Chemistry and Fe Isotopes of Magnetites in the Orbicular Bodies in the Tanling Diorite and Implications for the Skarn Iron Mineralization in the North China Craton
Abstract
1. Introduction
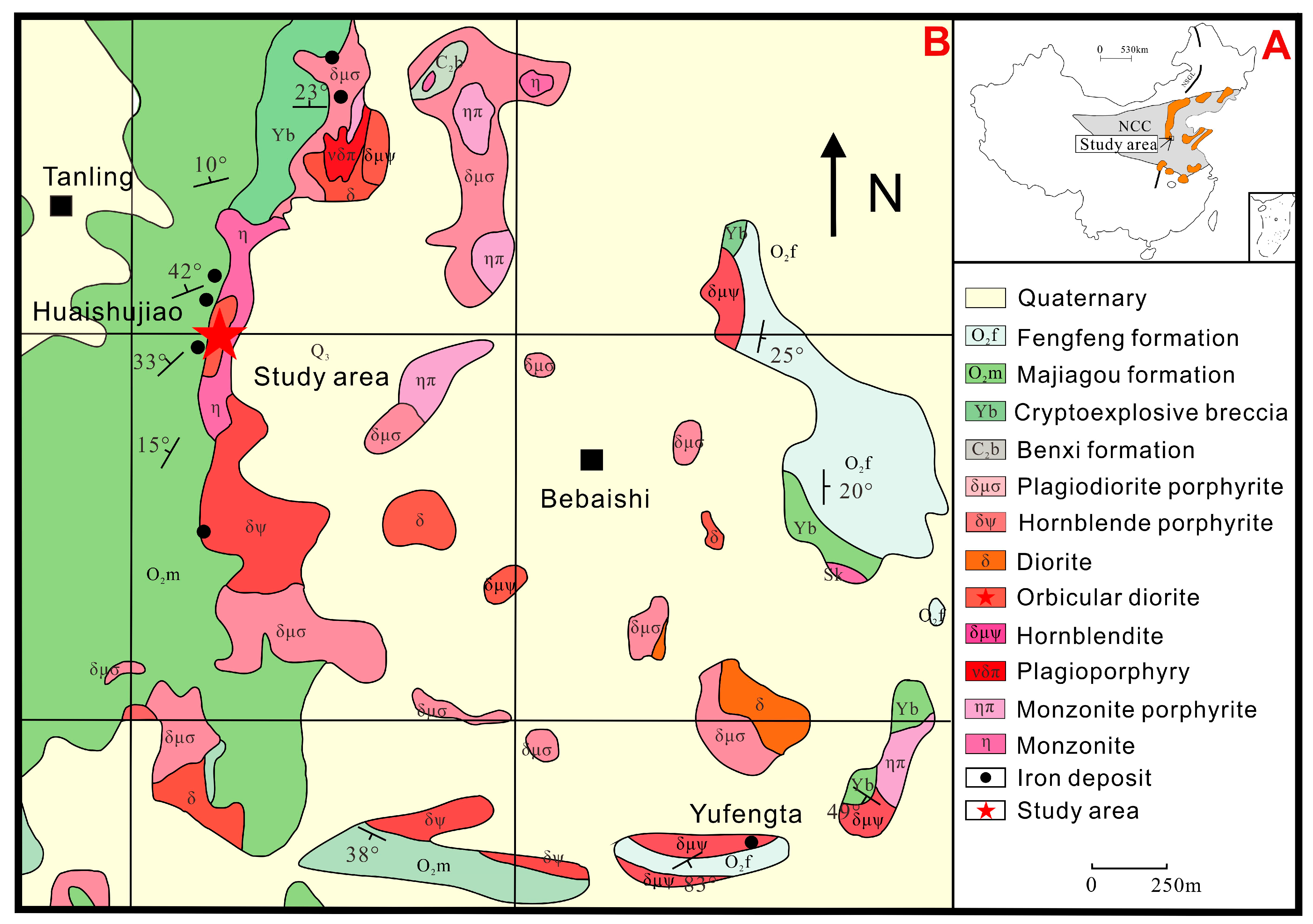
2. Regional Geology
3. Petrographic Characteristics
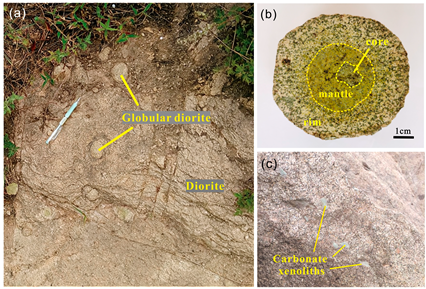
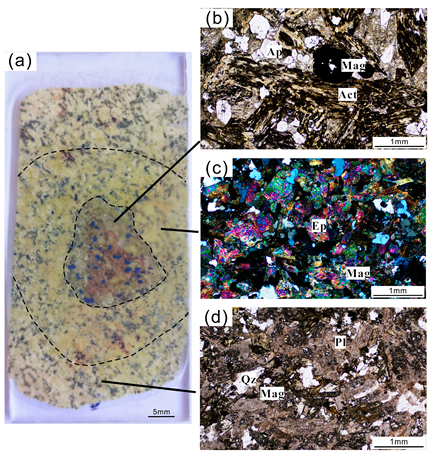
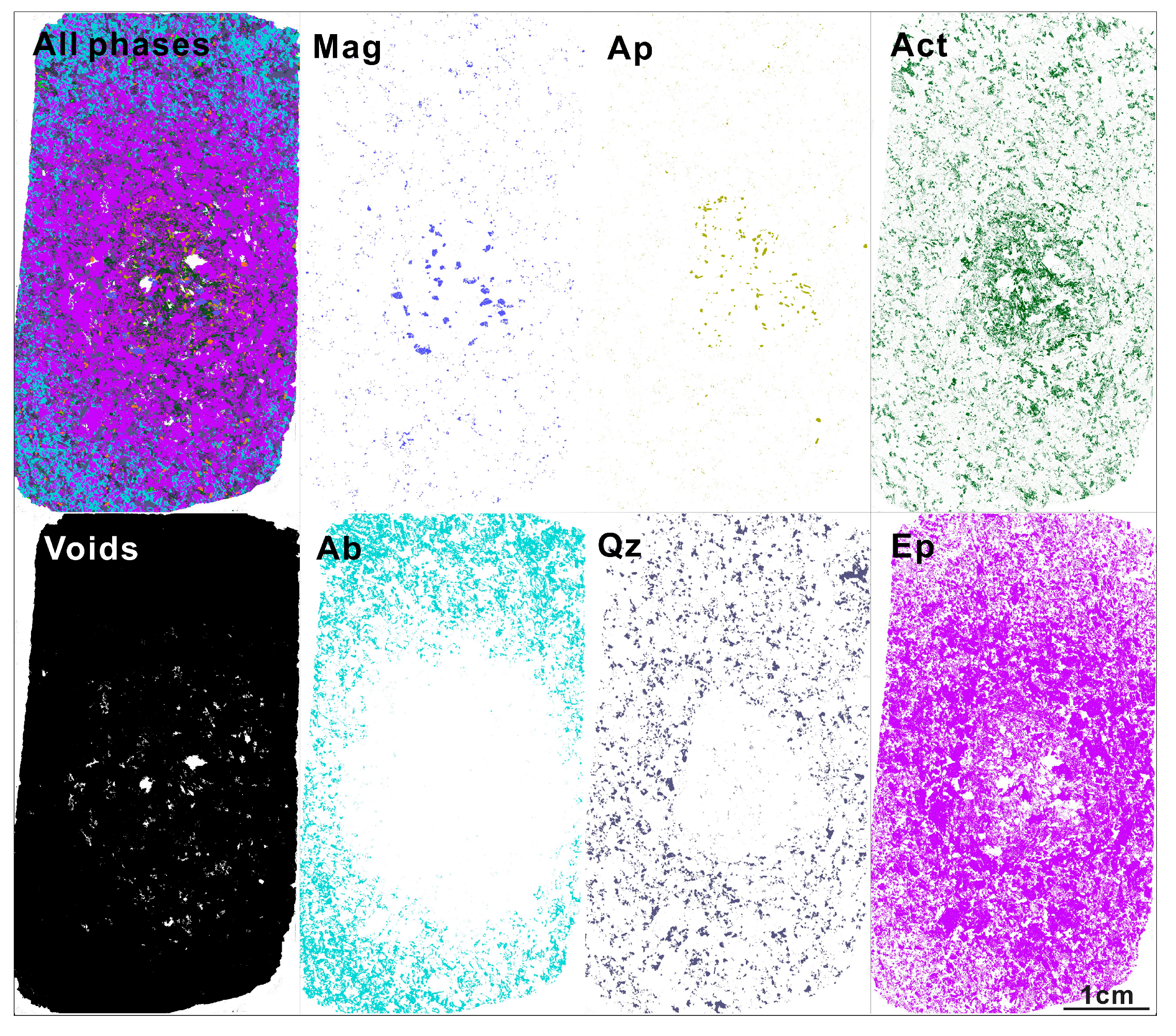
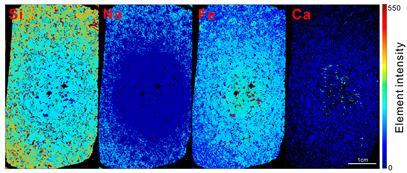
3.1. Orbicular Diorite
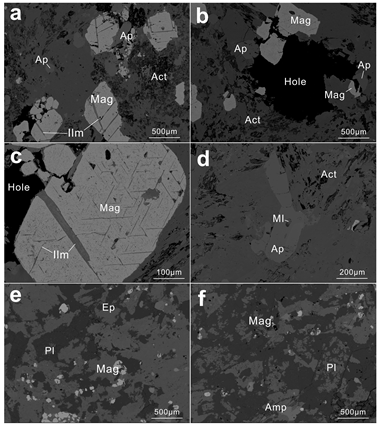
