The Application of the Radiotracer Techniques in Hydrometallurgy: A Method for Online Monitoring of Solvent Extraction Processes Using 181Hf
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reagents
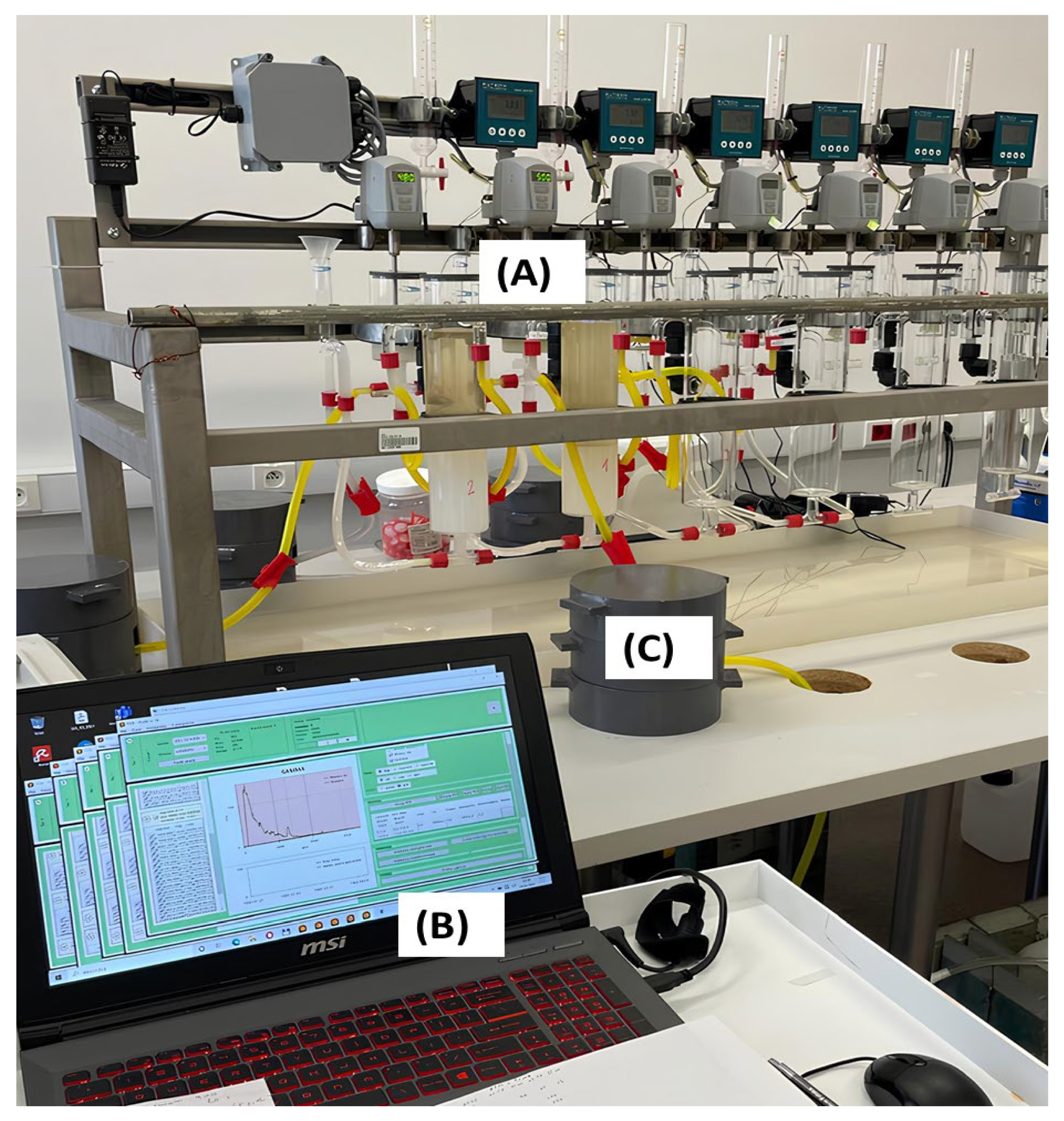
2.3. The Experimental Setup Based on a Mixer–Settler System
- It consists of a mixing chamber (mixer) with a capacity of 270 mL, allowing for precise control and manipulation of the solvent and feed phases during mixing.
