Abstract
Adsorption studies of ciprofloxacine (CPX) and lidocaine (LID) emerging contaminants were performed on two fibrous Mg clays from the Madrid basin and Senegal. The samples were characterized by X-ray diffraction, ICP major element analysis, infrared spectroscopy, thermal analysis, optical petrography, scanning and transmission electron microscopy, cation exchange capacity (CEC), and N2-BET analysis. Two mineral assemblages were established. Assemblage 1 mainly consists of sepiolite and minor trioctahedral smectite, while assemblage 2 is mostly composed of palygorskite, which is associated with dioctahedral smectite. The sorption was fast and reached equilibrium in 2 h. Fibrous Mg clays showed a higher adsorption capacity for CPX than for LID in the conditions studied. CPX adsorption on sepiolite and palygorskite can be the result of the combination of various mechanisms: ion exchange with permanently charged sites, electrostatic attractions with external surfaces, and an inner sphere complex with broken edges. LID adsorption mainly occurs by ion exchange and electrostatic interaction with the external surfaces of the clays. Dioctahedral smectite, as an associated phase, contributed to a higher removal percentage in palygorskite samples. By contrast, the trioctahedral smectite did not play a significant role in the adsorption of the samples with sepiolite. The mesoporous structure, high surface area, and moderate cation exchange of fibrous clays play a key role in the sorption process of CPX and LID.
1. Introduction
Pharmaceutical residues, which are considered emerging contaminants (ECs), have been detected frequently in surface waters [1]. Several recent reviews have also confirmed the presence of pharmaceuticals in drinking waters [2,3] as treatment processes largely fail to reduce the amounts of these substances to below current detection limits [4,5].
Ciprofloxacine (CPX), a fluoroquinolone antibiotic ubiquitously prescribed, and lidocaine (LID), a local anesthetic used also as an antiarrhythmic agent, are widely prescribed pharmaceuticals. These compounds are continuously discharged into surface waters due to a lack of human and animal metabolism or through the effluents of pharmaceutical companies [6,7,8]. Unused human pharmaceuticals may also enter the environment through landfill leachate [9]. The occurrence of antibiotics in low concentrations in waters is associated with bacterial resistance, and their presence in the aquatic environment could also portend serious toxicity for aquatic flora and fauna species [10]. In aquatic matrices, predicted no-effect concentrations for antibiotic resistance development for CPX are set at 100 ng·L−1; meanwhile, ecotoxicity risk is set at 1200 ng·L−1 [11]. Therefore, it is important to develop environmentally and economically sustainable methods for the removal of CPX and LID in the aqueous phase in order to prevent public health risks and ecotoxicological impacts.
Adsorption is suggested as a promising approach for the removal of CPX and LID with practical feasibility [7,9,10,12,13,14]. Clay minerals are sorbents with unique properties, such as a large surface area, low cost, and high abundance, that have been assessed for the removal of ECs as raw materials and after functionalizing their surfaces for improved performance [15,16,17].
Sepiolite and palygorskite belong to modulated phyllosilicates that consist of 2:1 ribbons (denominated as ‘polisomes’ by [18]) linked through the periodic inversion of the apical oxygen of the continuous tetrahedral sheet every six atoms of silicon for sepiolite and every four atoms of silicon for palygorskite [19]. The palygorskite–sepiolite minerals have channels that run the length of the fibers containing H2O molecules and exchangeable cations, similar to the zeolite minerals.
Both mineral fiber surfaces and the zeolite-like channels generate a relatively large sorption capacity. Mineral fiber surfaces have surficial oxygen atoms, silanol groups, and structural H2O (commonly called OH2 to differentiate it from zeolitic H2O), together with broken bonds [20]. The zeolite-like channels permit the exchange of cations; however, most organic molecules do not enter the channels because they are too large for exchange reactions. The interaction between organic molecules and zeolite-like channels is not well understood [21].
Sepiolite and palygorskite are widely distributed in continental deposits in Spain [22]. In Europe, these deposits represent 90% of the production of these clays [23]. The wide range of industrial applications of sepiolite and palygorskite can be classified as sorptive, rheological, and catalytic [24]. These fibrous clays have also been studied as potential pharmaceutical products [25]. Palygorskite has been used in antacids, gastrointestinal protectors, antidiarrhoeaics, cosmetic creams, powders, and emulsions, while sepiolite has been used as a therapeutic, functional, inert, and bulking agent in the pharmaceutical and cosmetic industries [26].
As an adsorbent, most studies on palygorskite were conducted on the removal of different kinds of pharmaceuticals (tetracycline, carbamazepine, sulfamethoxazole, ranitidine, amitriptyline, sodium diclofenac, and cephalosporin) [27,28,29,30,31,32]. In addition, this clay mineral has also been used for the removal of dyes [33,34,35], organic contaminants [36], and herbicides such as glyphosate [37].
Sepiolite has been widely used to remove pollutants from the environment. Several studies can be found on the feasibility of using sepiolite as an adsorbent for the removal of dyes [38,39,40,41]. Some of which have dealt with heavy metal sorption onto this clay [42,43]. The adsorption of pharmaceutical compounds (acetaminophen, diclofenac, tetracycline, oxytetracycline, ibuprofen, amoxicillin) has been mainly performed on organo-sepiolites [44,45,46].
The efficacy of a palygorskite–montmorillonite clay mix as a sewage treatment plant filter material for ciprofloxacine has been studied [12]. These authors evaluated the effect of granule size, solution chemistry, and temperature. They found that granules between 0.6 mm and 1.7 mm were appropriate for CPX removal and that CPX adsorption was strongly pH-dependent. On the other hand, the efficiency of the surfactant (cetyltri-methylammonium bromide)-modified sepiolite for ciprofloxacine antibiotics has also been evaluated by [9]. This study showed that the best test conditions were obtained at an initial concentration of CPX 10 mg·L−1 and an adsorbent dosage of 2 g·L−1 and that the percentage removal and the maximum adsorption capacity were found to be 99.1% and 63.84 mg·g−1, demonstrating the high potential of this material for the removal of CPX.
These findings suggest the potential of palygorskite and sepiolite in the development of sustainable solutions for the removal of pharmaceutical compounds.
The aim of this study was to evaluate the efficiency of raw fibrous Mg clays and associated clay minerals, widely distributed in the Madrid basin (Spain), for the removal of ciprofloxacine and lidocaine from water.
This study contributes to the knowledge of the interactions between natural clayey materials and anthropogenic contaminants. To date, there is an absence of scientific articles reporting the adsorption of LID on this type of clay mineral, whereas in the case of CPX, its adsorption has been scarcely studied on palygorskite samples, and no adsorption studies onto sepiolite have been reported. The results would add practical value to the use of clay minerals in the wastewater treatment of emerging compounds.
2. Materials and Methods
2.1. Materials
Four borehole samples from the Esquivias–Batallones sector located in the Madrid basin (Ad-1, Ad-2, Ad-3, Ad-4) and one outcrop sample from a deposit located in Senegal (Ad-9) were studied.
Hand sample description is as follows:
Ad-1: This is a mixture of three levels of earthy appearance, formed by brecciated to granular mudstones of a yellowish brown color (yellow grey 5Y 7/2 to greyish yellow 5Y 8/4) with the development of pedalization morphologies and bioturbation features (Figure 1A). Locally, there are calcite aggregates that act as cement in porosities and pyrolusite impregnations coating planes.
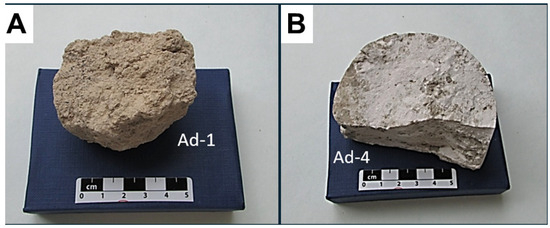
Figure 1.
(A) Hand sample of Ad.1, showing the earthy (clastic) appearance and massive aspect. (B) Hand sample of Ad.4, showing a very compact, massive aspect, with scattered darker inclusions.
Ad-2: It was not possible to examine the hand sample as the material was already granulated.
Ad-3: This is a mixture of three levels consisting of compact light brown- to salmon-colored lutites (very pale orange 10YR 8/2 to yellowish grey 5Y 8/1), massive to banded, and sometimes with intraclasts or millimeter-thick laminated pink intercalations.
Ad-4: Compact white to yellowish lutite (white N9 to yellowish grey 5Y 8/1), with dark relic material intercalated in a planar position (Figure 1B).
Ad-9: Laminated shale with black Mn-Fe oxide stains on planar surfaces. Scattered small ochre ferruginous nodules (<0.25 mm). Calcite is detected, sometimes concentrated locally, forming white patches on the laminar surfaces. Yellowish brown color (very pale orange 10YR 8/2).
The adsorbates studied were an anesthetic (LID) and an antibiotic (CPX), two pharmaceutical compounds with serious environmental impact. CPX and LID were employed in their hydrochloride forms as adsorbates. CPX.HCl, sourced from Magel S.A., Buenos Aires, Argentina, and LID.HCl.H2O, obtained from Parapharm (Parapharm Pharmaceuticals Group, Athens, Greece), were used, both with a purity of 99.9%. A detailed description of the characteristics of these molecules can be found in a previous paper by [14].
2.2. Methodology
2.2.1. Mineralogical and Physicochemical Characterization of Raw Mg Clays
The mineralogical composition of the samples was analyzed by X-ray diffraction using a Bruker D8 diffractometer (with Lynxeye XE-T detector) (Bruker, Billerica, MA, USA), operating with Cu-Kα radiation (40 kV, 20 mA) and a scanning speed of 1° 2θ/min. XRD studies were carried out on randomly oriented samples (bulk sample) and on the clay fraction (<2 μm). Powdered whole-rock samples were scanned from 2° to 65° 2θ. A dispersion of the clay fraction was dropped onto a glass slide to prepare oriented mounts. Identification of the clay minerals was carried out on the XRD patterns of oriented air-dried samples with ethylene glycol solvation (EG) and also after heating at 550 °C for 2 h. Quantitative estimation of the mineral content was carried out using the intensity factors calculated by [47,48]. Mineralogical characterization was complemented by infrared spectroscopy, thermal analysis, and scanning electron microscopy (SEM). Fourier-Transform Infrared (FTIR) spectroscopy was conducted on the samples using a Bruker IFS 66v instrument (Bruker, Billerica, MA, USA). The samples were milled with KBr and compressed into thin pellets. The spectrum was recorded with a spectral resolution of 2 cm−1 in transmission mode in the region of 4000–400 cm−1. Thermal analysis (DSC/DTA/TGA) was conducted using a TA Instruments Q600 system (TA Instruments, Inc., New Castle, DE, USA), with approximately 10 mg of powdered clay placed in a Pt sample holder. The measurements were carried out at an average heating rate of 10 °C/min, using alumina as the reference material. Petrographic analysis was performed on all samples using standard thin-section techniques. The samples were impregnated with epoxy before being cut and subsequently polished with petroleum to prevent their disintegration on contact with water. Undisturbed fragments were examined under a Hitachi S-3000 N Scanning Electron Microscope (SEM) (Hitachi, Chiyoda, Japan) after coating with gold (10 nm thick) in a sputtering chamber (coater system, Q150TS). The clay particle morphologies were examined in two selected samples by TEM using JEOL TEM 1016 equipment (JEOL, Akishima City, Japan).
Whole-rock chemical analyses were carried out using inductively coupled plasma atomic emission spectroscopy (ICP-AES and MS) for major elements at Actlabs Analytical Laboratories, Ltd. (Vancouver, BC, Canada).
