Methane in Fluid Inclusions in Ophiolitic Chromitites Revealed by Raman Spectroscopy: Preliminary Results
Abstract
1. Introduction
2. Sample Set
3. Methods
4. Results
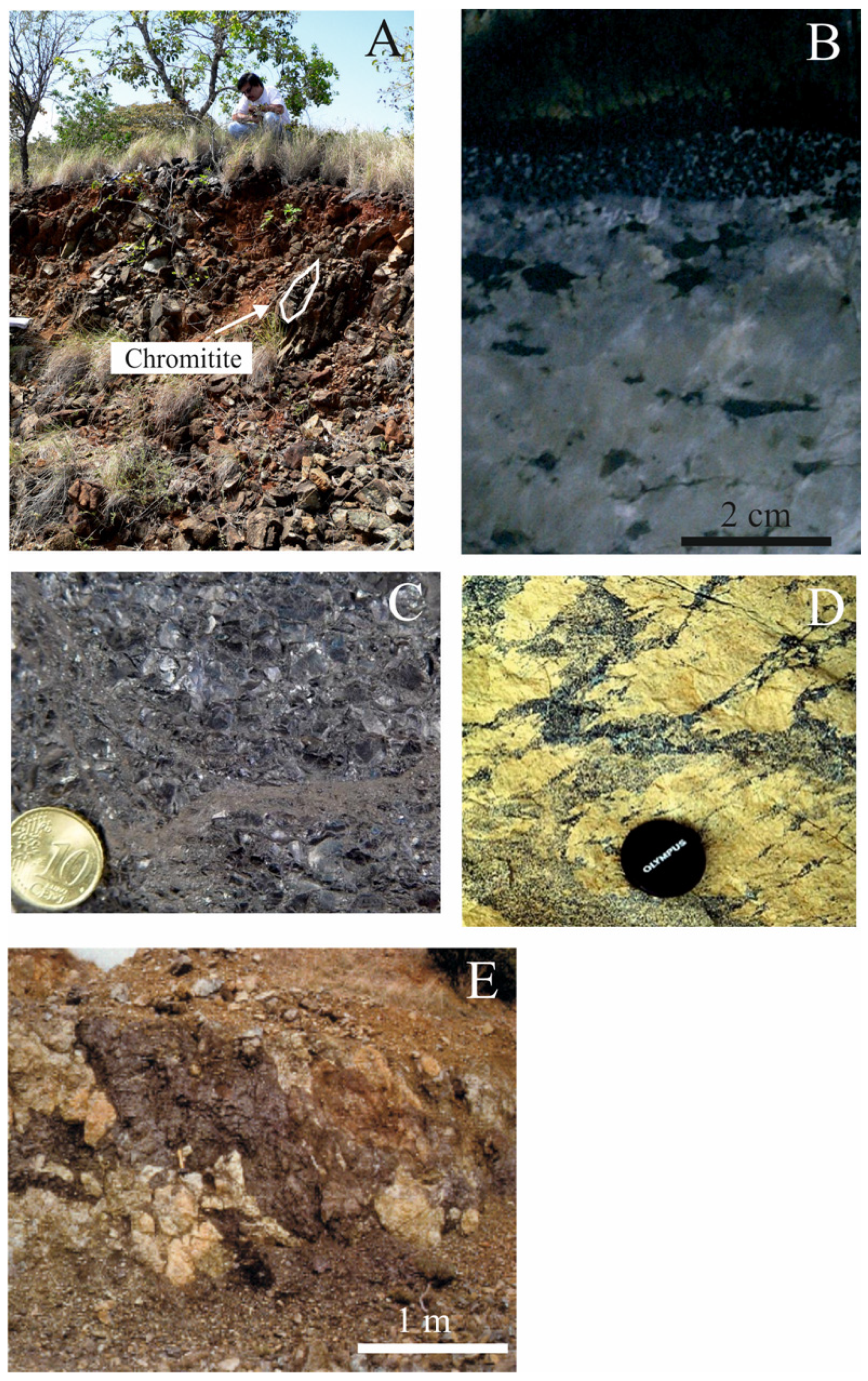
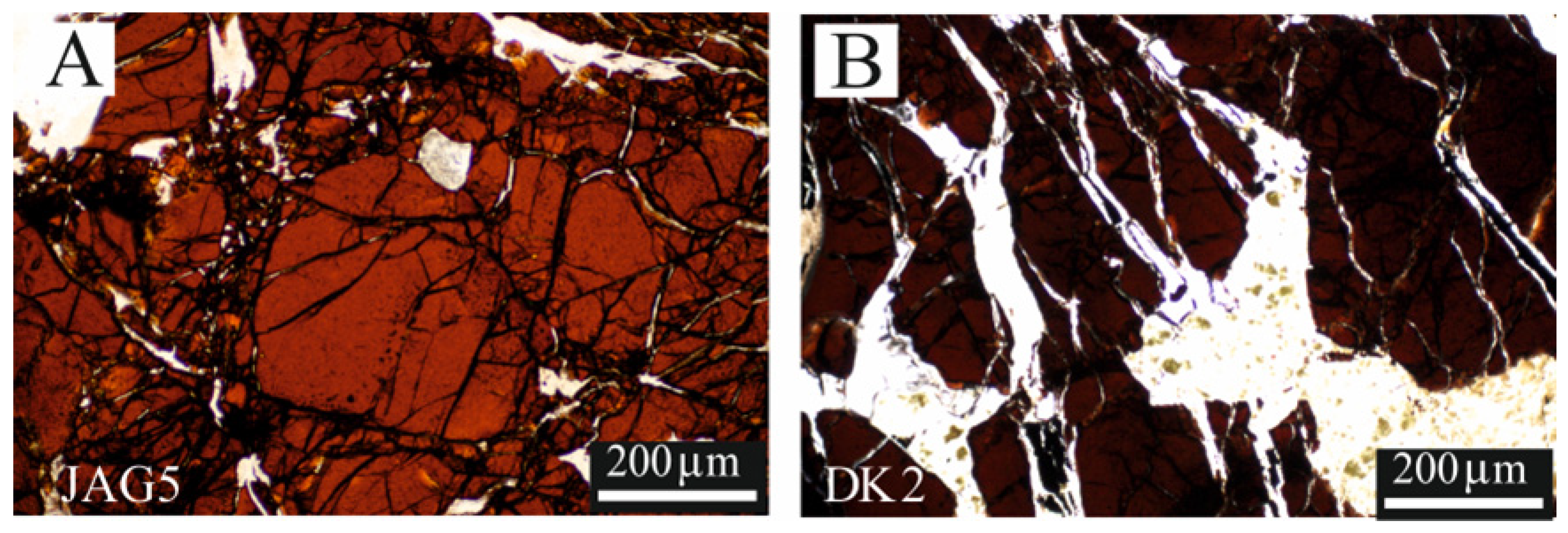
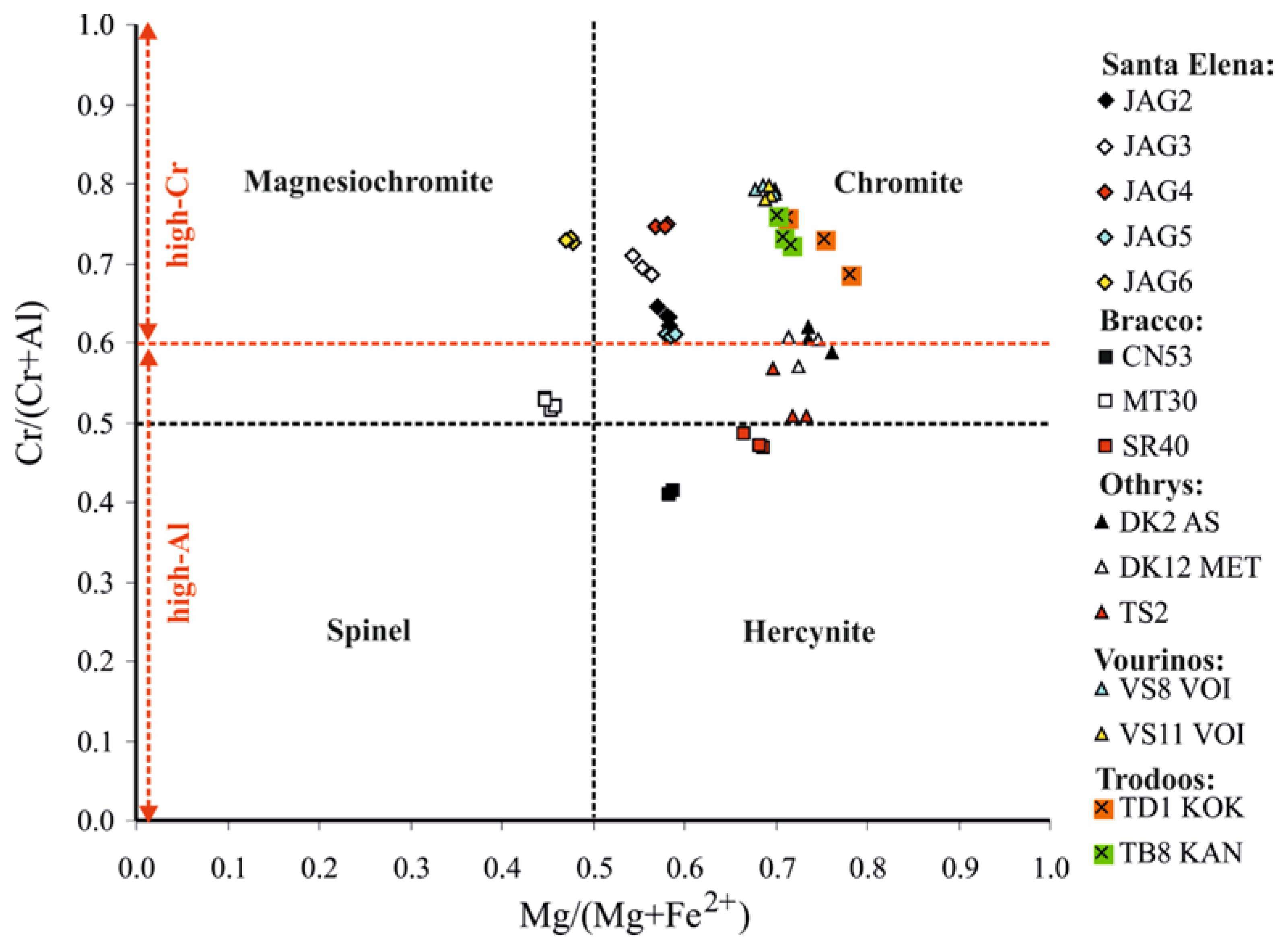
4.1. Texture of the Chromitites and the Chromite Composition
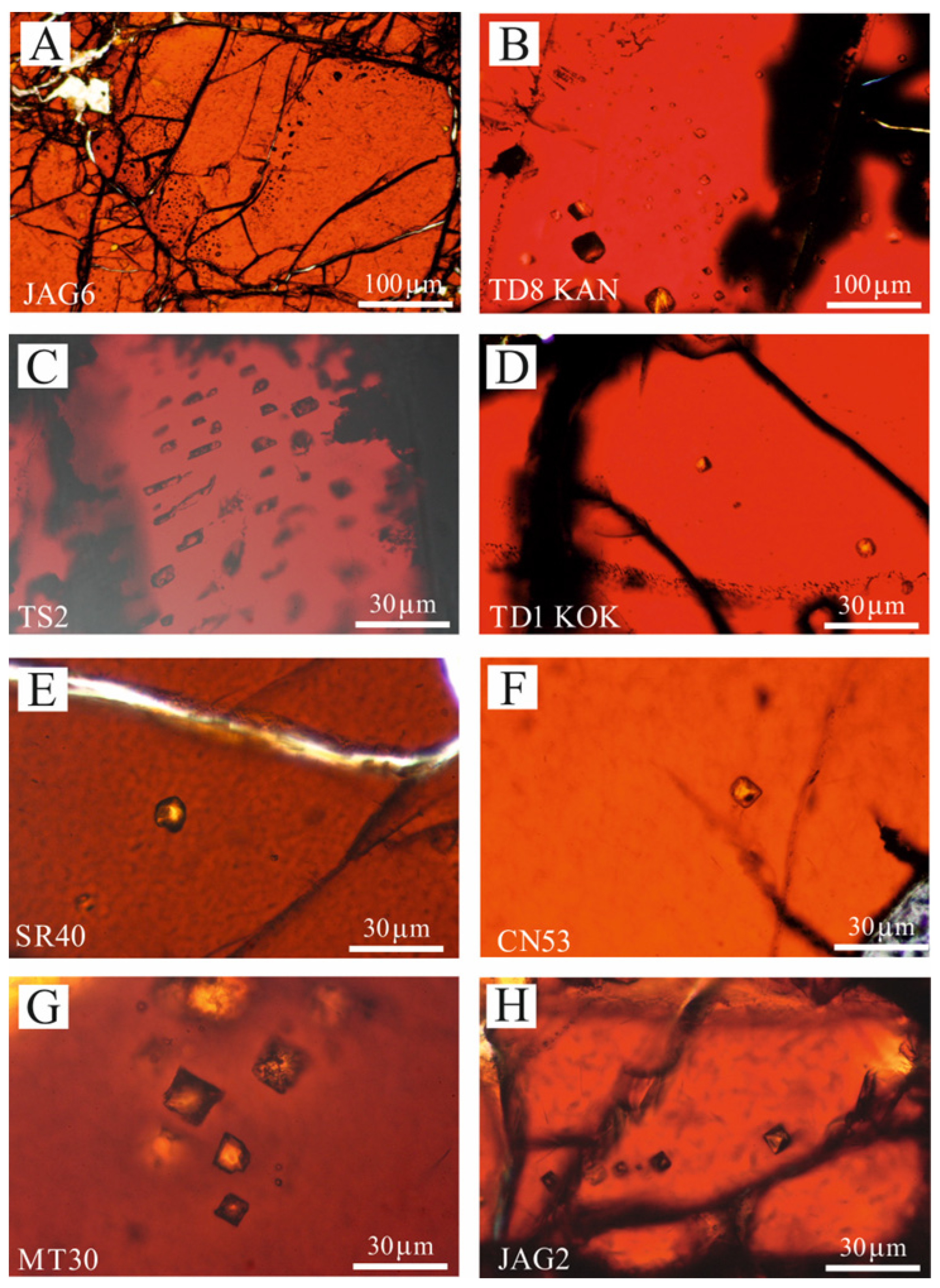
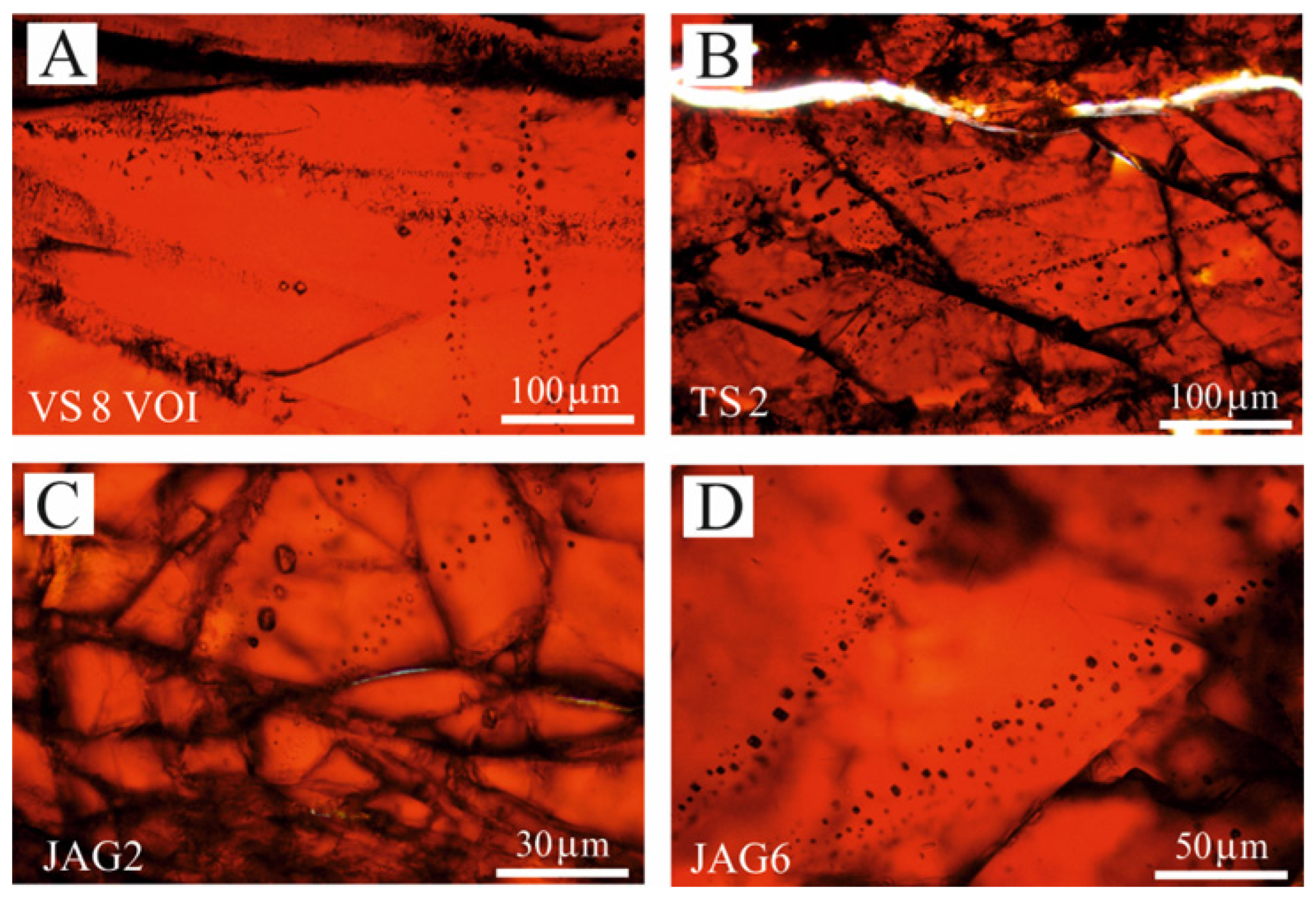
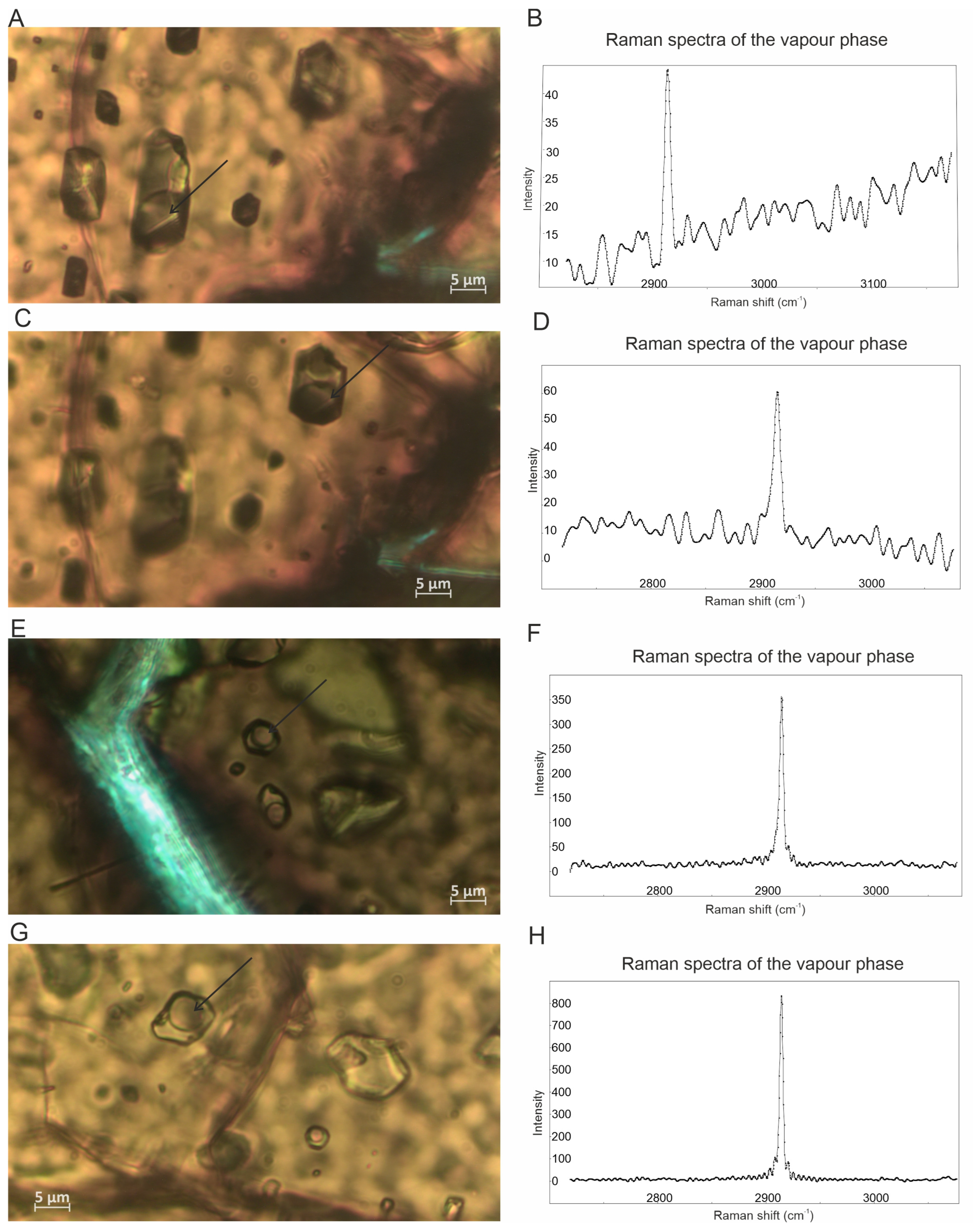
4.2. Fluid Inclusion Description and Raman Data
5. Discussion
6. Summary and Conclusions