- Decanter (settler): The system includes a decanter with a larger capacity of 1050 mL. The decanter is used for the settling phase of the solvent extraction process, where the phases separate based on their densities. This larger capacity allows for adequate settling time and separation of the phases.
- Peristaltic pumps: The system was equipped with two peristaltic pumps.
- Extraction and re-extraction system: The mixer–settler system is integrated with an extraction and re-extraction system, which involves the use of solvent phases to extract and separate desired components from the feed solution. This system is essential for carrying out solvent extraction processes effectively and efficiently in a maximum of six stages.
- Four scintillation probes with wound- and Pb-shielded tubes.
2.4. The Procedure for Sample Preparation and Analysis
- (a)
- Leaching
- (b)
- Solvent extraction process
- (c)
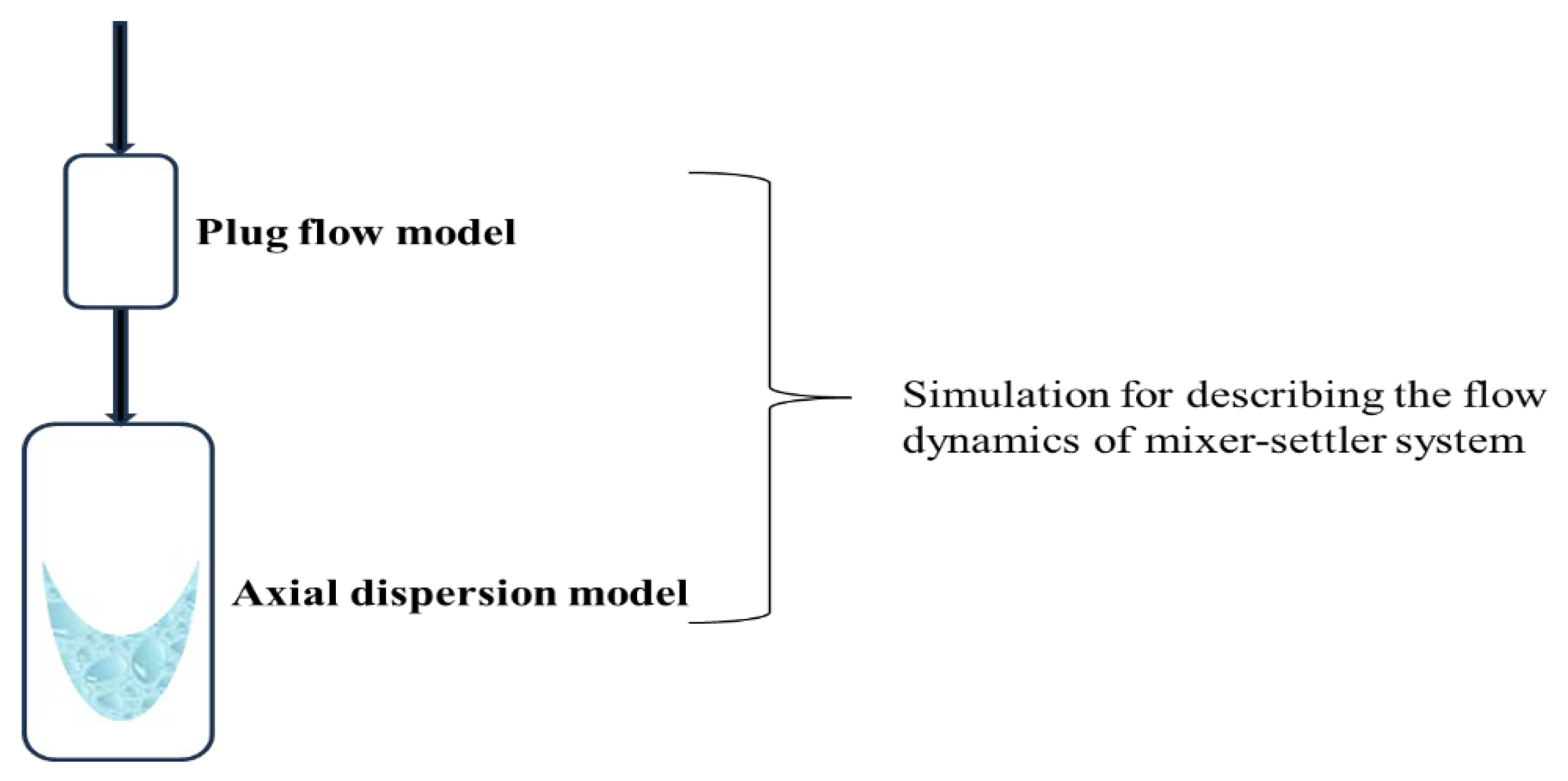
- Simulation of the flow dynamics
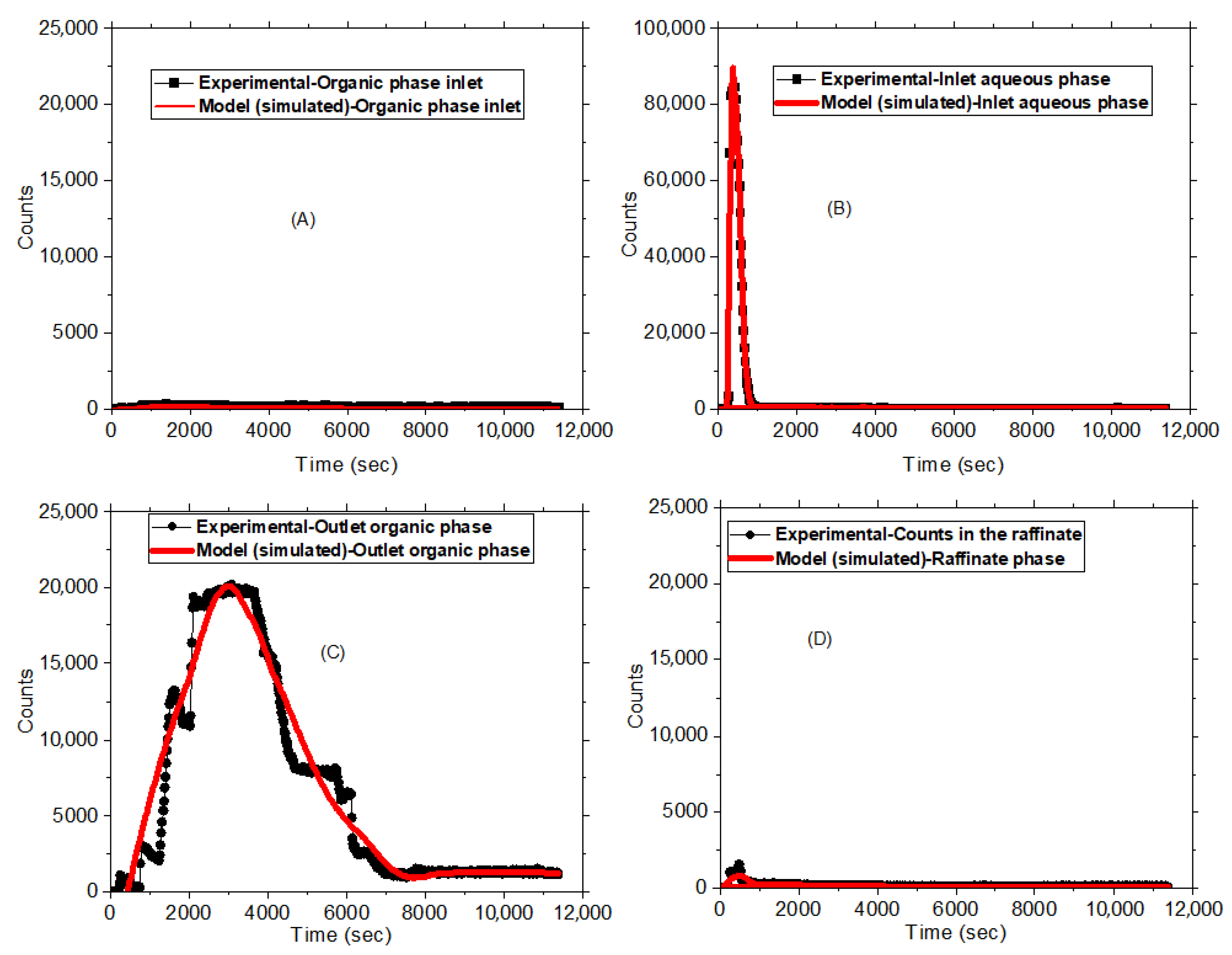
- Compute the response: This allows estimation of the response E∗H(t) of a model with respect to the specified inlet signal E(t). Here, H(t) stands for impulse response for the model while E∗H(t) refers to convolution of the inlet signal with the model’s impulse response.
- Optimize model parameters: If the actual system response S(t) has been acquired, the software can use that information to optimize the parameters of the model in such a manner that the calculated response E∗H(t) matches the actual S(t). This optimization is useful in making sure that the model reliably describes the behavior of