The cation exchange capacity (CEC) of the clays was measured by the copper triethylenetetramine [Cu(trien)]2+ method [49].
The textural properties were studied by nitrogen adsorption–desorption isotherms at −196.15 °C. The measurements were carried out using an ASAP manometer (Micromeritics Instrument, Norcross, GA, USA) on degassed samples at 200 °C for 12 h. The apparent specific surface area was calculated by the Brunauer, Emmett, and Teller (SBET) method. The micropore volume (Vµp) was calculated by applying the α-plot method [50]. The total pore volume (VT) was calculated using Gurvich’s rule at a relative pressure of 0.98, while the mesopore volume (Vmp) was determined using the Barrett–Joyner–Halenda (BJH) method [51]. The pore size classification followed the IUPAC guidelines [51], according to which pores larger than 50 nm are defined as macropores, those between 2 and 50 nm as mesopores, and pores smaller than 2 nm as micropores.
2.2.2. Batch Adsorption Studies
The adsorption tests were performed using 20 mg of adsorbent in contact with a drug solution at a ratio of 2.5 g·L−1. The studies were conducted at 20 °C under unadjusted pH conditions (pH values of around 5–6), and in all cases, separation was achieved by centrifugation at 8000 rpm for 20 min using a SOVALL ultracentrifuge (Thermo Fisher Scientific, Waltham, MA, USA). Initial concentrations of 0.3 mM for CPX and 1.69 mM for LID were used for the kinetic studies. Adsorption isotherms were obtained with concentrations ranging from 0.15 to 3 mM for CPX and 0.2 to 5 mM for LID, allowing the system to reach equilibrium over a 24 h contact period, both in accordance with previous studies [52,53].
CPX and LID concentrations were quantified using a UV-Vis T60 spectrophotometer at their respective maximum wavelengths. The adsorbed quantity was then calculated by Equation (1).
where Ci and Ce are the initial and equilibrium concentrations (mM), V is the solution volume (L), and w is the mass of adsorbent (g).
Langmuir, Freundlich, and Sips equations were employed to describe the adsorption behavior of CPX and LID (Table 1).

Table 1.
Equations employed to fit adsorption data.
3. Results
3.1. Characterization of Raw Mg Clays
3.1.1. X-Ray Diffraction (XRD)
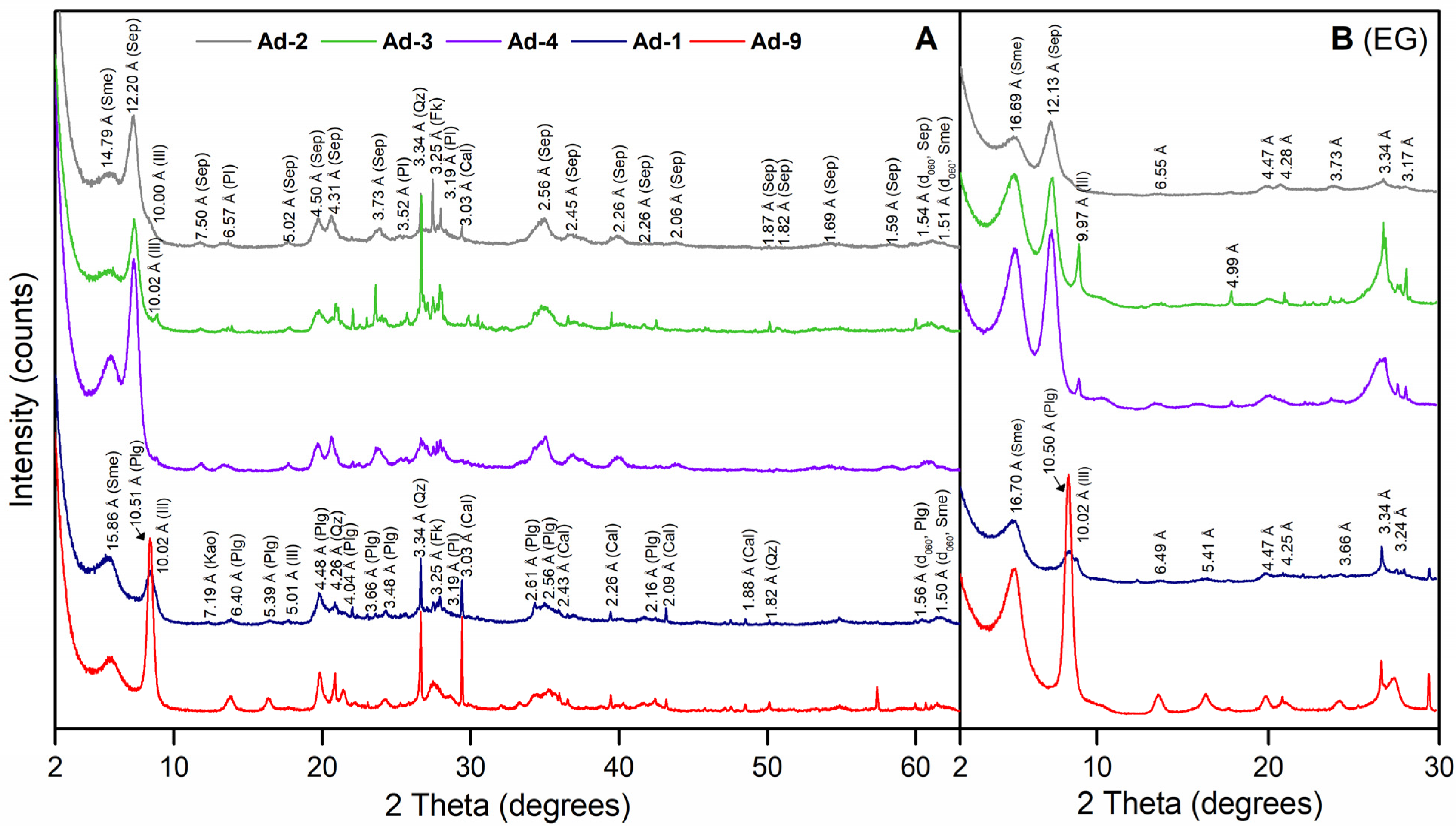
The mineralogical results of the samples are shown in Table 2. In the bulk samples, phyllosilicates were present in the highest proportions (>85%) with a mean value of 91%. Quartz (1%–8%), plagioclase (1%–3%), potassium feldspar (1%–4%), and calcite (2%–7%) occur subordinate. Within the phyllosilicates, XRD powder patterns with spacing sequences similar to those reported for palygorskite and sepiolite predominated in samples Ad-1/Ad-9 and Ad2/Ad3/Ad4, respectively (Figure 2A). The d060 spacing was predominantly trioctahedral in samples Ad-2, Ad-3, and Ad-4, with two reflections at 1.54 Å and 1.51–1.52 Å. However, in samples Ad-1 and Ad-9, the d060 reflection at 1.56 Å indicated the presence of a trioctahedral phase accompanied by a minor reflection at 1.505 Å, showing the presence of dioctahedral phases (Figure 2A) [54].

Table 2.
Bulk and clay mineralogy (% w/w). Tr < 0.1%.
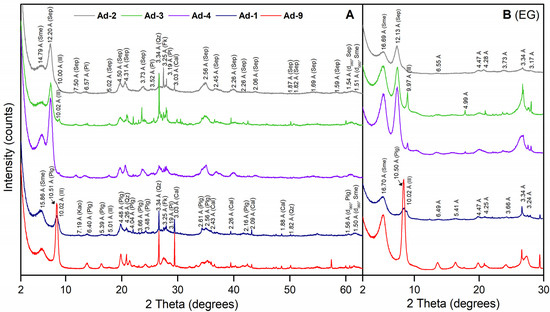
Figure 2.
(A) Powder XRD patterns of the fibrous Mg clays. (B) X-ray pattern of ethylene glycol-solvated (EG)-oriented mounts. Phy: phyllosilicates; Qz: quartz; Pl: plagioclase; Fk: potassium feldspar; Cal: calcite; Plg: palygorskite; Sep: sepiolite; Sme: smectite; Ill: illite; Kao: kaolinite.
The ethylene glycol-oriented mounts of the Ad-2, Ad-3, and Ad-4 clay fraction samples were characterized by a strong reflection (110) at about 12 Å, and several weak reflections at d-values of 6.55, 5.01, 4.47, 4.28, and 3.73 Å, which are indicative of sepiolite (Figure 2B). In these samples, another well-defined reflection was present at 15.1 Å (AD), which shifted to 16.69 Å in the ethylene glycol-solvated, -oriented mount, indicating the presence of smectite. Illite was identified in samples Ad-3 and Ad-4 by regular reflections at d-values of 10 and 5 Å (Figure 2B).
The samples Ad-1 and Ad-9 showed typical reflections of palygorskite (observed at d-values = 10.5, 5.41, 4.47, 3.66 Å) (Figure 2B). Smectite was also identified by the shift of the 15 Å reflection to 17 Å in the ethylene glycol-solvated, -oriented mount. In sample Ad-1, the presence of reflections at d-values of 10 and 5 Å indicated the occurrence of illite (Figure 2B).
Taking into account the bulk and clay mineralogy of the samples (Table 2), two mineralogical assemblages were established:
Assemblage 1 (Ad-2, Ad-3, Ad-4): Sepiolite was the main clay mineral (70%–86%), accompanied by trioctahedral smectite (14%–23%) (Figure 1B). Illite (1%–7%) was also present in samples Ad-3 and Ad-4. Non-clay minerals included quartz and feldspars.
Assemblage 2 (Ad-1 and Ad-9): It was mainly constituted by palygorskite (83%–66%) and dioctahedral smectite (17%–27%). Illite (7%) and traces of kaolinite were also identified in Ad-1. The non-clay minerals were quartz, calcite, and feldspars (only in Ad-1).
3.1.2. Chemical Analysis
Table 3 shows the chemical composition of the raw Mg clays. The results were in accordance with the mineralogy of the samples. The SiO2/MgO molar ratio was much higher in mineralogical assemblage 1 than in 2 (4.5 vs. 2.1) due to the higher MgO content in the latter, which was exclusively constituted by the trioctahedral phases. The Al2O3, Fe2O3, and TiO2 content was higher in the samples with palygorskite and dioctahedral smectite, such as Ad-1 and Ad-9. Ad-4 stood out by being the sample with the highest Mg content and the lowest Al2O3 and Fe2O3 content. The content of K2O was higher in the sample where illite was present.

Table 3.
Chemical analysis of major elements and LOI.
