Y.; Kusky, T.; Xiong, F. New SIMS zircon U-Pb ages and oxygen isotope data for ophiolite nappes in the Eastern Desert of Egypt: Implications for Gondwana assembly. Gondwana Res. 2022, 105, 450–467. [Google Scholar] [CrossRef]
- Longerich, H.P.; Jackson, S.E.; Günther, D. Inter-laboratory note. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Garbe-Schönberg, D.; Müller, S. Nano-particulate pressed powder tablets for LA-ICP-MS. J. Anal. At. Spectrom. 2014, 29, 990–1000. [Google Scholar] [CrossRef]
- El-Bayoumi, R. Ophiolites and melange complex of Wadi Ghadir area, Eastern Desert, Egypt. Precambrian Res. 1982, 16, A17–A18. [Google Scholar] [CrossRef]
- Basta, F.F.; Maurice, A.E.; Bakhit, B.R.; Ali, K.A.; Manton, W.I. Neoproterozoic contaminated MORB of Wadi Ghadir ophiolite, NE Africa: Geochemical and Nd and Sr isotopic constraints. J. Afr. Earth Sci. 2011, 59, 227–242. [Google Scholar] [CrossRef]
- Azer, M.K.; Gahlan, H.A.; Asimow, P.D.; Mubarak, H.S.; Al-Kahtany, K.M. Multiple stages of carbonation and element redistribution during formation of ultramafic-hosted magnesite in Neoproterozoic ophiolites of the Arabian-Nubian Shield, Egypt. J. Geol. 2019, 127, 81–107. [Google Scholar] [CrossRef]
- Evans, B.W.; Hattori, K.; Baronnet, A. Serpentinite: What, why, where? Elements 2013, 9, 99–106. [Google Scholar] [CrossRef]
- Xie, Z.; Hattori, K.; Dong, Y.; Wang, J. In situ characterization of forearc serpentinized peridotite from the Sulu ultrahigh-pressure terrane: Behavior of fluid-mobile elements in continental subduction zone. Geosci. Front. 2021, 12, 101139. [Google Scholar] [CrossRef]
- Boskabadi, A.; Pitcairn, I.K.; Leybourne, M.I.; Teagle, D.A.H.; Cooper, M.J.; Hadizadeh, H.; Nasiri Bezenjani, R.; Monazzami Bagherzadeh, R. Carbonation of ophiolitic ultramafic rocks: Listvenite formation in the Late Cretaceous ophiolites of eastern Iran. Lithos 2020, 352–353, 105307. [Google Scholar] [CrossRef]
- Gahlan, H.A.; Azer, M.K.; Asimow, P.D.; Al-Kahtany, K.M. Petrogenesis of gold-bearing listvenites from the carbonatized mantle section of the Neoproterozoic Ess ophiolite, Western Arabian Shield, Saudi Arabia. Lithos 2020, 372–373, 105679. [Google Scholar] [CrossRef]
- Akbulut, M.; Pïşkïn, Ö.; Karayïǧït, A.İ. The genesis of the carbonatized and silicified ultramafics known as listvenites: A case study from the Mihalıççık region (Eskişehir), NW Turkey. Geol. J. 2006, 41, 557–580. [Google Scholar] [CrossRef]
- Imai, N.; Mariko, T.; Shiga, Y. Supergene alteration of pentlandite to violarite Contribution ot the knowledge of secondary violarite. Min. Geol. 1978, 28, 1–11. [Google Scholar]
- Lorand, J.-P.; Luguet, A. Chalcophile and siderophile elements in mantle rocks: Trace elements controlled by trace minerals. Rev. Mineral. Geochem. 2016, 81, 441–488. [Google Scholar] [CrossRef]
- Rüpke, L.H.; Morgan, J.P.; Hort, M.; Connolly, J.A.D. Serpentine and the subduction zone water cycle. Earth Planet. Sci. Lett. 2004, 223, 17–34. [Google Scholar] [CrossRef]
- Groves, D.I.; Goldfarb, R.J.; Gebre-Mariam, M.; Hagemann, S.; Robert, F. Orogenic gold deposits: A proposed classification in the context of their crustal distribution and relationship to other gold deposit types. Ore Geol. Rev. 1998, 13, 7–27. [Google Scholar] [CrossRef]
- Deschamps, F.; Godard, M.; Guillot, S.; Hattori, K. Geochemistry of subduction zone serpentinites: A review. Lithos 2013, 178, 96–127. [Google Scholar] [CrossRef]
- Malvoisin, B. Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical. Earth Planet. Sci. Lett. 2015, 430, 75–85. [Google Scholar] [CrossRef]
- Naldrett, A.J. Magmatic Sulfide Deposits: Geology, Geochemistry and Exploration; Springer: Berlin/Heidelberg, Germany, 2004; pp. XVIII, 728. [Google Scholar]
- Brenan, J.M.; Andrews, D. High-temperature stability of laurite and Ru–Os–Ir alloy and their role in PGE fractionation in mafic magmas. Can. Mineral. 2001, 39, 341–360. [Google Scholar] [CrossRef]