3.2. Characteristics of Magnetite in Each Part of the Orbicular Diorite
4. Methods
5. Results
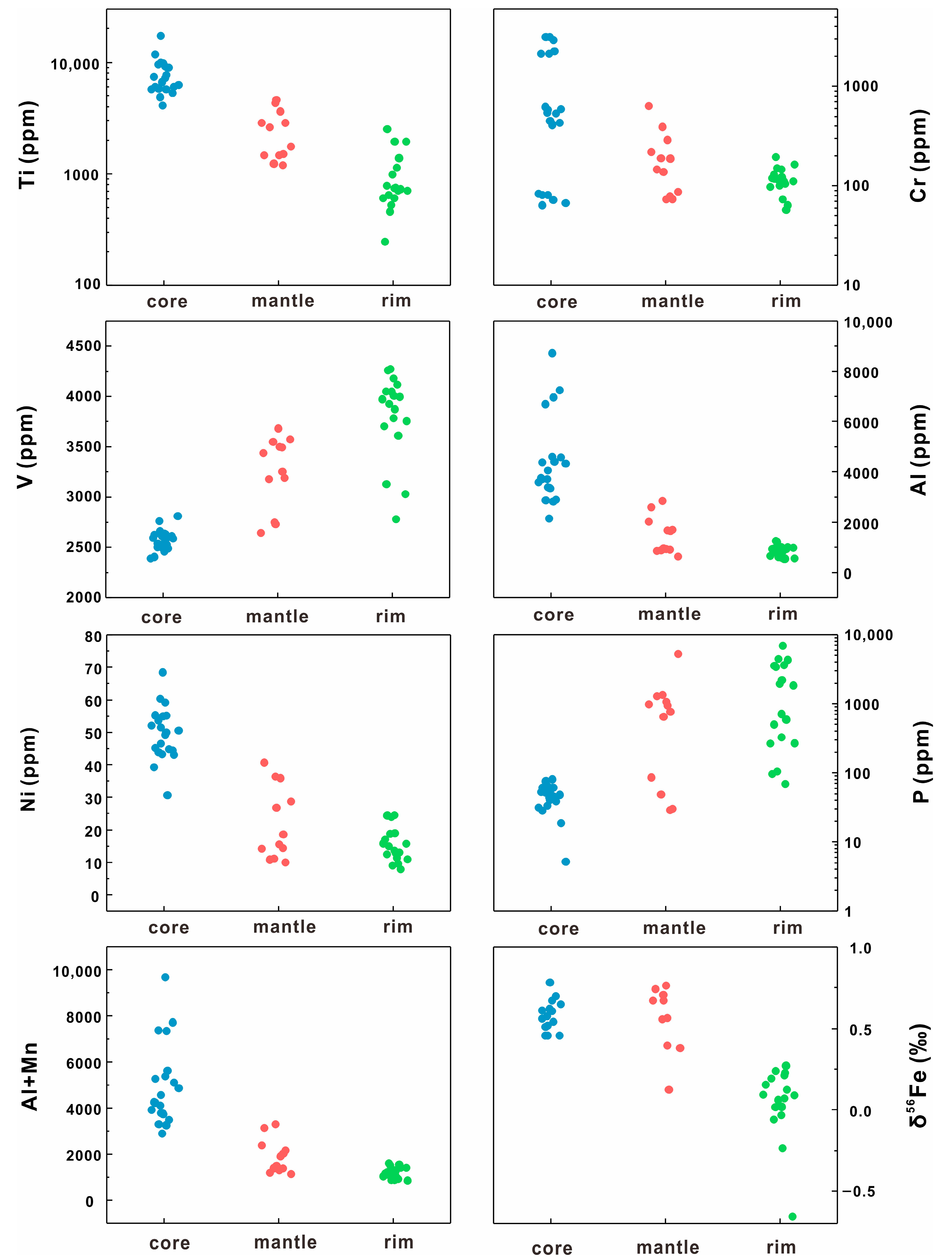
5.1. Major Elements of Magnetite
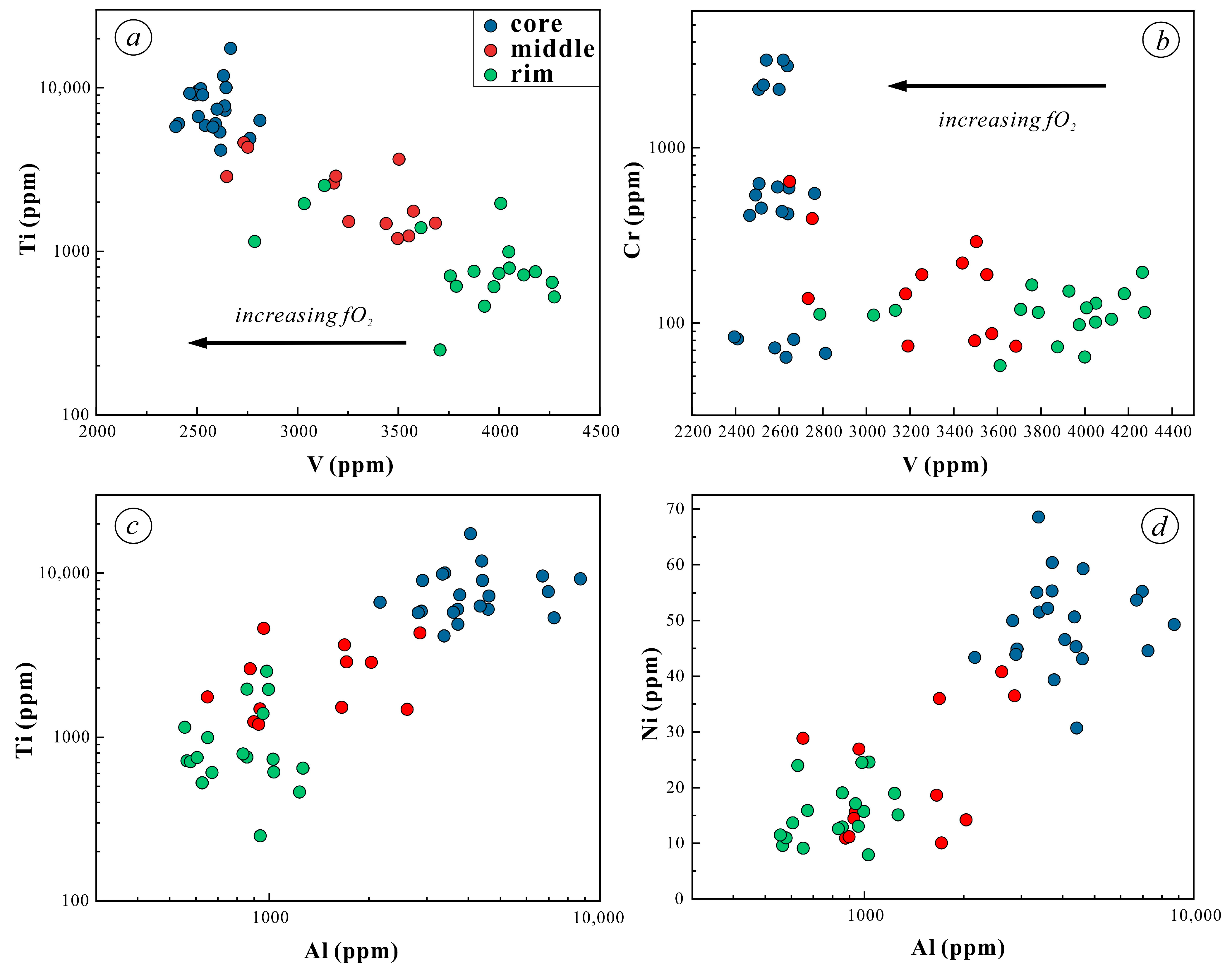
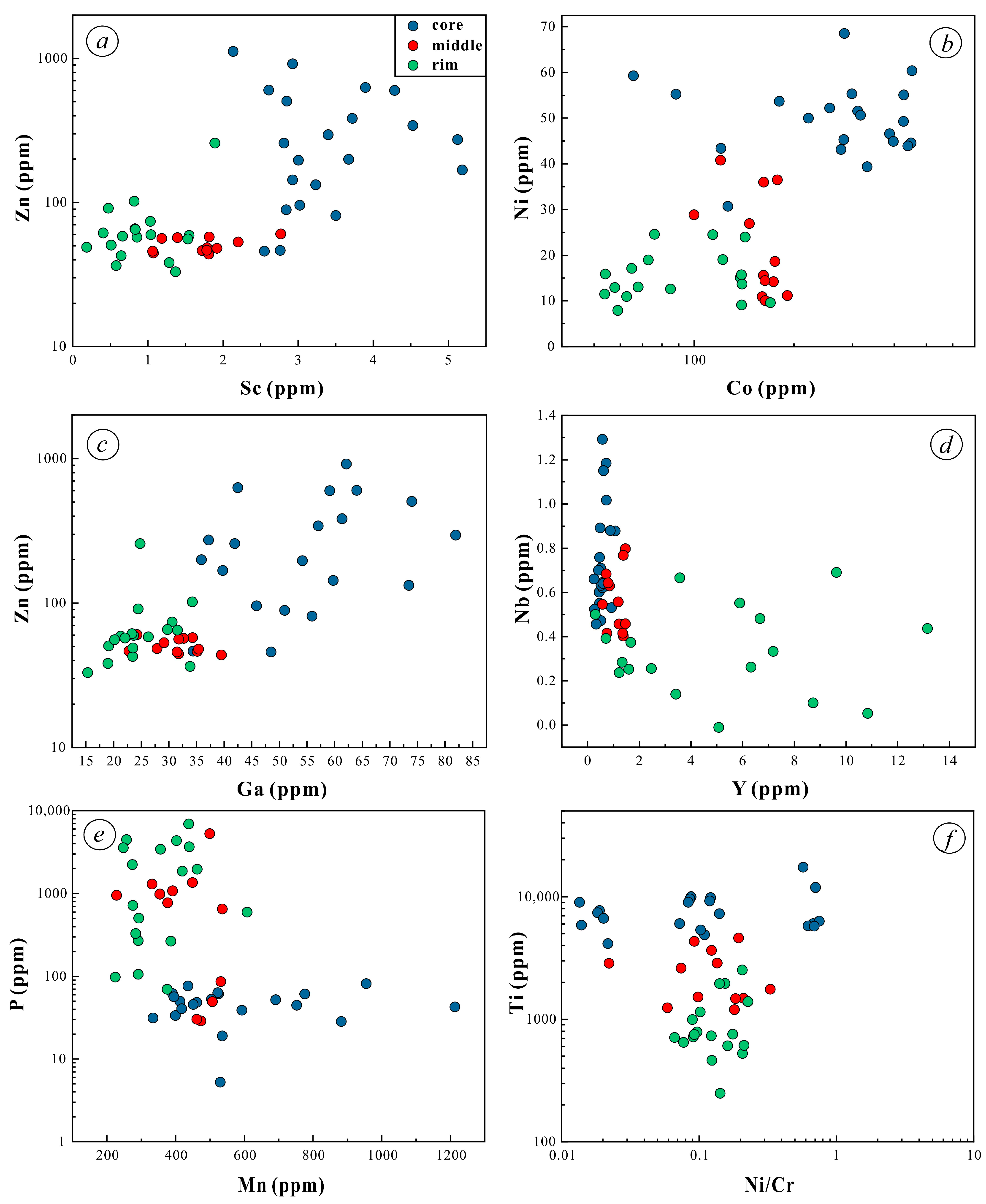
5.2. Trace Elements of Magnetite
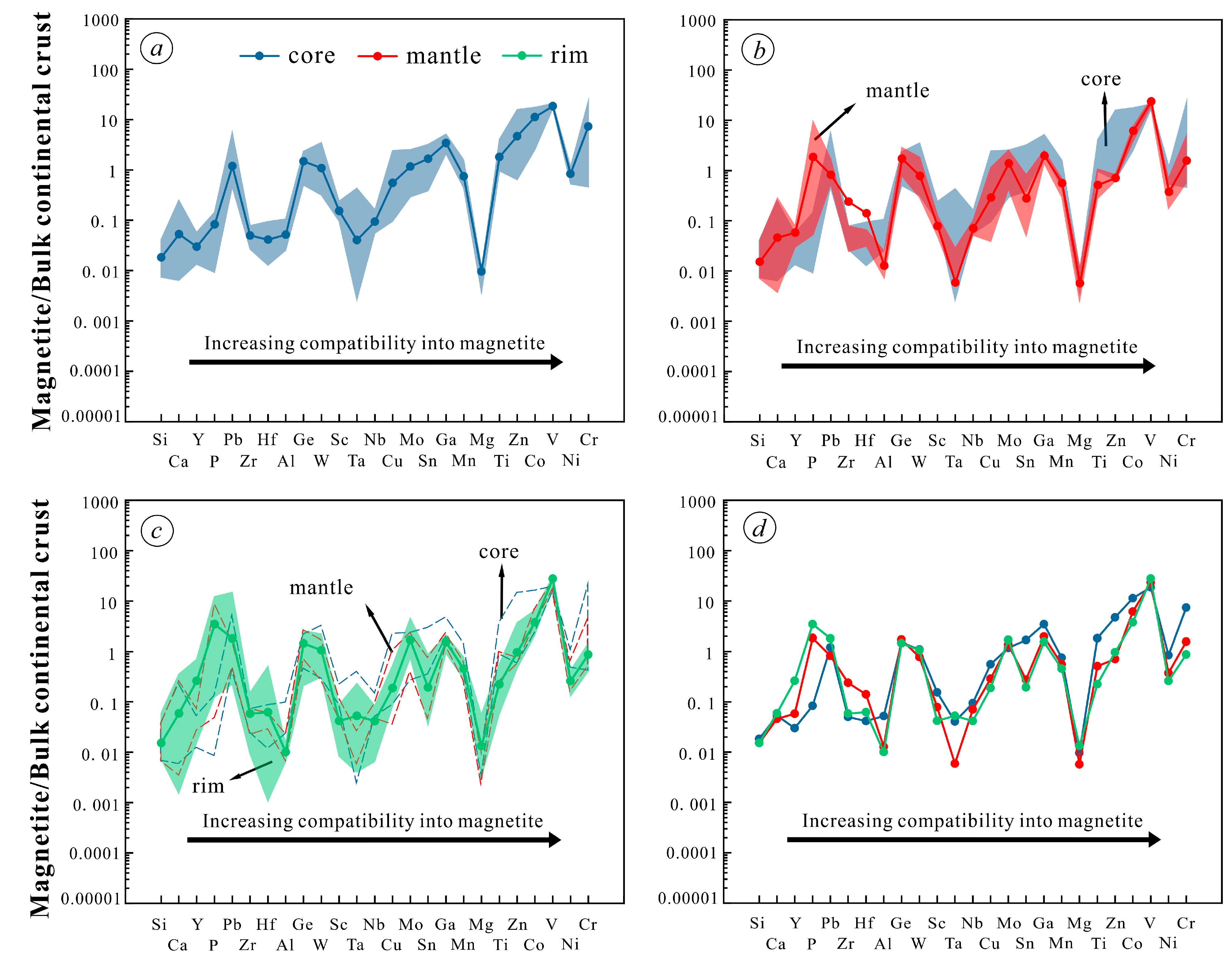
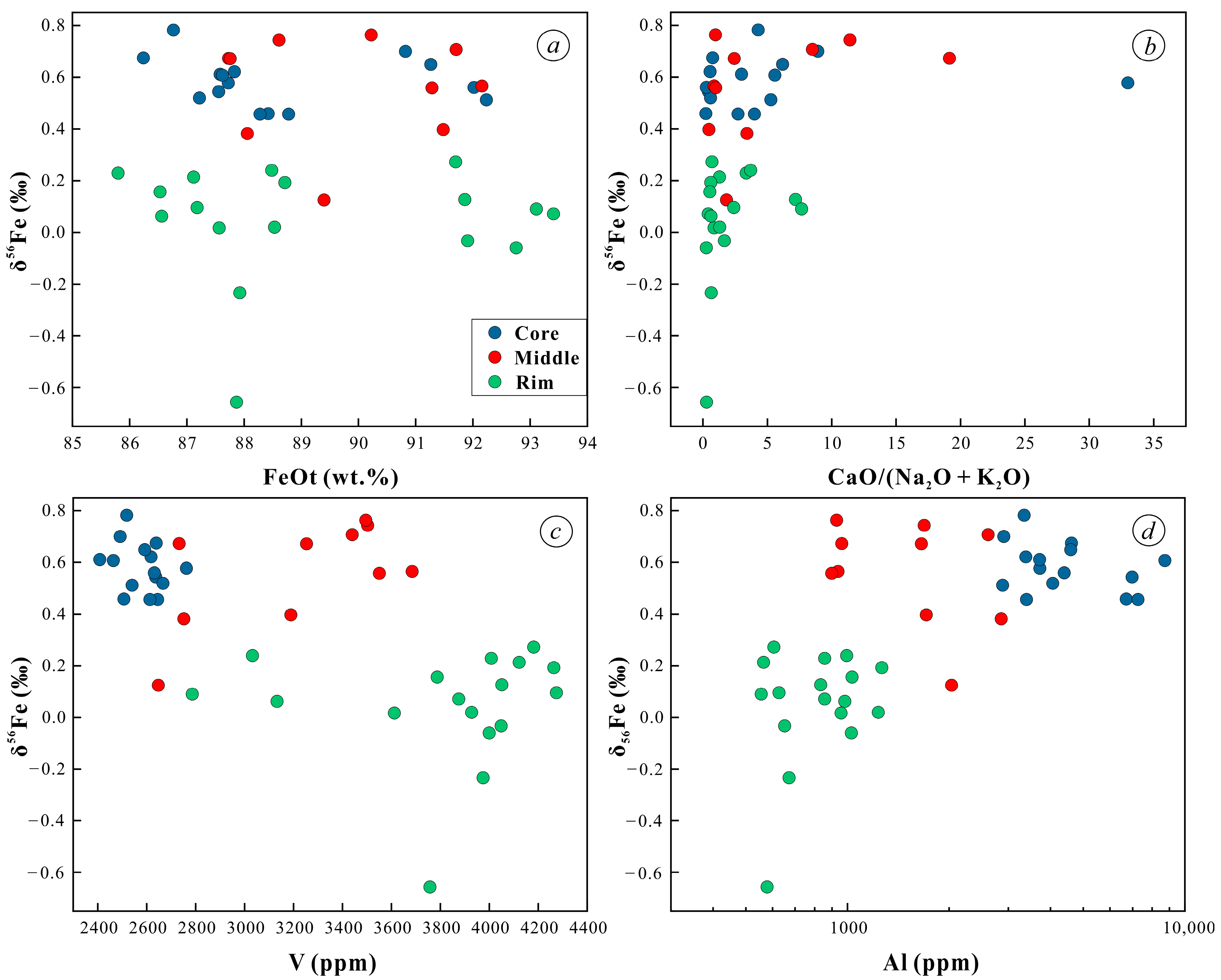
5.3. In Situ Fe Isotopes
- The δ56Fe values of magnetite in the orbicular diorite tend to decrease from the core to the rim (Figure 12).