3.1.3. Infrared Spectroscopy (FTIR)
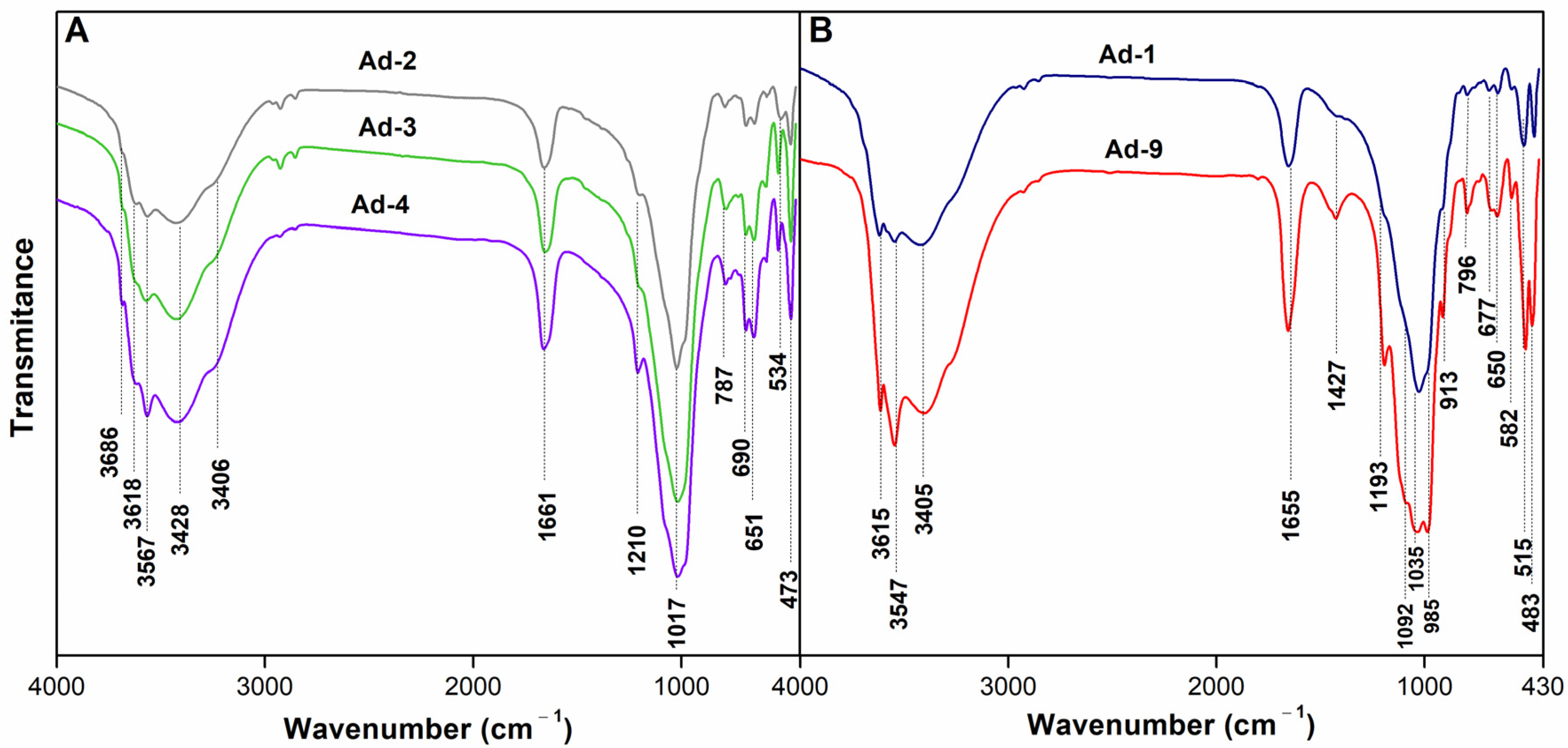
As seen in the FTIR spectrum shown in Figure 3A, the samples Ad-2, Ad-3, and Ad-4 revealed the typical absorption bands of sepiolite, exhibiting octahedral OH stretching weak bands at 3688–3685 cm−1, trioctahedral OH deformation band at 650 cm−1, and the SiO vibration band at 1210 cm−1 [55]. The strong band at 3567 cm−1 was due to water molecules bound to Mg at the edges of the discontinuous octahedral sheet inside the tunnels. The absorption band at 3618 cm−1 was due to the stretching vibrations of the structural OH groups of smectite. The broadband observed at 3406 cm−1 corresponded to the H–O–H vibrations of adsorbed water, and the OH-bending mode at 1660 cm−1 was also due to the deformation of the OH group of water coordinating with magnesium in the octahedral sheet. The bands at 473 and 1017 cm−1 were due to the bending vibration of Si–O and Si–O/Si–O–Si stretching of the tetrahedral sheet, respectively [56]. The band around 790 cm−1 was related to the Si-O-Si modes. The 691 cm−1 band was attributed to an out-of-plane Si-O mode. The absorption near 534 cm−1 corresponded to the perpendicular Mg-O vibration.
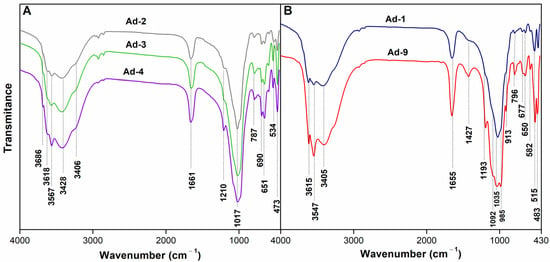
Figure 3.
FT-IR spectra of the bulk samples. (A): Sepiolite rich assemblage; (B): Palygorskite rich assemblage.
The FTIR spectrum of samples Ad-1 and Ad-9 has the characteristic bands of palygorskite (3615, 3547, 3405 cm−1) and smectite [57] (Figure 3B). The band at 3615 cm−1 corresponded to the OH stretching of structural hydroxyl groups of palygorskite. Meanwhile, the bands at 3547 cm−1 and 3405 cm−1 can be attributed to OH stretching of water coordinated with Al, Mg, and adsorbed-H2O OH stretching bands, respectively, in palygorskite. The band at 1655 cm−1 was due to the OH deformation of water in palygorskite [57]. Calcite impurities resulted in a 1427 cm−1 band in the Ad-9 sample [58].
The 2:1 ribbon layer structure of palygorskite also affected the shape of its FTIR spectra in the 1300–400 cm−1 region. The Si-O stretching region of both samples revealed a complex band with five more or less resolved absorptions at 1193, 1092, 1035, and 985 cm−1, which has been observed in other palygorskite spectra [57] (Figure 2B). The band at approximately 913 cm−1, which was more intense in Ad-9, was assigned to the bending vibration of Al–OH–Al in palygorskite.
Some quartz (797 cm−1) and possibly feldspar (582 cm−1) admixtures were revealed in the FTIR spectra.
The intensity of the shoulder at 670 cm−1 (Ad-9) is related to the Mg content in palygorskite. The vibration bands in the 550–400 cm−1 region corresponded to the mixed Al-O-Si and Si-O-Si bending vibrations of Al-rich palygorskite [57].
3.1.4. Thermal Analysis
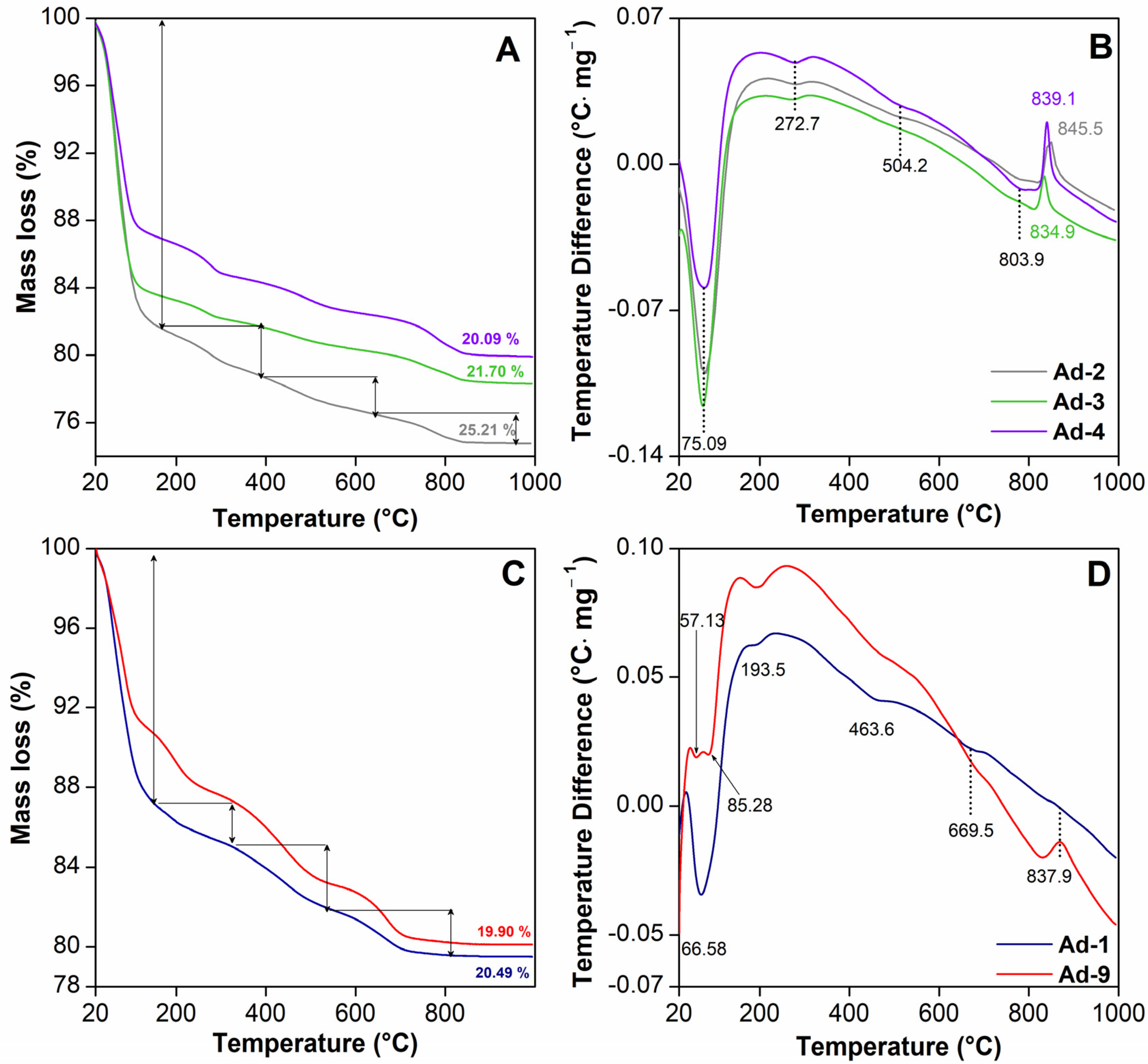
The TGA and DTA curves of the samples are shown in Figure 4 for the temperature range of 20–1000 °C. The TGA and DTA curve of sepiolite-rich assemblage 1 shows an endothermic peak at 75 °C, which corresponds to the removal of moisture water with a mass loss of 18.35% (Figure 4A,B). Another endothermic peak was observed at 272 °C, which represents the desorption of bound water in sepiolite [59]. There was an endothermic effect at 504 °C, with a weight loss of 2.22%, which can be attributed to the loss of water coordinated with the inner edges in the collapsed channels (Figure 4A,B). In the high temperature zones, there was a very marked endothermic effect at 803 °C, with a mass loss of 1.66% due to the loss of octahedral OH and probably the loss of OH of some edge SiOH [60]. This was followed by a phase change exothermic peak at 851 °C, which has also been observed by other authors in sepiolite [59] (Figure 4A,B). In the palygorskite-rich assemblage 2, the first endothermic mass loss of 12.55% between 20 and 140 °C, with a maximum rate at 66 °C, was due to the dehydration of adsorbed water (Figure 4C,D). The second endothermic mass loss of 2.78% between 140 and 320 °C, with a maximum rate at 193 °C, was due to the dehydration of the zeolitic water. In the range of 320–700 °C, two endothermic mass losses of 6.0% occurred due to the dehydration of the bound water in palygorskite and the dehydroxylation of the smectite (Figure 4C,D). The loss of bound water molecules occurred in two distinct steps, accompanied by a reversible structural transformation characteristic of sepiolite-like behavior. The fourth endothermic mass loss of 0.13% between 800 and 860 °C, with the maximum rate of change at 837 °C, originated from the dehydroxylation of palygorskite [61].