- Hattori, K.H.; Guillot, S. Geochemical character of serpentinites associated with high-to ultrahigh-pressure metamorphic rocks in the Alps, Cuba, and the Himalayas: Recycling of elements in subduction zones. Geochem. Geophys. Geosyst. 2007. [Google Scholar] [CrossRef]
- Augé, T. Chromite deposits in the northern Oman ophiolite: Mineralogical constraints. Miner. Depos. 1987, 22, 1–10. [Google Scholar] [CrossRef]
- Craig, J.R. Pyrite-pentlandite assemblages and other low temperature relations in the Fe-Ni-S system. Am. J. Sci. 1973, 273, 496–510. [Google Scholar]
- Naldrett, A.; Craig, J.; Kullerud, G. The central portion of the Fe-Ni-S system and its bearing on pentlandite exsolution in iron-nickel sulfide ores. Econ. Geol. 1967, 62, 826–847. [Google Scholar] [CrossRef]
- Gréau, Y.; Alard, O.; Griffin, W.L.; Huang, J.-X.; O’Reilly, S.Y. Sulfides and chalcophile elements in Roberts Victor eclogites: Unravelling a sulfide-rich metasomatic event. Chem. Geol. 2013, 354, 73–92. [Google Scholar] [CrossRef]
- Lorand, J.-P.; Alard, O. Pyrite tracks assimilation of crustal sulfur in Pyrenean peridotites. Mineral. Petrol. 2011, 101, 115–128. [Google Scholar] [CrossRef]
- Bortnikov, N.; Vikentyev, I.; Apollonov, V.; Stavrova, O.; Bogdanov, Y.; Lein, A.; Gurvich, E.; Sagalevich, A.; Simonov, V.; Ikorskii, S. The Rainbow serpentinite-related hydrothermal field, Mid-Atlantic Ridge, 36 14rN: Mineralogical and geochemical features. In Proceedings of the Joint Sixth Meeting. Mineral Deposits at the Beginning of the 21st Century, Krakow, Poland, 26–29 August 2001; pp. 265–268. [Google Scholar]
- Simon, A.C.; Pettke, T.; Candela, P.A.; Piccoli, P.M.; Heinrich, C.A. Magnetite solubility and iron transport in magmatic-hydrothermal environments. Geochim. Cosmochim. Acta 2004, 68, 4905–4914. [Google Scholar] [CrossRef]
- Tomkins, A.G. Windows of metamorphic sulfur liberation in the crust: Implications for gold deposit genesis. Geochim. Cosmochim. Acta 2010, 74, 3246–3259. [Google Scholar] [CrossRef]
- Melekestseva, I.; Maslennikov, V.; Tret’yakov, G.; Maslennikova, S.; Danyushevsky, L.; Kotlyarov, V.; Large, R.; Beltenev, V.; Khvorov, P. Trace element geochemistry of sulfides from the Ashadze-2 hydrothermal field (12°58′ N, Mid-Atlantic Ridge): Influence of host rocks, formation conditions or seawater? Minerals 2020, 10, 743. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Huang, X.; Qi, L.; Lyu, C. Trace element composition of magnetite from the Xinqiao Fe–S (–Cu–Au) deposit, Tongling, Eastern China: Constraints on fluid evolution and ore genesis. Acta Geochim. 2018, 37, 639–654. [Google Scholar] [CrossRef]
- Tenailleau, C.; Pring, A.; Etschmann, B.; Brugger, J.; Grguric, B.; Putnis, A. Transformation of pentlandite to violarite under mild hydrothermal conditions. Am. Mineral. 2006, 91, 706–709. [Google Scholar] [CrossRef]
- Pring, A.; Tenailleau, C.; Etschmann, B.; Brugger, J.; Grguric, B. The transformation of pentlandite to violarite under mild hydrothermal conditions: A dissolution-reprecipitation reaction. In Regolith 2005: Ten Years of CRC LEME. Proceedings of the CRC LEME Regional Regolith Symposia 2005; CRC LEME: Bentley, WA, USA, 2005; pp. 252–255. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=36c66679065e58a541fadf4953266dce47ea2b32 (accessed on 3 September 2024).
- Eckstrand, O.; Hulbert, L. Selenium and the source of sulfur in magmatic nickel and platinum deposits [abs.]. Geological Association of Canada-Mineralogical Association Canada Program with Abstracts. 1987, p. 40. Available online: https://scholar.google.com/scholar_lookup?title=Selenium%20and%20the%20source%20of%20sulfur%20in%20magmatic%20nickel%20and%20platinum%20deposits%20%3A%20Geological%20Association%20of%20CanadaMineralogical%20Association%20Canada%20Program%20with%20Abstracts&publication_year=1987&author=O.R.%20Eckstrand&author=L.J.%20Hulbert (accessed on 4 September 2024).
