Temperature: An Influencing Factor on the Rheological and Energetic Parameters of Acid Pressure Technology Operations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment
2.2. Preparation of Mineral Suspensions
2.3. Variable Selection
2.4. Rheological Measurements
3. Results and Discussion
3.1. Chemical Composition of Lateritic Suspensions
3.2. Granulometric Analysis
3.3. Experimental Results of Rheology in Raw and Preheated Pulp
3.4. Energy Parameters of the Pumping System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keenan, J.H.; Keyes, F.G. Thermodynamics Properties of Steam; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Treybal, R.E. Operaciones de Transferencia de Masa, 2nd ed.; McGraw-Hill: Mexico City, Mexico, 1990. [Google Scholar]
- Önal, M.A.R.; Topkaya, Y.A. Pressure acid leaching of Çaldağ lateritic nickel ore: An alternative to heap leaching. Hydrometallurgy 2014, 142, 98–107. [Google Scholar] [CrossRef]
- Mweene, L.; Gomez-Flores, A.; Jeong, H.E.; Ilyas, S.; Kim, H. Challenges and Future in Ni Laterite Ore Enrichment: A Critical Review. Miner. Process. Extr. Metall. Rev. 2023, 45, 539–563. [Google Scholar] [CrossRef]
- Castellanos, J.; Hernández, A.N. El futuro de la laterita de níquel. In Proceedings of the Reunión de Experto Interoceanmetal (IDM), Polonia y CUARTA CONVENCION DE LA TIERRA, GEOCIENCIAS, Havana, Cuba, 4–8 April 2011. [Google Scholar]
- Chaviano, L. Mejoras tecnologícas para las plantas con Lixiviación Ácida a Presión. In Proceedings of the Lixiviación Ácida a Presión, VI CONVENCION DE LA TIERRA, GEOCIENCIAS, Havana, Cuba, 4–8 May 2015. [Google Scholar]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review. Part I. Sulphuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Castellanos, S.J.; Hernández, M.N. Procesamiento de minerales niquel arcillosos y de mala sedimentación por lixiviación acida a presión. In Proceedings of the Congreso Internacional de Minería y Metalurgia (MINEMETAL 2016), Matanza, Cuba, 3–7 October 2016; ISBN 978-959.7117-73-5. [Google Scholar]
