Kinetics of Ion Exchange in Magnesium Sulfate Leaching of Rare Earths and Aluminum from Ionic Rare Earth Ores
Abstract
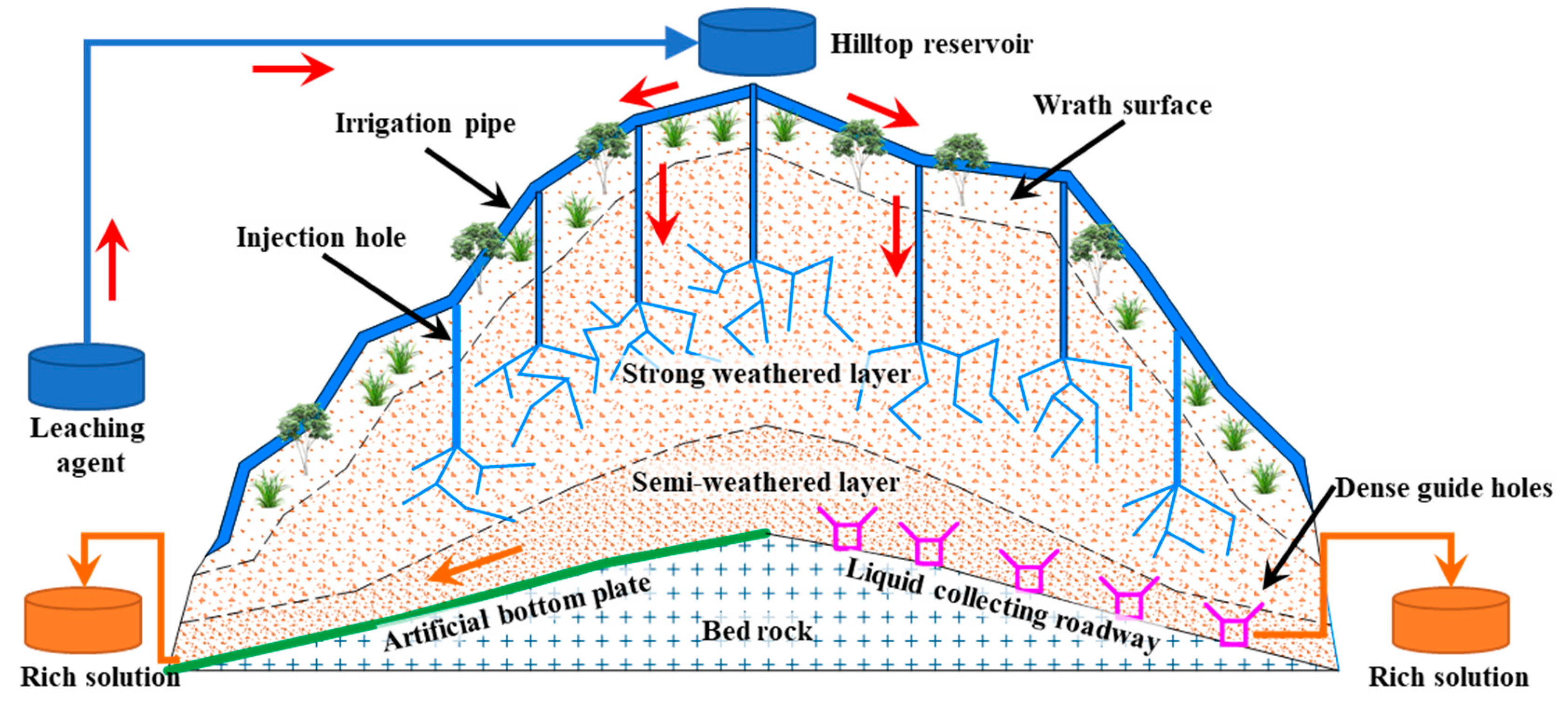
1. Introduction
2. Method
2.1. Ore Sample
2.2. Leaching Equilibrium Experiments
2.3. Leaching Kinetics Experiments
2.4. Test Methods for RE and Al Content
2.5. Theory-Based Analysis of Ion-Exchange Kinetics
3. Results and Discussion
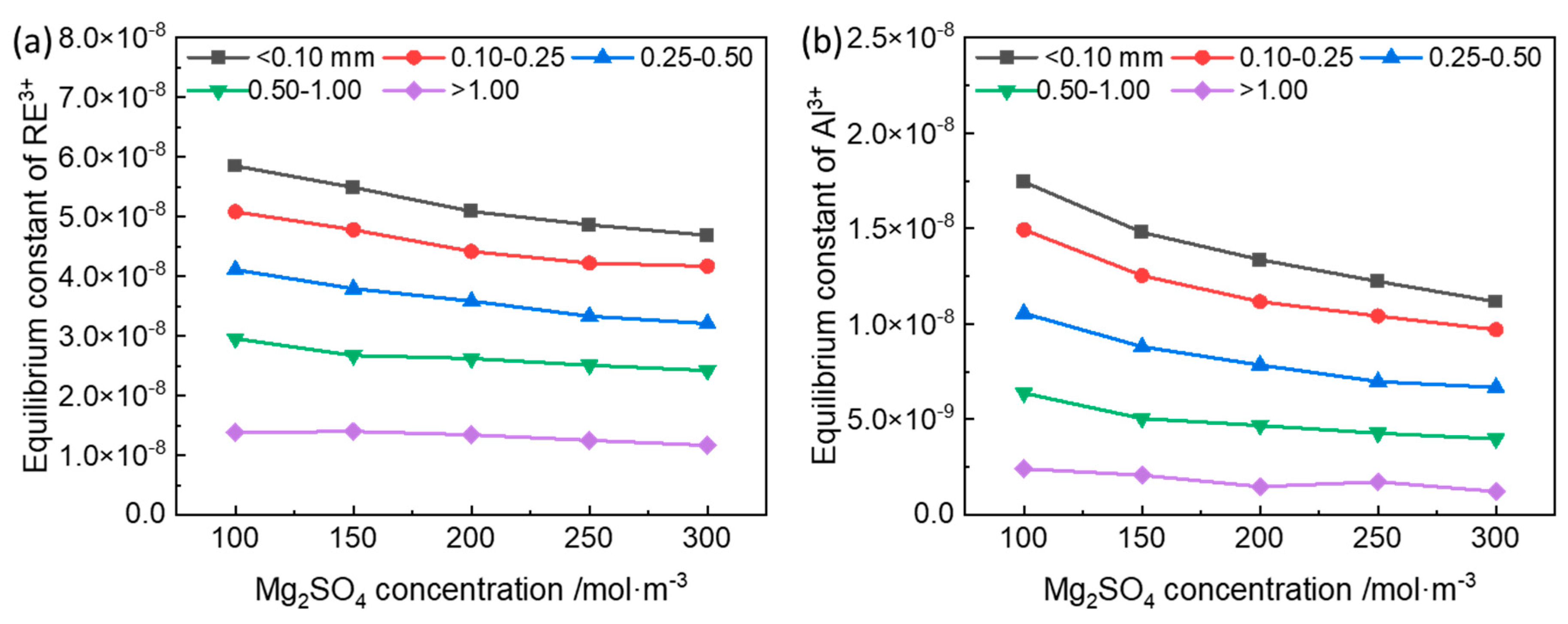
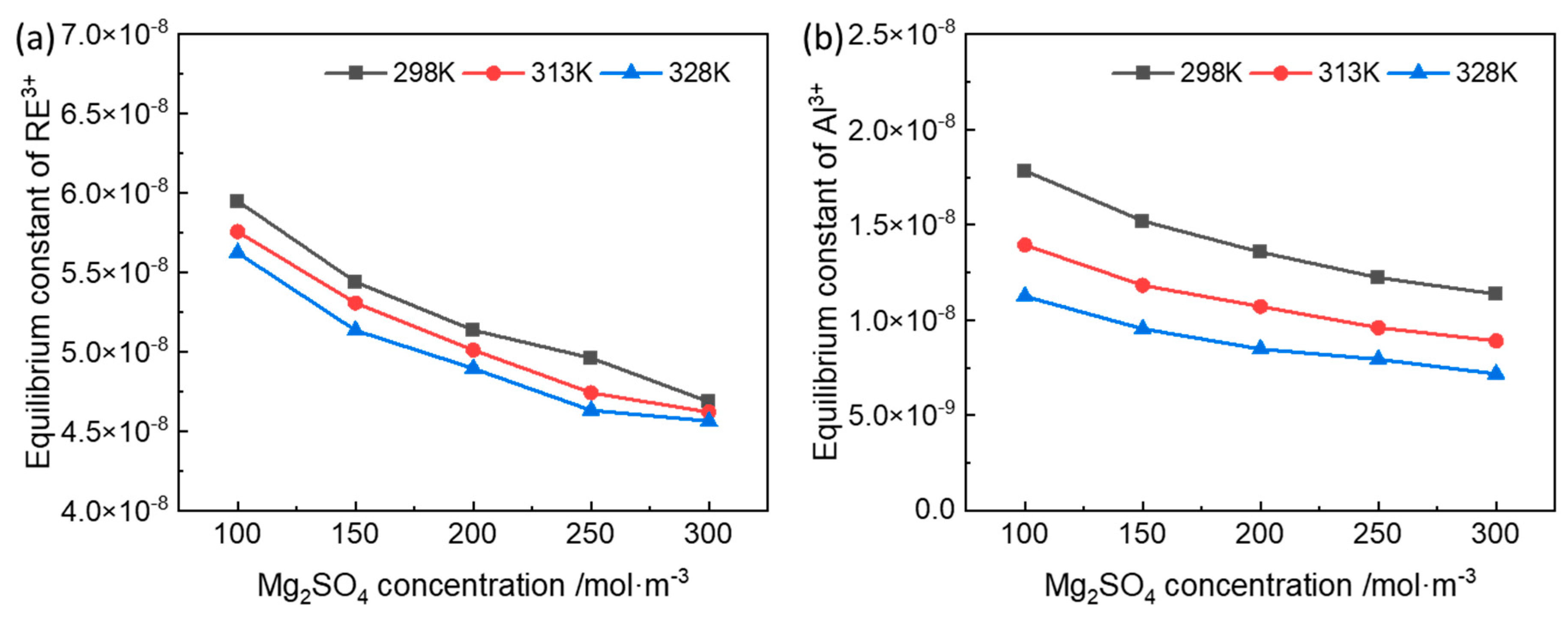
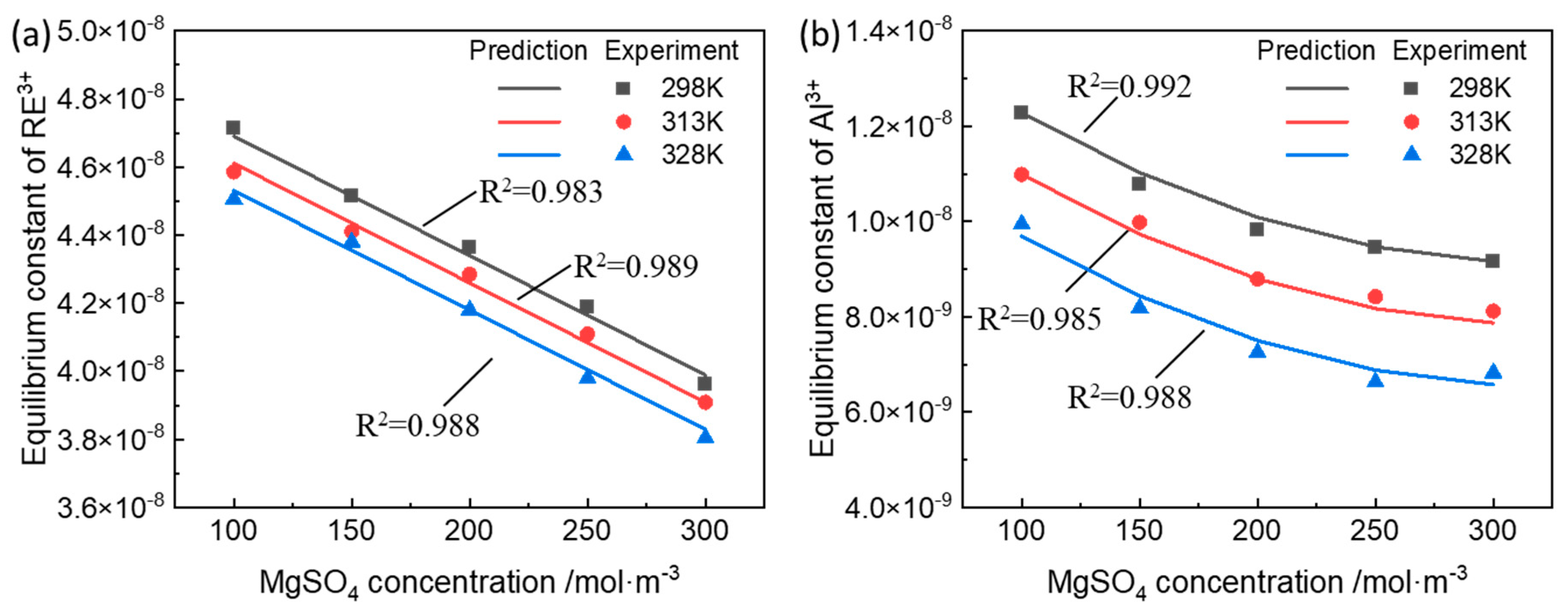
3.1. Equilibrium Constant of the Ion-Exchange Reactions
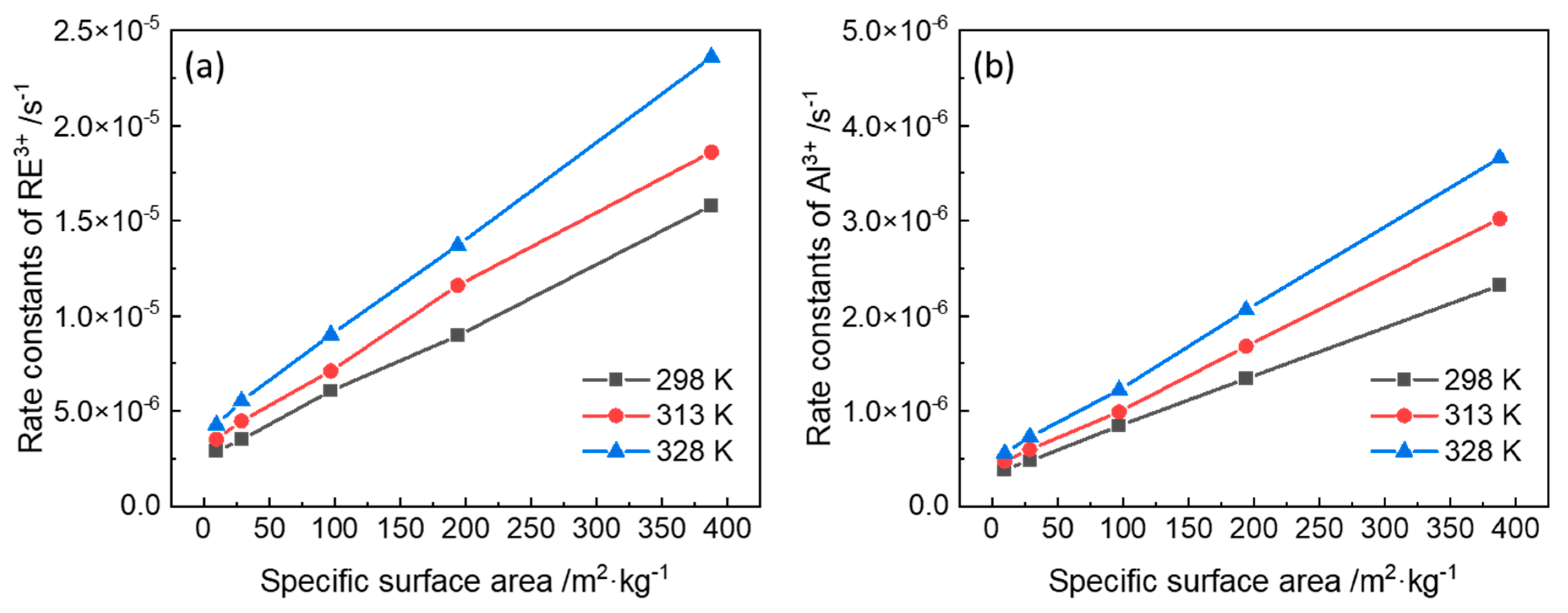