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Hattori, K.H.; Arai, S.; Clarke, D.B. Selenium, tellurium, arsenic and antimony contents of primary mantle sulfides. Can. Mineral. 2002, 40, 637–650. [Google Scholar] [CrossRef]
- Lorand, J.-P.; Alard, O.; Luguet, A.; Keays, R.R. Sulfur and selenium systematics of the subcontinental lithospheric mantle: Inferences from the Massif Central xenolith suite (France). Geochim. Cosmochim. Acta 2003, 67, 4137–4151. [Google Scholar] [CrossRef]
- Dreibus, G.; Palme, H.; Spettel, B.; Zipfel, J.; Wänke, H. Sulfur and selenium in chondritic meteorites. Meteoritics 1995, 30, 439–445. [Google Scholar] [CrossRef]
- Lodders, K. Solar system abundances and condensation temperatures of the elements. Astrophys. J. 2003, 591, 1220. [Google Scholar] [CrossRef]
- Leblanc, M. Platinum-Group Elements and Gold in Ophiolitic Complexes: Distribution and Fractionation from Mantle to Oceanic Floor. In Ophiolite Genesis and Evolution of the Oceanic Lithosphere: Proceedings of the Ophiolite Conference, Muscat, Oman, 7–18 January 1990; Springer: Dordrecht, The Netherlands, 1991; pp. 231–260. [Google Scholar]
- Hellmann, J.L.; Hopp, T.; Burkhardt, C.; Kleine, T. Origin of volatile element depletion among carbonaceous chondrites. Earth Planet. Sci. Lett. 2020, 549, 116508. [Google Scholar] [CrossRef]
- Holwell, D.A.; Fiorentini, M.L.; Knott, T.R.; McDonald, I.; Blanks, D.E.; Campbell McCuaig, T.; Gorczyk, W. Mobilisation of deep crustal sulfide melts as a first order control on upper lithospheric metallogeny. Nat. Commun. 2022, 13, 573. [Google Scholar] [CrossRef]
- Yamamoto, M. Relationship between Se/S and sulfur isotope ratios of hydrothermal sulfide minerals. Miner. Depos. 1976, 11, 197–209. [Google Scholar] [CrossRef]
- Savov, I.P.; Ryan, J.G.; D’Antonio, M.; Kelley, K.; Mattie, P. Geochemistry of serpentinized peridotites from the Mariana Forearc Conical Seamount, ODP Leg 125: Implications for the elemental recycling at subduction zones. Geochem. Geophys. Geosyst. 2005. [Google Scholar] [CrossRef]
- Rapp, R.P.; Watson, E.B.; Miller, C.F. Partial melting of amphibolite/eclogite and the origin of Archean trondhjemites and tonalites. Precambrian Res. 1991, 51, 1–25. [Google Scholar] [CrossRef]
- Fleet, M.E. XANES spectroscopy of sulfur in earth materials. Can. Mineral. 2005, 43, 1811. [Google Scholar] [CrossRef]
- Hanley, J.; Mungall, J.; Spooner, E.; Pettke, T. Fluid and melt inclusion evidence for platinum-group element transport by high salinity fluids and halide melts below the JM Reef, Stillwater Complex. In Proceedings of the Montana, USA: 10th International Platinum Symposium “Platinum-Group Elements-from Genesis to Beneficiation and Environmental Impact”, Oulu, Finland, 8–11 August 2005; pp. 94–97. [Google Scholar]
- Mungall, J.E.; Brenan, J.M. Partitioning of platinum-group elements and Au between sulfide liquid and basalt and the origins of mantle-crust fractionation of the chalcophile elements. Geochim. Cosmochim. Acta 2014, 125, 265–289. [Google Scholar] [CrossRef]
- O’Neil, H.S.C.; Mavrogenes, J.A. The sulfide capacity and the sulfur content at sulfide saturation of silicate melts at 1400 °C and 1 bar. J. Petrol. 2002, 43, 1049–1087. [Google Scholar] [CrossRef]
