New Insights for Improving Low-Rank Coal Flotation Performance via Tetrahydrofurfuryl Ester Collectors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
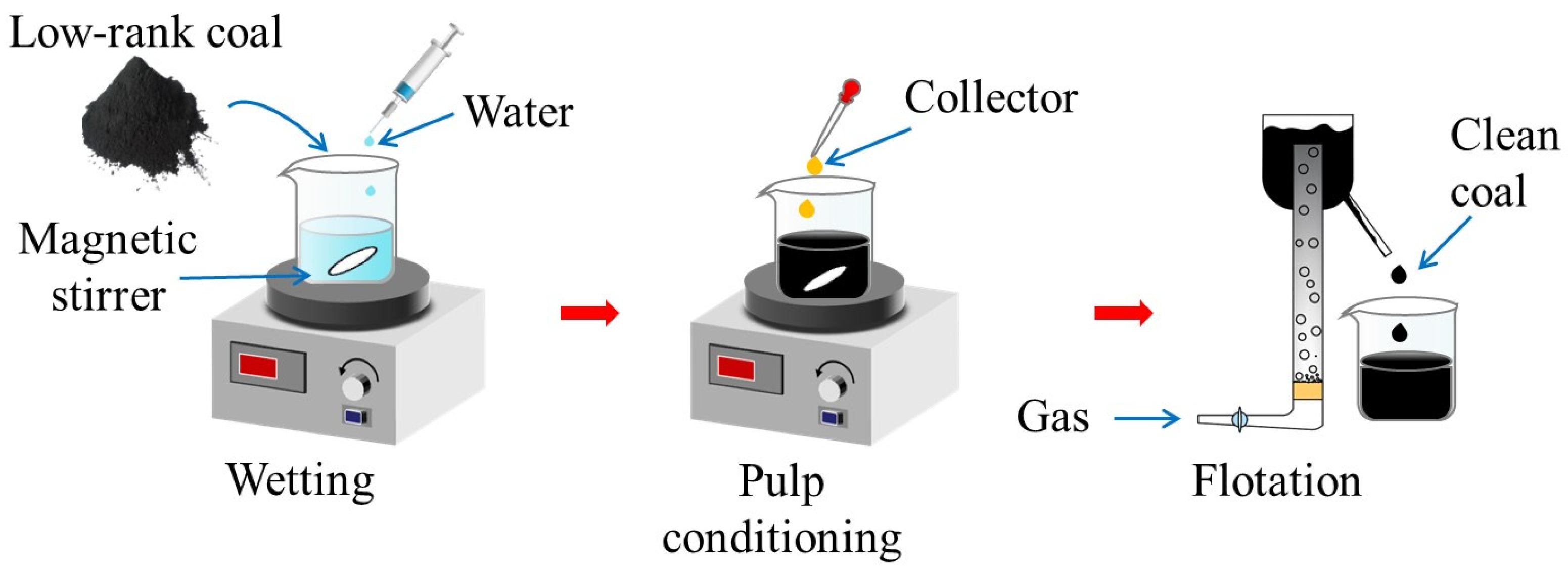
2.2. Micro-Flotation Tests
2.3. Characterizations
3. Results and Discussion