the system. Some of the common parameters include mean residence time (τ) which denotes the average time a particle stays in the system, helping in understanding flow dynamics and the performance of the mixers in the extraction processes. The exchange coefficient (N) measures the degree of particle exchange between system phases, with high values signifying more efficient mass transfer. The time constant (t) assesses the response of the system to changes, where a short Tm indicates a quick and stable reaction. Péclet number (Pe) offers insight into the relative significance of diffusion and advection (convection) in a fluid system.
3. Results and Discussion
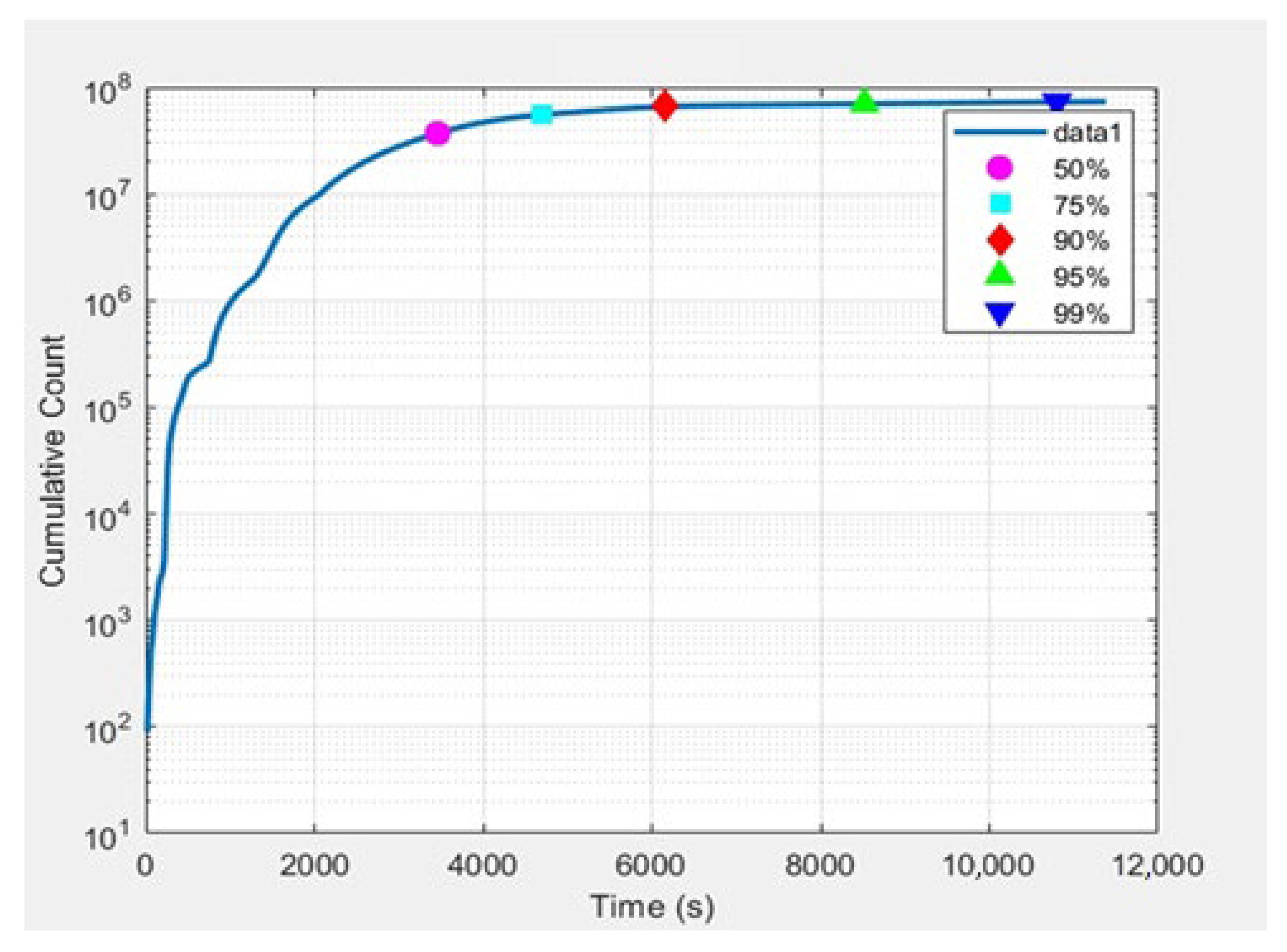
3.1. Online Monitoring of the Solvent Extraction Process of Hf
3.2. 181Hf4+ for the RTD Studies on the Mixer–Settler System
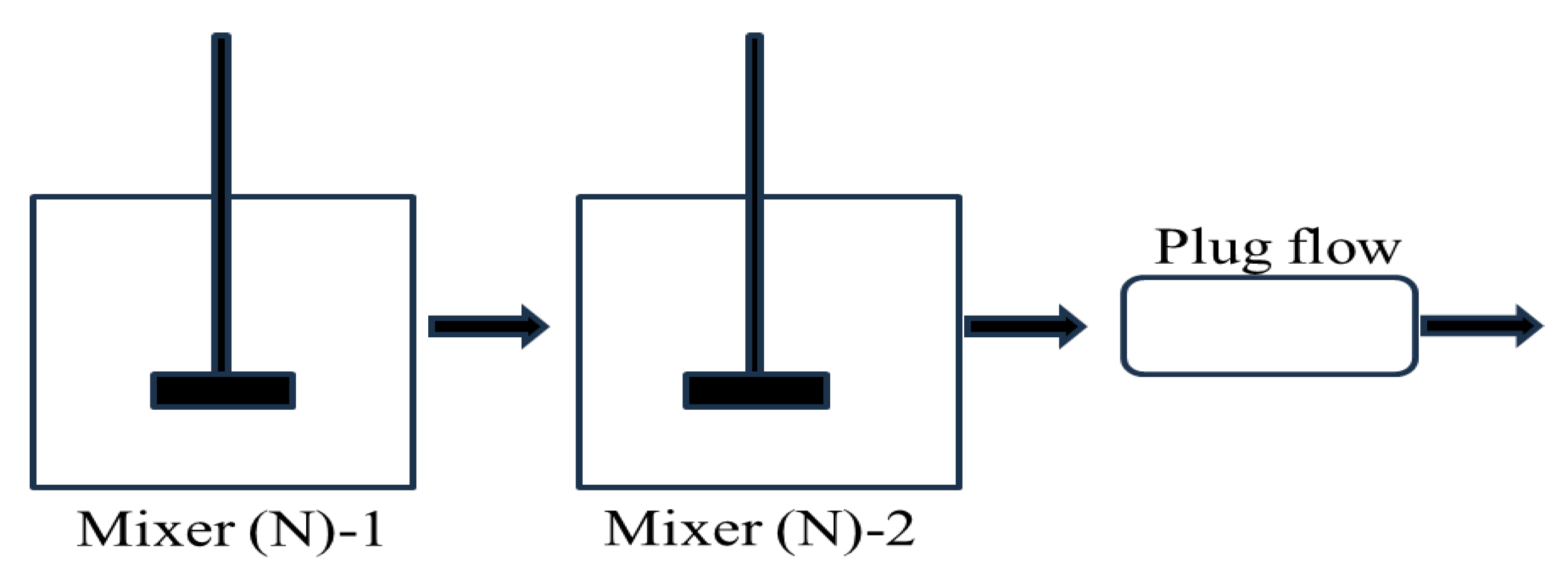
- (a)
- The mixer
- (b)
- The settler
3.3. Recommendations and Possibilities for the Potential Application of the Radiotracer Studies Based on 181Hf4+ in the Hydrometallurgical Processes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Guillaume, B.; Chung, J.; Hwang, Y. Critical and precious materials consumption and requirement in wind energy system in the EU 27. Appl. Energy 2015, 139, 327–334. [Google Scholar] [CrossRef]
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- Moss, R.L.; Tzimas, E.; Kara, H.; Willis, P.; Kooroshy, J. Critical Metals in Strategic Energy Technologies: Assessing rare metals as supply-chain bottlenecks in low-carbon energy technologies. In JRC-Scientific and Strategic Reports; European Commission Joint Research Centre