- Blanks, D.E.; Holwell, D.A.; Barnes, S.J.; Schoneveld, L.E.; Fiorentini, M.L.; Baublys, K.A.; Mbiri, L.; Knott, T.R. Mobilization and Fractionation of Magmatic Sulfide: Emplacement and Deformation of the Munali Ni-(Cu-Platinum Group Element) Deposit, Zambia. Econ. Geol. 2022, 117, 1709–1729. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Essene, E.J.; Kelly, W.C. A re-examination of the arsenopyrite geothermometer: Pressure considerations and applications to natural assemblages. J. Mineral. Assoc. Can. 1985, 23, 517–534. [Google Scholar]
- Evans, K.; Powell, R.; Frost, B. Using equilibrium thermodynamics in the study of metasomatic alteration, illustrated by an application to serpentinites. Lithos 2013, 168, 67–84. [Google Scholar] [CrossRef]
- Holwell, B.D.; McDonald, I. A review of the behaviour of platinum group elements within natural magmatic sulfide ore systems. Platin. Met. Rev. 2010, 54, 26–36. [Google Scholar] [CrossRef]
- Mansur, E.T.; Barnes, S.-J.; Duran, C.J. Textural and compositional evidence for the formation of pentlandite via peritectic reaction: Implications for the distribution of highly siderophile elements. Geology 2019, 47, 351–354. [Google Scholar] [CrossRef]
- Barnes, S.-J.; Cox, R.A.; Zientek, M.L. Platinum-group element, gold, silver and base metal distribution in compositionally zoned sulfide droplets from the Medvezky Creek Mine, Noril’sk, Russia. Contrib. Mineral. Petrol. 2006, 152, 187–200. [Google Scholar] [CrossRef]
- Dare, S.; Barnes, S. The origin of Pd in pentlandites from Creighton mine, Ni-Cu-PGE deposit, Sudbury, Canada. In Xi’an International Platinum Symposium, 174; Ontario Geological Survey, Miscellaneous Release–Data 269: Sudbury Ontario, ON, Canada, 2009. [Google Scholar]
- Wei, B.; Yan Wang, C.; Dong, Y. Behavior of palladium during fractionation of sulfide liquid: New constraints from the Kalatongke Cu-Ni sulfide-bearing intrusion in the central Asian orogenic belt, NW China. Ore Geol. Rev. 2023, 160, 105578. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Partitioning of trace elements in co-crystallized sphalerite–galena–chalcopyrite hydrothermal ores. Ore Geol. Rev. 2016, 77, 97–116. [Google Scholar] [CrossRef]
- Goldschmidt, V. Geochemistry; Clarendon: Oxford, UK, 1954; Volume 624, pp. 1–730. [Google Scholar]
- Helmy, H.M.; Ballhaus, C.; Berndt, J.; Bockrath, C.; Wohlgemuth-Ueberwasser, C. Formation of Pt, Pd and Ni tellurides: Experiments in sulfide–telluride systems. Contrib. Mineral. Petrol. 2007, 153, 577–591. [Google Scholar] [CrossRef]
- Mutschler, F.; Griffin, M.; Stevens, D.; Shannon, S., Jr. Precious metal deposits related to alkaline rocks in the North American Cordillera—An interpretive review: Transactions Geological Society of South Africa. South Afr. J. Geol. 1985, 88, 355–377. [Google Scholar]
- Mitchell, A.L.; Grove, T.L. Experiments on melt–rock reaction in the shallow mantle wedge. Contrib. Mineral. Petrol. 2016, 171, 1–21. [Google Scholar] [CrossRef]
- Qiu, K.-F.; Deng, J.; Laflamme, C.; Long, Z.-Y.; Wan, R.-Q.; Moynier, F.; Yu, H.-C.; Zhang, J.-Y.; Ding, Z.-J.; Goldfarb, R. Giant Mesozoic gold ores derived from subducted oceanic slab and overlying sediments. Geochim. Cosmochim. Acta 2023, 343, 133–141. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Special paper: Major gold deposits and belts of the North and South American Cordillera: Distribution, tectonomagmatic settings, and metallogenic considerations. Econ. Geol. 2008, 103, 663–687. [Google Scholar] [CrossRef]