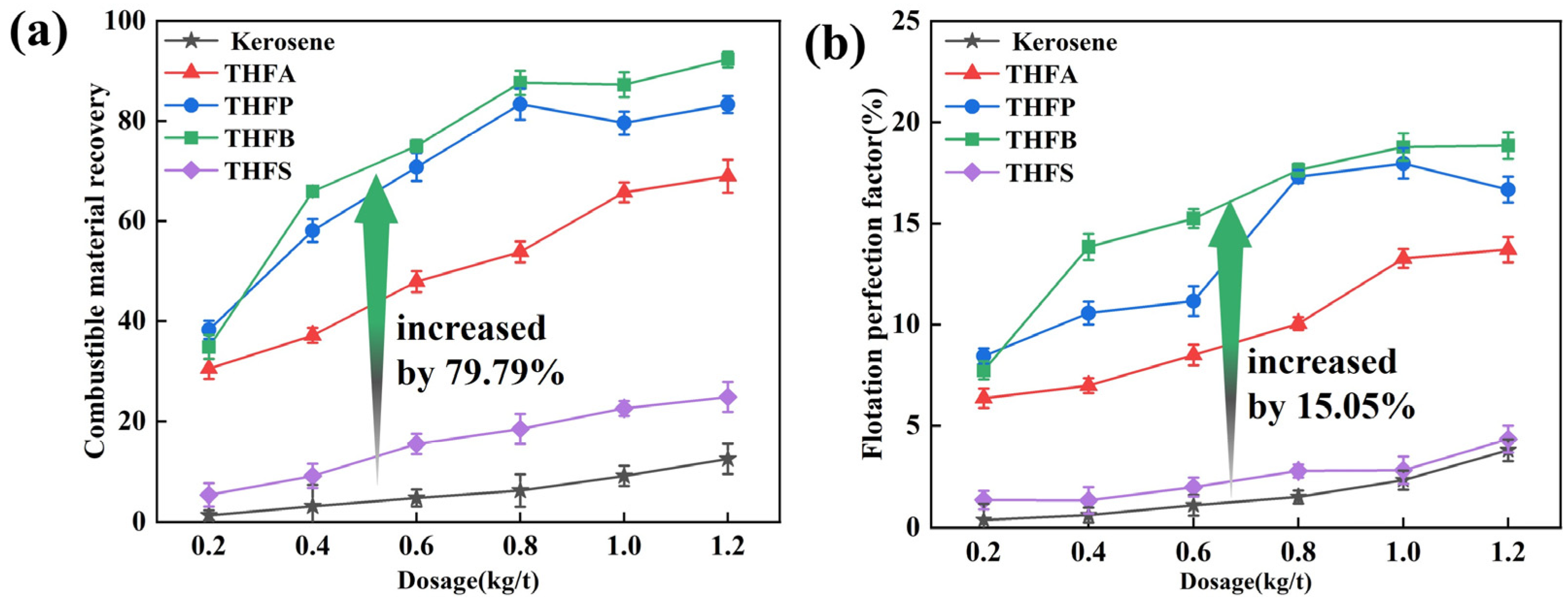
3.1. Flotation Performances
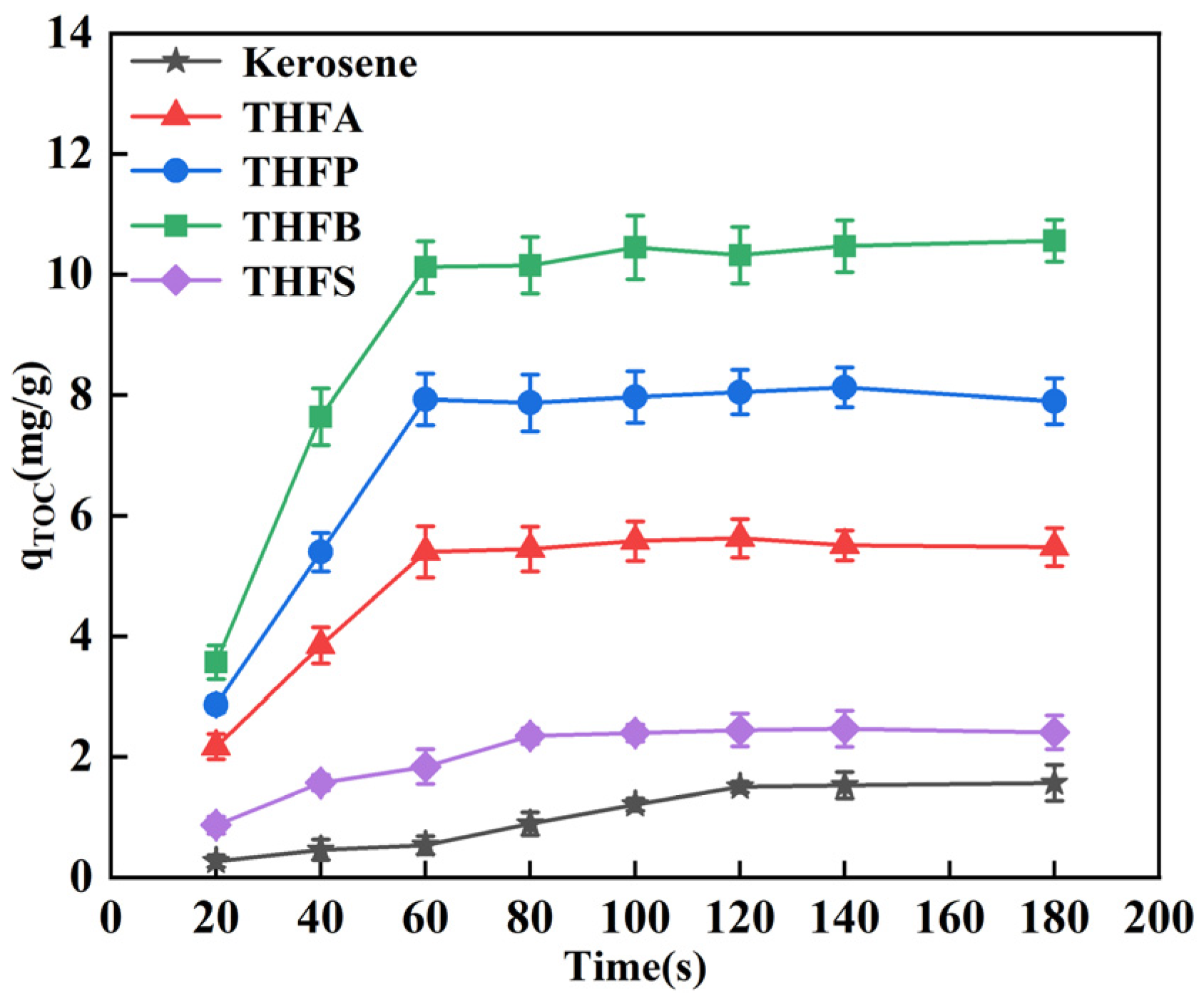
3.2. Adsorption Property Analysis
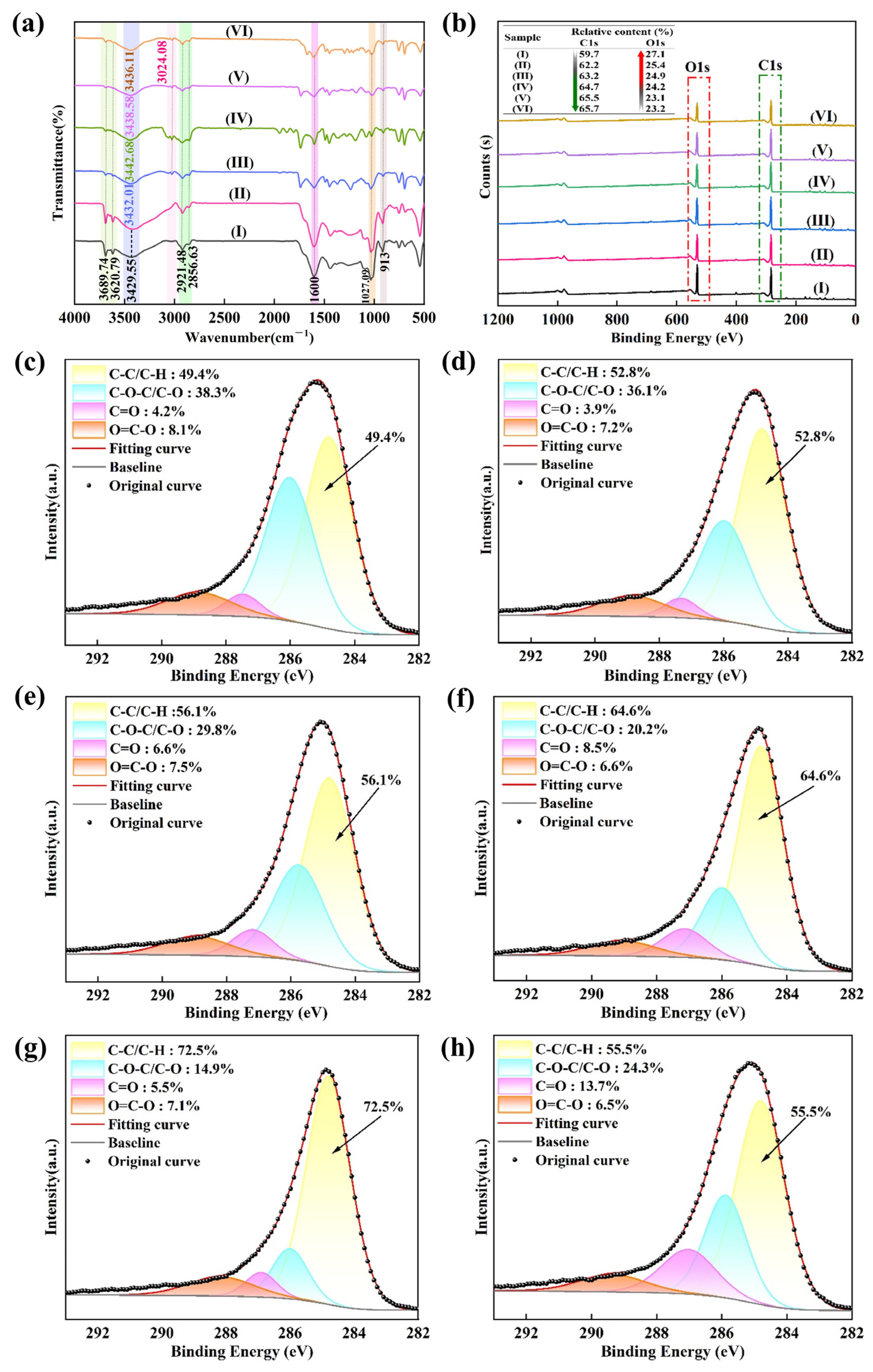
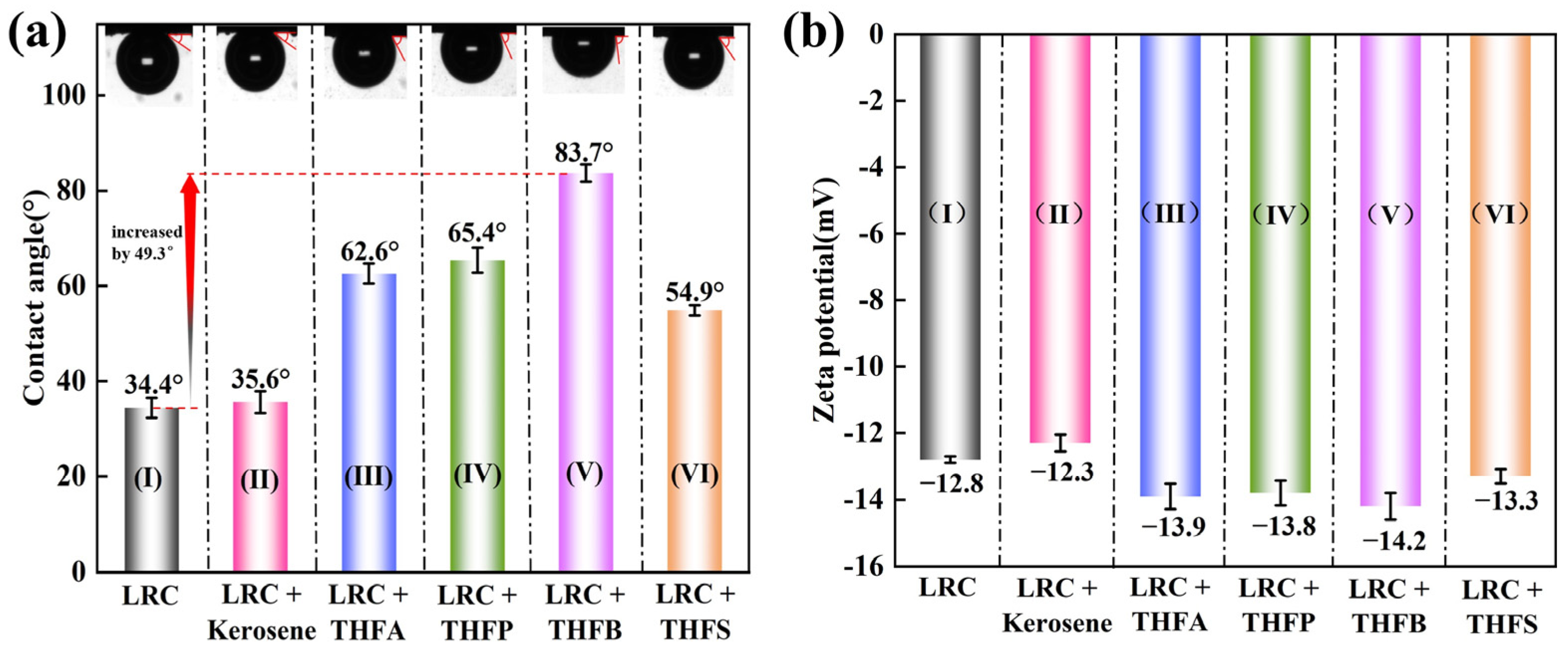
3.3. Changes in LRC Surface Properties
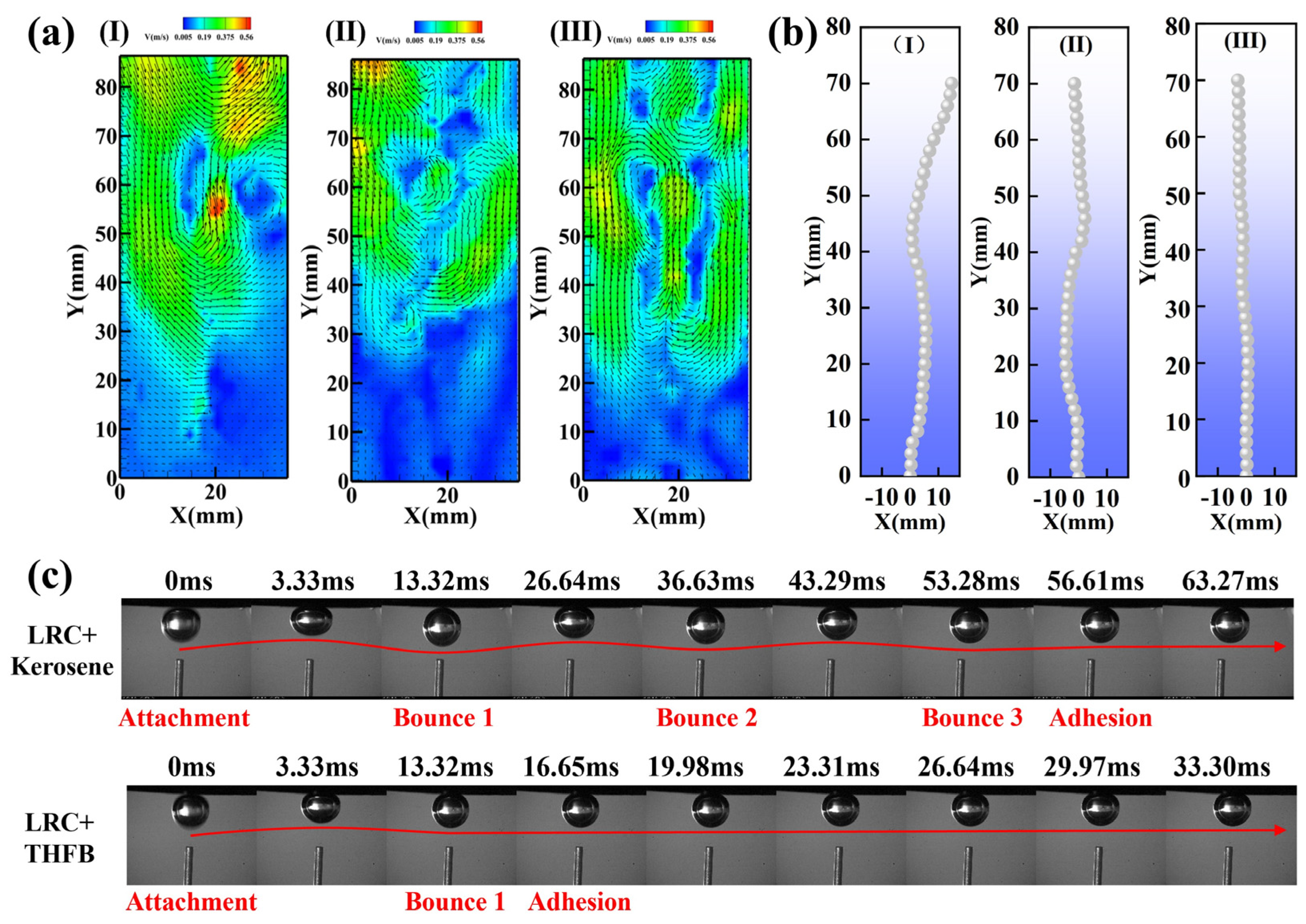
3.4. The Role of Bubbles in LRC Flotation
4. Conclusions
- (1)
- THF-series collectors exhibited superior flotation performance for LRC. Among them, THFB demonstrated the best effect, with combustible material recovery 79.79% higher than that of kerosene and a flotation perfection factor that increased by 15.05% at a dosage of 1.2 kg/t.
- (2)
- THF-series collectors can adsorb more quickly and efficiently onto the LRC surface to enhance its floatability. In this study, THFB reached adsorption equilibrium within 60 s, with a maximum adsorption capacity of 6.7 times that of kerosene. The THF-series collectors primarily interact with LRC through hydrogen bonding. After adsorption, THF-series collectors effectively mask the oxygen-containing sites on the LRC surface, reducing its wettability and enhancing its electronegativity.