Institute for Energy and Transport: Petten, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Zhuang, W.Q.; Fitts, J.P.; Ajo-Franklin, C.M.; Maes, S.; Alvarez-Cohen, L.; Hennebel, T. Recovery of critical metals using biometallurgy. Curr. Opin. Biotechnol. 2015, 33, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, G.J.P.; Chagnes, A.; Cote, G. Recent Advances in the Chemistry of Hydrometallurgical Methods. Sep. Purif. Rev. 2023, 52, 221–241. [Google Scholar] [CrossRef]
- European Commission. Study on the Critical Raw Materials for the Eu 2023. 2023. Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1 (accessed on 12 February 2025).
- Perks, C.; Mudd, G. Titanium, zirconium resources and production: A state of the art literature review. Ore Geol. Rev. 2019, 107, 629–646. [Google Scholar] [CrossRef]
- Pandey, G.; Mukhopadhyay, S.; Renjith, A.U.; Joshi, J.M.; Shenoy, K.T. Recovery of Hf and Zr from slurry waste of zirconium purification plant using solvent extraction. Hydrometallurgy 2016, 163, 61–68. [Google Scholar] [CrossRef]
- Galos, K.; Kot-Niewiadomska, A.; Kamyk, J. The Role of Poland in the European Union Supply Chain of Raw Materials, Including Critical Raw Materials. Mater. Proc. 2021, 5, 14. [Google Scholar] [CrossRef]
- Florek, J.; Giret, S.; Juère, E.; Larivière, D.; Kleitz, F. Functionalization of mesoporous materials for lanthanide and actinide extraction. Dalton Trans. 2016, 45, 14832. [Google Scholar] [CrossRef]
- Yadollahi, A.; Saberyan, K.; Torab-Mostaedi, M.; Charkhi, A.; Pourjavid, M.R. Solvent extraction separation of zirconium and hafnium from nitric acid solutions using mixture of Cyanex-272 and TBP. Radiochim. Acta 2018, 106, 675–684. [Google Scholar] [CrossRef]