- Begg, G.C.; Hronsky, J.A.; Arndt, N.T.; Griffin, W.L.; O’Reilly, S.Y.; Hayward, N. Lithospheric, cratonic, and geodynamic setting of Ni-Cu-PGE sulfide deposits. Econ. Geol. 2010, 105, 1057–1070. [Google Scholar] [CrossRef]
- Webber, A.; Roberts, S.; Taylor, R.; Pitcairn, I.K. Golden plumes: Substantial gold enrichment of oceanic crust during ridge-plume interaction. Geology 2013, 41, 87–90. [Google Scholar] [CrossRef]
- Core, D.P.; Kesler, S.E.; Essene, E.J. Unusually Cu-rich magmas associated with giant porphyry copper deposits: Evidence from Bingham, Utah. Geology 2006, 34, 41–44. [Google Scholar] [CrossRef]
- Dromgoole, E.L.; Pasteris, J.D. Interpretation of the sulfide assemblages in a suite of xenoliths. Mantle Metasomatism Alkaline Magmat. 1987, 215, 25. [Google Scholar]
- Szabó, C.; Bodnar, R. Chemistry and origin of mantle sulfides in spinel peridotite xenoliths from alkaline basaltic lavas, Nógraád-Gömör Volcanic Field, northern Hungary and southern Slovakia. Geochim. Cosmochim. Acta 1995, 59, 3917–3927. [Google Scholar] [CrossRef]
- Andersen, T.; Griffin, W.; O’Reilly, S.Y. Primary sulphide melt inclusions in mantle-derived megacrysts and pyroxenites. Lithos 1987, 20, 279–294. [Google Scholar] [CrossRef]
- Barnes, S.J.; Wells, M.A.; Verrall, M.R. Effects of magmatic processes, serpentinization, and talc-carbonate alteration on sulfide mineralogy and ore textures in the Black Swan disseminated nickel sulfide deposit, Yilgarn Craton. Econ. Geol. 2009, 104, 539–562. [Google Scholar] [CrossRef]
- Delacour, A.; Früh-Green, G.L.; Bernasconi, S.M.; Kelley, D.S. Sulfur in peridotites and gabbros at Lost City (30 N, MAR): Implications for hydrothermal alteration and microbial activity during serpentinization. Geochim. Cosmochim. Acta 2008, 72, 5090–5110. [Google Scholar] [CrossRef]
- Oberthuer, T.; Mumm, A.; Vetter, U.; Simon, K.; Amanor, J. Gold mineralization in the Ashanti Belt of Ghana; genetic constraints of the stable isotope geochemistry. Econ. Geol. 1996, 91, 289–301. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Peacock, S.M. Fluid processes in subduction zones. Science 1990, 248, 329–337. [Google Scholar] [CrossRef]
- Pearce, J.A. Supra-subduction zone ophiolites: The search for modern analogues. Spec. Pap.-Geol. Soc. Am. 2003, 373, 269–293. [Google Scholar]
- Bebout, G.E. Volatile transfer and recycling at convergent margins: Mass-balance and insights from high-P/T metamorphic rocks. Subduction Top Bottom 1996, 96, 179–193. [Google Scholar]
- Kerrich, R.; Goldfarb, R.; Groves, D.; Garwin, S. The Geodynamics of World-Class Gold Deposits: Characteristics, Space-Time Distribution, and Origins; Reviews in Economic Geology; GeoScienceWorld: McLean, VA, USA, 2000; Volume 13, pp. 501–551. [Google Scholar]
- Barnes, S.-J.; Lightfoot, P.C. Formation of Magmatic Nickel Sulfide Deposits and Processes Affecting Their Copper and Platinum Group Element Contents; GeoScienceWorld: McLean, VA, USA, 2005. [Google Scholar] [CrossRef]