- (3)
- THFB collectors can disperse more uniformly in the flotation system, reducing the lateral movement of bubbles during their ascent, decreasing the influence of bubble wakes on coal particles, and preventing entrainment effects. After treatment with THFB, bubbles could stably adhere to the LRC surface within 16.65 ms, significantly improving the flotation efficiency of LRC.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, K.; Zhang, W.; Li, Y.; Ping, A.; Wu, F.; Xie, G.; Xia, W. Enhancing flotation removal of unburned carbon from fly ash by coal tar-based collector: Experiment and simulation. Fuel 2023, 332, 126023. [Google Scholar] [CrossRef]
- Kang, W.; Ma, Z. Pyhsical and chemical properties of coal pyrite and flotation desulfurization. Clean Coal Technol. 2022, 28, 109–117. [Google Scholar]
- Karamaneas, A.; Koasidis, K.; Frilingou, N.; Xexakis, G.; Nikas, A.; Doukas, H. A stakeholder-informed modelling study of Greece’s energy transition amidst an energy crisis: The role of natural gas and climate ambition. Renew. Sustain. Energy Transit. 2023, 3, 100049. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Y.; Cao, Y.; Wang, X.; Li, E.; Guo, Y.; Lau, E.V. New Insights on the Understanding of Sulfur-Containing Coal Flotation Desulfurization. Minerals 2024, 14, 981. [Google Scholar] [CrossRef]
- Liao, Y.; Yang, Z.; An, M.; Ma, L.; Yang, A.; Cao, Y.; Chen, L.; Ren, H. Alkanes-esters mixed collector enhanced low rank coal flotation: Interfacial interaction between oil drop and coal particle. Fuel 2022, 321, 124045. [Google Scholar] [CrossRef]
- Xia, Y.; Xing, Y.; Gui, X. Oily collector pre-dispersion for enhanced surface adsorption during fine low-rank coal flotation. J. Ind. Eng. Chem. 2020, 82, 303–308. [Google Scholar] [CrossRef]
- Nyashina, G.S.; Kuznetsov, G.V.; Strizhak, P.A. Energy efficiency and environmental aspects of the combustion of coal-water slurries with and without petrochemicals. J. Clean. Prod. 2018, 172, 1730–1738. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, W.; Wang, X.; Cheng, H.; Cheng, F.; Miller, J.D. Lauryl phosphate flotation chemistry in barite flotation. Minerals 2020, 10, 280. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Tao, X.; Chen, L.; Yang, Z.; Li, L. Effect of oxidation processing on the surface properties and floatability of Meizhiyou long-flame coal. Fuel 2017, 210, 177–186. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Z.; Chen, L.; Tao, X.; Tang, L.; He, H. Wetting thermodynamics of low rank coal and attachment in flotation. Fuel 2017, 207, 214–225. [Google Scholar] [CrossRef]
- Chen, S.; Tao, X.; Wang, S.; Tang, L.; Liu, Q.; Li, L. Comparison of air and oily bubbles flotation kinetics of long-flame coal. Fuel 2019, 236, 636–642. [Google Scholar] [CrossRef]
- Huang, Y.; Takaoka, M.; Takeda, N.; Oshita, K. Partial removal of PCDD/Fs, coplanar PCBs, and PCBs from municipal solid waste incineration fly ash by a column flotation process. Environ. Sci. Technol. 2007, 41, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Xia, W.; Sokolovic, J.M. Recent advances in effective collectors for enhancing the flotation of low rank/oxidized coals. Powder Technol. 2017, 319, 1–11. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Tao, X.; He, H.; Chen, L.; Yang, Z. Enhancing flotation performance of low rank coal by improving its hydrophobicity and the property of oily bubbles using 2-ethylhexanol. Int. J. Miner. Process. 2017, 167, 61–67. [Google Scholar] [CrossRef]
- Lam, N.L.; Chen, Y.; Weyant, C.; Venkataraman, C.; Sadavarte, P.; Johnson, M.A.; Smith, K.R.; Brem, B.T.; Arineitwe, J.; Ellis, J.E. Household light makes global heat: High black carbon emissions from kerosene wick lamps. Environ. Sci. Technol. 2012, 46, 13531–13538. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Xiao, X.; Wang, X.; Pan, Z.; Qin, Y.; Gao, G.; Du, Z.; Cheng, F. Interfacial interaction of emulsion collector in enhancing low-rank coal flotation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133965. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Performance of used lubricating oil as flotation collector for the recovery of clean low-rank coal. Fuel 2019, 239, 717–725. [Google Scholar] [CrossRef]