- Chalkley, M.E.; Matos, W.R.; Ellenwood, S. Successfully adapting to continuing challenges at the moa joint venture. In Proceedings of the Congreso Internacional de Minería y Metalurgia (MINEMETAL 2016), Mantanza, Cuba, 3–7 October 2016. [Google Scholar]
- Mackenzie, M.; Virnig, M.; Feather, A. The recovery of nickel from high-pressure acid leach solutions using mixed hydroxide product LIX®84-INS technology. Miner. Eng. 2006, 19, 1220–1233. [Google Scholar] [CrossRef]
- Romanovskaia, E.; Romanovski, V.; Kwapinski, W.; Kurilo, I. Selective recovery of vanadium pentoxide from spent catalysts of sulfuric acid production: Sustainable approach. Hydrometallurgy 2021, 200, 105568. [Google Scholar] [CrossRef]
- Coniglio, A.; Fierro, A.; Herrmann, H.J.; Nicodemi, M. Unifying Concepts in Granular Media and Glasses; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Balagui, S.; Mohammadifar, M.; Zargaraan, A. Physicochemical and rheological characterization of gum tragacanth exudates from six species of iranian astragalus. J. Food Biophys. 2010, 15, 59–71. [Google Scholar] [CrossRef]
- Bourbon, A.; Pinheiro, A.; Ribeiro, C.; Miranda, C.; Maia, J.; Teixeira, J.; Vicente, A. Characterization of galactomannans extracted from seeds of gleditsia triacanthos and sophorajaponica through shear and extensional rheology. J. Food Hydrocolloid 2010, 12, 184–192. [Google Scholar] [CrossRef]
- Colby, R. Structure and linear viscoelasticity of flexible polymer solutions: Comparison of polyelectrolyte and neutral polymer solutions. J. Rheol. 2010, 24, 425–442. [Google Scholar] [CrossRef]
- Andrade, R.; Torres, R.; Montes, E. Efecto de la temperatura en el comportamiento reológico de pulpas orgánicas. Rev. Fac. Agron. 2009, 26, 599–612. [Google Scholar]