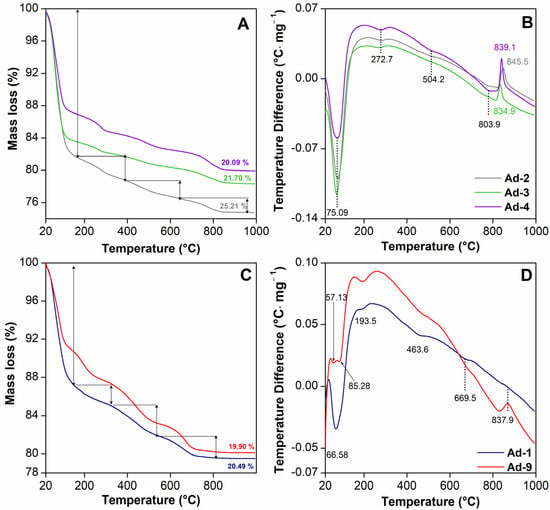
Figure 4.
Thermal analysis of the fibrous Mg clays studied. TGA and DTA curves of sepiolite-rich samples (assemblage 1) (A,B) and palygorskite-rich samples (assemblage 2) (C,D).
3.1.5. Petrography
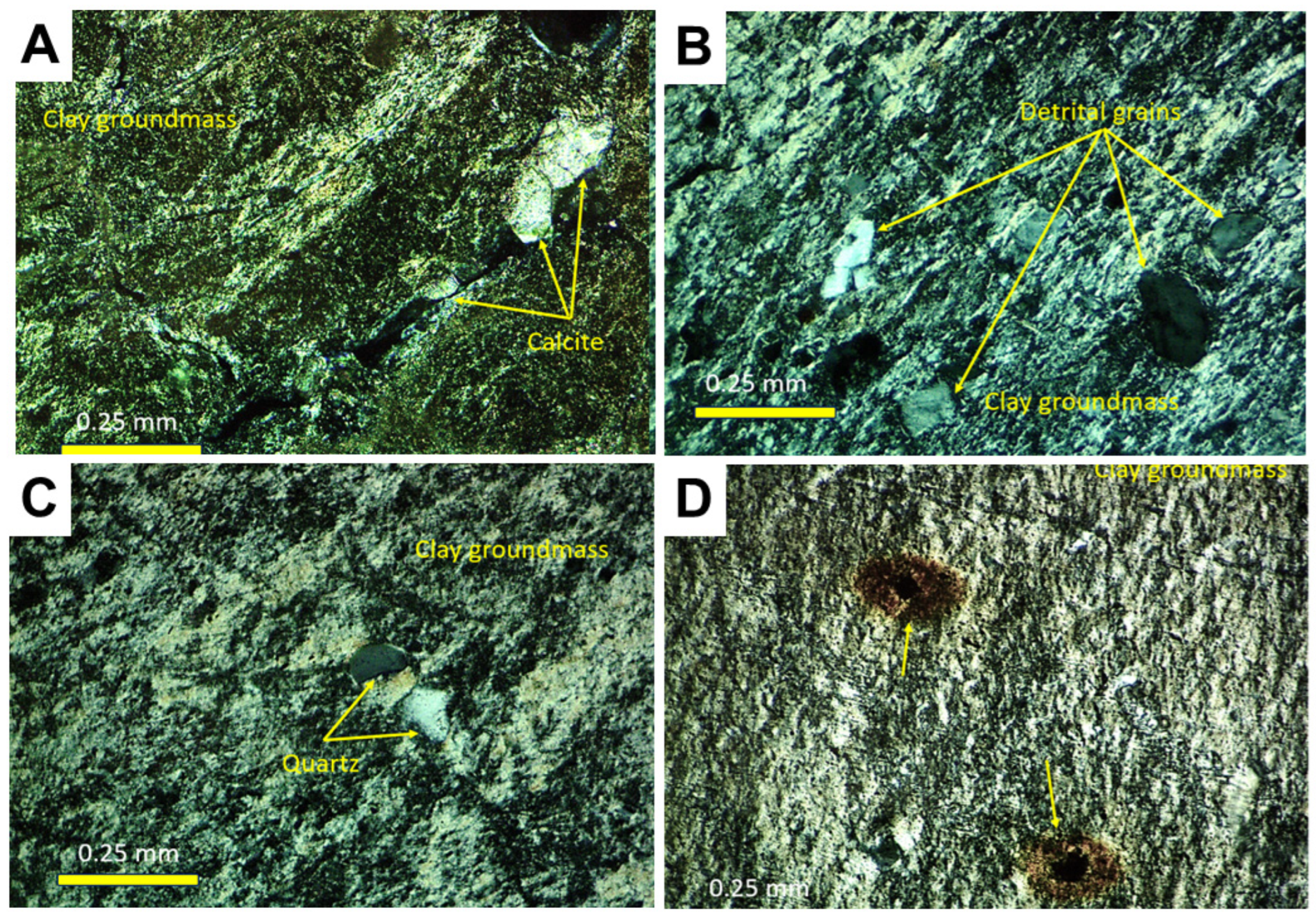
The petrographic features of the samples can be observed in Figure 5 and are described as follows:
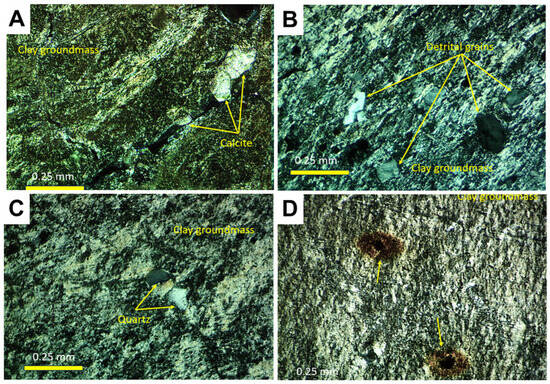
Figure 5.
Petrography. (A) Petrographic appearance of sample Ad.1, showing the presence of calcite sparitic crystals cementing cracks in the clay mass and oriented clays in coatings (N+). (B) Thin section detail of Ad.3, showing frequent detrital grains (mainly quartz) in the birefringent clay mass (N+). (C) Birefringent fabric and scattered quartz grains in sample Ad.4 (N+). (D) Textural features of sample Ad.9, showing lamination and opaque inclusions (yellow arrows) releasing iron phases around them (N+).
Ad-1. This consists of mudstones with evidence of pedality and the development of intraclastic morphologies (granules). They show evidence of bioturbation, birefringent fabrics, and sparitic calcite cements. The non-clay components include quartz, orthoclase, microcline, plagioclase, muscovite, biotite, zircon, pyrolusite, and calcite (Figure 5A).
Ad-3. This is formed by claystones with desiccation morphologies (brecciation), abundant scattered detrital grains, and birefringent fabrics. Non-clay components include quartz, orthoclase, microcline, plagioclase, muscovite, biotite, zircon, tourmaline, apatite, and calcite (Figure 5B).
Ad-4. Claystone with isotropic relict inclusions, root bioturbation, and birefringent fabrics. The non-clay grain content is low, consisting of minor quartz, orthoclase, plagioclase, and tourmaline (Figure 5C).
Ad-9. Shale with opaque inclusions surrounded by orange halos and evidence of recrystallization of the clay material. Detrital grains are barely identifiable (Figure 5D).
3.1.6. SEM-TEM
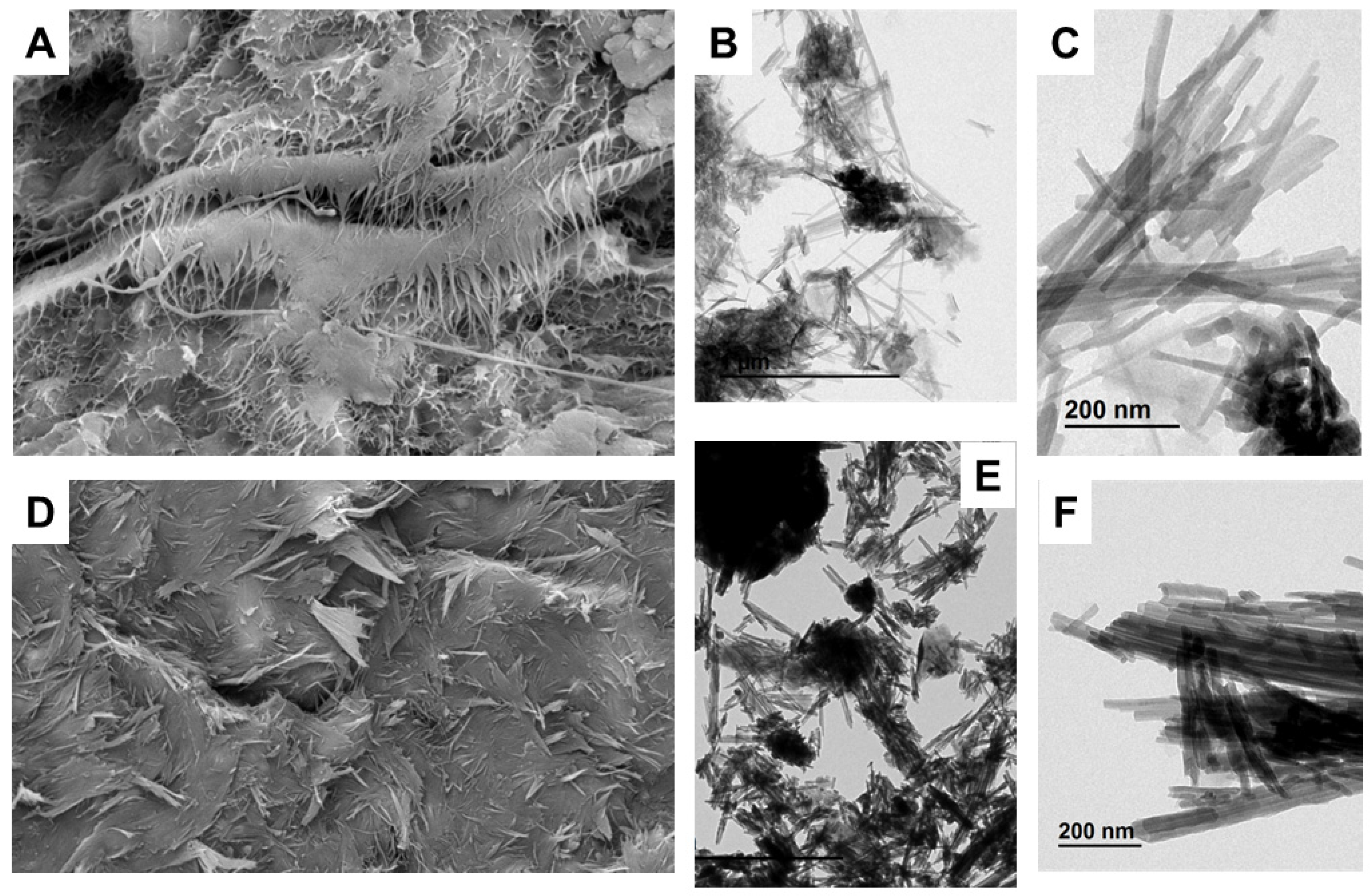
The microfabric characteristics of the clays studied are shown in Figure 6. Two samples (Ad-9 and Ad-4) representative of the associations I and II were examined.