6. Discussion
6.1. Controls on Compositions of Magnetite
6.1.1. Magma Composition and Coexisting Minerals
6.1.2. Oxygen Fugacity
6.2. Reasons for High δ56Fe in Magnetites in the Cores of Orbicules
6.2.1. Crystallization of Light δ56Fe Silicate Minerals
6.2.2. Hydrothermal Alteration
6.2.3. High Oxygen Fugacity
6.2.4. Magma Differentiation/Immiscibility
6.2.5. Exsolution Lamellae of Ilmenite
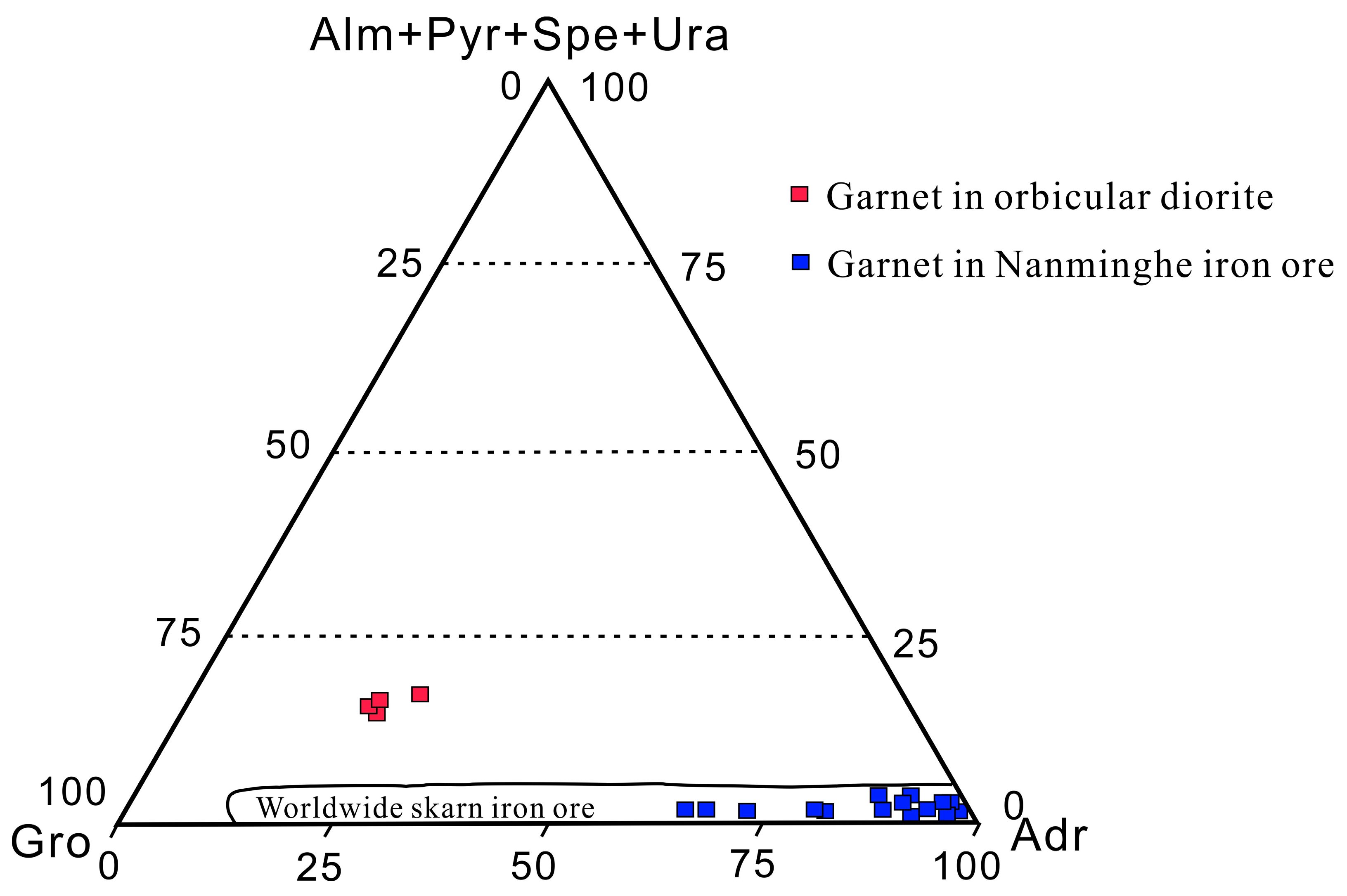
6.3. Origin of Skarn Iron Ore
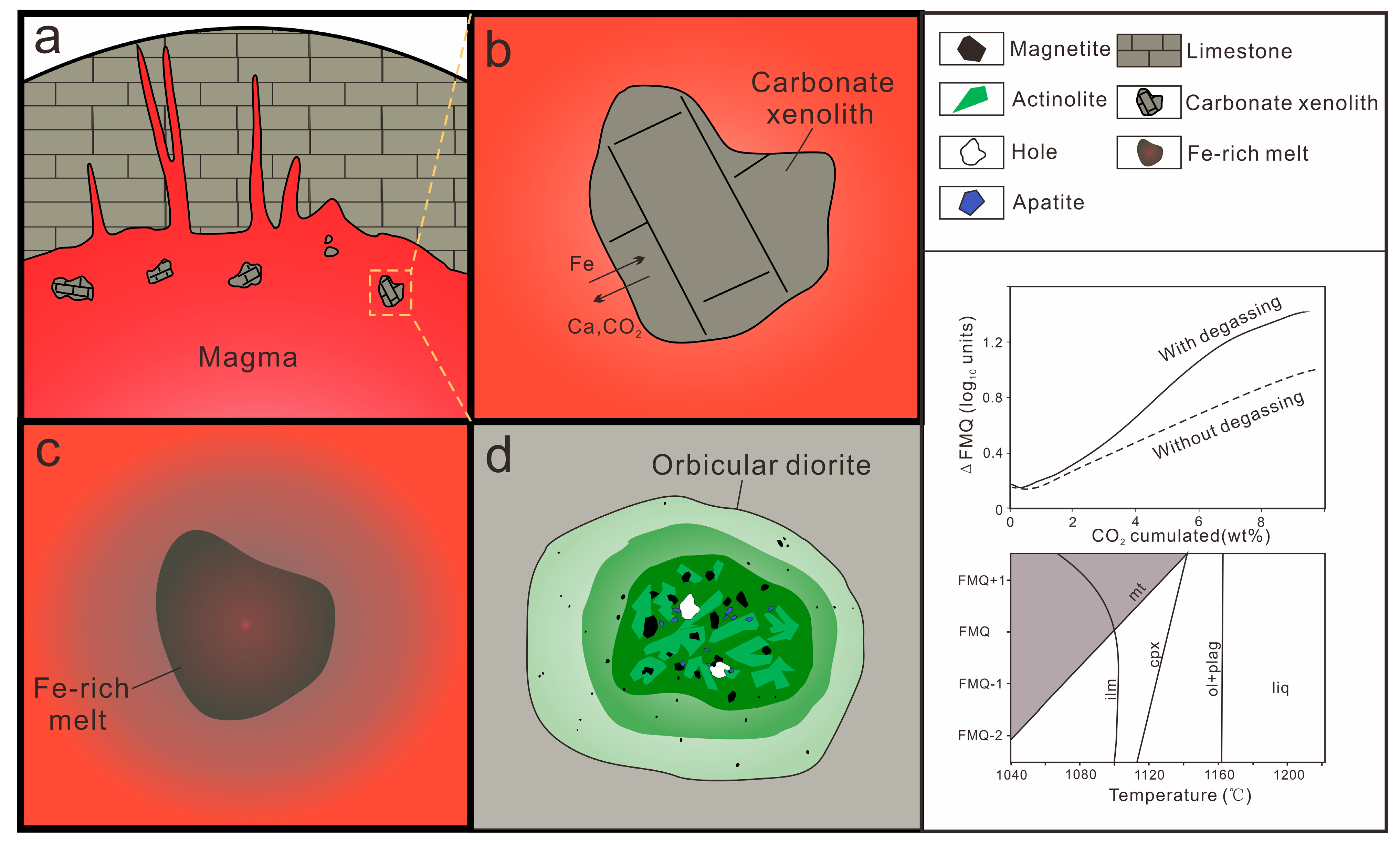
6.3.1. Melting of Carbonate Xenoliths by Magma
6.3.2. Formation Mechanism of Fe-Rich Melt
7. Conclusions
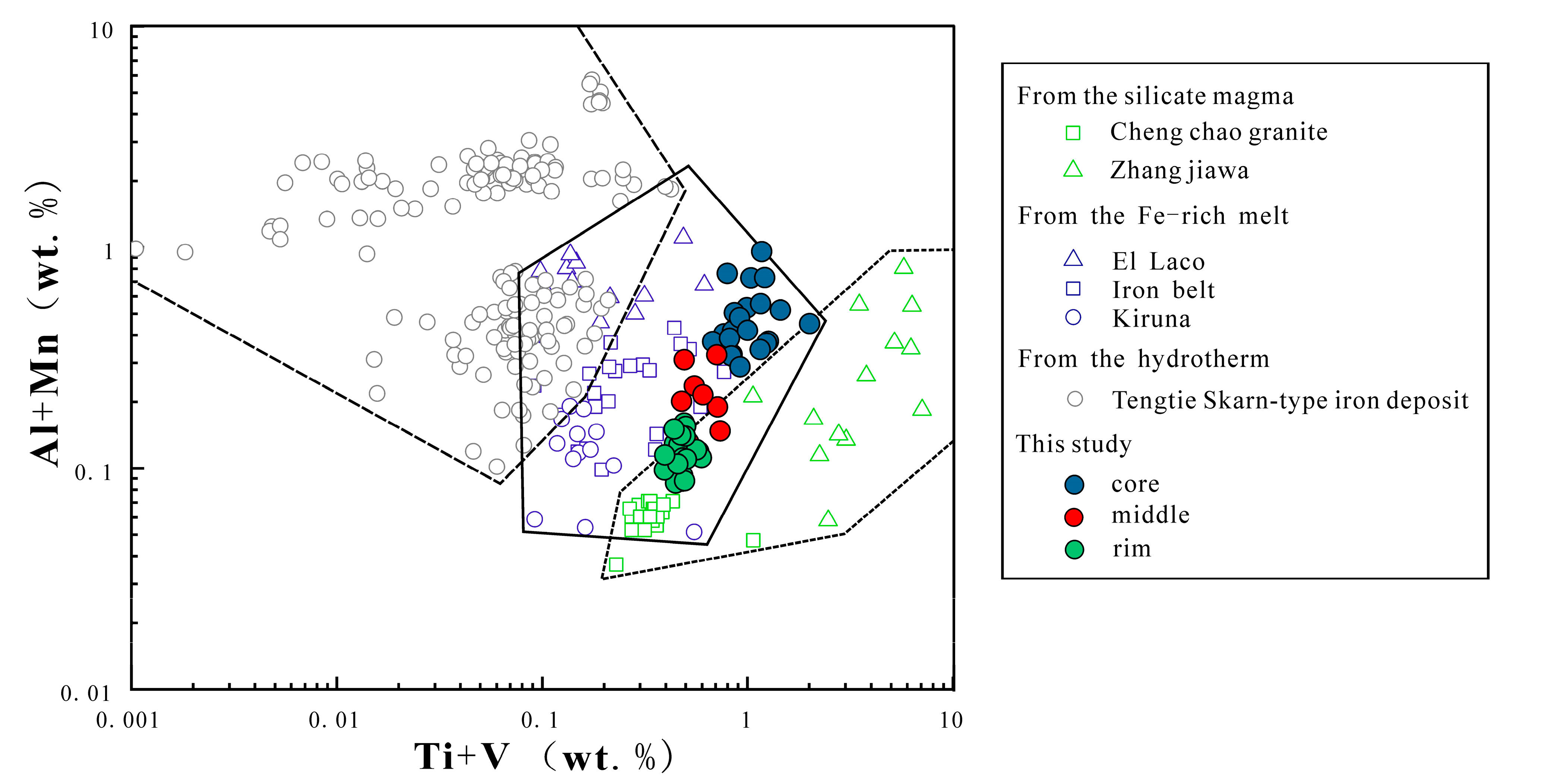
- Magnetite composition varies significantly across the orbicule: rim magnetite has a small particle size (about 200 μm) and is associated mainly with plagioclase and hornblende, indicating that it crystallizes from normal diorite magma. The magnetite in the core has a large particle size (>1000 μm), and is associated with apatite and actinolite, with apatite inclusions and a large number of vesicles in the core. The magnetites in the cores of orbicules have higher Ti, Al, Ni, Cr, Sc, Zn, Co, Ga, and Nb than those in the rim. The multi-peak distribution of Cr and the (Al + Mn) vs. (Ti + V) diagram of the magnetites in the cores of orbicules indicate that they crystallized from iron-rich melts.