- Vandresen, S.; Quadri, M.; De Souza, J.; Hotza, D. Temperature effect on the rheological behavior of carrot juices. J. Food Eng. 2009, 29, 269–274. [Google Scholar] [CrossRef]
- Garcell, L. Composición Mineralógica de las Suspensiones de Limonita de Moa, en Períodos de Sedimentación Normal y Crítica; Informe Investigative; Facultad de Ingeniería Química, ISPJAM: Santiago de Cuba, Cuba, 1993. [Google Scholar]
- Laurencio, H.; Delgado, Y. Influencia de la temperatura en las propiedades reológicas de la emulsión de petróleo pesado. Minería Geol. 2008, 24, 12. [Google Scholar]
- Trapeznikov, S. Fundamentación de los Regímenes de Temperaturas de Trabajo de los Oleoductos Superficiales en Caliente. Ph.D. Thesis, Instituto de Minas de San Petersburgos, San Petersburgos, Russia, 2011. [Google Scholar]
- Avotins, A.P.; Ahlschlager, S.S.; Wicker, R.G. The Rheology and Handling of Laterite Slurries. In Proceedings of the International Lateritic Symposium, New Orleans, LA, USA, 19–21 February 1979. [Google Scholar]
- Pérez, L.; Cardero, Y.; Lamoth, Y.; Garcell, L. Estudio del comportamiento reológico de una suspensión industrial de laterita. Rev. Tecnol. Química 2008, 28, 22–33. [Google Scholar]
- Kaya, Ş.; Topkaya, Y.A. High pressure acid leaching of a refractory lateritic nickel ore. Miner. Eng. 2011, 24, 1188–1197. [Google Scholar] [CrossRef]
- Dak, M.; Verma, R.; Jaaffrey, S. Effect of temperatures and concentration on rheological properties of kesar. J. Food Eng. 2007, 28. [Google Scholar] [CrossRef]
- Ma, B.; Yang, W.; Yang, B.; Wang, C.; Chen, Y.; Zhang, Y. Pilot-scale plant study on the innovative nitric acid pressure leaching technology for laterite ores. Hydrometallurgy 2015, 155, 88–94. [Google Scholar] [CrossRef]
- Bienvenido, J. Modelo de un sistema de bomba, tanque y red. Volunt. Hidráulica 1973, 3, 11–32. [Google Scholar]
- Laurencio, A.H. Método para la Determinación de Parámetros Racionales de Transporte por Tuberías del Combustible Cubano Crudo Mejorado 650. Ph.D. Thesis, Ingeniería Mecánica, Instituto Superior Minero Metalúrgico de Moa, Moa, Cuba, 2012. [Google Scholar]