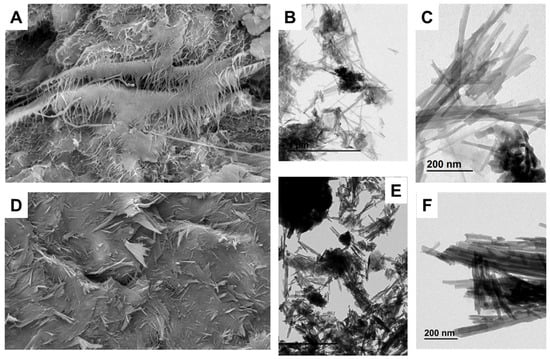
Figure 6.
SEM, TEM, and backscattered electron (BSE) images of the fibrous Mg clays studied. (A) SEM image of a sample of the assemblage with sepiolite (Ad-4), showing the laminar arrangement of the fiber aggregates (BSE). (B) TEM image showing the small fiber aggregates and the presence of magnesium smectite flake morphologies (Ad-4). (C) TEM detail of the fiber aggregates formed by sepiolite laths (Ad-4). (D) SEM image of a sample of the assemblage with palygorskite (Ad-9), showing the dense distribution of fibers with evidence of compaction in one of the layers forming the clay material (BSE). (E) TEM image showing fiber aggregates of palygorskite and flake morphologies belonging to aluminum smectites (Ad-9). (F) TEM detail of fiber aggregates formed by palygorskite laths (Ad-9).
Sample Ad.4 showed fibrous aggregates characteristic of sepiolite with a laminar to matrix microstructure and interlaminar porosity (Figure 6A). TEM revealed the small size of the fibrous aggregates and the presence of flake morphologies belonging to smectite (Figure 6B). In detail, the fiber aggregates are formed by laths that group together to form the fibers (Figure 6C).
Sample Ad.9 showed very dense aggregates of palygorskite fibers parallel to the laminated structure with low porosity (Figure 6D). In TEM, the fiber aggregates appeared alongside the flake morphologies corresponding to the smectites (Figure 6E). In detail, the fiber aggregates are formed by palygorskite laths, similar to those of sepiolite but larger in size.
3.1.7. Physicochemical and Textural Properties
The CEC values of assemblage 2 were between 32 and 53 cmol+·kg−1, being higher in the Ad-1 sample (Table 4), which was related to the dioctahedral smectite content of this sample. In assemblage 1, the CEC values ranged between 37 and 48 cmol+·kg−1, the maximum in the sample, with the higher quantity of sepiolite (Ad-2) indicating the lower effect of trioctahedral smectite content on this property.

Table 4.
Physicochemical and textural properties.
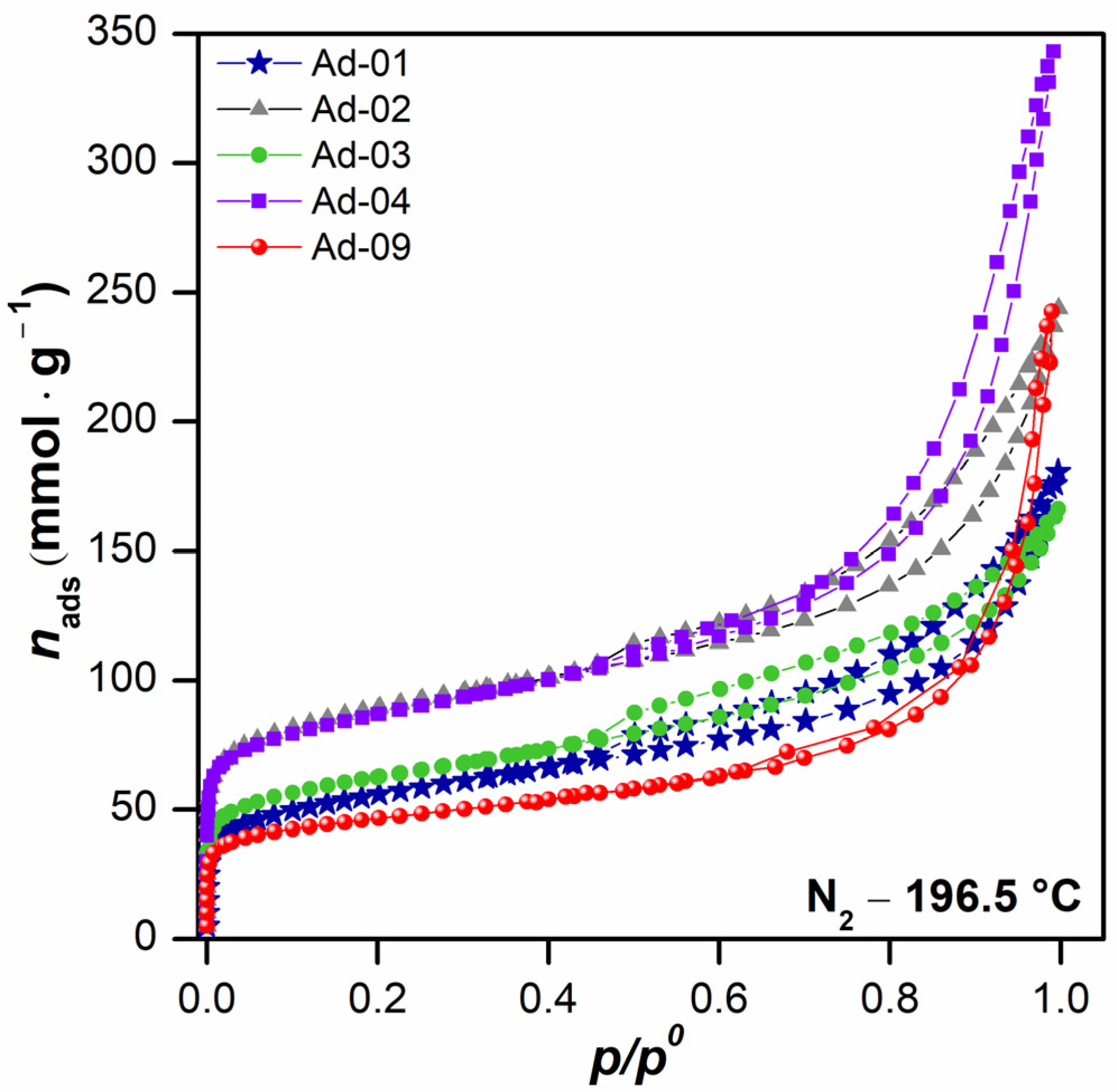
N2 adsorption–desorption isotherms at 196.5 °C are shown in Figure 7. The isotherms can be classified as type II according to the IUPAC classification, associated in this case with the presence of large mesopores, in which there is an important adsorption increase at high relative pressures [51]. However, the hysteresis cycles exhibited different classifications. For instance, Ad-2, Ad-4, and Ad-9 are considered an H3-type cycle, which is associated with the presence of non-rigid aggregates of plate-like particles. Meanwhile, fibrous Mg clays Ad-1 and Ad-3 exhibit an H4-type cycle, which is associated with the presence of narrow slit-shaped pores, indicating a lower quantity of large mesopores [51,62,63]. The almost negligible hysteresis loop in Ad-9 is noteworthy, as this suggests that this clay has a low quantity of intermediate mesopores. The comparison between the volume of mesopores revealed that Ad-4 (sepiolite-rich) is the clay with the highest quantity of large-sized pores, followed by Ad-9, which belongs to association 2, palygorskite-rich, which is consistent with the results reported in the literature [62,63].
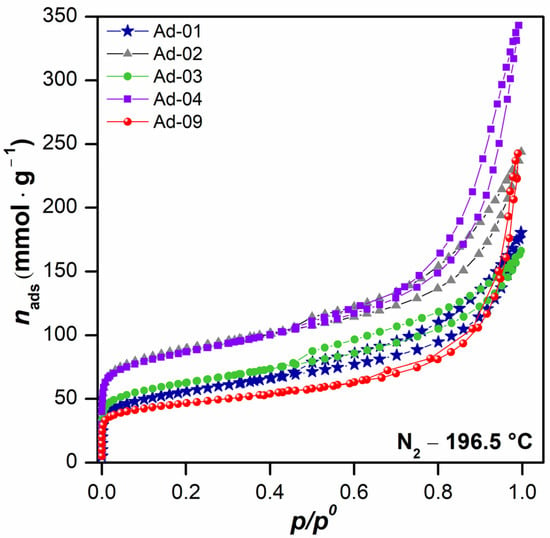
Figure 7.
N2 adsorption–desorption isotherms at −196.15 °C of fibrous Mg clays.
The textural properties are shown in Table 4. The specific surface area (SBET) of sepiolite-rich samples was higher than that obtained for palygorskite-rich samples. In assemblage 1 (sepiolite-rich), a positive correlation was observed between the sepiolite content and the SBET values. This is in agreement with the shape of their isotherms, where an overlap can be seen at low relative pressure values (Figure 7). This was also associated with the presence of a small amount of microporosity, as is shown by the Vμp values obtained. In these samples, Ad-4 adsorbed the highest total volume, as was expected due to its isotherm behavior at high p/p0 values. Similar SBET values were previously observed for this type of clay [62,63].
In the palygorskite-rich samples, Ad-1 presented a higher SBET than Ad-9, with a similar contribution of micropores in both samples. The total volume adsorbed, Vt, indicated that Ad-9 was made up of pores with a larger size, and therefore, this parameter is higher. Similar results have been reported by other authors using these kinds of clay [36,64].
3.2. Adsorption Studies
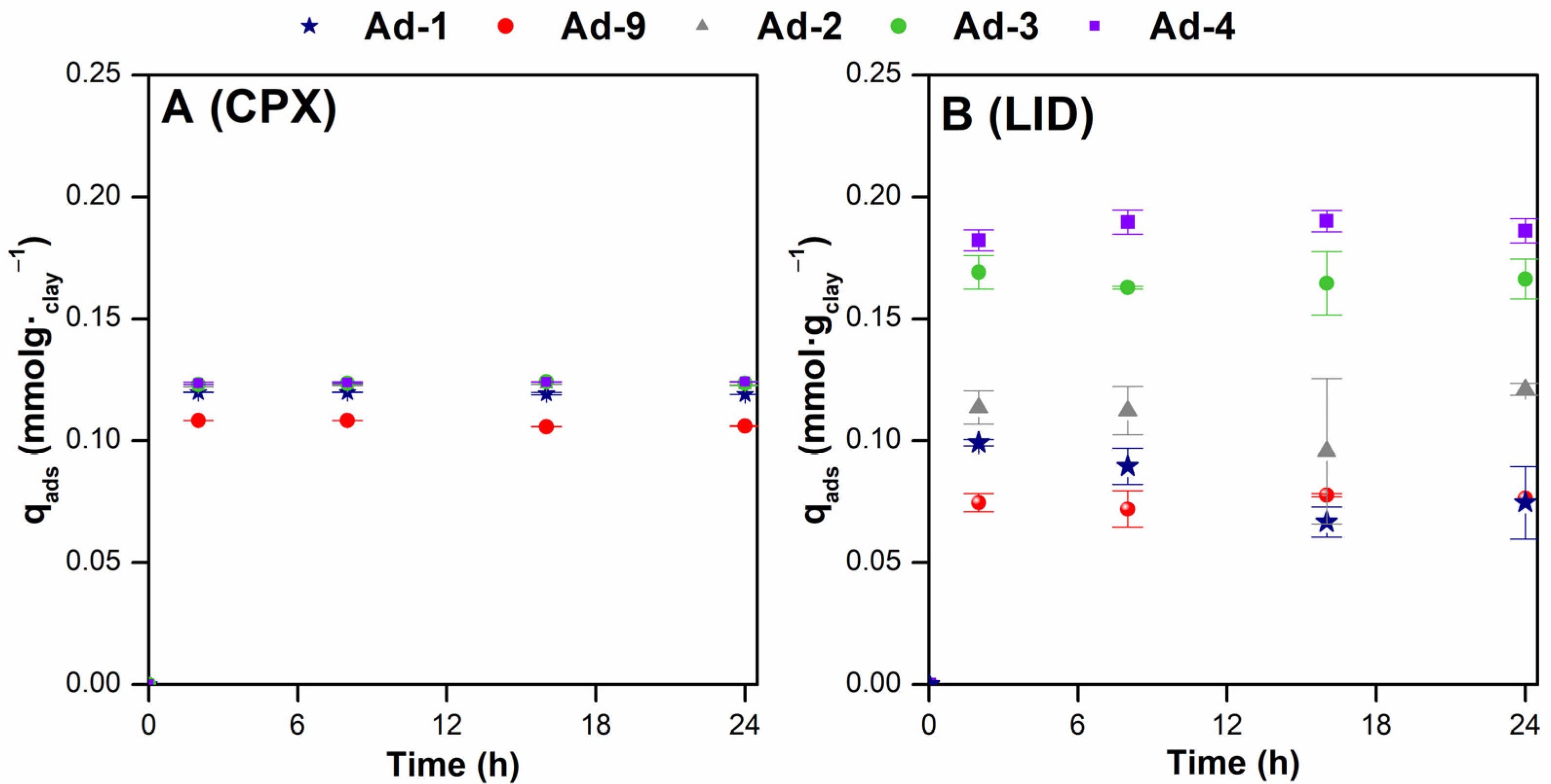
From Figure 8, it can be observed that the adsorption capacity of CIP and LID reached equilibrium in 2 h. Any increase in contact time after 2 h did not enhance the adsorption onto the fibrous Mg clays. This indicated that all adsorption data from this study were obtained under equilibrium conditions. The results showed that the CPX adsorption is similar in all of the samples analyzed (Figure 8A). Conversely, at the concentration tested in the kinetic experiment, LID adsorption onto sepiolite was higher compared to the adsorption onto palygorskite (Figure 8B). Moreover, differences in the LID adsorption among the samples of association 1 (sepiolite-rich) were also recorded and were lowest in the Ad-2 sample.