- The δ56Fe value of the core magnetite (0.46‰–0.78‰) is much higher than that of the mantle and rim magnetite in orbicules. The δ56Fe values of magnetite in different parts have no correlation with Fetotal and CaO/(Na2O + K2O), but they increase as the V content of magnetite gradually decreases. This large fractionation of iron isotopes is not caused by mineral fractionation, crystallization, or fluid alteration, but may be driven by liquid immiscibility that forms iron-rich melts under high oxygen fugacity.
- The iron enrichment mechanism of the Wuan orbicular diorite is as follows: During upward intrusion of magma, hot magma reacts with the carbonate xenoliths [(Ca, Mg) CO3] to generate abundant CO2, which greatly increases the oxygen fugacity of the magmatic system; Under the action of CO2 and other volatile components, liquid immiscibility occurs in the magma chamber, and the Fe-rich oxide melt is formed by melting away. Iron oxides (Fe3O4/Fe2O3) will crystallize close to the liquidus due to high oxygen fugacity.
- The aforementioned characteristics of magnetite in the Tanling orbicular diorite (Wuan, Hebei Province) indicate that diorite magma reacts with carbonate xenoliths to form “Fe-rich melts”; furthermore, skarn iron ore deposits are probably formed by the reaction of intermediate-to-basic magma with carbonate rocks to generate such “Fe-rich melts”. A possible reaction is as follows:
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Edwards, R.; Atkinson, K. Ore Deposit Geology and Its Influence on Mineral Exploration; Springer: Dordrecht, The Netherlands, 1986; pp. 143–174. [Google Scholar]
- Hall, D.L.; Cohen, L.H.; Schiffman, P. Hydrothermal alteration associated with the iron hat iron skarn deposit, eastern Mojave Desert, San Bernardino County, California. Econ. Geol. 1988, 83, 568–587. [Google Scholar] [CrossRef]
- Zheng, J.M.; Mao, J.W.; Chen, M.H.; Li, G.D.; Ban, C.Y. Geological characteristics and metallogenic model of skarn iron deposits in the Handan- Xingtai area, southern Hebei, China. Geol. Bull. China 2007, 26, 150–154. (In Chinese) [Google Scholar]
- Shi, Z.L.; Jin, Z.M.; Xiong, P.F.; Wang, D.Y.; Huang, K.K. A Preliminary Discussion on Problems of ore-magma mineralization of “Daye-Type” iron deposits at Tieshan, Hubei. Earth Sci. 1981, 2, 145–154. (In Chinese) [Google Scholar]
- Liu, P.P.; Zhou, M.F.; Chen, W.T.; Gao, J.F.; Huang, X.W. In-situ LA-ICP-MS trace elemental analyses of magnetite: Fe-Ti-(V) oxide-bearing mafic-ultramafic layered intrusions of the Emeishan Large Igneous Province, SW China. Ore Geol. Rev. 2015, 65, 853–871. [Google Scholar] [CrossRef]
- Li, Y.H.; Xie, G.Q.; Duan, C.; Han, D.; Wang, C.Y. Effect of Sulfate Evaporate Salt Layer over the Formation of Skarn-Type Iron Ore. Acta Geol. Sin. 2013, 87, 1324–1334. (In Chinese) [Google Scholar]
- Wang, W.; Su, S.; Santosh, M.; Lu, X.; Hou, X.; Li, X. Formation mechanism of skarn iron deposit: Evidence from Fe-rich enclave in porphyritic monzonite. Ore Geol. Rev. 2024, 165, 105902. [Google Scholar] [CrossRef]
- Maia, M.; Barrulas, P.; Nogueira, P.; Mirao, J.; Noronha, F. In-situ LA-ICP-MS trace element analysis of magnetite as a vector towards mineral exploration: A comparative case study of Fe-skarn deposits from SW Iberia (Ossa-Morena Zone). J. Geochem. Explor. 2022, 234, 106941. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, M.; Gao, J.; Hu, R. Geochemistry of magnetite from Proterozoic Fe-Cu deposits in the Kangdian metallogenic province, SW China. Miner. Depos. 2015, 50, 795–809. [Google Scholar]
- Huang, X.W.; Gao, J.F.; Qi, L.; Zhou, M.F. In-situ LA-ICP-MS trace elemental analyses of magnetite and Re-Os dating of pyrite: The Tianhu hydrothermally remobilized sedimentary Fe deposit, NW China. Ore Geol. Rev. 2015, 65, 900–916. [Google Scholar] [CrossRef]
- Stergiou, C.L.; Melfos, V.; Papadopoulou, L.; Ladas, A.D.; Aidona, E. Volcanic Rocks from Western Limnos Island, Greece: Petrography, Magnetite Geochemistry, and Magnetic Susceptibility Constraints. Minerals 2025, 15, 673. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.K.; Mao, J.W.; Li, Z.H.; Cheng, Y.B. Iron isotope fractionation during skarn-type metallogeny: A case study of Xinqiao Cu-S-Fe-Au deposit in the Middle-Lower Yangtze valley. Ore Geol. Rev. 2011, 43, 194–202. [Google Scholar] [CrossRef]
- Anbar, A.D. Iron stable isotopes: Beyond biosignatures. Earth Planet. Sci. Lett. 2004, 217, 223–236. [Google Scholar] [CrossRef]
- Dziony, W.; Horn, I.; Lattard, D. In-situ Fe isotope ratio determination in Fe–Ti oxides and sulfides from drilled gabbros and basalt from the IODP Hole 1256D in the eastern equatorial Pacific. Chem. Geol. 2014, 363, 101–113. [Google Scholar]
- Bilenker, L.D.; VanTongeren, J.A.; Lundstrom, C.C.; Simon, A.C. Iron isotopic evolution during fractional crystallization of the uppermost Bushveld Complex layered mafic intrusion. Geochem. Geophys. Geosyst. 2017, 18, 956–972. [Google Scholar]
- Liu, P.P.; Zhou, M.F.; Luais, B.; Cividini, D.; Rollion-Bard, C. Disequilibrium iron isotopic fractionation during the high-temperature magmatic differentiation of the Baima Fe–Ti oxide-bearing mafic intrusion, SW China. Earth Planet. Sci. Lett. 2014, 399, 21–29. [Google Scholar]
- Luo, Z.H.; Deng, J.F.; Zhao, G.C.; Cao, Y.Q. Characteristics of magmatic activities and orogenic process of Taihangshan intraplate orogen. Earth Sci. 1997, 22, 57–62. (In Chinese) [Google Scholar]
- Chen, B.; Chen, C.J.; He, J.B.; Liu, A.K. Origin of Mesozoic high-Mg adakitic rocks from northeastern China:Petrological and Nd-Sr-Os isotopic constraints. Chin. Sci. Bull. 2013, 58, 1941–1953. (In Chinese) [Google Scholar]
- Li, Y.H.; Duan, C.; Dan, H.; Chen, X.; Wang, C.; Yang, B.; Zhang, C.; Liu, F. Effect of sulfate evaporate salt layer for formation of porphyrite iron ores in the Middle-Lower Yangtze River area. Acta Petrol. Sin. 2014, 30, 1355–1368. [Google Scholar]
- Shen, J.; Santosh, M.; Li, S.; Zhang, H.; Yin, N.; Dong, G.; Wang, Y.; Ma, G.; Yu, H. The Beiminghe skarn iron deposit, eastern China: Geochronology, isotope geochemistry and implications for the destruction of the North China Craton. Lithos 2013, 156, 218–229. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, L.; Zhu, D.; Wu, T.; Deng, X.; Bai, M.; Wen, G. Geochemical, geochronological, and sr-nd-hf isotopic constraints on the petrogenesis of the qicun intrusive complex from the handan-xingtai district: Implications for the mechanism of lithospheric thinning of the north china craton. Ore Geol. Rev. 2014, 57, 363–374. [Google Scholar] [CrossRef]
- Menzies, M.A.; Xu, Y.G.; Zhang, H.F.; Fan, W.M. Integration of geology, geophysics and geochemistry: A key to understanding the North China Craton. Lithos 2007, 96, 1–21. [Google Scholar] [CrossRef]
- Zhang, K.J. Destruction of the North China Craton: Lithosphere folding-induced removal of lithospheric mantle? J. Geodyn. 2012, 53, 8–17. [Google Scholar] [CrossRef]
- Su, S.G.; Hou, J.G.; Luo, Z.H.; Wu, G.Y.; Chen, Y.J. Genetic mechanism of skarn iron deposit: Evidence from Xishimen Iron deposit in Wu’an, Hebei Province. J. Jilin Univ. (Earth Sci. Ed.) 2015, 201, 346–347. (In Chinese) [Google Scholar]
- Liu, L.L.; Su, S.G.; Wang, N.; Wang, W.B. Deep magmatic processes and shallow responses during the lithospheric thinning of North China Craton: Taking Tanling intrusive complex as an example. Acta Petrol. Sin. 2019, 35, 2873–2892. (In Chinese) [Google Scholar]
- McFarlane, C.R.M.; Luo, Y. U-Pb Geochronology Using 193 nm Excimer LA-ICP-MS Optimized for In Situ Accessory Mineral Dating in Thin Sections. Geosci. Can. 2012, 39, 158–172. [Google Scholar]
- Zhang, W.; Hu, Z.C.; Liu, Y.S. Iso-Compass: New freeware software for isotopic data reduction of LA-MC-ICP-MS. J. Anal. At. Spectrom. 2020, 35, 1087–1096. [Google Scholar] [CrossRef]
- Feng, Y.T.; Zhang, W.; Hu, Z.C.; Luo, T.; Li, M.; Liu, Y.S.; Liu, H.; Li, Q.L. A new synthesis program of pyrite and chalcopyrite reference materials for in situ iron and sulfur isotope analysis using LA-MC-ICP-MS. J. Anal. At. Spectrom. 2022, 37, 551–562. [Google Scholar]
- Toplis, M.J.; Corgne, A. An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contrib. Mineral. Petrol. 2002, 144, 22–37. [Google Scholar]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Bordage, A.; Balan, E.; Villiers, J.P.R.d.; Cromarty, R.; Juhin, A.; Carvallo, C.; Calas, G.; Raju, P.V.S.; Glatzel, P. V oxidation state in Fe–Ti oxides by high-energy resolution fluorescence-detected X-ray absorption spectroscopy. Phys. Chem. Miner. 2011, 38, 449–458. [Google Scholar] [CrossRef]
- Barnes, S.J.; Godel, B.; Gürer, D.; Brenan, J.M.; Robertson, J.; Paterson, D. Sulfide-Olivine Fe-Ni Exchange and the Origin of Anomalously Ni Rich Magmatic Sulfides. Econ. Geol. 2013, 108, 1971–1982. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. Treatise Geochem. 2003, 3, 1–64. [Google Scholar]