- León, A.; Percy, F. Ahorro de energía por control de velocidad en el sistema de bombeo de Guarapo. Centroazúcar 2000, 1, 27–38. [Google Scholar]
- Santos, F.; Martin, M. Modelos matemáticos para la determinación aproximada de la forma de la caracteristica de trabajo de una bomba centrífuga. Cent. Azucar. 1999, 1, 123–167. [Google Scholar]
- Turiño, I.M. Determinación aproximada de la característica de funcionamiento de una bomba centrifuga. Cent. Azúcar 1999, 3, 1–7. [Google Scholar]
- Almaguer, F. Composición de las pulpas limoníticas de la planta Pedro Sotto Alba” (Parte II). Período de Crisis de Sedimentación. Rev. Minería Geol. 1996, 13, 27–30. [Google Scholar]
- Rojas, A.; Beyris, P. Influencia de la composición mineralógica en la sedimentación del material limonítico de frentes de explotación de la industria “Pedro Sotto Alba”. Minería Geol. 1994, 11, 13–17. [Google Scholar]
- Colectivo, D.A. Manual de Operaciones Planta de Espesadores; Alba, E.P.S.: Caracas, Venecuela, 2010. [Google Scholar]
- Levenspiel, O. Engineering Flow and Heat Exchange; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Slatter, P.T. The rheological characterisation of sludges. IAW J. Water Sci. Technol. 1997, 36, 9–18. [Google Scholar] [CrossRef]
- Büyükakinci, E.; Topkaya, Y.A. Extraction of nickel from lateritic ores at atmospheric pressure with agitation leaching. Hydrometallurgy 2009, 97, 33–38. [Google Scholar] [CrossRef]
- Hernández, R.G. Modelación de los Parámetros Reológicos de las Pulpas Lateríticas y su Influencia en el Sistema de Bombeo. Ph.D. Thesis, Departamento de Ingeniería Eléctrica, Instituto Superior Minero Metarlúrgico de Moa, Moa, Cuba, 2017. [Google Scholar]
- Borland. Delphi, 7th ed.; Borland: Scotts Valley, CA, USA, 2002. [Google Scholar]