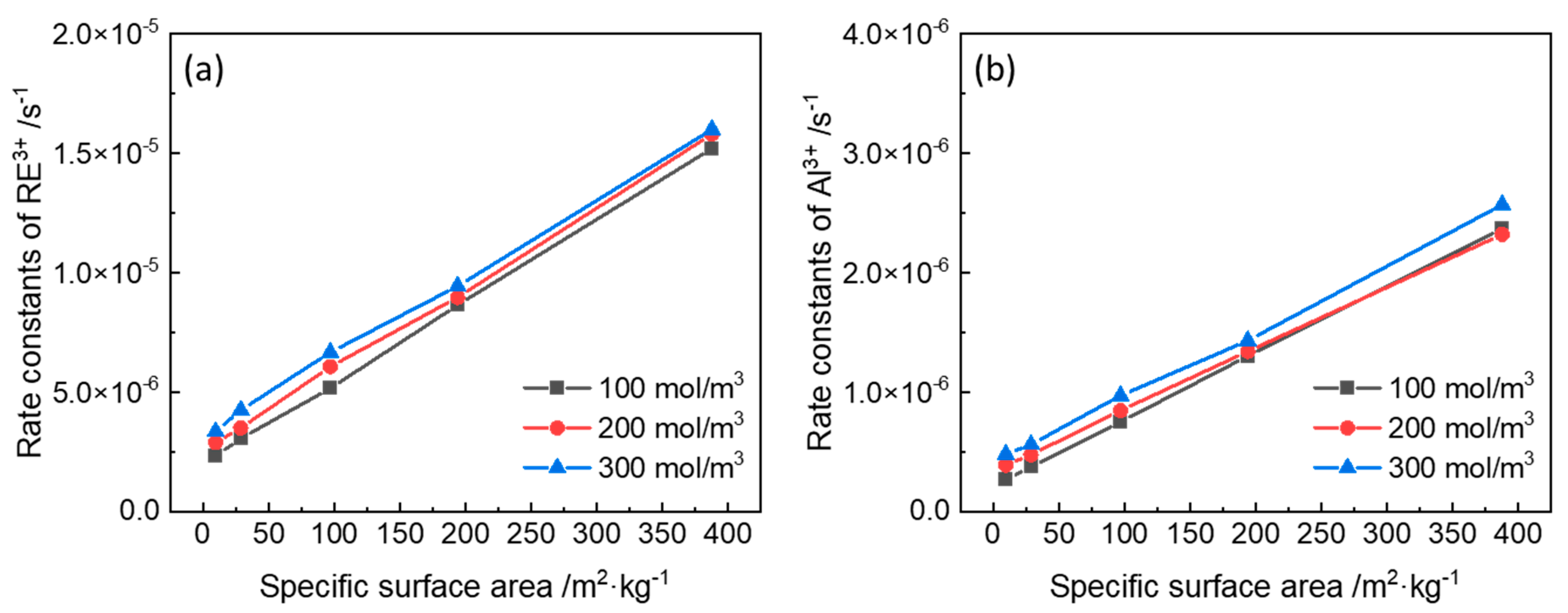
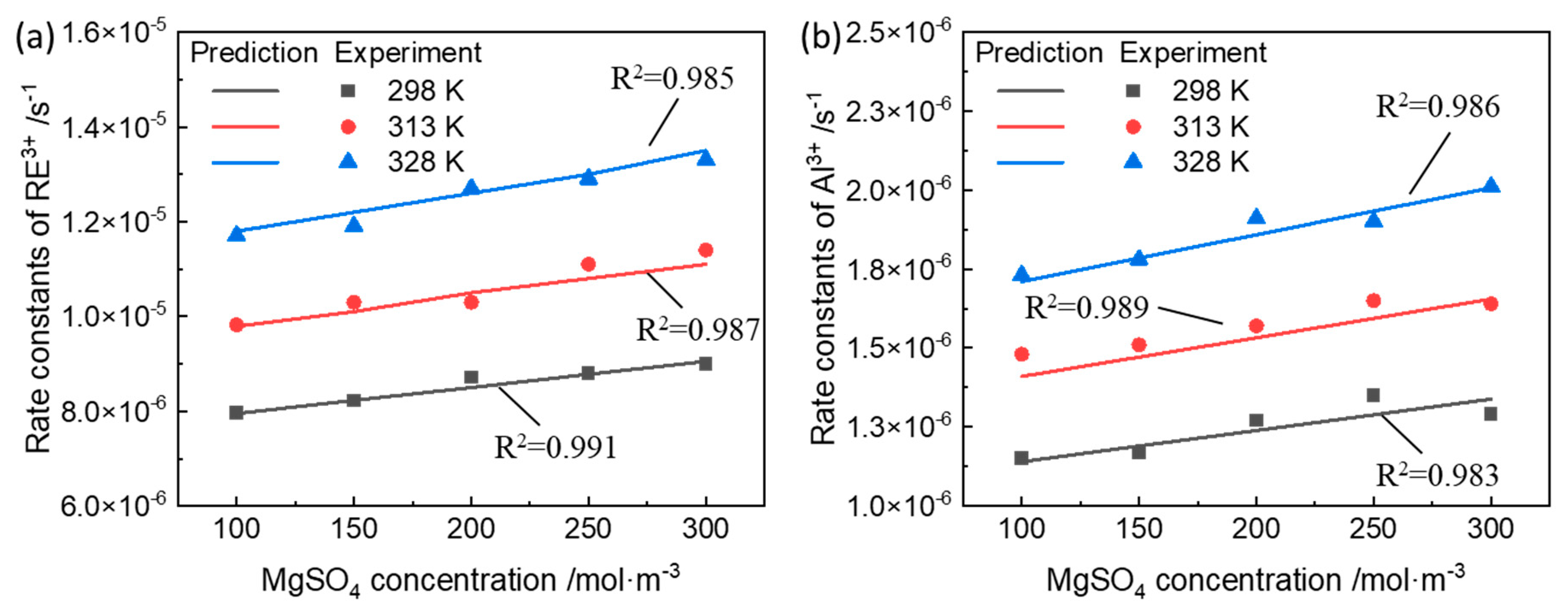
3.2. Rate Constants of Ion-Exchange Reactions
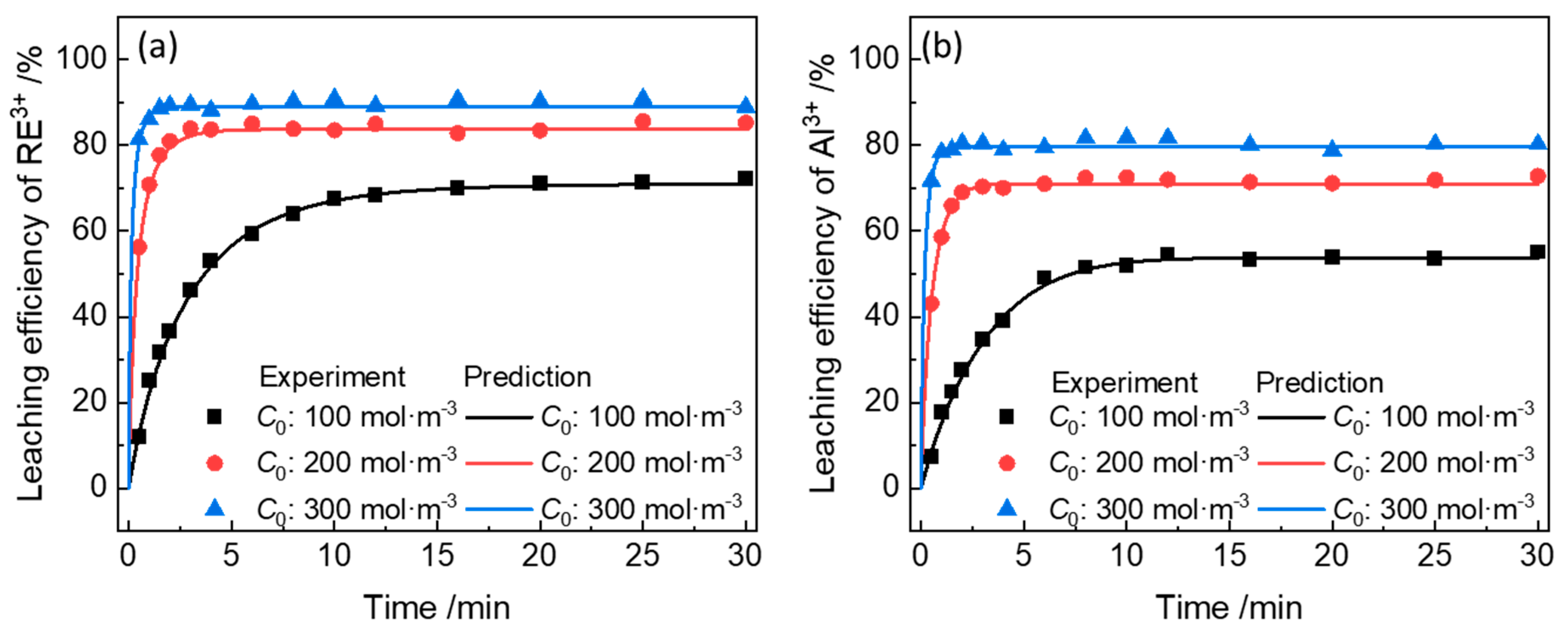
3.3. Model Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yin, S.; Chen, X.; Yan, R.; Wang, L. Pore Structure Characterization of Undisturbed Weathered Crust Elution-Deposited Rare Earth Ore Based on X-Ray Micro-CT Scanning. Minerals 2021, 11, 236. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, L.; Wang, Z.; Zhang, Y.; Chi, R.; Wu, X. Application of Surfactant for Improving Leaching Process of Weathered Crust Elution-Deposited Rare Earth Ores. J. Rare Earths 2024, 42, 181–190. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Liu, Q.; Luo, F.; Liu, Y. Microstructure Evolution Law of Ionic Rare Earth at Different Depths in In Situ Leaching Mine Site. Minerals 2024, 14, 570. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Z.; Hu, G.; Huang, L.; Huang, X.; Chen, Y.; Li, M. Leaching and Mass Transfer Characteristics of Elements from Ion-Adsorption Type Rare Earth Ore. Rare Met. 2015, 34, 357–365. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Y.; Feng, X.; Wang, J.; Ma, Z.; Yao, R.; Zhai, Y.; Tian, L. Leaching of Ion Adsorption Rare Earths and the Role of Bioleaching in the Process: A Review. J. Clean. Prod. 2024, 468, 143067. [Google Scholar] [CrossRef]
- Feng, Z.; Huang, X.; Wang, M.; Zhang, G. Progress and Trend of Green Chemistry in Extraction and Separation of Typical Rare Earth Resources. Chin. J. Rare Met. 2017, 41, 604–612. (In Chinese) [Google Scholar] [CrossRef]
- Lai, F.; Gao, G.; Huang, L.; Xiao, Y.; Yang, R.; Li, K. Compound Leaching of Rare Earth from the Ion-Adsorption Type Rare Earth Ore with Magnesium Sulfate and Ascorbic Acid. Hydrometallurgy 2018, 179, 25–35. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; Deng, Y.; Wang, L.; Wang, H.; Huang, Z. Leaching Kinetics of Ionic Rare Earth Ore with Magnesium Sulfate. Chin. J. Rare Met. 2022, 46, 265–272. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, W.; Kuang, J.; Hu, H.; Liang, B.; Yu, M. Research Progress on Formation and Removal of Impurity in Leaching Process of Ion-Adsorbed Rare Earth Ore. Matel Mine 2024, 196, 119–128. (In Chinese) [Google Scholar] [CrossRef]
- Qin, L.; Wang, G.S.; Luo, S.H.; Peng, C.L.; Qi, J.; Song, C.X. Calculation model of leaching agent dosage in ion-type rare earth ore. Chin. J. Nonferr. Met. 2019, 29, 1781–1789. (In Chinese) [Google Scholar] [CrossRef]
- He, Q.; Qiu, J.; Rao, M.; Xiao, Y. Leaching Behaviors of Calcium and Aluminum from an Ionic Type Rare Earth Ore Using MgSO4 as Leaching Agent. Minerals 2021, 11, 716. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, S.; Long, P.; Shi, Y.; Wang, G. Ion exchange model with the unsteady selectivity coefficient for the leaching process of ion- adsorption type rare earth ores. J. Chin. Soc. Rare Earth 2025, 1–13. Available online: https://link.cnki.net/urlid/11.2365.TG.20250115.1645.002 (accessed on 10 February 2025). (In Chinese).