- Dare, S.A.S.; Barnes, S.J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Nystroem, J.O.; Henriquez, F. Magmatic features of iron ores of the Kiruna type in Chile and Sweden; ore textures and mag-netite geochemistry. Econ. Geol. 1994, 89, 820–839. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zhou, M.F. In-situ LA-ICP-MS trace elemental analyses of magnetite: The Mesozoic Tengtie skarn Fe deposit in the Nanling Range, South China. Ore Geol. Rev. 2015, 65, 872–883. [Google Scholar] [CrossRef]
- Chen, L.; Song, X.; Zhu, X.; Zhang, X.; Yu, S.; Yi, J. Iron isotope fractionation during crystallization and sub-solidus re-equilibration: Constraints from the Baima mafic layered intrusion, SW China. Chem. Geol. 2014, 380, 97–109. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, C.Y.; Huang, F.; Zhang, Z. Iron Isotope Systematics of the Panzhihua Mafic Layered Intrusion Associated With Giant Fe-Ti Oxide Deposit in the Emeishan Large Igneous Province, SW China. J. Geophys. Res. 2019, 124, 358–375. [Google Scholar]
- Wang, Y.; Zhu, X.; Tang, C.; Mao, J.; Chang, Z. Discriminate between magmatic- and magmatic-hydrothermal ore deposits using Fe isotopes. Ore Geol. Rev. 2021, 130, 103946. [Google Scholar] [CrossRef]
- Heimann, A.E.A.; Beard, B.L.; Johnson, C.M. View Correspondence The role of volatile exsolution and sub-solidus fluid/rock interactions in producing high (Heimann)Fe/(Heimann)Fe ratios in siliceous igneous rocks. Geochim. Cosmochim. Acta 2009, 72, 4379–4396. [Google Scholar] [CrossRef]
- Bilenker, L.D.; Simon, A.C.; Reich, M.; Lundstrom, C.C.; Gajos, N.; Bindeman, I.; Barra, F.; Munizaga, R. Fe-O stable isotope pairs elucidate a high-temperature origin of Chilean iron oxide-apatite deposits. Geochim. Cosmochim. Acta 2016, 177, 94–104. [Google Scholar] [CrossRef]
- Weis, F. Oxygen and Iron Isotope Systematics of the Grängesberg Mining District (GMD), Central Sweden. Ph.D. Thesis, Uppsala University, Uppsala City, Sweden, 2017. [Google Scholar]
- Su, S.G.; Lu, X.; Santosh, M.; Hou, J.G.; Cui, Y.; Cui, X.L. Geochemical and Fe-isotope characteristics of the largest Mesozoic skarn deposit in China: Implications for the mechanism of Fe skarn formation. Ore Geol. Rev. 2021, 138, 104400. [Google Scholar] [CrossRef]
- Severmann, S.; Anbar, A.D. Reconstructing Paleoredox Conditions through a Multitracer Approach: The Key to the Past Is the Present. Elements 2009, 5, 359–364. [Google Scholar] [CrossRef]
- Sossi, P.A.; Foden, J.D.; Halverson, G.P. Redox-controlled iron isotope fractionation during magmatic differentiation: An example from the Red Hill intrusion, S. Tasmania. Contrib. Mineral. Petrol. 2012, 164, 757–772. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Buddington, A.F.; Lindsley, D.H. Iron-titanium oxide minerals and synthetic equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Frost, B.R.; Lindsley, D.H. Occurrence of iron-titanium oxides in igneous rocks. Rev. Miner. Geochem. 1991, 25, 433–468. [Google Scholar]
- Ghiorso, M.S.; Sack, O. Iron-titanium oxide geothermometry: Thermodynamic formulation and the estimation of intensive variables in silicic magmas. Contrib. Mineral. Petrol. 1991, 108, 485–510. [Google Scholar] [CrossRef]
- Keller, T.; Tornos, F.; Hanchar, J.M.; Pietruszka, D.K.; Soldati, A.; Dingwell, D.B.; Suckale, J. Genetic model of the El Laco magnetite-apatite deposits by extrusion of iron-rich melt. Nat. Commun. 2022, 13, 6114. [Google Scholar] [CrossRef]
- Wang, S.; Hou, T.; Xie, Q. Formation of orthopyroxene-magnetite symplectites by reactive melt flow: Insights into the Shangzhuang layered intrusion in Beijing, China. J. Asian Earth Sci. 2024, 273, 106262. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Z.; Hou, T.; Cheng, Z.; Campos, E.; Wang, Z.; Fei, X. New insights for the formation of Kiruna-type iron deposits by immiscible hydrous Fe-P melt and high-temperature hydrothermal processes: Evidence from El Laco deposit. Econ. Geol. 2019, 114, 35–46. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, M.; Lightfoot, P.; Wang, C.; Qi, L.; Sun, M. Sulfide Saturation and Magma Emplacement in the Formation of the Permian Huangshandong Ni-Cu Sulfide Deposit, Xinjiang, Northwestern China. Econ. Geol. 2013, 108, 1833–1848. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, M.; Qi, L.; Gao, J.; Wang, Y. Re–Os isotopic ages of pyrite and chemical composition of magnetite from the Cihai magmatic–hydrothermal Fe deposit, NW China. Miner. Depos. 2013, 48, 925–946. [Google Scholar] [CrossRef]
- McCarthy, T.S.; Cawthorn, R.G. The geochemistry of vanadiferous magnetite in the bushveld complex: Implications for crystallization mechanisms in layered complexes. Miner. Depos. 1983, 18, 505–518. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, M. New textural and mineralogical constraints on the origin of the Hongge Fe-Ti-V oxide deposit, SW China. Miner. Depos. 2013, 48, 787–798. [Google Scholar] [CrossRef]
- McCarthy, T.S.; Cawthorn, R.G.; Wright, C.J.; McIver, J.R. Mineral layering in the Bushveld Complex: Implications of chromium abundances in magnetite from closely spaced magnetitite and intervening silicate-rich layers. Econ. Geol. 1985, 80, 1062–1074. [Google Scholar] [CrossRef]
- He, D.T.; Liu, Y.; Chen, C.; Foley, S.F.; Ducea, M.N. Oxidization of the mantle caused by sediment recycling may contribute to the formation of iron-rich mantle melts. Sci. Bull. 2020, 65, 519–521. [Google Scholar] [CrossRef]
- Goldstein, S.B.; Francis, D. The Petrogenesis and Mantle Source of Archaean Ferropicrites from the Western Superior Province, Ontario, Canada. J. Petrol. 2008, 49, 1729–1753. [Google Scholar] [CrossRef]
- Liu, P.P.; Zhou, M.; Chen, W.; Boone, M.; Cnudde, V. Using Multiphase Solid Inclusions to Constrain the Origin of the Baima Fe-Ti-(V) Oxide Deposit, SW China. J. Petrol. 2014, 55, 951–976. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, H.; Zhang, L.; Li, D.; Zhang, W.; Wang, C.; Yang, J.; Yan, X. Magnetite geochemistry of the Heijianshan Fe–Cu (–Au) deposit in Eastern Tianshan: Metallogenic implications for submarine volcanic-hosted Fe–Cu deposits in NW China. Ore Geol. Rev. 2018, 100, 422–440. [Google Scholar] [CrossRef]
- Yang, R.N. Genesis of Orbicular Diorite in Tanling, Wuan, Hebei. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2017. (In Chinese). [Google Scholar]
- Ganino, C.; Arndt, N.; Zhou, M.F.; Gaillard, F.; Chauvel, C. Interaction of magma with sedimentary wall rock and magnetite ore genesis in the Panzhihua mafic intrusion, SW China. Miner. Depos. 2008, 43, 677–694. [Google Scholar] [CrossRef]
- Ganino, C.; Harris, C.; Arndt, N.T. Assimilation of carbonate country rock by the parent magma of the Panzhihua Fe-Ti-V deposit (SW China): Evidence from stable isotopes. Geosci. Front. 2013, 4, 547–554. [Google Scholar] [CrossRef]
- Pecher, A.; Arndt, N.; Jean, A. Structure of the Panzhihua intrusion and its Fe-Ti-V deposit, China. Geosci. Front. 2013, 4, 571–581. [Google Scholar] [CrossRef]
- Dauphas, N.; Roskosz, M.; Alp, E.E.; Neuville, D.R.; Hu, M.Y.; Sio, C.K.; Tissot, F.L.; Zhao, J.; Tissandier, L.; Médard, E. Magma redox and structural controls on iron isotope variations in Earth’s mantle and crust. Earth Planet. Sci. Lett. 2014, 398, 127–140. [Google Scholar] [CrossRef]
- Prissel, K.B.; Krawczynski, M.J.; Nie, N.X.; Dauphas, N.; Couvy, H.; Hu, M.Y.; Alp, E.E.; Roskosz, M. Experimentally determined effects of olivine crystallization and melt titanium content on iron isotopic fractionation in planetary basalts. Geochim. Cosmochim. Acta 2018, 238, 580–598. [Google Scholar] [CrossRef]
- Shahar, A.; Young, E.D.; Manning, C.E. Equilibrium high-temperature Fe isotope fractionation between fayalite and magnetite: An experimental calibration. Earth Planet. Sci. Lett. 2008, 268, 330–338. [Google Scholar] [CrossRef]
- Poitrasson, F.; Freydier, R. Heavy iron isotope composition of granites determined by high resolution MC-ICP-MS. Chem. Geol. 2005, 222, 132–147. [Google Scholar] [CrossRef]
- Wang, Y.J.; Hu, Y.Y.; Shen, J.F.; Qu, K.; Yin, N.; Yu, H.J.; Ma, G.G. Sulfur and lead isotope composition and tracing for sources of ore-forming materials in Beiming river iron deposits, southern Taihang mountains. Nat. Geosci. 2011, 25, 846–852. [Google Scholar]
- Tang, D.; Qin, K.; Mao, Y.; Evans, N.J. Magnetite geochemistry and iron isotope signature of disseminated and massive mineralization in the Kalatongke magmatic Cu-Ni sulfide deposit, northwest China. Chem. Geol. 2022, 605, 120965. [Google Scholar] [CrossRef]
- Dauphas, N.; Zuilen, M.V.; Wadhwa, M.; Davis, A.M.; Marty, B.; Janney, P.E. Clues from Fe Isotope Variations on the Origin of Early Archean BIFs from Greenland. Science 2004, 306, 2077–2080. [Google Scholar] [CrossRef]
- Dauphas, N.; Cates, N.L.; Mojzsis, S.J.; Busigny, V. Identification of chemical sedimentary protoliths using iron isotopes in the >3750 Ma Nuvvuagittuq supracrustal belt, Canada. Earth Planet. Sci. Lett. 2007, 254, 358–376. [Google Scholar] [CrossRef]