- Garcell, L. Flujo por Tuberías de Suspensiones Minerales no Newtonianas; Universidad de Oriente: Puerto La Cruz, Venezuela, 2001. [Google Scholar]
- Rabinovich, Z. Hidraúlica; Editorial Pueblo y Educación: La Habana, Cuba, 1987. [Google Scholar]
- Nekrasov, B. Hidráulica; Editorial Mir: Moscow, Rusia, 1990. [Google Scholar]
- Streeter, V.; Benjamin, E.; Bedford, K. Mecánica de Fluidos; Novena Edición; Mc Graw Hill: Madrid, Spain, 2000. [Google Scholar]
- Turiño, I.M. Procedimientos Metodológicos para la Determinación del Punto de Operación en Sistemas de Bombeo Mediante Modelos Matemáticos. Ph.D. Thesis, Mecánica, Universidad Central de las Villas, Santa Clara, Cuba, 1996. [Google Scholar]
- Hernández Ramírez, G.; Legrá-Lobaina, A.A.; Rojas Hidalgo, L.; Ramírez-Serrano, B.; Mariño Pérez, A. Modelos matemáticos de parámetros reológicos y su influencia en el sistema de bombeo de fluidos no newtonianos. Colomb. Química 2018, 47, 52–60. [Google Scholar] [CrossRef]
- Pérez-García, L.; Hernández-Ramírez, G.; Laffita, Q.R.; Garcell-Puyáns, L.R.; Legrá-Lobaina, A.A. Influencia del comportamiento reológico de las pulpas lateríticas en la eficiencia de bombeo. Tecnol. Química 2021, 42, 619. [Google Scholar]
- Pérez García, C.L.; Garcell Puyáns, C.L.R.; Ramírez, G.H. Modelo granulométrico y de viscosidad relativa de pulpa limonítica del proceso de lixiviación ácida. Rev. Tecnol. Química 2020, 40, 356–374. [Google Scholar]
- Hernández-Ramírez, G.; León-Segovia, M.Á.; Salazar, E.; Beltran-Reyna, R.; Pino-Tarragó, J.C. Mathematical modeling of the load correction coefficient of the lateritic of hydromixtures pumping. Dyna 2019, 86, 19–27. [Google Scholar] [CrossRef]
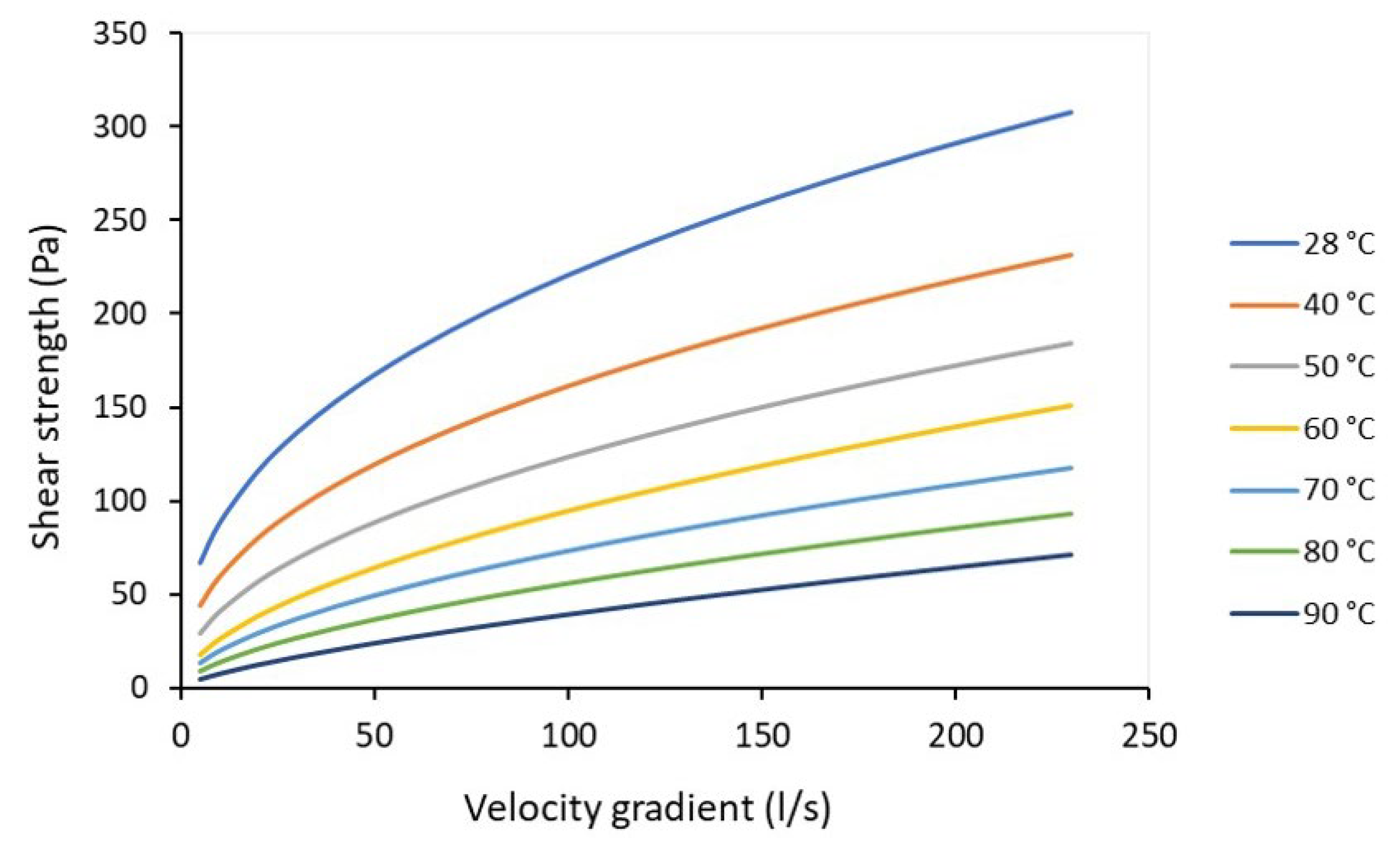
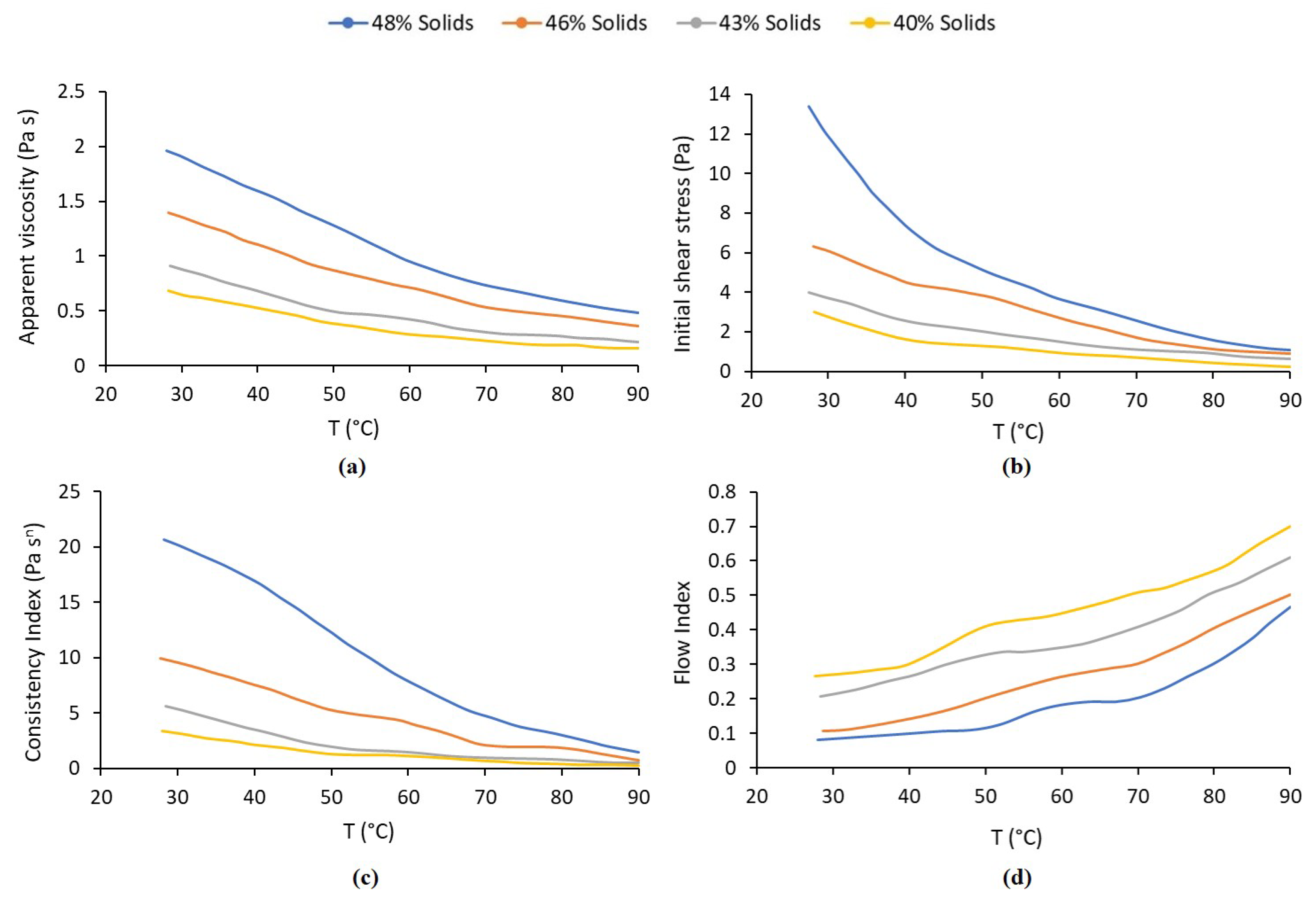
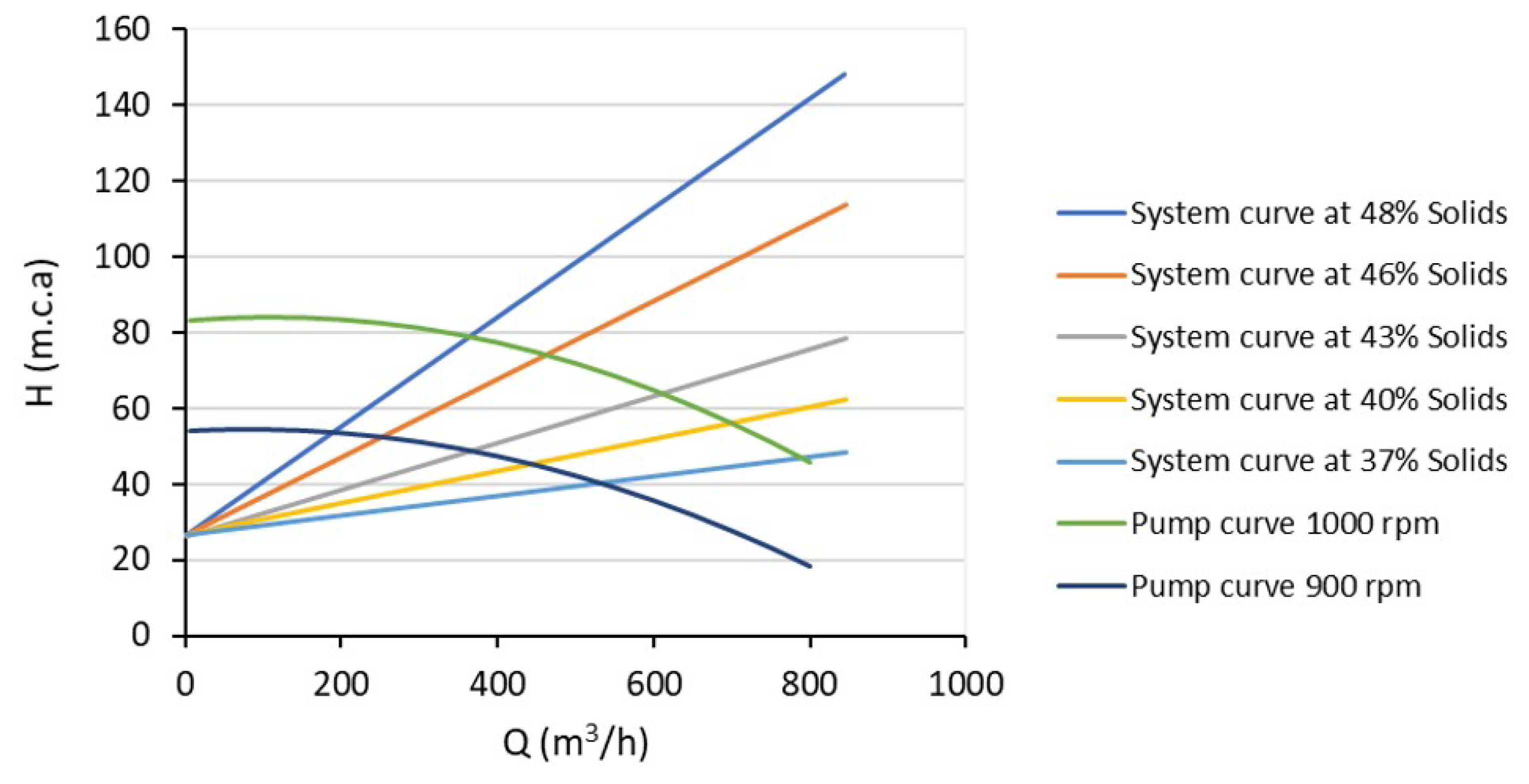
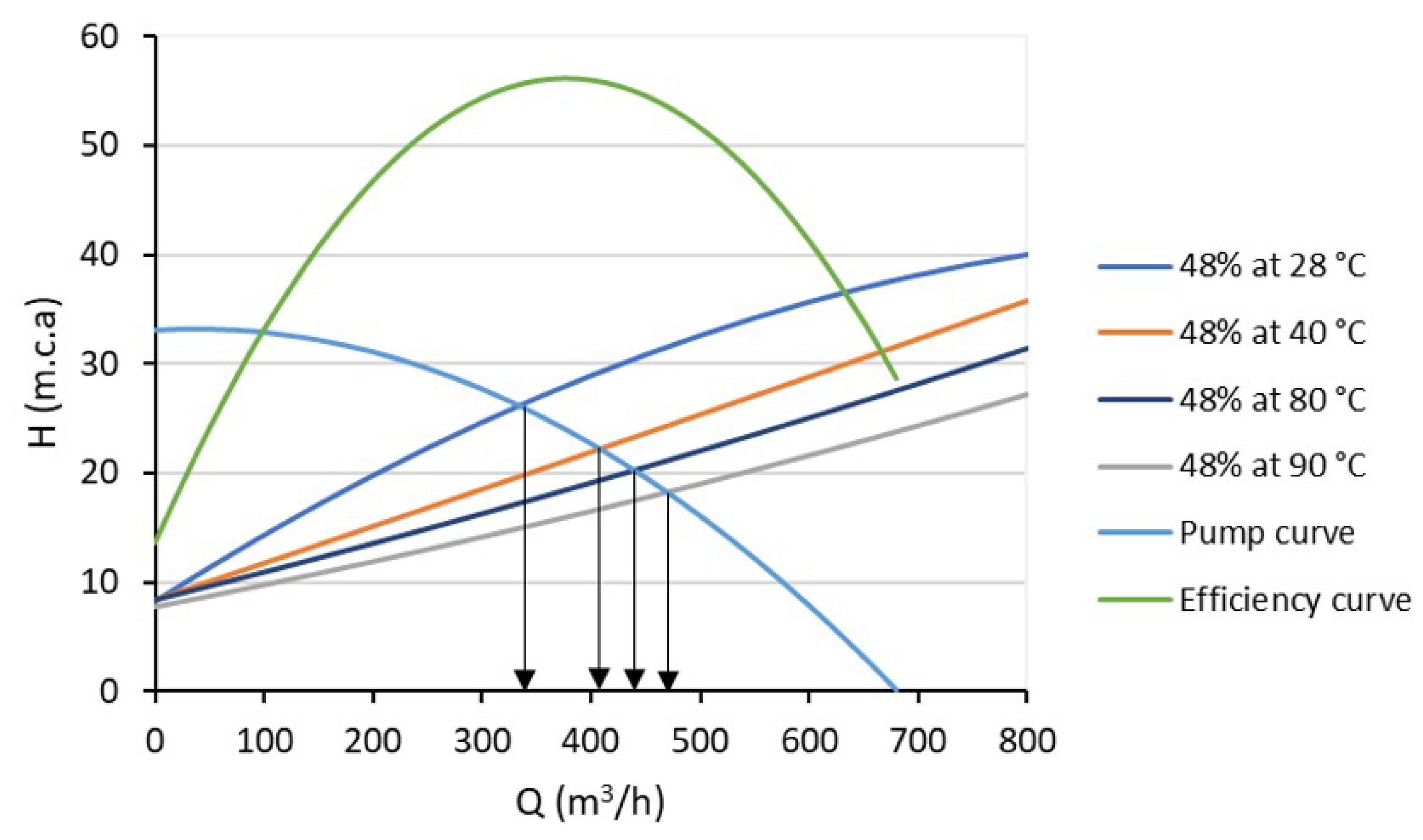
| Statistical Analysis | Raw Pulp | Preheated Pulp | ||
|---|---|---|---|---|
| Value | % | Value | % | |
| Minimum value | 36.97 | - | 34.44 | - |