- Goswami, S.; Manna, S.; Suman, S.K.; Sharma, V.K.; Satpati, S.K.; Sahu, M.L.; Pant, H.J. Investigation of aqueous phase dynamics in a uranium stripping unit using radioactive tracer. Appl. Radiat. Isot. 2022, 189, 110404. [Google Scholar] [CrossRef]
- Domínguez, J.; Abreu, A.M.; McCalla, R.; Borroto, J.; Ortueta, M.; Pérez, E. Mixing characterization in batch reactors using the radiotracer technique. J. Radioanal. Nucl. Chem. 1999, 241, 337–340. [Google Scholar] [CrossRef]
- Rahman, M.S. Advancements in solvent extraction of metal ions: Mechanisms, kinetics, and efficiency with diverse extractants. Int. J. Sci. Res. Arch. 2024, 12, 2374–2396. [Google Scholar] [CrossRef]
- Kiprono, N.R.; Kawalec, A.; Klis, B.; Smolinski, T.; Rogowski, M.; Kalbarczyk, P.; Samczynski, Z.; Norenberg, M.; Ostachowicz, B.; Adamowska, M.; et al. Radiation Techniques for Tracking the Progress of the Hydrometallurgical Leaching Process: A Case Study of Mn and Zn. Metals 2024, 14, 744. [Google Scholar] [CrossRef]
- Parent, M.; Cornelis, R.; Dams, R.; Alt, F. Investigation of extraction and back-extraction behaviour of platinum (IV) with rubeanic acid in tributyl phosphate, with tributyl phosphate and with thenoyltrifuoroacetone in n-butyl alcohol-acetophenone by means of platinum-191 radiotracer for platinum-enrichment purposes. Anal. Chim. Acta 1993, 281, 153–160. [Google Scholar] [CrossRef]
- Gloe, K.; Mühl, P. Determination of Metal Extraction Process Parameters Using Tracer Technique. Isot. Isot. Environ. Health Stud. 1983, 19, 257–260. [Google Scholar] [CrossRef]