- Dauphas, N.; van Zuilen, M.; Busigny, V.; Lepland, A.; Wadhwa, M.; Janney, P.E. View Correspondence Iron isotope, major and trace element characterization of early Archean supracrustal rocks from SW Greenland: Protolith identification and metamorphic overprint. Geochim. Cosmochim. Acta 2008, 71, 4745–4770. [Google Scholar] [CrossRef]
- Frost, C.D.; Blanckenburg, F.v.; Schoenberg, R.; Frost, B.R.; Swapp, S.M. Preservation of Fe isotope heterogeneities during diagenesis and metamorphism of banded iron formation. Contrib. Mineral. Petrol. 2007, 153, 211–235. [Google Scholar] [CrossRef]
- Hyslop, E.; Valley, J.W.; Johnson, C.M.; Beard, B.L. The effects of metamorphism on O and Fe isotope compositions in the Biwabik Iron Formation, northern Minnesota. Contrib. Mineral. Petrol. 2008, 155, 313–328. [Google Scholar] [CrossRef]
- Ruan, C.T. The Gem-Mineralogy Characteristics of Garnet in the Nanminghe Skarn Iron Deposit in Wuan, Hebei. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2020. (In Chinese). [Google Scholar]
- Troll, V.R.; Weis, F.A.; Jonsson, E.; Andersson, U.B.; Majidi, S.A.; Högdahl, K.; Harris, C.; Millet, M.-A.; Chinnasamy, S.S.; Kooijman, E.; et al. Global Fe–O isotope correlation reveals magmatic origin of Kiruna-type apatite-iron-oxide ores. Nat. Commun. 2019, 10, 1712. [Google Scholar] [CrossRef]
- Telus, M.; Dauphas, N.; Moynier, F.; Tissot, F.L.H.; Teng, F.Z.; Nabelek, P.I.; Craddock, P.R.; Groat, L.A. Iron, zinc, magnesium and uranium isotopic fractionation during continental crust differentiation: The tale from migmatites, granitoids, and pegmatites. Geochim. Cosmochim. Acta 2012, 97, 247–265. [Google Scholar] [CrossRef]
- Mungall, J.E.; Brenan, J.M.; Godel, B.; Barnes, S.J.; Gaillard, F. Transport of metals and sulphur in magmas by flotation of sulphide melt on vapour bubbles. Nat. Geosci. 2015, 8, 216–219. [Google Scholar] [CrossRef]
- Su, S.G.; Tang, Z.L.; Luo, Z.H.; Deng, J.F.; Wu, G.Y.; Zhou, M.F.; Song, C.; Xiao, Q.H. Magmatic Conduit Metallogenic System. Acta Petrol. Sin. 2014, 11, 3120–3130. (In Chinese) [Google Scholar]
- Clechenko, C.C.; Valley, J.W. Oscillatory zoning in garnet from the Willsboro Wollastonite Skarn, Adirondack Mts, New York: A record of shallow hydrothermal processes preserved in a granulite facies terrane. J. Metamorph. Geol. 2003, 21, 771–784. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, J.; Zhang, H.; Wang, J.; Su, L.; Yang, X.; Wu, S. Origin of oscillatory zoned garnets from the Xieertala Fe–Zn skarn deposit, northern China: In situ LA–ICP-MS evidence. Lithos 2014, 190, 279–291. [Google Scholar] [CrossRef]
- Mirnejad, H.; Hasannejad, M.; Miller, N.; Hassanzadeh, J.; Bocchio, R.; Modabberi, S. Origin and Evolution of Oscillatory Zoned Garnet from Kasva Skarn, Northeast Tafresh, Iran. Can. Mineral. 2018, 56, 15–37. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Xie, G.Q.; Li, W. In-situ analysis of garnets from the Jingshandian iron skarn deposit, Hubei Province, and its geological implications. Geol. China 2014, 41, 1944–1963. (In Chinese) [Google Scholar]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Bayukov, O.A. Wüstite stability in the presence of a CO2-fluid and a carbonate-silicate melt: Implications for the graphite/diamond formation and generation of Fe-rich mantle metasomatic agents. Lithos 2016, 244, 20–29. [Google Scholar] [CrossRef]
- Qiu, J.X. Magmatic Petrology; Geological Publishing House: Beijing, China, 1985; p. 340. (In Chinese) [Google Scholar]
- Fan, Q.C. Zircon chronology and rare earth geochemistry of xenolith granulites in Hanuoba. Chin. Sci. Bull. 1998, 2, 133–137. (In Chinese) [Google Scholar]
- Chen, B.; Zhai, M.G.; Tian, W.; Jiang, B.M. Petrogenesis of the Mesozoic Intrusive Complexes from the Southern Taihang Orogen, North China Craton: Elemental and Sr-Nd-Pb Isotopic Constraints. Bull. Mineral. Petrol. Geochem. 2005, 24, 10. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, J.M.; Xie, G.Q.; Liu, J.; Chen, M.H.; Wang, S.M.; Guo, S.F.; Gao, X.; Li, G.D. 40Ar-39Ar dating of phologopite from the Xishimen skarn iron deposit in the Handan-Xingtai area, southern Hebei, and its implications. Acta Petrol. Sinca 2007, 23, 2513–2518. (In Chinese) [Google Scholar]
- Förster, H.; Bachtiar, I.; Borumandi, H. Petrographische Detailuntersuchungen im Bereich der Eisenerzlagerstätten von Bafq/Zentraliran. Z. Dtsch. Geol. Ges. 1973, 124, 121–134. [Google Scholar]
- Park, C.F. A magnetite “flow” in northern Chile. Econ. Geol. 1961, 56, 431–436. [Google Scholar] [CrossRef]
- Philpotts, A.R. Origin of certain iron-titanium oxide and apatite rocks. Econ. Geol. 1967, 62, 303–315. [Google Scholar] [CrossRef]
- Lledo, H.L.; Jenkins, D.M. Experimental Investigation of the Upper Thermal Stability of Mg-rich Actinolite; Implications for Kiruna-Type Iron Deposits. J. Petrol. 2008, 49, 225–238. [Google Scholar] [CrossRef]
- Philpotts, A.R. Compositions of immiscible liquids in volcanic rocks. Contrib. Mineral. Petrol. 1982, 80, 201–218. [Google Scholar] [CrossRef]
- Su, L.H. The importance of liquid immiscibility in petrology and mineral deposits. J. Earth Sci. 1984, 1, 1–12. (In Chinese) [Google Scholar]
- Yu, X.H. The geological significance and the phase equilibrium experiments of wustite-fluor-phlogopite-diopsied melt system atonebar and high temperature. J. Earth Sci. 1984, 1, 13–18+135–136. (In Chinese) [Google Scholar]
- Li, Y.H.; Duan, C.; Han, D.; Liu, F.; Wan, D.F.; Wang, C.Y. Oxygen isotopic discriminant marker of magmatic iron deposits: Ningwu porphyrite iron ore as an example. Acta Petrol. Sin. 2017, 33, 3411–3421. (In Chinese) [Google Scholar]
| Core Magnetite | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Name | K2O | CaO | TiO2 | CoO | P2O5 | Na2O | MgO | Al2O3 | SiO2 | Cr2O3 | MnO | FeO | Total | CaO/(Na2O + K2O) |
| Q22-A-Q1-1 | 0.03 | 0.07 | 0.16 | 0.06 | 0.01 | 0.06 | 0.05 | 0.53 | 0.47 | 1.48 | 0.02 | 86.24 | 89.68 | 0.76 |
| Q22-A-Q1-2 | 0.02 | 0.03 | 0.20 | 0.12 | n.d. | 0.04 | n.d. | 0.42 | 0.22 | 0.50 | 0.08 | 87.56 | 89.72 | 0.43 |
| Q22-A-Q1-3 | n.d. | 0.03 | 0.14 | 0.14 | 0.02 | 0.00 | 0.03 | 0.31 | 0.24 | 0.20 | 0.04 | 87.73 | 89.43 | 33.00 |
| Q22-A-Q2-1 | n.d. | 0.04 | 0.15 | 0.14 | 0.01 | 0.01 | 0.03 | 0.22 | 0.19 | 0.23 | n.d. | 88.78 | 90.35 | 2.73 |
| Q22-A-Q2-2 | n.d. | 0.01 | 0.11 | 0.13 | n.d. | 0.06 | 0.04 | 0.36 | 0.20 | 0.19 | 0.09 | 88.43 | 90.12 | 0.24 |
| Q22-A-Q2-3 | n.d. | 0.05 | 0.15 | 0.30 | 0.03 | n.d. | 0.02 | 0.42 | 0.39 | 0.80 | 0.02 | 88.17 | 90.87 | - |
| Q22-A-Q3-1 | n.d. | 0.03 | 0.16 | 0.09 | n.d. | n.d. | n.d. | 0.36 | 0.18 | 0.07 | 0.06 | 88.73 | 90.16 | - |
| Q22-A-Q3-2 | n.d. | 0.04 | 0.18 | 0.11 | n.d. | n.d. | 0.03 | 0.42 | 0.21 | 0.10 | 0.04 | 88.04 | 89.70 | - |
| Q22-A-Q3-3 | n.d. | 0.04 | 0.12 | 0.07 | 0.01 | n.d. | 0.03 | 0.19 | 0.22 | 0.38 | 0.06 | 88.52 | 90.08 | - |
| Q22-A-Q4-1 | n.d. | 0.03 | 0.09 | 0.06 | n.d. | 0.04 | n.d. | 0.38 | 0.17 | 0.34 | 0.07 | 87.83 | 89.53 | 0.55 |
| Q22-A-Q4-2 | n.d. | 0.04 | 0.14 | 0.13 | n.d. | 0.01 | 0.02 | 0.30 | 0.05 | 0.14 | 0.05 | 87.58 | 88.93 | 3.00 |
| Q22-A-Q4-3 | n.d. | 0.06 | 0.13 | 0.16 | 0.02 | n.d. | 0.02 | 0.22 | 0.06 | 0.08 | 0.05 | 87.92 | 89.20 | - |
| Q22-A-Q5-1 | n.d. | 0.04 | 0.16 | 0.09 | 0.02 | 0.06 | 0.06 | 0.15 | 0.16 | 0.06 | 0.16 | 87.22 | 88.47 | 0.60 |
| Q22-A-Q5-2 | n.d. | 0.04 | 0.12 | n.d. | n.d. | 0.15 | n.d. | 0.21 | 0.12 | 0.10 | 0.03 | 92.02 | 92.98 | 0.28 |
| Q22-A-Q6-1 | n.d. | 0.13 | 0.07 | n.d. | n.d. | 0.01 | n.d. | 0.07 | 0.16 | 0.03 | 0.02 | 91.27 | 92.02 | 6.19 |
| Q22-A-Q6-2 | n.d. | 0.12 | 0.03 | n.d. | n.d. | 0.01 | n.d. | 0.05 | 0.03 | 0.26 | 0.03 | 90.82 | 91.51 | 8.92 |
| Q22-A-Q6-3 | 0.01 | 0.07 | 0.16 | 0.06 | 0.01 | n.d. | 0.04 | 0.13 | 0.18 | 0.41 | 0.04 | 87.62 | 89.28 | 5.58 |
| Q22-A-Q7-1 | n.d. | 0.02 | 0.12 | 0.23 | 0.05 | n.d. | n.d. | 0.38 | 0.22 | 0.13 | 0.08 | 88.28 | 90.10 | 4.00 |
| Q22-A-Q7-2 | n.d. | 0.07 | 0.03 | n.d. | n.d. | 0.01 | n.d. | 0.03 | 0.04 | 0.03 | 0.04 | 92.24 | 92.66 | 5.29 |
| Q22-A-Q7-3 | n.d. | 0.04 | 0.10 | 0.25 | n.d. | n.d. | 0.08 | 0.46 | 0.29 | 0.46 | 0.06 | 86.77 | 89.12 | 4.30 |
| Middle Magnetite | ||||||||||||||
| Sample Name | K2O | CaO | TiO2 | CoO | P2O5 | Na2O | MgO | Al2O3 | SiO2 | Cr2O3 | MnO | FeO | Total | CaO/(Na2O + K2O) |
| Q22-B-1 | n.d. | 0.13 | 0.11 | 0.06 | n.d. | n.d. | 0.02 | 0.13 | 0.33 | 0.78 | 0.03 | 87.73 | 89.75 | 19.14 |
| Q22-B-2 | 0.02 | 0.16 | 0.14 | 0.12 | 0.03 | 0.03 | 0.04 | 0.27 | 0.56 | 0.71 | 0.03 | 88.06 | 90.53 | 3.42 |
| Q22-B-3 | n.d. | 0.06 | 0.05 | n.d. | n.d. | 0.06 | n.d. | n.d. | 0.09 | 0.04 | n.d. | 92.16 | 92.62 | 0.88 |
| Q22-B-4 | n.d. | 0.22 | 0.06 | 0.08 | n.d. | 0.02 | 0.04 | 0.21 | 0.53 | 0.51 | 0.06 | 88.61 | 90.70 | 11.42 |
| Q22-B-5 | n.d. | 0.07 | 0.14 | 0.08 | n.d. | n.d. | 0.03 | 0.18 | 0.25 | 0.56 | 0.03 | 88.34 | 90.16 | - |
| Q22-B-6 | n.d. | 0.11 | 0.25 | 0.13 | 0.01 | 0.04 | 0.03 | 0.28 | 0.42 | 0.72 | 0.08 | 87.76 | 90.31 | 2.43 |