| Maximum value | 46.85 | - | 45.33 | - |
| Average value | 43.17 | - | 40.90 | - |
| Standard deviation | 1.62 | - | 1.646 | - |
| Coefficient of variation | 3.76 | - | 4.02 | - |
| Greater than 45 | 99.0 | 12.0 | 4.0 | 0.5 |
| Less than 40 | 383.0 | 47.9 | 238.0 | 29.8 |
| Between 40 and 45 | 421.0 | 40.1 | 559.0 | 69.7 |
| Total | 100 | 100 | ||
| Statistical Analysis | Value °C | % |
|---|---|---|
| Minimum value | 75.48 | - |
| Maximum value | 94.07 | - |
| Average value | 90.02 | - |
| Standard deviation | 1.88 | - |
| Coefficient of variation | 2.10 | - |
| Greater than 90 °C | 434 | 54.32 |
| Less than 82 °C | 8 | |
| Between 82 and 90 °C | 357 | 44.68 |
| Total | 100 |
| Sampling Points | Composite Samples | Solids Concentration, % | Mineral Number |
|---|---|---|---|
| Point 1. Discharge Thickener A | MC1 | 44.70 | 3.97 |
| MC5 | 45.41 | 9.74 | |
| MC9 | 44.11 | 9.16 | |
| Point 2. Discharge Thickener D | MC2 | 39.34 | 9.07 |
| MC6 | 37.64 | 9.65 | |
| MC10 | 37.23 | 9.23 | |
| Point 3. 508 mm transfer line | MC3 | 43.56 | 9.75 |
| MC7 | 43.28 | 9.41 | |
| MC11 | 42.54 | 9.48 | |
| Point 4. At the suction of the volumetric pump | MC4 | 40.28 | 12.19 |
| MC8 | 41.17 | 9.21 | |
| MC12 | 40.78 | 9.06 |
| Components | Content (%) | Components | Content (%) |
|---|---|---|---|
| Nickel (Ni) | 1.20–1.33 | Copper (Cu) | 0.02 |
| Cobalt (Co) | 0.12–0.13 | Aluminum (Al) | 4.0–4.9 |
| Iron (Fe) | 47.5 | Zinc (Zn) | 0.040 |
| Magnesium (Mg) | 0.4–0.8 | Chromium (Cr) | 1.5–2.3 |
| Manganese (Mn) | 0.65–1.0 | Silica (SiO2) | 3.5–4.5 |
| Elements (%) | Samples Composites | |||
|---|---|---|---|---|
| MC1 | MC6 | MC11 | MC4 | |
| Ni | 1.21 | 1.3 | 1.22 | 1.21 |
| Co | 0.11 | 0.121 | 0.115 | 0.112 |
| Fe | 42.9 | 43.9 | 43.2 | 43.1 |
| Mg | 1.77 | 1.94 | 1.72 | 1.61 |
| Al | 4.43 | 4.32 | 4.42 | 4.44 |
| SiO2 | 6.61 | 5.21 | 6.39 | 6.52 |
| Cr | 1.55 | 1.68 | 1.56 | 1.57 |
| Mn | 0.75 | 0.79 | 0.74 | 0.73 |