- Hu, S.; Cao, X.; Wang, G.; Long, P.; Zhou, X. An Ion exchange Model for Leaching Process of Weathered Crust Elution-deposited Rare Earth. Min. Metall. Eng. 2018, 38, 1–5. [Google Scholar] [CrossRef]
- Guo, J.; Qin, L.; Wang, G.; Luo, S.; Peng, C.; Long, P. Study on the periodic changes of chemical equilibrium constants of ionic rare earth ores. J. Chin. Soc. Rare Earth 2024, 1–18. Available online: https://link.cnki.net/urlid/11.2365.TG.20240119.1450.008 (accessed on 10 February 2025). (In Chinese).
- Long, P.; Wang, G.; Zhang, C.; Yang, Y.; Cao, X.; Shi, Z. Kinetics Model for Leaching of Ion-Adsorption Type Rare Earth Ores. J. Rare Earths 2020, 38, 1354–1360. [Google Scholar] [CrossRef]
- Tian, J.; Yin, J.; Chi, R.; Rao, G.; Jiang, M.; Ouyang, K. Kinetics on Leaching Rare Earth from the Weathered Crust Elution-Deposited Rare Earth Ores with Ammonium Sulfate Solution. Hydrometallurgy 2010, 101, 166–170. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, X.; Feng, Z.; Huang, X.; Huang, L.; Chen, Y.; Wu, W. Role of Minerals Properties on Leaching Process of Weathered Crust Elution-Deposited Rare Earth Ore. J. Rare Earths 2015, 33, 545–552. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Sun, N.; Zhang, H.; Liu, Z. Leaching Kinetics of Weathered Crust Elution-Deposited Rare Earth Ore with Magnesium Salt. Matel Mine 2018, 10, 84–91. (In Chinese) [Google Scholar] [CrossRef]
- GBT19587-2017; Determination of the Specific Surface Area of Solids by Gas Adsorption Using the BET Method. State Administration of Quality Supervision and Quarantine of PRC and Standardization Administration of PRC: Beijing, China, 2017. Available online: https://www.ncrm.org.cn/Web/News/SpecificationView?id=295 (accessed on 10 February 2025).
- Xiao, Y.; Chen, Y.; Feng, Z.; Huang, X.; Huang, L.; Long, Z.; Cui, D. Leaching characteristics of ion-adsorption type rare earths ore with magnesium sulfate. Trans. Nonferrous Met. Soc. China 2015, 25, 3784–3790. [Google Scholar] [CrossRef]
- Wu, X.; Feng, J.; Zhou, F.; Liu, C.; Chi, R. Optimization of a Rare Earth and Aluminum Leaching Process from Weathered Crust Elution-Deposited Rare Earth Ore with Surfactant CTAB. Minerals 2024, 14, 321. [Google Scholar] [CrossRef]
- GB/T 14635-2020; Rare Earth Metals and Their Compounds Determination of Total Rare Earth Content. State Administration of Quality Supervision and Quarantine of PRC and Standardization Administration of PRC: Beijing, China, 2020. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=6CE39DE72F319773961072256064A339 (accessed on 10 February 2025).
- Nie, W.; Zhang, R.; He, Z.; Zhou, J.; Wu, M.; Xu, Z.; Chi, R.; Yang, H. Research Progress on Leaching Technology and Theory of Weathered Crust Elution-Deposited Rare Earth Ore. Hydrometallurgy 2020, 193, 105295. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, G.; Huang, L.; Feng, Z.; Lai, F.; Long, Z. A Discussion on the Leaching Process of the Ion-Adsorption Type Rare Earth Ore with the Electrical Double Layer Model. Miner. Eng. 2018, 120, 35–43. [Google Scholar] [CrossRef]
- Chi, R.; Tian, J.; Zhu, G.; Wu, Y.; Li, S.; Wang, C.; Zhou, Z.A. Kinetics of Rare Earth Leaching from a Manganese-removed Weathered Rare-earth Mud in Hydrochloric Acid Solutions. Sep. Sci. Technol. 2006, 41, 1099–1113. [Google Scholar] [CrossRef]
- COMSOL Finding Kinetic Arrhenius Parameters Using Parameter Estimation 2024. Available online: https://cn.comsol.com/model/finding-kinetic-arrhenius-parameters-using-parameter-estimation-10305 (accessed on 10 February 2025).