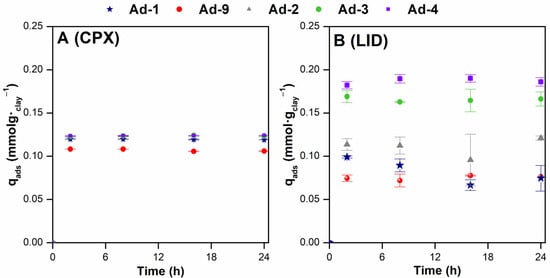
Figure 8.
Effect of contact time on the adsorption of CPX (A) and LID (B) onto the fibrous Mg clays studied under the following experimental conditions: dosage 2.5 g·L−1, C0 = 110 (CPX) and 500 (LID) mg·L−1, T = 20 °C, rpm = 3600, and pH ~ 6.
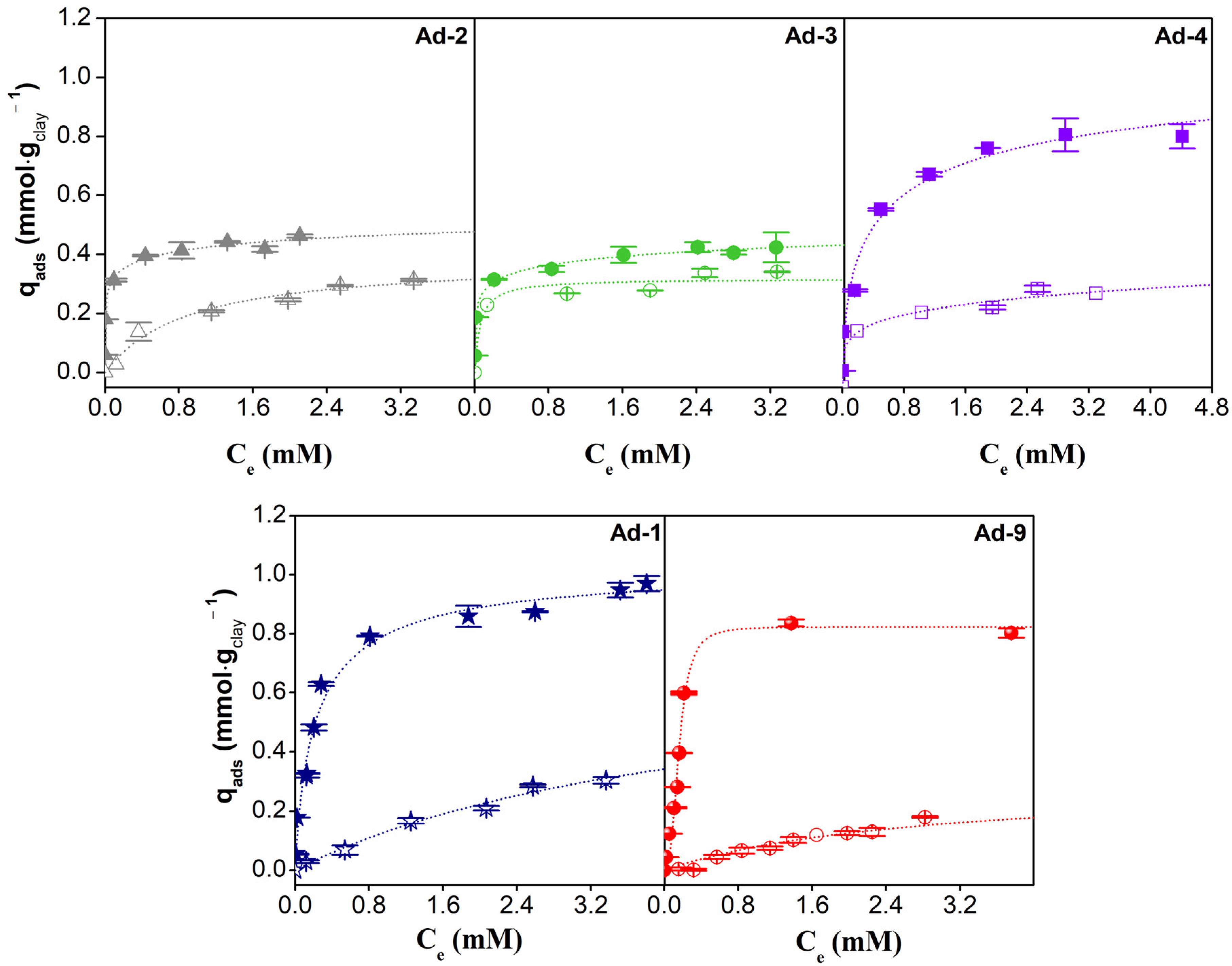
The experimental results and fitted isotherms for CPX and LID adsorbed on the fibrous Mg clays studied are shown in Figure 9. All the adsorption isotherms were of the L type (Langmuir) according to [65]. However, it is outstanding that the higher affinity for CPX and LID species is present in solutions at lower equilibrium concentration values in sepiolite-rich samples. The palygorskite-rich samples exhibited a higher CPX adsorption capacity when compared to sepiolite. However, the adsorption capacity of Ad-4 (sepiolite-rich) stood out among the other sepiolite samples and is even of the order of the palygorskite samples. On the other hand, LID adsorption capacity was higher for sepiolite-rich samples.
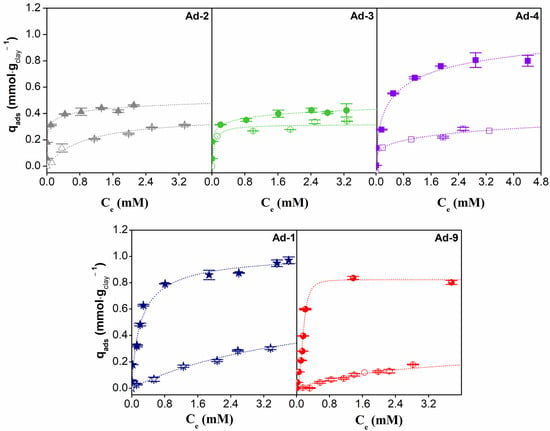
Figure 9.
Adsorption isotherms of CPX (full points) and LID (empty points) at 20 °C on fibrous Mg clay and their best fit.
The values obtained from fitting the parameters of the Freundlich, Langmuir, and Sips models are given in Table 5. The Sips model is the best fit for CPX adsorption data, suggesting highly heterogeneous systems. The adsorption capacities (qmáx) were higher in palygorskite-rich samples (Ad-1 and Ad-9) and the Ad-4 sepiolite sample.

Table 5.
Langmuir, Freundlich, and Sips parameters for CPX and LID adsorption on fibrous Mg clays.
In the case of LID adsorption, the best fit was obtained for the Langmuir model in the case of assemblage 1 and the Freundlich model in the case of assemblage 2, suggesting that for these systems, the LID species was adsorbed in the same type of available site. Higher adsorption capacities were observed in the sepiolite assemblage, where the amounts adsorbed were very similar among the samples and followed the order Ad4 > Ad-2 > Ad-3.
The Sips model parameters indicated a higher affinity (b) for both CPX and LID species present in solution in sepiolite samples, which would be related to a higher adsorption capacity at a low concentration. This is consistent with the isotherm shape and is particularly important in environmental protection uses of natural clays because most contaminants are present in very low concentrations.
If the same analysis were to be carried out for the parameter n, which represents the heterogeneity of the system (or adsorption intensity), it would be observed that in all cases, the values would be greater than unity for CPX adsorption. The n values greater than 1 might suggest either a homogeneous surface or the presence of cooperative adsorption, potentially indicating that in the systems studied, once a molecule had been adsorbed onto the surface, it might become a new adsorption site for subsequent molecules.
In the case of LID adsorption, the parameter obtained (b) was, in all cases, lower than that obtained for CPX systems, which indicated a lower affinity of the LID species for the surface of the clays. On the other hand, the n values indicated that adsorption would occur on a single type of available site, which is consistent with the fact that these systems fitted better to the Langmuir and the Freundlich models.
4. Discussion
4.1. Assemblage 1 (Sepiolite + Trioctahedral Smectite)
With regard to CPX adsorption, no relationship was observed between the CEC and qm values obtained by the Sips model. Moreover, all the samples showed maximum adsorption capacities much higher than their CEC values (2.4 times more in the case of Ad-4). The LID adsorption showed a positive correlation with CEC values, and, in this case, the Langmuir maximum adsorption capacities were slightly lower than their CEC.
At the pH (5.90) used in this analysis, the species present in solution were positively charged CPX+ or LID+ and the deprotonated carboxylate group of CPX±. In addition, the point of zero charge for natural sepiolite, as determined by other studies, was 3.22 [46,66,67]. Therefore, the external surface of sepiolite would be negatively charged, increasing electrostatic attractions between positively charged CPX+/LID+ and the adsorption sites. According to this, ion exchange and electrostatic interactions between positively charged CPX+ and LID+ species and the negatively charged clay surface would be the main mechanism for LID adsorption. This is in agreement with kinetic results that indicated an instantaneous interaction. Also, the high affinity observed at low concentrations for this species in solution could reaffirm this process. This kind of interaction would occur mainly with sepiolite because trioctahedrical smectite does not contribute to the CEC of this group of clays, as there was an inverse relationship between CEC and smectite content.