| Cu | 0.029 | 0.03 | 0.028 | 0.028 |
| Zn | 0.036 | 0.036 | 0.036 | 0.036 |
| Ni + Co | 1.32 | 1.421 | 1.335 | 1.322 |
| Nr min | 9.74 | 7.85 | 9.48 | 9.65 |
| Shows 1 TK-A | Herschel Bulkley Pseudoplastic Mathematical Model: | |||||||
|---|---|---|---|---|---|---|---|---|
| % Solid | Parameters | T = 28 °C | T = 40 °C | T = 50 °C | T = 60 °C | T = 70 °C | T = 80 °C | T = 90 °C |
| 37 | 2.978 | 1.648 | 1.283 | 0.969 | 0.68 | 0.421 | 0.207 | |
| K | 3.438 | 2.211 | 1.406 | 1.144 | 0.64 | 0.436 | 0.328 | |
| n | 0.63 | 0.65 | 0.7 | 0.72 | 0.75 | 0.78 | 0.85 | |
| 40 | 4.006 | 2.586 | 1.994 | 1.453 | 1.099 | 0.87 | 0.567 | |
| K | 5.721 | 3.528 | 1.934 | 1.45 | 0.989 | 0.846 | 0.542 | |
| n | 0.6 | 0.63 | 0.66 | 0.67 | 0.7 | 0.75 | 0.8 | |
| 43 | 6.357 | 4.622 | 3.842 | 2.669 | 1.681 | 1.092 | 0.833 | |
| K | 10.023 | 7.651 | 4.258 | 4.278 | 2.407 | 1.844 | 0.654 | |
| n | 0.55 | 0.57 | 0.6 | 0.63 | 0.65 | 0.7 | 0.75 | |
| 46 | 12.95 | 7.419 | 5.166 | 3.755 | 2.549 | 1.497 | 0.963 | |
| K | 20.611 | 16.81 | 12.146 | 7.812 | 4.696 | 2.932 | 1.345 | |
| n | 0.54 | 0.55 | 0.56 | 0.59 | 0.6 | 0.65 | 0.73 | |
| 48 | 16.109 | 9.503 | 7.752 | 7.412 | 7.3081 | 2.7717 | 1.887 | |
| K | 28.943 | 17.952 | 13.075 | 8.939 | 5.637 | 3.752 | 2.893 | |
| n | 0.5 | 0.52 | 0.53 | 0.54 | 0.56 | 0.6 | 0.65 | |
| Solids Concentration, % (p/p) | Reynolds Number Downlod | Fanning’s Friction Factor | Darcy’s Friction Factor | Net Required Height, m.c.a | Pump Head, m.c.a. | Pump Power, kW |
|---|---|---|---|---|---|---|
| 37 | 207.62 | 0.077 | 0.308 | 8.59 | 38.60 | 114.33 |
| 40 | 131.00 | 0.122 | 0.488 | 13.17 | 43.17 | 134.26 |
| 43 | 78.71 | 0.203 | 0.813 | 20.77 | 50.78 | 166.22 |
| 46 | 40.40 | 0.396 | 1.584 | 40.06 | 70.06 | 242.10 |
| 48 | 29.879 | 0.535 | 2.141 | 52.09 | 82.10 | 294.58 |
| Solids Concentration % (p/p) | Temperature, °C | Reynolds Number Downlod | Fanning’s Friction Factor | Darcy’s Friction Factor | Net Required Height, m.c.a | Pump Head, m.c.a | Pump Power, kW |