- Yunos, M.A.S.M.; Hussain, S.A.; Yusoff, H.M.; Abdullah, J. Industrial radiotracer technology for process optimizations in chemical industries—A review. Pertanika J. Sch. Res. Rev. 2016, 2, 20–40. [Google Scholar]
- Pant, H.J.; Sharma, V.K.; Naik, S.V.; Singh, G.; Kalgutkar, D.B.; Patil, S.P.; Jayachandran, N.; Unni, V.K.P. Investigation of leaching of an antifouling agent from marine paint formulations using radiotracer technique. J. Radioanal. Nucl. Chem. 2012, 294, 65–69. [Google Scholar] [CrossRef]
- Goswami, S.; Biswal, J.; Samantray, J.; Gupta, D.F.; Pant, H.J. Measurement of mixing time and holdup of solids in gas–solid fluidized bed using radiotracer technique. J. Radioanal. Nucl. Chem. 2014, 302, 845–850. [Google Scholar] [CrossRef]
- Kasban, H.; Arafa, H.; Elaraby, S.M.S. Principle component analysis for radiotracer signal separation. Appl. Radiat. Isot. 2016, 112, 20–26. [Google Scholar] [CrossRef]
- Pant, H.J.; Sharma, V.K.; Singh, G.; Raman, V.K.; Bornare, J.; Sonde, R.R. Radiotracer investigation in a rotary fluidized bioreactor. J. Radioanal. Nucl. Chem. 2012, 294, 59–63. [Google Scholar] [CrossRef]
- El Korchi, K.; Alami, R.; Saadaoui, A.; Mimount, S.; Chaouch, A. Residence time distribution studies using radiotracers in a lab-scale distillation column: Experiments and modeling. Appl. Radiat. Isot. 2019, 154, 108889. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.J.; Sharma, V.K.; Shenoy, K.T.; Sreenivas, T. Measurements of liquid phase residence time distributions in a pilot-scale continuous leaching reactor using radiotracer technique. Appl. Radiat. Isot. 2015, 97, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Aminian, H.; Bazin, C.; Hodouin, D. Residence time distributions in SX–EW equipment. Int. J. Miner. Process. 1998, 54, 235–242. [Google Scholar] [CrossRef]