- Li, M.; Xia, Y.; Zhang, Y.; Ding, S.; Rong, G.; Cao, Y.; Xing, Y.; Gui, X. Mechanism of shale oil as an effective collector for oxidized coal flotation: From bubble–particle attachment and detachment point of view. Fuel 2019, 255, 115885. [Google Scholar] [CrossRef]
- Xia, W.; Ni, C.; Xie, G. Effective flotation of lignite using a mixture of dodecane and 4-dodecylphenol (DDP) as a collector. Int. J. Coal Prep. Util. 2016, 36, 262–271. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y.; Wang, D.; Zhang, H. Clean low-rank-coal purification technique combining cyclonic-static microbubble flotation column with collector emulsification. J. Clean. Prod. 2017, 153, 657–672. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.H.; Fuerstenau, D.W. An improved class of universal collectors for the flotation of oxidized and/or low-rank coal. Int. J. Miner. Process. 2000, 58, 99–118. [Google Scholar] [CrossRef]
- Hancer, M.; Celik, M.; Miller, J.D. The significance of interfacial water structure in soluble salt flotation systems. J. Colloid Interface Sci. 2001, 235, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, Y.; Du, Z.; Li, D.; Cheng, F. Bubbles facilitate ODA adsorption and improve flotation recovery at low temperature during KCl flotation. Chem. Eng. Res. Des. 2017, 117, 557–563. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Li, W. Recycling of waste plastic combustion soot and diesel oil mixing to prepare a new collector to improve the performance of low-rank coal flotation. Powder Technol. 2024, 439, 119727. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Z.; Ma, Z.; Cao, Y.; Sun, L.; Jiang, Z. Optimization of collector and its action mechanism in lignite flotation. Powder Technol. 2019, 345, 182–189. [Google Scholar] [CrossRef]
- He, J.; Liu, C.; Yao, Y. Flotation intensification of the coal slime using a new compound collector and the interaction mechanism between the reagent and coal surface. Powder Technol. 2018, 325, 333–339. [Google Scholar] [CrossRef]
- Ge, W.; Liu, J.; Ren, H.; Zhu, Y.; Han, W.; Han, Y. Enhanced mixed flotation of copper–molybdenum ore using dodecyl dimethyl betaine-emulsified kerosene as environmentally friendly collector. J. Clean. Prod. 2024, 447, 141576. [Google Scholar] [CrossRef]
- Lyu, X.; You, X.; He, M.; Zhang, W.; Wei, H.; Li, L.; He, Q. Adsorption and molecular dynamics simulations of nonionic surfactant on the low rank coal surface. Fuel 2018, 211, 529–534. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.; Peng, Y.; Xie, G. A novel coal tar-based collector for effective flotation cleaning of low rank coal. J. Clean. Prod. 2020, 273, 123172. [Google Scholar] [CrossRef]
- Ban, Y.; Jin, L.; Zhu, J.; Liu, F.; Hu, H. Insights into effect of Ca(OH)2 on pyrolysis behaviors and products distribution of Hongshaquan coal. Fuel 2022, 307, 121791. [Google Scholar] [CrossRef]
- Zhu, X.-n.; Wang, D.-z.; Ni, Y.; Wang, J.-x.; Nie, C.-c.; Yang, C.; Lyu, X.-j.; Qiu, J.; Li, L. Cleaner approach to fine coal flotation by renewable collectors prepared by waste oil transesterification. J. Clean. Prod. 2020, 252, 119822. [Google Scholar] [CrossRef]
- Xu, M.; Xing, Y.; Cao, Y.; Gui, X. Waste colza oil used as renewable collector for low rank coal flotation. Powder Technol. 2019, 344, 611–616. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Ma, X.; Tao, X. Comparison of flotation performances of low-rank coal with lower ash content using air and oily bubbles. Powder Technol. 2020, 374, 443–448. [Google Scholar] [CrossRef]