- Andreani, M.; Muñoz, M.; Marcaillou, C.; Delacour, A. μXANES study of iron redox state in serpentine during oceanic serpentinization. Lithos 2013, 178, 70–83. [Google Scholar] [CrossRef]
- Garuti, G.; Pushkarev, E.V.; Zaccarini, F.; Cabella, R.; Anikina, E. Chromite composition and platinum-group mineral assemblage in the Uktus Uralian-Alaskan-type complex (Central Urals, Russia). Miner. Depos. 2003, 38, 312–326. [Google Scholar] [CrossRef]
- Lorand, J.-P.; Alard, O. Platinum-group element abundances in the upper mantle: New constraints from in situ and whole-rock analyses of Massif Central xenoliths (France). Geochim. Cosmochim. Acta 2001, 65, 2789–2806. [Google Scholar] [CrossRef]



| Rock Type | Serpentinite | Birbirite | Listvenite | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | AD_SP1 | AD_SP2 | AD_S1 | AD_S2 | AD_S3 | AD_B1 | AD_B2 | AD_L1 | AD_L2 |
| SiO2 | 41.07 | 40.48 | 36.05 | 37.55 | 36.82 | 50.92 | 49.15 | 43.03 | 44.46 |
| TiO2 | 0.05 | 0.08 | 0.03 | 0.02 | 0.03 | 0.05 | 0.06 | 0.07 | 0.08 |
| Al2O3 | 1.21 | 1.42 | 0.81 | 0.90 | 0.85 | 0.15 | 0.18 | 1.51 | 1.61 |
| Fe2O3t | 8.03 | 7.55 | 7.48 | 6.99 | 6.60 | 10.23 | 10.45 | 8.97 | 8.53 |
| MnO | 0.15 | 0.10 | 0.07 | 0.08 | 0.07 | 0.07 | 0.09 | 0.10 | 0.12 |
| MgO | 38.99 | 37.48 | 40.05 | 39.04 | 41.30 | 20.01 | 22.80 | 35.05 | 33.97 |
| CaO | 0.61 | 0.79 | 0.15 | 0.10 | 0.18 | 7.14 | 6.85 | 0.79 | 0.85 |
| Na2O | 0.05 | 0.08 | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 | 0.05 | 0.06 |
| K2O | 0.02 | 0.03 | 0.03 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 |
| P2O₅ | 0.03 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 |
| Cr2O3 | 0.32 | 0.45 | 0.21 | 0.24 | 0.23 | 0.31 | 0.34 | 0.51 | 0.55 |
| NiO | 0.25 | 0.35 | 0.20 | 0.15 | 0.22 | 0.08 | 0.10 | 0.20 | 0.25 |
| LOI | 11.05 | 10.53 | 13.97 | 14.97 | 14.21 | 10.53 | 9.05 | 8.02 | 9.02 |
| Total | 101.83 | 99.36 | 99.08 | 100.09 | 100.56 | 99.53 | 99.12 | 98.33 | 99.56 |
| Pentlandite: n = 14 | Nickeloan Pyrrhotite: n = 12 | Chalcopyrite: n = 10 | Millerite: n = 8 | Violarite: n = 11 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | Min. | Max. | Average | wt.% | Min. | Max. | Average | wt.% | Min. | Max. | Average | wt.% | Min. | Max. | Average | wt.% | Min. | Max. | Average |
| Fe | 27.35 | 28.33 | 27.99 | Fe | 53.51 | 60.92 | 55.67 | Fe | 29.13 | 30.41 | 30.08 | Fe | 0.11 | 0.17 | 0.14 | Fe | 13.48 | 31.10 | 19.90 |
| S | 32.25 | 33.73 | 33.17 | S | 34.66 | 39.11 | 36.29 | S | 34.42 | 35.39 | 35.03 | S | 32.69 | 36.05 | 33.96 | S | 36.97 | 41.74 | 38.55 |
| As | 0.07 | 0.13 | 0.11 | As | 0.00 | 0.50 | 0.06 | Ni | 0.00 | 0.06 | 0.01 | As | 0.11 | 0.33 | 0.19 | As | 0.00 | 0.56 | 0.17 |
| Co | 0.00 | 0.32 | 0.17 | Co | 0.00 | 0.47 | 0.19 | Cu | 33.74 | 34.67 | 34.35 | Co | 0.04 | 0.34 | 0.19 | Ni | 24.85 | 44.38 | 38.61 |
| Ni | 36.66 | 39.28 | 37.16 | Ni | 0.44 | 10.48 | 7.30 | Sum | 97.35 | 100.15 | 99.47 | Ni | 62.70 | 66.51 | 64.08 | Te | 0.11 | 0.25 | 0.20 |
| Zn | 0.00 | 0.05 | 0.03 | Sum | 97.83 | 100.43 | 99.50 | atom % | Te | 0.29 | 0.35 | 0.33 | Pb | 0.04 | 0.18 | 0.11 | |||
| Sum | 97.51 | 99.88 | 98.62 | atom % | Fe | 24.45 | 25.03 | 24.80 | Sum | 97.26 | 100.42 | 98.89 | Sum | 96.08 | 98.22 | 97.54 | |||
| atom % | Fe | 42.63 | 47.08 | 44.15 | S | 50.10 | 50.66 | 50.30 | atom % | atom % | |||||||||