- Dams, R. Selection of short-lived isotopes for activation analysis with respect to sensitivity—A review. J. Radioanal. Chem. 1981, 61, 13–36. [Google Scholar] [CrossRef]
- Shikov, A.K.; Bocharov, O.V.; Arzhakova, V.M.; Bezumov, V.N.; Perlovich, Y.A.; Isaenkova, M.G. Use of hafnium in control elements of nuclear reactors and power units. Met. Sci. Heat Treat. 2003, 45, 300–303. [Google Scholar] [CrossRef]
- Broerman, B.; Laubenstein, M.; Nagorny, S.; Song, N.; Vincent, A.C. A search for rare and induced nuclear decays in hafnium. Nucl. Phys. A 2020, 1012, 122212. [Google Scholar] [CrossRef]
- IAEA. Radiotracer Residence Time Distribution Method for Industrial and Environmental Applications: Material for Education and On-the-Job Training for Practitioners of Radiotracer Technology. 2008. Available online: https://www-pub.iaea.org/mtcd/publications/pdf/tcs-31_web.pdf (accessed on 27 August 2024).
- Rotich, N.K.; Herdzik-Koniecko, I.; Smolinski, T.; Kalbarczyk, P.; Sudlitz, M.; Rogowski, M.; Stosnach, H.; Chmielewski, A.G. Recovery of Metals from Titanium Ore Using Solvent Extraction Process: Part 1—Transition Metals. Minerals 2024, 14, 1212. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end-of-life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Kiprono, N.R.; Smolinski, T.R.; Rogowski, M.; Chmielewski, A.G. The State of Critical and Strategic Metals Recovery and the Role of Nuclear Techniques in the Separation Technologies Development: Review. Separations 2023, 10, 112. [Google Scholar] [CrossRef]