- Yang, H.; Sha, A.; He, Z.; Wu, C.; Xu, Y.; Hu, J.; Xu, Z.; Chi, R. Leaching Kinetics and Permeability of Polyethyleneimine Added Ammonium Sulfate on Weathered Crust Elution-Deposited Rare Earth Ores. J. Rare Earths 2024, 42, 1610–1619. [Google Scholar] [CrossRef]
- Chai, X.; Li, G.; Zhang, Z.; Chi, R.; Chen, Z. Leaching Kinetics of Weathered Crust Elution-Deposited Rare Earth Ore with Compound Ammonium Carboxylate. Minerals 2020, 10, 516. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Z.; Chi, R.; Chen, Z.; Chi, X.; Wu, M.; Chi, R. Enhancement of the Leaching Process of Weathered Crust Elution-deposited Rare Earth Ore with Composite Magnesium Salt. Met. Mine 2020, 43, 95–101. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, H.; Han, H.; Jiang, X.; Yang, Y.; Li, T. Differential Leaching Mechanisms and Ecological Impact of Organic Acids on Ion-Adsorption Type Rare Earth Ores. Sep. Purif. Technol. 2025, 362, 131701. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, J.; Kang, S.; Wang, X.; Yu, H.; Wan, Y. Enhancing Leaching Efficiency of Ion Adsorption Rare Earths by Ameliorating Mass Transfer Effect of Rare Earth Ions by Applying an Electric Field. J. Rare Earths 2024, 42, 172–180. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, J.; Liang, X.; Ling, B.; Xu, J.; Yang, Y.; Kang, S.; Tan, W.; Xu, Y.; Zou, X.; et al. Industrial-Scale Sustainable Rare Earth Mining Enabled by Electrokinetics. Nat. Sustain. 2025, 8, 182–189. [Google Scholar] [CrossRef]

| Composition | SiO2 | Al2O3 | K2O | P2O5 | Fe2O3 | TiO | MgO | MnO | CaO | TREO |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 45.802 | 29.863 | 4.575 | 2.276 | 3.388 | 0.523 | 0.247 | 0.091 | 0.035 | 0.096 |
| Composition | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 14.59 | 0.79 | 10.06 | 13.43 | 2.93 | 0.41 | 4.63 | 0.74 | 6.13 | 0.97 | 3.57 | 0.36 | 3.41 | 0.30 | 37.68 |
| Particle Size/mm | Mass Distribution/% | Specific Surface Area/(m2/kg) | RE2O3 Distribution/% | RE2O3 Grade/‰ | Al2O3 Distribution/% | Al2O3 Grade/‰ |
|---|---|---|---|---|---|---|
| <0.10 | 27.35 | 387.71 | 45.38 | 1.12 | 60.38 | 0.73 |
| 0.10–0.25 | 21.02 | 193.85 | 21.56 | 0.87 | 16.56 | 0.55 |
| 0.25–0.50 | 16.18 | 96.93 | 14.87 | 0.57 | 14.87 | 0.37 |
| 0.50–1.00 | 14.90 | 28.71 | 12.86 | 0.44 | 6.86 | 0.35 |
| >1.00 | 20.54 | 9.6 | 5.33 | 0.14 | 1.33 | 0.10 |
| Full-particle grade | 174.32 | 0.83 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Shao, Y.; Chen, G. Kinetics of Ion Exchange in Magnesium Sulfate Leaching of Rare Earths and Aluminum from Ionic Rare Earth Ores. Minerals 2025, 15, 290. https://doi.org/10.3390/min15030290
Hu M, Shao Y, Chen G. Kinetics of Ion Exchange in Magnesium Sulfate Leaching of Rare Earths and Aluminum from Ionic Rare Earth Ores. Minerals. 2025; 15(3):290. https://doi.org/10.3390/min15030290
Chicago/Turabian StyleHu, Mingbing, Yajian Shao, and Guoliang Chen. 2025. "Kinetics of Ion Exchange in Magnesium Sulfate Leaching of Rare Earths and Aluminum from Ionic Rare Earth Ores" Minerals 15, no. 3: 290. https://doi.org/10.3390/min15030290
APA StyleHu, M., Shao, Y., & Chen, G. (2025). Kinetics of Ion Exchange in Magnesium Sulfate Leaching of Rare Earths and Aluminum from Ionic Rare Earth Ores. Minerals, 15(3), 290. https://doi.org/10.3390/min15030290