| Q22-B-7 | n.d. | 0.10 | 0.04 | 0.09 | 0.05 | 0.05 | 0.01 | 0.05 | 0.11 | 0.04 | n.d. | 89.40 | 90.29 | 1.83 |
| Q22-B-8 | 0.01 | 0.12 | 0.06 | 0.05 | 0.01 | 0.03 | 0.03 | 0.14 | 0.22 | 0.02 | 0.04 | 87.73 | 88.81 | 3.03 |
| Q22-B-9 | n.d. | 0.03 | 0.03 | 0.11 | 0.02 | n.d. | 0.01 | n.d. | 0.07 | 0.03 | 0.03 | 91.71 | 92.35 | 8.50 |
| Q22-B-10 | 0.01 | 0.07 | 0.05 | 0.11 | n.d. | 0.01 | n.d. | 0.04 | 0.09 | 0.02 | 0.06 | 91.89 | 92.65 | 3.25 |
| Q22-B-12 | n.d. | 0.05 | 0.06 | 0.16 | n.d. | 0.05 | 0.03 | 0.02 | 0.05 | n.d. | n.d. | 91.29 | 92.07 | 1.00 |
| Q22-B-13 | 0.01 | 0.04 | 0.07 | 0.09 | n.d. | 0.03 | n.d. | 0.04 | 0.06 | 0.05 | 0.03 | 90.22 | 90.99 | 0.97 |
| Q22-B-14 | n.d. | 0.12 | 0.06 | 0.09 | 0.01 | n.d. | 0.04 | 0.06 | 0.19 | 0.05 | 0.03 | 88.39 | 89.34 | - |
| Q22-B-15 | n.d. | 0.04 | 0.03 | 0.09 | n.d. | 0.08 | n.d. | 0.01 | 0.03 | 0.01 | 0.02 | 91.48 | 92.11 | 0.47 |
| Rim Magnetite | ||||||||||||||
| Sample Name | K2O | CaO | TiO2 | CoO | P2O5 | Na2O | MgO | Al2O3 | SiO2 | Cr2O3 | MnO | FeO | Total | CaO/(Na2O + K2O) |
| Q22-C-Q1-1 | 0.01 | 0.16 | 0.18 | 0.04 | 0.06 | 0.11 | 0.04 | 0.19 | 0.36 | 0.15 | 0.05 | 87.12 | 88.85 | 1.28 |
| Q22-C-Q1-2 | n.d. | 0.16 | 0.16 | 0.07 | n.d. | 0.07 | 0.02 | 0.09 | 0.27 | 0.13 | 0.03 | 87.18 | 88.62 | 2.41 |
| Q22-C-Q1-3 | n.d. | 0.07 | 0.12 | 0.06 | n.d. | 0.11 | n.d. | 0.11 | 0.19 | 0.30 | 0.09 | 88.72 | 90.15 | 0.61 |
| Q22-C-Q1-4 | 0.03 | 0.07 | 0.07 | 0.08 | n.d. | 0.08 | n.d. | 0.08 | 0.39 | 0.06 | 0.03 | 86.56 | 87.83 | 0.63 |
| Q22-C-Q1-5 | n.d. | 0.14 | 0.05 | 0.12 | 0.02 | 0.04 | 0.04 | 0.08 | 0.33 | 0.03 | 0.03 | 88.49 | 89.71 | 3.71 |
| Q22-C-Q1-6 | n.d. | 0.05 | 0.06 | n.d. | n.d. | n.d. | 0.01 | 0.08 | 0.05 | 0.01 | n.d. | 93.11 | 93.54 | 7.67 |
| Q22-C-Q1-7 | 0.02 | 0.03 | 0.02 | 0.11 | 0.02 | 0.03 | 0.03 | 0.13 | 0.06 | 0.05 | 0.06 | 91.70 | 92.59 | 0.71 |
| Q22-C-Q2-1 | 0.08 | 0.11 | 0.05 | 0.06 | 0.02 | 0.32 | 0.02 | 0.18 | 0.42 | 0.45 | n.d. | 87.87 | 89.88 | 0.28 |
| Q22-C-Q2-2 | n.d. | 0.06 | 0.04 | 0.17 | n.d. | 0.00 | n.d. | n.d. | 0.03 | n.d. | 0.02 | 92.01 | 92.55 | - |
| Q22-C-Q2-3 | 0.02 | 0.08 | 0.24 | 0.13 | n.d. | 0.13 | 0.08 | 0.24 | 0.41 | 1.12 | 0.06 | 86.53 | 89.48 | 0.54 |
| Q22-C-Q2-4 | n.d. | 0.09 | 0.06 | n.d. | n.d. | 0.05 | 0.02 | 0.06 | 0.06 | 0.07 | n.d. | 91.91 | 92.43 | 1.67 |
| Q22-C-Q2-5 | n.d. | 0.05 | 0.07 | 0.10 | n.d. | 0.07 | n.d. | 0.06 | 0.12 | 0.04 | 0.05 | 88.84 | 89.78 | 0.66 |
| Q22-C-Q2-6 | 0.02 | 0.04 | 0.07 | 0.08 | 0.02 | 0.05 | 0.03 | 0.16 | 0.30 | 0.02 | 0.06 | 87.93 | 89.11 | 0.65 |
| Q22-C-Q3-1 | n.d. | 0.10 | 0.10 | 0.13 | n.d. | 0.11 | 0.03 | 0.07 | 0.17 | 0.10 | 0.02 | 87.57 | 88.78 | 0.87 |
| Q22-C-Q3-2 | n.d. | 0.03 | 0.13 | 0.02 | 0.01 | 0.07 | n.d. | 0.02 | 0.06 | 0.05 | 0.01 | 93.41 | 94.20 | 0.42 |
| Q22-C-Q3-3 | 0.01 | 0.07 | 0.04 | 0.08 | 0.01 | n.d. | n.d. | 0.01 | 0.04 | 0.01 | 0.02 | 91.86 | 92.51 | 7.20 |
| Q22-C-Q3-4 | n.d. | 0.07 | 0.04 | 0.12 | 0.01 | 0.02 | 0.01 | 0.05 | 0.06 | n.d. | 0.04 | 85.80 | 86.58 | 3.36 |
| Q22-C-Q3-5 | n.d. | 0.02 | 0.05 | 0.14 | 0.02 | 0.06 | n.d. | 0.07 | 0.02 | 0.04 | 0.03 | 92.76 | 93.60 | 0.28 |
| Q22-C-Q3-6 | n.d. | 0.11 | 0.06 | 0.10 | n.d. | 0.08 | 0.01 | 0.04 | 0.03 | 0.05 | 0.02 | 88.53 | 89.39 | 1.32 |
| Sample Name | Remarks | δ56FeIRMM(‰) | Delta-2SE |
|---|---|---|---|
| MT-8 | Standards | −0.20 | 0.06 |
| MT-8 | Standards | −0.19 | 0.06 |
| MT-10 | Standards | 0.25 | 0.06 |
| MT-10 | Standards | 0.24 | 0.06 |
| MT-8 | Standards | −0.08 | 0.06 |
| MT-10 | Standards | 0.30 | 0.04 |
| MT-8 | Standards | −0.13 | 0.06 |
| Q22-A-Q1-1 | Core magnetite | 0.67 | 0.05 |
| Q22-A-Q1-2 | Core magnetite | 0.54 | 0.04 |
| Q22-A-Q1-3 | Core magnetite | 0.58 | 0.04 |
| MT-8 | Standards | −0.19 | 0.05 |
| Q22-A-Q2-1 | Core magnetite | 0.46 | 0.05 |
| Q22-A-Q2-2 | Core magnetite | 0.46 | 0.05 |
| Q22-A-Q4-1 | Core magnetite | 0.62 | 0.04 |
| MT-8 | Standards | −0.18 | 0.06 |
| Q22-A-Q4-2 | Core magnetite | 0.61 | 0.04 |
| Q22-A-Q5-1 | Core magnetite | 0.52 | 0.04 |
| Q22-A-Q5-2 | Core magnetite | 0.56 | 0.05 |
| MT-8 | Standards | −0.11 | 0.05 |
| Q22-A-Q6-1 | Core magnetite | 0.65 | 0.05 |
| Q22-A-Q6-2 | Core magnetite | 0.78 | 0.04 |
| Q22-A-Q6-3 | Core magnetite | 0.70 | 0.04 |
| MT-8 | Standards | −0.18 | 0.07 |
| Q22-A-Q7-1 | Core magnetite | 0.61 | 0.06 |
| Q22-A-Q7-2 | Core magnetite | 0.46 | 0.05 |
| Q22-A-Q7-3 | Core magnetite | 0.51 | 0.05 |
| MT-8 | Standards | −0.13 | 0.06 |
| MT-10 | Standards | 0.27 | 0.06 |
| MT-8 | Standards | −0.10 | 0.06 |
| Q22-B-1 | Middle magnetite | 0.67 | 0.05 |
| Q22-B-2 | Middle magnetite | 0.38 | 0.06 |
| Q22-B-3 | Middle magnetite | 0.57 | 0.05 |
| MT-8 | Standards | −0.14 | 0.05 |
| Q22-B-4 | Middle magnetite | 0.74 | 0.04 |
| Q22-B-6 | Middle magnetite | 0.67 | 0.06 |
| MT-8 | Standards | −0.23 | 0.07 |
| Q22-B-7 | Middle magnetite | 0.12 | 0.05 |
| Q22-B-9 | Middle magnetite | 0.71 | 0.04 |
| MT-8 | Standards | −0.11 | 0.06 |
| MT-8 | Standards | −0.20 | 0.04 |
| MT-8 | Standards | −0.09 | 0.06 |
| MT-10 | Standards | 0.31 | 0.06 |
| MT-8 | Standards | −0.12 | 0.06 |
| Q22-B-12 | Middle magnetite | 0.56 | 0.05 |
| MT-8 | Standards | −0.10 | 0.04 |
| Q22-B-13 | Middle magnetite | 0.76 | 0.06 |
| Q22-B-15 | Middle magnetite | 0.40 | 0.05 |
| MT-8 | Standards | −0.09 | 0.07 |
| MT-10 | Standards | 0.31 | 0.05 |
| MT-10 | Standards | 0.30 | 0.05 |
| MT-8 | Standards | −0.14 | 0.06 |
| MT-10 | Standards | 0.34 | 0.09 |
| MT-10 | Standards | 0.32 | 0.07 |
| MT-8 | Standards | −0.07 | 0.05 |
| MT-8 | Standards | −0.09 | 0.06 |
| MT-10 | Standards | 0.23 | 0.07 |
| MT-10 | Standards | 0.23 | 0.06 |
| MT-8 | Standards | −0.10 | 0.06 |
| MT-10 | Standards | 0.28 | 0.05 |
| MT-8 | Standards | −0.17 | 0.06 |
| Q22-C-Q1-1 | Rim magnetite | 0.21 | 0.06 |
| Q22-C-Q1-2 | Rim magnetite | 0.10 | 0.05 |
| Q22-C-Q1-3 | Rim magnetite | 0.19 | 0.06 |
| MT-8 | Standards | −0.11 | 0.08 |
| Q22-C-Q2-1 | Rim magnetite | −0.66 | 0.04 |
| Q22-C-Q2-3 | Rim magnetite | 0.16 | 0.05 |
| MT-8 | Standards | −0.07 | 0.05 |
| Q22-C-Q3-1 | Rim magnetite | 0.02 | 0.08 |
| Q22-C-Q3-2 | Rim magnetite | 0.07 | 0.09 |
| Q22-C-Q3-3 | Rim magnetite | 0.13 | 0.08 |
| MT-8 | Standards | −0.17 | 0.06 |
| MT-8 | Standards | −0.15 | 0.05 |
| Q22-C-Q3-4 | Rim magnetite | 0.23 | 0.07 |
| Q22-C-Q1-4 | Rim magnetite | 0.06 | 0.08 |
| Q22-C-Q1-5 | Rim magnetite | 0.24 | 0.05 |
| MT-8 | Standards | −0.17 | 0.07 |
| Q22-C-Q1-6 | Rim magnetite | 0.09 | 0.06 |
| Q22-C-Q2-4 | Rim magnetite | −0.03 | 0.07 |
| MT-8 | Standards | −0.18 | 0.07 |
| MT-10 | Standards | 0.27 | 0.07 |
| MT-10 | Standards | 0.23 | 0.05 |
| MT-8 | Standards | −0.11 | 0.06 |
| MT-8 | Standards | −0.12 | 0.05 |
| Q22-C-Q1-7 | Rim magnetite | 0.27 | 0.07 |
| Q22-C-Q2-6 | Rim magnetite | −0.23 | 0.06 |
| Q22-C-Q3-5 | Rim magnetite | −0.06 | 0.07 |
| Q22-C-Q3-6 | Rim magnetite | 0.02 | 0.10 |
| MT-8 | Standards | −0.19 | 0.06 |
| MT-10 | Standards | 0.21 | 0.09 |
| MT-8 | Standards | −0.14 | 0.06 |
| Sample | Sample Description | Sample Provenance | δ56Fe in ‰ | 2σ |
|---|---|---|---|---|
| Apatite-iron oxide ore | ||||
| Kiruna Mining District (KMD), Northern Sweden | ||||
| Kiruna-17NY28 | Banded massive (Ap-)magnetite ore | Kiirunavaara mine, Kiruna | 0.19 | ±0.03 |
| K-Mt-1 (907/75) | Massive magnetite ore | Kiirunavaara mine, Kiruna | 0.2 | ±0.03 |
| Ki-Mi-2a (1079/251) | Massive magnetite ore | Kiirunavaara mine, Kiruna | 0.21 | ±0.02 |
| Ki-Mi-2b (1079/251) | Massive magnetite ore | Kiirunavaara mine, Kiruna | 0.22 | ±0.04 |
| K-Mt-1079/303 | Massive magnetite ore | Kiirunavaara mine, Kiruna | 0.27 | ±0.04 |
| K-Mt-1079/437 | Massive magnetite ore | Kiirunavaara mine, Kiruna | 0.16 | ±0.02 |
| M1931 | Massive magnetite ore | Kiirunavaara mine, Kiruna | - | - |
| M1937 | Skeletal magnetite ore | Kiirunavaara mine, Kiruna | - | - |
| LVA-3 | Massive magnetite ore | Luossavaara mine, Kiruna | 0.23 | ±0.02 |
| LVA-FW-1 | Massive magnetite ore | Luossavaara mine, Kiruna | 0.12 | ±0.04 |
| LVA-FW-2 | Massive magnetite ore | Luossavaara mine, Kiruna | 0.27 | ±0.03 |
| K-Mt-3 | Massive magnetite ore | Mertainen mine, Kiruna | 0.41 | ±0.03 |
| K-Mt-4 | Massive magnetite ore | Mertainen mine, Kiruna | 0.29 | ±0.03 |
| M7557* | Massive magnetite ore | Rektorn mine, Kiruna | - | - |
| Grängesberg Mining District (GMD), Central Sweden | ||||
| DC717-KES090068 | Massive (Ap-)magnetite ore | Grängesberg mine, Grängesberg | 0.4 | ±0.03 |
| DC717-KES090070 | Massive (Ap-)magnetite ore | Grängesberg mine, Grängesberg | 0.24 | ±0.03 |
| DC717-KES090072 | Massive (Ap-)magnetite ore | Grängesberg mine, Grängesberg | 0.33 | ±0.03 |
| DC717-KES090084 | Magnetite vein in intermediate volcanic rock | Grängesberg mine, Grängesberg | 0.11 | ±0.03 |
| DC690-KES090011 | Massive (Ap-)magnetite ore | Grängesberg mine, Grängesberg | 0.31 | ±0.03 |
| DC690-KES090012 | Massive (Ap-)magnetite ore | Grängesberg mine, Grängesberg | 0.31 | ±0.04 |
| DC690-KES090020 | Massive Ap-veined magnetite ore | Grängesberg mine, Grängesberg | 0.3 | ±0.04 |