| Fe | 22.58 | 23.20 | 23.06 | S | 48.81 | 52.78 | 50.14 | Ni | 0.00 | 0.05 | 0.01 | Fe | 0.09 | 0.14 | 0.12 | Fe | 11.05 | 24.36 | 16.00 |
| S | 46.38 | 47.90 | 47.60 | As | 0.00 | 0.30 | 0.03 | Cu | 24.78 | 25.00 | 24.89 | S | 47.21 | 50.71 | 49.00 | S | 52.39 | 56.94 | 54.13 |
| As | 0.04 | 0.08 | 0.07 | Co | 0.00 | 0.35 | 0.14 | Sum | 100 | 100 | 100 | As | 0.07 | 0.21 | 0.12 | As | 0.00 | 0.34 | 0.10 |
| Co | 0.00 | 0.25 | 0.13 | Ni | 0.33 | 7.91 | 5.54 | formula | Fe0.99Cu1.0S2.01 | Co | 0.03 | 0.27 | 0.15 | Ni | 18.51 | 34.20 | 29.67 | ||
| Ni | 28.85 | 30.85 | 29.13 | Sum | 100 | 100 | 100 | Fe | 0.98 | 1.00 | 0.99 | Ni | 48.91 | 52.45 | 50.50 | Te | 0.04 | 0.09 | 0.07 |
| Zn | 0.00 | 0.04 | 0.02 | formula | Fe0.88Ni0.11S1.00 | S | 2.00 | 2.03 | 2.01 | Te | 0.11 | 0.13 | 0.12 | Pb | 0.01 | 0.04 | 0.02 | ||
| Sum | 100 | 100 | 100 | Fe | 0.85 | 0.94 | 0.88 | Cu | 0.99 | 1.00 | 1.00 | Sum | 100 | 100 | 100 | Sum | 100 | 100 | 100 |
| formula | Fe3.92Ni4.95Co0.02As0.01S8.09 | S | 0.98 | 1.06 | 1.00 | Sum | 4 | 4 | 4 | formula | Ni1.01S0.98 | formula | Fe1.12Ni2.08As0.01S3.79 | ||||||
| Fe | 3.84 | 3.94 | 3.92 | Ni | 0.01 | 0.16 | 0.11 | S | 0.94 | 1.01 | 0.98 | Fe | 0.77 | 1.70 | 1.12 | ||||
| S | 7.89 | 8.14 | 8.09 | Sum | 2 | 2 | 2 | Ni | 0.98 | 1.05 | 1.01 | S | 3.67 | 3.99 | 3.79 | ||||
| As | 0.01 | 0.01 | 0.01 | Sum | 2 | 2 | 2 | As | 0.00 | 0.02 | 0.01 | ||||||||
| Co | 0.00 | 0.04 | 0.02 | Ni | 1.30 | 2.39 | 2.08 | ||||||||||||
| Ni | 4.90 | 5.24 | 4.95 | Sum | 7 | 7 | 7 | ||||||||||||
| Sum | 17 | 17 | 17 | ||||||||||||||||
| LOD | Pentlandite: n = 14 | Nickeloan Pyrrhotite: n = 12 | Chalcopyrite: n = 10 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Average | STDV | Min | Max | Average | STDV | Min | Max | Average | STDV | |||
| 47Ti | 2.26 | 12.55 | 96.9 | 37.2 | 22.9 | 0.59 | 12.6 | 3.93 | 5.11 | |||||
| 51V | 0.18 | 5.94 | 156 | 25.2 | 38.1 | 0.03 | 1.9 | 0.48 | 0.68 | 1.12 | 3.85 | 2.77 | 1.45 | |
| 52Cr | 2.08 | 15.02 | 1271 | 238 | 323 | |||||||||
| 55Mn | 1.11 | 57.8 | 662 | 353 | 196 | 0.02 | 55.9 | 14.43 | 19.9 | 6.45 | 28.31 | 13.95 | 9.8 | |
| 59Co | 0.1 | 448 | 15,553 | 2855 | 3894 | 1113 | 4801 | 2715 | 1228 | 0.62 | 7.35 | 3.15 | 2.04 | |
| 63Cu | 0.3 | 3.03 | 390 | 142 | 135 | 3.06 | 170 | 28.8 | 50.3 | |||||
| 66Zn | 0.36 | 1.66 | 26.5 | 7.28 | 7.43 | 1.67 | 41.16 | 6.9 | 11.89 | 468 | 1050 | 607 | 166 | |
| 75As | 0.61 | 651 | 1053 | 836 | 112 | 3735 | 78,733 | 12,867 | 22,851 | |||||
| 77Se | 0.32 | 14.3 | 39.5 | 19.18 | 6.33 | |||||||||
| 95Mo | 0.11 | 0.68 | 33.2 | 3.49 | 8.57 | 0.11 | 2.34 | 0.7 | 0.87 | |||||
| 105Pd | 0.14 | 0.02 | 0.18 | 0.08 | 0.05 | 0.01 | 0.2 | 0.06 | 0.08 | 10.9 | 14.53 | 12.62 | 1.18 | |
| 109Ag | 0.08 | 0.25 | 6.64 | 1.39 | 1.65 | 0 | 6.51 | 1.01 | 1.92 | 1.58 | 67.46 | 15.72 | 24.5 | |
| 111Cd | 0.32 | 0.03 | 0.09 | 0.06 | 0.02 | 0 | 0.05 | 0.02 | 0.02 | 64.6 | 187 | 114 | 40.0 | |
| 120Sn | 0.15 | 0.04 | 0.08 | 0.05 | 0.02 | 1.74 | 44.67 | 13.5 | 13.59 | |||||
| 123Sb | 0.35 | 3.7 | 34.38 | 15.02 | 8.62 | 3.32 | 115.66 | 19.26 | 33.94 | 1.34 | 10.48 | 5.1 | 4.78 | |
| 184W | 0.08 | 2.86 | 30.12 | 11.58 | 7.88 | 0 | 0.08 | 0.03 | 0.03 | |||||
| 193Ir | 0.05 | 0 | 0.01 | 0.01 | 0 | 0.02 | 0.34 | 0.09 | 0.12 | 0.12 | 0.35 | 0.23 | 0.17 | |
| 195Pt | 0.26 | 0.02 | 0.26 | 0.08 | 0.08 | |||||||||