| Model Parameters | Before Optimization | Optimized Parameters |
|---|---|---|
| τ | 200 | 1811 |
| N | 10 | 5.55 |
| t | 300 | 140 |
| Model Parameters | Before Optimization | Optimized Parameters |
|---|---|---|
| τ | 70 | 4165 |
| Pe | 10 | 6.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiprono, N.R.; Herdzik-Koniecko, I.; Smolinski, T.; Rogowski, M.; Chmielewski, A.G. The Application of the Radiotracer Techniques in Hydrometallurgy: A Method for Online Monitoring of Solvent Extraction Processes Using 181Hf. Minerals 2025, 15, 268. https://doi.org/10.3390/min15030268
Kiprono NR, Herdzik-Koniecko I, Smolinski T, Rogowski M, Chmielewski AG. The Application of the Radiotracer Techniques in Hydrometallurgy: A Method for Online Monitoring of Solvent Extraction Processes Using 181Hf. Minerals. 2025; 15(3):268. https://doi.org/10.3390/min15030268
Chicago/Turabian StyleKiprono, Nelson Rotich, Irena Herdzik-Koniecko, Tomasz Smolinski, Marcin Rogowski, and Andrzej G. Chmielewski. 2025. "The Application of the Radiotracer Techniques in Hydrometallurgy: A Method for Online Monitoring of Solvent Extraction Processes Using 181Hf" Minerals 15, no. 3: 268. https://doi.org/10.3390/min15030268
APA StyleKiprono, N. R., Herdzik-Koniecko, I., Smolinski, T., Rogowski, M., & Chmielewski, A. G. (2025). The Application of the Radiotracer Techniques in Hydrometallurgy: A Method for Online Monitoring of Solvent Extraction Processes Using 181Hf. Minerals, 15(3), 268. https://doi.org/10.3390/min15030268