|---|---|---|---|---|---|---|---|
| 48 | 28 | 29.87 | 0.535 | 2.141 | 52.09 | 82.10 | 294.58 |
| 40 | 48.17 | 0.332 | 1.328 | 32.94 | 62.94 | 225.85 | |
| 70 | 153.41 | 0.104 | 0.417 | 10.77 | 40.77 | 146.30 | |
| 80 | 230.48 | 0.069 | 0.277 | 7.48 | 37.49 | 134.52 | |
| 90 | 298.92 | 0.053 | 0.214 | 6.11 | 36.12 | 129.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ramírez, G.; Bernardo-Sánchez, A.; Legrá-Lobaina, A.A.; Álvarez de Prado, L.; Martínez-Rojas, R.; Pérez-García, L.; Garcell-Puyáns, L.; Fernández-Ordás, J.; Menéndez, J. Temperature: An Influencing Factor on the Rheological and Energetic Parameters of Acid Pressure Technology Operations. Minerals 2025, 15, 86. https://doi.org/10.3390/min15010086
Hernández-Ramírez G, Bernardo-Sánchez A, Legrá-Lobaina AA, Álvarez de Prado L, Martínez-Rojas R, Pérez-García L, Garcell-Puyáns L, Fernández-Ordás J, Menéndez J. Temperature: An Influencing Factor on the Rheological and Energetic Parameters of Acid Pressure Technology Operations. Minerals. 2025; 15(1):86. https://doi.org/10.3390/min15010086
Chicago/Turabian StyleHernández-Ramírez, Gabriel, Antonio Bernardo-Sánchez, Aristides Alejandro Legrá-Lobaina, Laura Álvarez de Prado, Rodney Martínez-Rojas, Liudmila Pérez-García, Leonel Garcell-Puyáns, Jose Fernández-Ordás, and Javier Menéndez. 2025. "Temperature: An Influencing Factor on the Rheological and Energetic Parameters of Acid Pressure Technology Operations" Minerals 15, no. 1: 86. https://doi.org/10.3390/min15010086
APA StyleHernández-Ramírez, G., Bernardo-Sánchez, A., Legrá-Lobaina, A. A., Álvarez de Prado, L., Martínez-Rojas, R., Pérez-García, L., Garcell-Puyáns, L., Fernández-Ordás, J., & Menéndez, J. (2025). Temperature: An Influencing Factor on the Rheological and Energetic Parameters of Acid Pressure Technology Operations. Minerals, 15(1), 86. https://doi.org/10.3390/min15010086