Conversely, CPX adsorption values exceeding the CEC would indicate the presence of other mechanisms. In this case, elevated adsorption values could be attributed to the high values of SBET, suggesting that physisorption in the mesoporous structure of sepiolite could be an important mechanism because it was highest in the sample with the highest total porosity (VT) and mesoporosity (Vmp) volume (Ad-4). This sample is the purest from a mineralogical point of view because it is composed of 99% phyllosilicates and recorded the highest CPX adsorption values among all the analyzed samples in this study. These results agree with previous studies on magnesium non-fibrous clays reported by the research team, where in kerolite/smectite composite samples, it was observed that kerolite mesoporosity was mainly responsible for CPX adsorption [14].
4.2. Assemblage 2 (Palygorskite + Dioctahedral Smectite)
In this assemblage, higher adsorption capacities for both EC, CPX, and LID were observed in the Ad-1 sample. This sample was characterized by having a higher quantity of dioctahedral smectite compared to Ad-9, which resulted in a higher CEC value. The CEC of palygorskite clays ranges from 4 to 40 cmol+·kg−1 and is typically in the range of 20–30 cmol+·kg−1, but the greater values are likely to be related to impurities, particularly smectite [30,68], as it would be in the case of Ad-1.
Cation exchange with dioctahedral smectite surfaces could be partly responsible for CPX sorption at the pH (5.9) used because the predominant CPX species are CPX+ and the zwitterionic form (with its protonated amine group and deprotonated carboxylic group), which can be adsorbed onto the negatively charged structural sites of smectite clays. Indeed, cation exchange with sodium was identified as the main mechanism contributing to the adsorptive removal of CPX by clay minerals [45,69,70]. Also, at the pH values examined in the present study, the predominant species of LID would be its cationic form (LID+), favoring electrostatic attraction processes.
With regard to the interactions with palygorskite, the channel size of this mineral is 3.7 Å × 6.4 Å [68], while the molecular size of CPX is 13.5 Å long, 7.4 Å high, and 3.0 Å thick, and LID is 10.4 Å long, 5.9 Å high, and 6.0 Å thick. Thus, the channel size of palygorskite was too small to allow access to CPX or LID. In such a case, the adsorbed CPX or LID can only be retained on the sites available on the external surface of this clay mineral. Palygorskite has negative surface charges in the entire pH range from 2 to 12 due to a large number of Si-O− sites [35]. Therefore, the processes of adsorption of CPX or LID on palygorskite would also be by electrostatic interactions between the negative surface charge of their layers and positively charged CPX+ or LID+. However, both samples adsorbed quantities of CPX much higher than their CEC values, and this can be explained by the occurrence of another type of interaction. The presence of the deprotonated carboxylate group of CPX± species could favor CPX interaction with the broken edge groups on the surface (Si-OH) to form inner sphere complexes, increasing the total amount adsorbed for these clays [71]. This kind of adsorption mechanism has been reported for CPX adsorption on other natural and modified clays [52]. Conversely, LID sorption capacities were lower than the CEC of the samples and higher in the sample with a higher CEC, indicating that ion exchange would be the main mechanism for the adsorption of this molecule onto the surface of the clays.
All the samples showed a higher adsorption capacity for CPX than for LID in the conditions studied. This has already been seen in a previous study of CPX and LID adsorption onto non-fibrous Mg clay [14]. CPX is a flat-shaped molecule with a lower thickness than LID. In addition, the positive charge of the CPX is located on its amine group situated at one end of the molecule, which would facilitate the interaction with the solids’ surface. Additionally, at the pH analyzed, the zwitterionic form of CPX was also present and can therefore be adsorbed on different surface reactive sites.
In the LID molecule, voluminous carbon chains surround the protonated group, hindering interaction with the solid surface. Also, the larger size of the LID molecule could make its interaction with the mesoporous structure of fibrous clays difficult.
Finally, the adsorbent performance for the uptake of CPX from aqueous media by the fibrous Mg clays, in terms of the maximum adsorption capacity (qmax), was compared with the results reported in [72] for a large quantity of natural and synthetic adsorbents. The studied fibrous Mg clays showed higher adsorption capacity than several types of industrial adsorbents listed in [72], being surpassed only by microporous synthetic materials such as magnetic N-doped porous carbon, Fe-based MOF, AC from bamboo, etc. This is a very important finding since these clays are a low-cost, natural, eco-friendly, and widespread material that has not been used before for this kind of application.
5. Conclusions
Fibrous Mg clays showed a higher adsorption capacity for CPX than for LID in the conditions studied. CPX adsorption on sepiolite and palygorskite could be the result of the combination of various mechanisms: ion exchange with permanently charged sites, electrostatic attractions with external surface, and an inner sphere complex with broken edges. Dioctahedral smectite as an associated phase contributed to a higher removal percentage in the palygorskite samples. By contrast, the trioctahedral smectite did not play a significant role in the adsorption of the samples with sepiolite.
LID adsorption occurred, in all cases, by ion exchange and electrostatic interaction with the external surfaces of the clays. LID was more effectively removed by sepiolite samples because they have a higher specific surface and more available reactive sites. The size and the location of the charge in the LID molecule make its interaction with the surface and mesoporous structure of the clays more difficult.
The shape, the location of the charge, and the presence of two types of species in the CPX molecule enhanced its adsorption onto the clays analyzed. The mesoporous structure of fibrous clays played a key role in CPX adsorption, particularly the presence of mesopores of an intermediate size.
While additional studies—such as those involving pH variation, dosage optimization, and other operational parameters—are necessary to evaluate the scalability and practical implementation of these materials, the present work provides a foundational framework for investigating the adsorption of emerging contaminants onto fibrous clay minerals. To the best of our knowledge, this is the first study that reports adsorption data of LID onto fibrous clays.
Author Contributions
Conceptualization, T.B.M., M.E.R.-J. and M.P.; methodology, M.E.R.-J., V.R.-A., M.S. and A.M.; formal analysis, T.B.M., M.E.R.-J., M.P., V.R.-A., M.S. and A.M.; investigation, M.P., T.B.M., G.P., M.T.B. and M.E.R.-J.; resources, M.E.R.-J. and M.P.; data curation, V.R.-A., M.S., A.M., T.B.M., G.P. and M.P.; writing—original draft preparation, T.B.M., M.E.R.-J. and M.P.; writing—review and editing, T.B.M., M.E.R.-J., A.M. and M.P.; project administration, M.E.R.-J. and M.P.; funding acquisition, M.P., L.V., M.T.B., A.P.-A. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Autonomous University of Madrid (FUAM 2023/006) and the National University of Comahue (Argentina) under the Funding Project I04-249, entitled “Natural and Modified Clay Minerals for Applications in Health and Environment”.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are grateful to Minersa Group, the National University of Comahue (UNCo), PROBIEN Institute (CONICET-UNCo), and the Autonomous University of Madrid for providing technology and financial support.
Conflicts of Interest
Luis Villa and Alejandro Pérez-Abad are employed by Minersa Group. The paper reflects the views of the scientists and not the companies.
References
- Undabeytia, T.; Madrid, F.; Vázquez, J.; Pérez-Martínez, J.I. Grafted sepiolites for the removal of pharmaceuticals in water treatment. Clays Clay Miner. 2019, 67, 173–182. [Google Scholar] [CrossRef]
- Caban, M.; Białk-Bielińska, A.; Stepnowski, P.; Kumirska, J. Current issues in pharmaceutical residues in drinking water. Curr. Anal. Chem. 2016, 12, 249–257. [Google Scholar] [CrossRef]
- Praveena, S.M.; Mohd Rashid, M.Z.; Mohd Nasir, F.A.; Sze Yee, W.; Aris, A.Z. Occurrence and potential human health risk of pharmaceutical residues in drinking water from Putrajaya (Malaysia). Ecotoxicol. Environ. Saf. 2019, 180, 549–556. [Google Scholar] [CrossRef]
- Rodil, R.; Quintana, J.B.; Concha-Graña, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Meffe, R.; de Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Degradation of lidocaine, tramadol, venlafaxine and the metabolites O-desmethyltramadol and O-desmethylvenlafaxine in surface waters. Chemosphere 2013, 90, 1952–1959. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Q.; Huang, B.; Cheng, H.; Wang, L.; He, Q. Removal of ciprofloxacin as an emerging pollutant: A novel application for bauxite residue reuse. J. Clean. Prod. 2020, 253, 120049. [Google Scholar] [CrossRef]
- Ghosh, S.; Harsha, N.V.M.S.; Singh, S.P.; Shriwastav, A. Simultaneous removal of ciprofloxacin and disinfection from wastewater by combined photocatalytic reactor (PCR) and membrane bioreactor (MBR) system. J. Environ. Chem. Eng. 2023, 11, 110855. [Google Scholar] [CrossRef]
- Balarak, D.; Zafariyan, M.; Siddiqui, S. Investigation of adsortive properties of surfactant modified sepiolite for removal of ciprofloxacin. Int. J. Life Sci. Pharma Res. 2020, 10, 12–19. [Google Scholar]
- Jara-Cobos, L.; Peñafiel, M.E.; Montero, C.; Menendez, M.; Pinos-Vélez, V. Ciprofloxacin removal using pillared clays. Water 2023, 15, 2056. [Google Scholar] [CrossRef]
- Kelly, K.R.; Brooks, B.W. Global Aquatic Hazard Assessment of Ciprofloxacin: Exceedances of Antibiotic Resistance Development and Ecotoxicological Thresholds. Prog. Mol. Biol. Transl. Sci. 2018, 159, 59–77. [Google Scholar]
- Berhane, T.M.; Levy, J.; Krekeler, P.S.; Danielson, N.D. Adsorption of bisphenol A and ciprofloxacin by palygorskite-montmorillonite: Effect of granule size, solution chemistry and temperature. Appl. Clay Sci. 2016, 132–133, 518–527. [Google Scholar] [CrossRef]
- Gulen, B.; Demircivi, P. Adsorption properties of flouroquinolone type antibiotic ciprofloxacin into 2:1 dioctahedral clay structure: Box-Behnken experimental design. J. Mol. Struct. 2020, 1206, 127659. [Google Scholar] [CrossRef]
- Roca-Jalil, M.E.; Musso, T.; Rodriguez-Ameijide, V.; Sanchez, M.; Maggio, A.; Baschini, M.T.; Pettinari, G.; Villa, L.; Pozo, M.; Pérez-Abad, A. Adsorption of ciprofloxacin and lidocaine by non-fibrous raw Mg-clays: The role of composition and texture. Minerals 2024, 14, 966. [Google Scholar] [CrossRef]
- Rodriguez, O.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Abdulsalam, A.; Ibrahim, Y.S.; Yaro, N.A.; Olanrewaju, O.A.; Alhassan, B.; Iyayosa, D.A.; Lawal, K. Emerging contaminants removal from wastewater using organo-modified bentonite clay. J. Eng. Res. Rep. 2023, 25, 121–144. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, P.; Hrichi, A.; Garrido-Zoido, J.M.; Alvarez-Torrellas, S.; Larriba, M.; Gil, M.V.; Ben Amor, H.; García, J. Natural clays as adsorbents for the efficient removal of antibiotic ciprofloxacin from wastewaters: Experimental and theoretical studies using DFT method. J. Ind. Eng. Chem. 2024, 134, 137–151. [Google Scholar] [CrossRef]
- Krekeler, M.P.S.; Guggenheim, S. Defects in microstructure in palygorskite-sepiolite minerals: A transmission electron microscopy (TEM) study. Appl. Clay Sci. 2008, 39, 98–105. [Google Scholar] [CrossRef]
- Bailey, S.W. Structure of layer silicates. In Crystal Structures of Clay Minerals and Their X-Ray Identification; Brindley, G.W., Brown, G., Eds.; Mineralogical Society: London, UK, 1980; pp. 1–123. [Google Scholar]
- Galán, E. Industrial applications of sepiolite from Vallecas-Vicalvaro, Spain: A review. In Proceedings of the International Clay Conference, Denver, CO, USA, 28 July–2 August 1985; Schultz, L.C., Van Olphen, H., Mumpton, F.A., Eds.; Bloomington, IN, USA, 1987; pp. 400–404. Available online: https://pubs.geoscienceworld.org/clays/books/edited-volume/2670/chapter-abstract/145119976/Industrial-Applications-of-Sepiolite-from-Vallacas?redirectedFrom=fulltext (accessed on 10 October 2025).