| 197Au | 0.05 | 0.17 | 6.11 | 1.99 | 1.83 | 0.03 | 0.41 | 0.11 | 0.12 | |||||
| 205Tl | 0.06 | 0.29 | 3.87 | 1.38 | 1 | |||||||||
| 208Pb | 0.13 | 9.68 | 26.1 | 18.4 | 5.3 | 10.55 | 361 | 60.1 | 106 | 1.14 | 86.2 | 23.52 | 25.23 | |
| 209Bi | 0.07 | 0.55 | 2.41 | 1.24 | 0.47 | 0.02 | 0.80 | 0.13 | 0.24 | 0.26 | 14.32 | 3.55 | 4.33 | |
| 60Ni | 0.05 | 3770 | 98,018 | 15,683 | 28,278 | 1.61 | 74.31 | 17.2 | 31.94 | |||||
| S/Se | 8543 | 23,174 | 18,381 | 3779 | ||||||||||
| Millerite: n = 8 | Violarite: n = 11 | |||||||||||||
| Min | Max | Average | STDV | Min | Max | Average | STDV | |||||||
| 20.9 | 267 | 114 | 91.4 | 10.7 | 234 | 79.8 | 68.8 | |||||||
| 47Ti | 20.4 | 105 | 52.3 | 33.2 | 2.79 | 124 | 54.4 | 40.7 | ||||||
| 51V | 45.2 | 797 | 322 | 293 | 7.59 | 1973 | 300 | 635 | ||||||
| 52Cr | 380 | 2619 | 886 | 725 | 203 | 607 | 440 | 126 | ||||||
| 55Mn | 1492 | 13,776 | 8443 | 5397 | 25,354 | 42,679 | 33,623 | 5336 | ||||||
| 59Co | 66.6 | 1351 | 542 | 379 | 8.45 | 538 | 230 | 177 | ||||||
| 63Cu | 3.16 | 215 | 44.7 | 74.5 | 2.02 | 54.8 | 15.5 | 20.1 | ||||||
| 66Zn | 1082 | 3342 | 1870 | 755 | 939 | 2052 | 1294 | 325 | ||||||
| 75As | 37.44 | 75.1 | 50.19 | 12.96 | 2.52 | 81.69 | 24.3 | 24.0 | ||||||
| 77Se | 1.6 | 87.42 | 30.55 | 38.53 | 1.72 | 247 | 57.02 | 76.3 | ||||||
| 95Mo | 0.05 | 0.61 | 0.25 | 0.25 | ||||||||||
| 105Pd | 0.23 | 18.29 | 5.73 | 6.24 | 0.34 | 2.3 | 1.23 | 0.58 | ||||||
| 109Ag | 0.07 | 0.83 | 0.4 | 0.39 | ||||||||||
| 111Cd | 0.06 | 0.27 | 0.12 | 0.1 | ||||||||||
| 120Sn | 11.2 | 50.8 | 25.6 | 13.5 | 5.47 | 80.8 | 26.2 | 20.8 | ||||||
| 123Sb | 5.87 | 47.5 | 19.8 | 14.8 | 2.82 | 120 | 34.2 | 33.0 | ||||||
| 184W | ||||||||||||||
| 193Ir | 0.08 | 0.13 | 0.11 | 0.04 | ||||||||||
| 195Pt | 0.06 | 0.32 | 0.19 | 0.13 | 0.09 | 0.12 | 0.11 | 0.02 | ||||||
| 197Au | 0.72 | 4.91 | 2.47 | 1.78 | 0.2 | 6.89 | 1.77 | 2.21 | ||||||
| 205Tl | 17.2 | 114 | 45.2 | 32.1 | 5.94 | 60.7 | 32.5 | 18.7 | ||||||
| 208Pb | 1.44 | 3.82 | 2.71 | 0.8 | 0.62 | 4.04 | 1.99 | 1 | ||||||
| 209Bi | ||||||||||||||
| 60Ni | 4526 | 9177 | 7129 | 1667 | 4546 | 160,925 | 45,133 | 505,245 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoheir, B.; Holzheid, A.; Diab, A.; Ragab, A.; Deshesh, F.; Abdelnasser, A. Recycling of Au during Serpentinization of Ultramafic Rocks: A Case Study from Neoproterozoic Forearc Ophiolites, Egypt. Minerals 2024, 14, 916. https://doi.org/10.3390/min14090916
Zoheir B, Holzheid A, Diab A, Ragab A, Deshesh F, Abdelnasser A. Recycling of Au during Serpentinization of Ultramafic Rocks: A Case Study from Neoproterozoic Forearc Ophiolites, Egypt. Minerals. 2024; 14(9):916. https://doi.org/10.3390/min14090916
Chicago/Turabian StyleZoheir, Basem, Astrid Holzheid, Aliaa Diab, Azza Ragab, Fatma Deshesh, and Amr Abdelnasser. 2024. "Recycling of Au during Serpentinization of Ultramafic Rocks: A Case Study from Neoproterozoic Forearc Ophiolites, Egypt" Minerals 14, no. 9: 916. https://doi.org/10.3390/min14090916
APA StyleZoheir, B., Holzheid, A., Diab, A., Ragab, A., Deshesh, F., & Abdelnasser, A. (2024). Recycling of Au during Serpentinization of Ultramafic Rocks: A Case Study from Neoproterozoic Forearc Ophiolites, Egypt. Minerals, 14(9), 916. https://doi.org/10.3390/min14090916