- Wang, C.; Xing, Y.; Xia, Y.; Zhang, R.; Wang, S.; Shi, K.; Tan, J.; Gui, X. Investigation of interactions between oxygen-containing groups and water molecules on coal surfaces using density functional theory. Fuel 2021, 287, 119556. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, C.; Cheng, W.; Zhang, Q.; Nie, W. Effects of oxygen element and oxygen-containing functional groups on surface wettability of coal dust with various metamorphic degrees based on XPS experiment. J. Anal. Methods Chem. 2015, 2015, 467242. [Google Scholar] [CrossRef]
- Wan, H.; Hu, X.; Luukkanen, S.; Qu, J.; Zhang, C.; Xue, J.; Li, H.; Yang, W.; Yang, S.; Bu, X. Effect of the oxygen-containing functional group on the adsorption of hydrocarbon oily collectors on coal surfaces. Physicochem. Probl. Miner. Process. 2022, 58, 149937. [Google Scholar] [CrossRef]
- Grzybek, T.; Pietrzak, R.; Wachowska, H. X-ray photoelectron spectroscopy study of oxidized coals with different sulphur content. Fuel Process. Technol. 2002, 77, 1–7. [Google Scholar] [CrossRef]
- Pietrzak, R.; Wachowska, H. The influence of oxidation with HNO3 on the surface composition of high-sulphur coals: XPS study. Fuel Process. Technol. 2006, 87, 1021–1029. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Enhancement of the surface hydrophobicity of low-rank coal by adsorbing DTAB: An experimental and molecular dynamics simulation study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]
- Xia, W.; Niu, C.; Li, Y. Effect of heating process on the wettability of fine coals of various ranks. Can. J. Chem. Eng. 2017, 95, 475–478. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.; Pan, L.; Tian, F.; Peng, Y.; Xie, G.; Li, Y. Flotation of low-rank coal using sodium oleate and sodium hexametaphosphate. J. Clean. Prod. 2020, 261, 121216. [Google Scholar] [CrossRef]
- Ji, D.G.; Cai, Y.H.; Guo, X.L.; Peng, S.Q.; Wei, S.G. Research of Optimize Performance of Coal Flotation Collectors. Adv. Mater. Res. 2012, 361, 296–300. [Google Scholar] [CrossRef]
- Gui, X.; Xing, Y.; Wang, T.; Cao, Y.; Miao, Z.; Xu, M. Intensification mechanism of oxidized coal flotation by using oxygen-containing collector α-furanacrylic acid. Powder Technol. 2017, 305, 109–116. [Google Scholar] [CrossRef]
- Pan, G.; Zhu, H.; Shi, Q.; Zhang, Y.; Zhu, J.; Ou, Z.; Gao, L. Effect of bubble trailing vortex on coal slime motion in flotation. Fuel 2023, 334, 126802. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, H.; Shen, T.; Qin, Z.; Zhu, J.; Gao, L.; Ou, Z.; Zhang, Y.; Pan, G. Effect of frother on bubble entraining particles in coal flotation. Energy 2024, 288, 129711. [Google Scholar] [CrossRef]
- Lu, Y.; Li, E.; Cheng, H.; Wang, X.; Du, Z.; Cheng, F.; Miller, J.D. Effect of oxygen functional groups on the surface properties and flotation response of fine coal, comparison of rank with oxidation. Int. J. Coal Prep. Util. 2018, 41, 290–306. [Google Scholar] [CrossRef]

| Samples | Proximate Analysis (%) | Ultimate Analysis (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Cdaf | Hdaf | Odaf | Ndaf | Sdaf | |
| Raw coal | 4.63 | 17.64 | 35.58 | 42.15 | 64.95 | 4.26 | 27.93 | 1.27 | 1.39 |
| Clean coal | 4.57 | 10.68 | 28.36 | 56.39 | 68.21 | 4.38 | 24.71 | 1.29 | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ding, R.; Cui, X.; Qin, Y.; Cheng, G.; Abaka-Wood, G.; Li, E. New Insights for Improving Low-Rank Coal Flotation Performance via Tetrahydrofurfuryl Ester Collectors. Minerals 2025, 15, 78. https://doi.org/10.3390/min15010078
Wang X, Ding R, Cui X, Qin Y, Cheng G, Abaka-Wood G, Li E. New Insights for Improving Low-Rank Coal Flotation Performance via Tetrahydrofurfuryl Ester Collectors. Minerals. 2025; 15(1):78. https://doi.org/10.3390/min15010078
Chicago/Turabian StyleWang, Xin, Rui Ding, Xinyu Cui, Yonghong Qin, Gan Cheng, George Abaka-Wood, and Enze Li. 2025. "New Insights for Improving Low-Rank Coal Flotation Performance via Tetrahydrofurfuryl Ester Collectors" Minerals 15, no. 1: 78. https://doi.org/10.3390/min15010078
APA StyleWang, X., Ding, R., Cui, X., Qin, Y., Cheng, G., Abaka-Wood, G., & Li, E. (2025). New Insights for Improving Low-Rank Coal Flotation Performance via Tetrahydrofurfuryl Ester Collectors. Minerals, 15(1), 78. https://doi.org/10.3390/min15010078