- Ruiz-Hitzky, E. Molecular access to intracrystalline tunnels of sepiolite. J. Mater. Chem. 2001, 11, 86–91. [Google Scholar] [CrossRef]
- Galán, E.; Pozo, M. Palygorskite and sepiolite deposits in continental environments. Description, genetic patterns and sedimentary settings. In Developments in Clay Science; Elsevier B.V.: Amsterdam, The Netherlands, 2011; pp. 125–173. [Google Scholar]
- Song, N.; Hursthouse, A.; McLellan, I.; Wang, Z. Treatment of environmental contamination using sepiolite: Current approaches and future potential. Environ. Geochem. Health 2021, 43, 2679–2697. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Santarén, J.; Esteban-Cubillo, A.; Aparicio, P. Current industrial applications of palygorskite and sepiolite. In Developments in Clay Science; Elsevier B.V.: Amsterdam, The Netherlands, 2011; pp. 281–298. [Google Scholar]
- Viseras, C.; Lopez-Galindo, A. Pharmaceutical applications of some spanish clays (sepiolite, palygorskite, bentonite): Some preformulation studies. Appl. Clay Sci. 1999, 14, 69–82. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Berhane, T.M.; Levy, J.; Krekeler, M.P.S.; Danielson, N.D.; Stalcup, A. Sorption-desorption of carbamazepine by palygorskite-montmorillonite (PM) filter medium. J. Hazard. Mater. 2015, 282, 183–193. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Yu, T.-L.; Munkhbayer, S.; Kuo, T.-H.; Hung, Y.-C.; Jean, J.-S.; Lin, K.-H. Sorptive removal of tetracycline from water by palygorskite. J. Hazard. Mater. 2009, 165, 148–155. [Google Scholar] [CrossRef]
- Li, Z.; Fitzgerald, N.M.; Jiang, W.T.; Lv, G. Palygorskite for the uptake and removal of pharmaceuticals for wastewater treatment. Process Saf. Environ. Prot. 2016, 101, 80–87. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Chang, P.H.; Gao, Z.Y.; Xu, X.Y.; Chen, Y.H.; Wang, Z.H.; Chen, X.Y.; Yang, Z.Y.; Wang, T.H.; Jean, J.-S.; et al. Amitriptyline removal using palygorskite clay. Chemosphere 2016, 155, 292–299. [Google Scholar] [CrossRef]
- Sousa, M.U.; Rodrigues, A.M.; Araujo, M.E.B.; Menezes, R.R.; Neves, G.A.; Lira, H.L. Adsorption of sodium diclofenac in functionalized palygoskite clays. Materials 2022, 15, 2708. [Google Scholar] [CrossRef]
- Perelomov, L.; Gertsen, M.; Mandzhieva, S.; Sychev, V.; Dudnikova, T.; Khaidanov, I.; Perelomova, I.; Minkina, T.; Atroshchenko, Y. Adsorption of antibiotics by natural clay minerals. Minerals 2025, 15, 733. [Google Scholar] [CrossRef]
- Mu, B.; Wang, A. Adsorption of dyes onto palygorskite and its composites: A review. J. Environ. Chem. Eng. 2016, 4, 1274–1294. [Google Scholar] [CrossRef]
- Silva, V.C.; Araújo, M.E.B.; Rodrigues, A.M.; Cartaxo, J.M.; Menezes, R.R.; Neves, G.A. Adsorption behavior of acid-treated Brazilian palygorskite for cationic and anionic dyes removal from the water. Sustainability 2021, 13, 3954. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Leal, M.; Martínez-Hernández, V.; Meffe, R.; Lillo, J.; de Bustamante, I. Clinoptilolite and palygorskite as sorbents of neutral emerging organic contaminants in treated wastewater: Sorption-desorption studies. Chemosphere 2017, 175, 534–542. [Google Scholar] [CrossRef]
- Rodrigues, P.V.; Silva, F.A.N.G.; Pontes, F.V.M.; Barbato, C.N.; Teixeira, V.G.; de Assis, T.C.; Brandão, V.S.; Bertolino, L.C. Adsorption of glyphosate by palygorskite. Mater. Res. 2023, 26, e20220335. [Google Scholar] [CrossRef]
- Eren, E.; Afsin, B. Investigation of a basic dye adsorption from aqueous solution onto raw and pre-treated sepiolite surfaces. Dye. Pigment. 2007, 73, 162–167. [Google Scholar] [CrossRef]
- Özdemir, Y.; Doǧan, M.; Alkan, M. Adsorption of cationic dyes from aqueous solutions by sepiolite. Microporous Mesoporous Mater. 2006, 96, 419–427. [Google Scholar] [CrossRef]
- Santos, S.C.R.; Boaventura, R.A.R. Adsorption modelling of textile dyes by sepiolite. Appl. Clay Sci. 2008, 42, 137–145. [Google Scholar] [CrossRef]
- Küncek, I.; Şener, S. Adsorption of methylene blue onto sonicated sepiolite from aqueous solutions. Ultrason. Sonochem. 2010, 17, 250–257. [Google Scholar] [CrossRef]
- Kara, M.; Yuzer, H.; Sabah, E.; Celik, M. Adsorption of cobalt from aqueous solutions onto sepiolite. Water Res. 2003, 37, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Kocaoba, S. Adsorption of Cd(II), Cr(III) and Mn(II) on natural sepiolite. Desalination 2009, 244, 24–30. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Bedia, J.; Belver, C. Simultaneous adsorption of acetaminophen, diclofenac and tetracycline by organo-sepiolite: Experiments and statistical physics modelling. Chem. Eng. J. 2021, 404, 126601. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Wu, Z.; Gao, Y.; Li, X. Adsorption properties and mechanism of sepiolite modified by anionic and cationic surfactants on oxytetracycline from aqueous solutions. Sci. Total Environ. 2020, 708, 134409. [Google Scholar] [CrossRef]
- Bilgin, N.; Bulut, E.; Sabah, E. Mechanistic insight into amoxicillin removal by natural sepiolite. Int. J. Environ. Sci. Technol. 2023, 20, 8897–8912. [Google Scholar] [CrossRef]
- Schultz, L.G. Quantitative Interpretation of Mineralogical Composition from X-Ray and Chemical Data for the Pierre Shale. Geological Survey Professional Paper 391-C; United States Government Printing Office: Washington, DC, USA, 1964. [Google Scholar]
- Van der Marei, H.W. Quantitative analysis of clay minerals and their admixtures. Contrib. Mineral. Petrol. 1966, 12, 96–138. [Google Scholar] [CrossRef]
- Meier, L.P.; Kahr, G. Determination of the cation exchange capacity (cec) of clay minerals using the complexes of copper(ii) ion with triethylenetetramine and tetraethylenepentamine. Clays Clay Miner. 1999, 47, 38–388. [Google Scholar] [CrossRef]
- Villarroel-Rocha, J.; Barrera, D.; Blanco, A.A.; Jalil, M.E.R.; Sapag, K. Importance of the αs-plot Method in the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2013, 31, 165–183. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Maggio, A.A.; Roca Jalil, M.E.; Villarroel-Rocha, J.; Sapag, K.; Baschini, M.T. Fe- and SiFe-pillared clays from a mineralogical waste as adsorbents of ciprofloxacin from water. Appl. Clay Sci. 2022, 220, 106458. [Google Scholar] [CrossRef]
- Roca Jalil, M.E.; Toschi, F.; Baschini, M.; Sapag, K. Silica pillared montmorillonites as possible adsorbents of antibiotics from water media. Appl. Sci. 2018, 8, 1403. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Mineralogical Society: Oxford, MS, USA, 1997. [Google Scholar]
- Russell, J.D.; Fraser, A.R. Infrared methods. In Clay Mineralogy: Spectroscopic and Chemical Determinative Methods; Wilson, M.J., Ed.; Chapman & Hall: London, UK, 1994; pp. 11–67. [Google Scholar]
- Sabah, E.; Ouki, S. Sepiolite and sepiolite-bound humic acid interactions in alkaline media and the mechanism of the formation of sepiolite-humic acid complexes. Int. J. Miner. Process. 2017, 162, 69–80. [Google Scholar] [CrossRef]
- Madejová, J.; Gates, W.P.; Petit, S. IR Spectra of clay minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–149. [Google Scholar]
- Gunasekaran, S.; Anbalagan, G. Spectroscopic characterization of natural calcite minerals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Tabak, A.; Eren, E.; Afsin, B.; Caglar, B. Determination of adsorptive properties of a Turkish Sepiolite for removal of Reactive Blue 15 anionic dye from aqueous solutions. J. Hazard. Mater. 2009, 161, 1087–1094. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, J.L.; Galán, E. Determination of impurity in sepiolite by thermal analysis. J. Therm. Anal. 1994, 42, 131–141. [Google Scholar] [CrossRef]
- Önal, M.; Sarikaya, Y. Some physicochemical properties of a clay containing smectite and palygorskite. Appl. Clay Sci. 2009, 44, 161–165. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benítez-Guerrero, M.; Pozo, E.; Martín Rubí, J.A. Microwave assisted acid treatment of sepiolite: The role of composition and ‘crystallinity’. Appl. Clay Sci. 2014, 102, 15–27. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romero, E. Variability of the surface properties of sepiolite. Appl. Clay Sci. 2012, 67–68, 72–82. [Google Scholar] [CrossRef]
- He, D.; Huang, H.; Xu, W.; Qin, F.; Liu, S. Adsorption properties and mechanism of purified palygorskite on methylene blue. Arab. J. Geosci. 2018, 11, 658. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Santarén, J.; Rasteiro, M.G.; Gamelas, J.A.F. Improving colloidal stability of sepiolite suspensions: Effect of the mechanical disperser and chemical dispersant. Minerals 2020, 10, 779. [Google Scholar] [CrossRef]
- Tonkur, H.; Can, M.F.; Sabah, E. Rheological behavior of sepiolite suspensions homogenized by ultra-turrax high-speed homogenizer. Physicochem. Probl. Miner. Process. 2022, 58, 153415. [Google Scholar] [CrossRef]
- Galan, E. Properties and applications of palygorskite-sepiolite clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Li, Z.; Hong, H.; Liao, L.; Ackley, C.J.; Schulz, L.A.; MacDonald, R.A.; Mihelich, A.L.; Emard, S.M. A mechanistic study of ciprofloxacin removal by kaolinite. Colloids Surf. B Biointerfaces 2011, 88, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Roca Jalil, M.E.; Baschini, M.; Sapag, K. Influence of pH and antibiotic solubility on the removal of ciprofloxacin from aqueous media using montmorillonite. Appl. Clay Sci. 2015, 114, 69–76. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, C.; Zheng, X.; Liu, S.; Hu, F.; Xu, L.; Xu, G.; Peng, X. Removal of ciprofloxacin with aluminum-pillared kaolin sodium alginate beads (CA-Al-KABs): Kinetics, isotherms, and BBD model. Water 2020, 12, 905. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of ciprofloxacin from water: A comprehensive review. Korean Soc. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).