New Technology of Zinc Oxide Concentrate Flotation + Mechanical Stirring Defoaming Zinc Leaching
Abstract
:1. Introduction
2. Preparation Methods
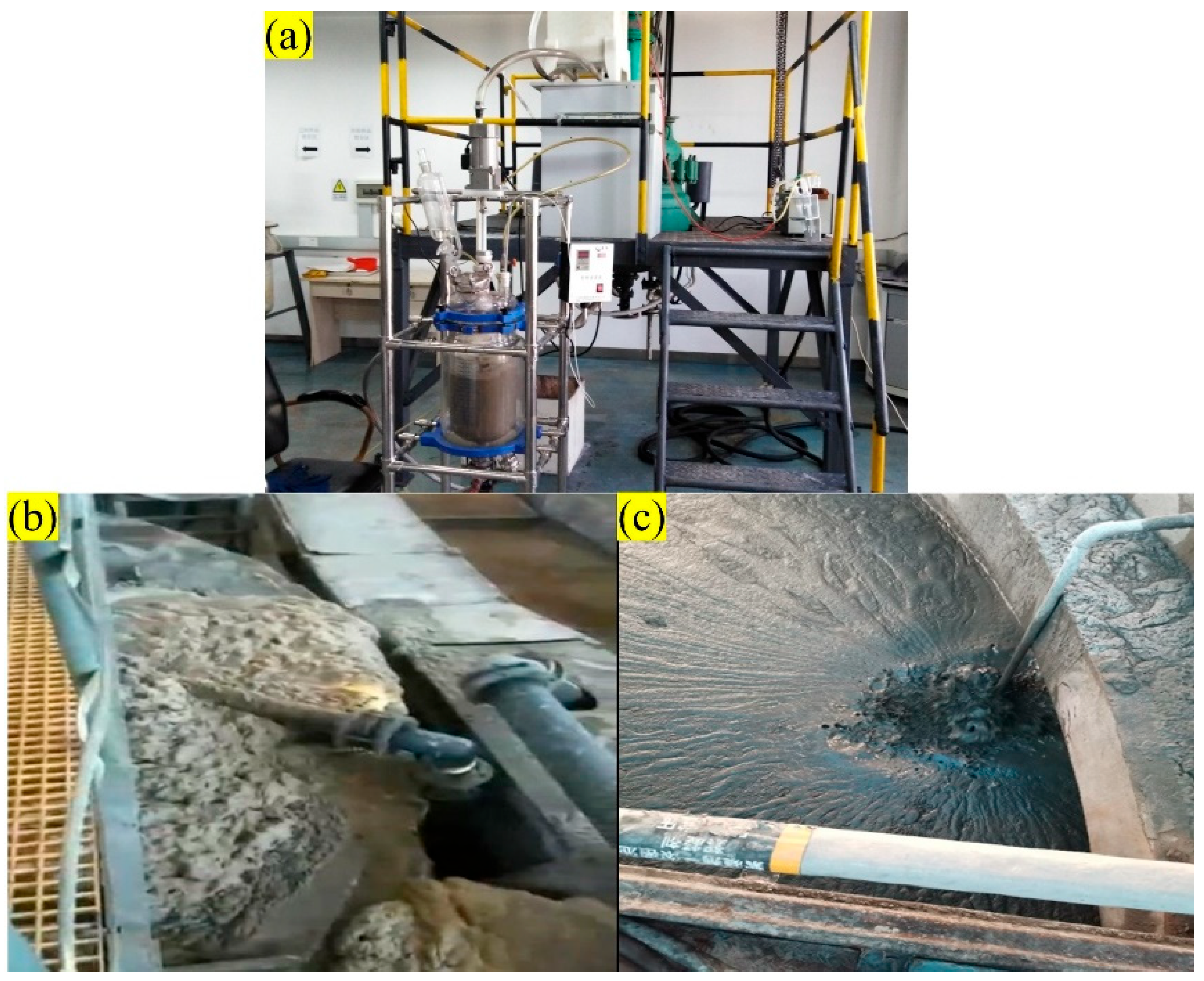
2.1. Experimental Materials and Equipment
2.2. Outcome Representation
3. Results and Discussion
3.1. Leaching Results
3.2. H2S Dilution Experiment
- ①
- Theoretical Calculation
- ②
- Experimental Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kania, H.; Saternus, M. Evaluation and current state of primary and secondary zinc production—A review. Appl. Sci. 2023, 13, 2003. [Google Scholar] [CrossRef]
- Kaya, M.; Hussaini, S.; Kursunoglu, S. Critical review on secondary zinc resources and their recycling technologies. Hydrometallurgy 2020, 195, 105362. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. A critical review of material flow, recycling technologies, challenges and future strategy for scattered metals from minerals to wastes. J. Clean. Prod. 2018, 202, 1001–1025. [Google Scholar] [CrossRef]
- Nayak, A.; Jena, M.S.; Mandre, N.R. Beneficiation of lead-zinc ores–a review. Miner. Process. Extr. Metall. Rev. 2022, 43, 564–583. [Google Scholar] [CrossRef]
- Hoal, K.O.; McNulty, T.P.; Schmidt, R. Metallurgical Advances and Their Impact on Mineral Exploration and Mining. In Wealth Creation in the Minerals Industry: Integrating Science, Business, and Education; Doggett, M.D., Parry, J.R., Eds.; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2005. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2015. [Google Scholar]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.H. Recovery of Magnesium Sulfate from a Zinc Ore Flotation Tailing Using Hydrometallurgical Route; Universidade de São Paulo: São Paulo, Brazil, 2020. [Google Scholar]
- Tao, M.; Zhang, X.; Wang, S.; Cao, W.; Jiang, Y. Life cycle assessment on lead–zinc ore mining and beneficiation in China. J. Clean. Prod. 2019, 237, 117833. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Han, Y. Efficient flotation separation of lead–zinc oxide ores using mineral sulfidation reconstruction technology: A review. Green Smart Min. Eng. 2024, 1, 175–189. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Zhou, P.; Wu, D.; Wu, C. An Overview of Flashing Phenomena in Pressure Hydrometallurgy. Processes 2023, 11, 2322. [Google Scholar] [CrossRef]
- Levchuk, I. Role of Oxidation-Reduction Cycle of Iron in Direct Leaching of Zinc Concentrate; LUT University: Lappeenranta, Finland, 2010. [Google Scholar]
- Zhao, L.; Zhang, Q. A significant review of froth stability in mineral flotation. Chem. Eng. Sci. 2024, 302, 120738. [Google Scholar] [CrossRef]
- Ettel, V.A.; Tilak, B.V. Electrolytic refining and winning of metals. In Comprehensive Treatise of Electrochemistry: Electrochemical Processing; Springer: Boston, MA, USA, 1981; pp. 327–380. [Google Scholar]