| DC690-KES090024 | Massive (Ap-)magnetite ore | Grängesberg mine, Grängesberg | 0.26 | ±0.03 |
| DC690-KES090027 | Ap-veined/banded massive magnetite ore | Grängesberg mine, Grängesberg | 0.29 | ±0.03 |
| DC690-KES090030 | Silicate-spotted massive (Ap-)magnetite ore | Grängesberg mine, Grängesberg | 0.39 | ±0.04 |
| DC690-KES090034 | Coarse-grained Ap-spotted massive magnetite ore | Grängesberg mine, Grängesberg | 0.27 | ±0.03 |
| DC690-KES090044 | Disseminated magnetite in intermediate volcanic rock | Grängesberg mine, Grängesberg | 0.24 | ±0.03 |
| DC575-KES103011 | Magnetite-dominated massive ore | Grängesberg mine, Grängesberg | 0.31 | ±0.03 |
| DC575-KES103016 | Coarse, massive (Ap-)magnetite ore | Grängesberg mine, Grängesberg | 0.27 | ±0.04 |
| DC575-KES103003 | Magnetite-dominated massive ore | Grängesberg mine, Grängesberg | 1 | ±0.03 |
| KES091013b | Massive (Ap-)magnetite ore | Blötberget mine, Blötberget | 0.33 | ±0.03 |
| El Laco Ap-Fe-oxide deposit, Chile | ||||
| EJ-LS-11-1 | Massive magnetite ore | Laco Sur, El Laco | 0.28 | ±0.03 |
| EJ-LS-11-2 | Massive magnetite ore | Laco Sur, El Laco | 0.24 | ±0.03 |
| EJ-LS-11-3 | Massive magnetite ore | Laco Sur, El Laco | 0.36 | ±0.03 |
| EJ-LS-11-4 | Massive magnetite ore | Laco Sur, El Laco | 0.34 | ±0.03 |
| LS-2 | Massive magnetite ore | Laco Sur, El Laco | 0.27 | ±0.03 |
| LS-52 | Massive magnetite ore | Laco Sur, El Laco | 0.28 | ±0.03 |
| Plutonic reference material | ||||
| Ruoutevare | Ti-magnetite, layered igneous intrusion | Kvikjokk, Norrbotten, Sweden | 0.31 | ±0.03 |
| Ulvön | Ti-magnetite, layered igneous intrusion | Ulvön island, Ångermanland, Sweden | 0.13 | ±0.03 |
| Taberg | Ti-magnetite, layered igneous intrusion | Iron mine, Taberg, Småland, Sweden | 0.23 | ±0.04 |
| EM419 | Massive Fe-Ti magnetite ore | Northern pit, Panzhihua, China | 0.61 | ±0.05 |
| EM424 | Massive Fe-Ti magnetite ore | Nalahe, Panzhihua, China | 0.12 | ±0.04 |
| Bushveld | Massive magnetite ore | Upper Zone, Bushveld Complex, South Africa | - | - |
| Gabbrobomb | Magnetite from a gabbro xenolith | NW-Flank, Skjaldbreiður, Iceland | 0.46 | ±0.03 |
| Volcanic reference material | ||||
| TEF-NER-18 | Magnetite from an ankaramite dyke | NE Rift Zone, Tenerife, Spain | 0.07 | ±0.05 |
| TEF-NER-57B | Magnetite from an ankaramite dyke | NE Rift Zone, Tenerife, Spain | 0.16 | ±0.02 |
| TEF-NER-70 | Magnetite from a pyroxene phyric dyke | NE Rift Zone, Tenerife, Spain | 0.1 | ±0.02 |
| MG-07 | Igneous magnetite from dacite | S-Flank, Mt. Ruapehu, New Zealand | 0.32 | ±0.03 |
| MG-09 | Igneous magnetite from dacite | S-Flank, Mt. Ruapehu, New Zealand | 0.29 | ±0.03 |
| Kelut A1 | Igneous magnetite from basaltic andesite | Mt. Kelut, Java, Indonesia | 0.1 | ±0.04 |
| GD-D-2 | Igneous magnetite from basaltic andesite | Gede Dome, Java, Indonesia | 0.12 | ±0.03 |
| AK-B1 | Igneous magnetite from basaltic andesite | SE-Flank, Anak Krakatau, Indonesia | 0.06 | ±0.03 |
| AK-B3 | Igneous magnetite from basaltic andesite | SE-Flank, Anak Krakatau, Indonesia | 0.16 | ±0.03 |
| A-BA-1 | Igneous magnetite from basaltic andesite | Mt. Agung, Bali, Indonesia | 0.18 | ±0.05 |
| M-BA06-KA-3 | Igneous magnetite from basaltic andesite | Mt. Merapi, Java, Indonesia | 0.17 | ±0.03 |
| 83/CRS/6 | Igneous magnetite from a dolerite dyke | Agros, Troodos Massif, Cyprus | 0.34 | ±0.03 |
| Low-temperature or hydrothermal magnetites | ||||
| KES091007B | Calcite bearing-magnetite ore | Björnberget, Sweden | −0.02 | ±0.03 |
| DM-1 | Iron-skarn magnetite ore | Botenhäll, Dannemora, Sweden | −0.36 | ±0.03 |
| DM-2 | Iron-skarn magnetite ore | Norrnäs 3, Dannemora, Sweden | 0.01 | ±0.03 |
| DM-3 | Iron-skarn magnetite ore | Konstäng, Dannemora, Sweden | −0.43 | ±0.03 |
| DM-4 | Iron-skarn magnetite ore | Strömsmalmen, Dannemora, Sweden | −0.35 | ±0.03 |
| EJ092008 | Magnetite from a banded iron formation deposit | Striberg, Bergslagen, Sweden | −0.57 | ±0.03 |
| Panzhihua Layered Intrusion | ||||
| LJ1482 | Leuco-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.34 | 0.05 |
| LJ1487a | Leuco-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.36 | 0.03 |
| LJ1487 | Leuco-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.32 | 0.01 |
| LJ1434 | Ap-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.29 | 0.03 |
| LJ1438 | Ap-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.4 | 0.04 |
| LJ1453 | Ap-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.57 | 0.01 |
| LJ1457 | Ap-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.3 | 0.03 |
| LJ1458 | Ap-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.62 | 0.02 |
| LJ1461 | Mela-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.53 | 0.04 |
| LJ1462 | Mela-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.46 | 0.04 |
| LJ1463 | Mela-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.49 | 0.03 |
| LJ1430a | Mela-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.23 | 0.03 |
| LJ1430 | Mela-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.25 | 0.04 |
| LJ1427 | Mela-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.28 | 0.01 |
| LJ1426 | Oxide ore | the Lanjiahuoshan open pit, Panzhihua, China | 0.22 | 0.01 |
| LJ1424 | Oxide ore | the Lanjiahuoshan open pit, Panzhihua, China | 0.22 | 0.02 |
| LJ1420 | Oxide ore | the Lanjiahuoshan open pit, Panzhihua, China | 0.17 | 0.05 |
| LJ1410 | Micro-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.28 | 0.05 |
| LJ1407 | Micro-gb | the Lanjiahuoshan open pit, Panzhihua, China | 0.44 | 0.03 |
| Gushan iron deposit, China | ||||
| 10GS_4_Hm | Massive ore(vesicular structure) | Gushan iron deposit, China | 0.24 | |
| 10GS_5_Hm | Massive ore (vesicular structure) | Gushan iron deposit, China | 0.32 | |
| 10GS_6_Hm | Massive ore (vesicular structure) | Gushan iron deposit, China | 0.53 | |
| 10GS_17_Hm | Massive ore (vesicular structure) | Gushan iron deposit, China | 0.24 | |
| 10GS_19_Hm | Massive ore (vesicular structure) | Gushan iron deposit, China | 0.09 | |
| 10GS_20_Hm | Massive ore (vesicular structure) | Gushan iron deposit, China | 0.49 | |
| Xishimen iron deposit | ||||
| XSM2-130503-01 | Massive magnetite ore | Xishimen iron deposit, China | 0.008 | 0.04 |
| XSM3-130504-06 | Massive magnetite ore | Xishimen iron deposit, China | 0.034 | 0.04 |
| XSM3-130504-09 | Massive magnetite ore | Xishimen iron deposit, China | 0.115 | 0.04 |
| XSM3-130504-14 | Massive magnetite ore | Xishimen iron deposit, China | 0.047 | 0.04 |
| XSM3-130504-16 | Massive magnetite ore | Xishimen iron deposit, China | 0.054 | 0.04 |
| XSM3-130504-18 | Massive magnetite ore | Xishimen iron deposit, China | 0.111 | 0.04 |
| XSM3-130504-33 | Massive magnetite ore | Xishimen iron deposit, China | 0.113 | 0.04 |
| XSM4-130506-22 | Massive magnetite ore | Xishimen iron deposit, China | 0.105 | 0.052 |
| XSM4-130506-23 | Massive magnetite ore | Xishimen iron deposit, China | 0.079 | 0.052 |
| XSM4-130506-26 | Massive magnetite ore | Xishimen iron deposit, China | 0.06 | 0.052 |
| XSM4-130506-29 | Massive magnetite ore | Xishimen iron deposit, China | 0.049 | 0.04 |
| XSM4-130506-34 | Massive magnetite ore | Xishimen iron deposit, China | 0.067 | 0.052 |
| Sample Name | SiO2 | TiO2 | Al2O3 | Cr2O3 | P2O5 | V2O3 | FeO | MnO | MgO | CaO | NiO | Na2O | K2O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grt1 | 40.20 | n.d. | 26.02 | n.d. | n.d. | n.d. | 10.17 | 0.22 | 0.02 | 23.28 | 0.02 | 0.06 | 0.02 | 100 |
| Grt2 | 40.01 | n.d. | 23.88 | n.d. | n.d. | n.d. | 12.94 | 0.06 | 0.02 | 23.04 | 0.01 | 0.04 | n.d. | 100 |
| Grt3 | 40.14 | 0.04 | 25.54 | 0.01 | n.d. | n.d. | 11.02 | 0.02 | n.d. | 23.16 | n.d. | 0.03 | 0.03 | 99.99 |
| Grt4 | 39.59 | 0.02 | 26.20 | 0.05 | n.d. | n.d. | 10.68 | 0.42 | 0.02 | 23.03 | 0.01 | n.d. | n.d. | 99.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Su, S.; Wang, P. Chemistry and Fe Isotopes of Magnetites in the Orbicular Bodies in the Tanling Diorite and Implications for the Skarn Iron Mineralization in the North China Craton. Minerals 2025, 15, 1061. https://doi.org/10.3390/min15101061
Li R, Su S, Wang P. Chemistry and Fe Isotopes of Magnetites in the Orbicular Bodies in the Tanling Diorite and Implications for the Skarn Iron Mineralization in the North China Craton. Minerals. 2025; 15(10):1061. https://doi.org/10.3390/min15101061
Chicago/Turabian StyleLi, Ruipeng, Shangguo Su, and Peng Wang. 2025. "Chemistry and Fe Isotopes of Magnetites in the Orbicular Bodies in the Tanling Diorite and Implications for the Skarn Iron Mineralization in the North China Craton" Minerals 15, no. 10: 1061. https://doi.org/10.3390/min15101061
APA StyleLi, R., Su, S., & Wang, P. (2025). Chemistry and Fe Isotopes of Magnetites in the Orbicular Bodies in the Tanling Diorite and Implications for the Skarn Iron Mineralization in the North China Craton. Minerals, 15(10), 1061. https://doi.org/10.3390/min15101061