- Gomez-Flores, A.; Heyes, G.W.; Ilyas, S.; Kim, H. Effects of artificial impeller blade wear on bubble–particle interactions using CFD (k–ε and LES), PIV, and 3D printing. Miner. Eng. 2022, 186, 107766. [Google Scholar] [CrossRef]
- Kracht, W.; Finch, J.A. Bubble break-up and the role of frother and salt. Int. J. Miner. Process. 2009, 92, 153–161. [Google Scholar] [CrossRef]
- Sovechles, J.M.; Lepage, M.R.; Johnson, B.; Waters, K.E. Effect of gas rate and impeller speed on bubble size in frother-electrolyte solutions. Miner. Eng. 2016, 99, 133–141. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, B.B.; Gao, Z.L.; Fang, D.M. Stress corrosion crack tests of 16MnR low alloy steel in anhydrous ammonia environment. Key Eng. Mater. 2005, 297, 974–979. [Google Scholar] [CrossRef]


| Zn | Znd | Fe | SiO2 | Cd | Co | Na2S | MgO | CaO | C | Moisture | Pb | Oxidation Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14.75 | 10.63 | 13.88 | 27.12 | 0.18 | 0.004 | 0.2 | 0.28 | 7.18 | 6.8 | 15.6 | 0.87 | 72.07 |
| ZnSO4 | ZnO | ZnS | Zinc Iron Spinel and Other | Total Zn |
|---|---|---|---|---|
| 1.54 | 9.09 | 4.08 | 0.044 | 14.75 |
| No. | Oxidation Rate (%) | Quantity of Slag (g) | Leaching Liquid (g/L) | Leaching Rate of ZnO (%) | Slag Rate (%) | Barometric Pressure (Mpa) | Amount of Wind (L/min) | Leaching Method |
|---|---|---|---|---|---|---|---|---|
| 1 | 76.59% | 603.83 | 2240 | 93.14 | 53.52 | 0.02 | 7.5 | air flotation |
| 2 | 596.54 | 4500 | 96.56 | 59.65 | 0.05 | 15 | air flotation | |
| 3 | 702.89 | 4610 | 93.33 | 62.30 | 0.08 | 22.5 | air flotation | |
| Stirring rate r/min | Problem | |||||||
| 4 | 557.85 | 4610 | 91.93 | 65.73 | 100 | A large amount of foam is generated during the acid leaching process, and the foam is viscous and difficult to break down. | mechanical stirring | |
| 5 | 579.44 | 4770 | 94.21 | 61.95 | 300 | mechanical stirring | ||
| 6 | 610.21 | 4900 | 93.05 | 67.02 | 500 | mechanical stirring | ||
| 7 | 607.89 | 4900 | 98.15 | 61.02 | 0.05 | 15 | air flotation+ mechanical stirring (300 r/min) |
| No. | H2S (mg/m3) |
|---|---|
| 2 | 10.30 |
| 5 | 5.21 |
| 7 | 1.16 |
| 8 | 44.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Xu, Q.; Xu, R.; He, X.; Lin, S. New Technology of Zinc Oxide Concentrate Flotation + Mechanical Stirring Defoaming Zinc Leaching. Minerals 2025, 15, 313. https://doi.org/10.3390/min15030313
Yang C, Xu Q, Xu R, He X, Lin S. New Technology of Zinc Oxide Concentrate Flotation + Mechanical Stirring Defoaming Zinc Leaching. Minerals. 2025; 15(3):313. https://doi.org/10.3390/min15030313
Chicago/Turabian StyleYang, Chen, Qingxin Xu, Ruidong Xu, Xiaocai He, and Shengnan Lin. 2025. "New Technology of Zinc Oxide Concentrate Flotation + Mechanical Stirring Defoaming Zinc Leaching" Minerals 15, no. 3: 313. https://doi.org/10.3390/min15030313
APA StyleYang, C., Xu, Q., Xu, R., He, X., & Lin, S. (2025). New Technology of Zinc Oxide Concentrate Flotation + Mechanical Stirring Defoaming Zinc Leaching. Minerals, 15(3), 313. https://doi.org/10.3390/min15030313