- The research and Raman investigation of fluid inclusions in podiform chromitites is very challenging due to their lack of or poor transparency, although they may host millions of μm-sized fluid inclusions per cubic centimetre. A maximum thickness of 30 μm in thin, polished sections is considered to be optimal, because in some cases, enough light can pass through. This thickness prevents the application of the traditional methods for fluid inclusion studies in the analyzed samples. Furthermore, during the sample preparation, only very tiny fluid inclusions may preserve their original content of fluid, vapour, gas and solid phases.
- Despite the analytical challenges, the Raman spectra reveal the systematic presence of CH4 in certain fluid inclusions. Considering the exceptionally high number of fluid inclusions that potentially contain CH4 found in the investigated chromites as well as the CH4-rich fluid inclusions previously reported in the New Caledonia chromitites [6,18], we suggest that fluid inclusions in chromite crystals and their leaching can be a possible alternative source of the high amount of CH4 detected in some podiform chromitites and not only through the Sabatier reaction as previously proposed by Etiope et al. [11] and Portella et al. [12] based on whole-rock analyses. Therefore, a careful mineralogical and petrographic study is strongly recommended in order to support the whole-rock geochemical data.
- The mode of the occurrences of the studied CH4 bearing fluid inclusions, i.e., entrapped in unaltered chromite crystals formed at a magmatic temperature, suggest their origin from mantle-derived fluids, rather than those related to low-temperature serpentinization processes. Fluids with a mantle origin responsible for the presence of fluid inclusions were also described in the Oman chromitites [17].
- The fluid inclusions in high-Cr chromitites are exceedingly numerous compared to the high-Al ones from the Bracco complex, Italy, suggesting more abundant fluids in chromitites related to a subduction zone rather than those in the mid-ocean ridge. This is in agreement with the model proposed for the formation of podiform chromitites from basaltic melts with primary H2O contents high enough to exsolve a H2O-rich fluid phase during decompressing in the mantle, in a supra subduction geodynamic setting, in contrast to basaltic magmas in mid-ocean ridges, which are too dry to exsolve a H2O-rich fluid [9].
- Taking into consideration the wide distribution of podiform chromitites, the investigation of fluid inclusions, although difficult and challenging or even impossible when the chromite is opaque, can be applicable to other chromitites worldwide in order to verify the presence of H2O, CH4 and other volatile species. This information will greatly improve our understanding of the nature of the fluid phases during the formation of podiform chromitites.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arai, S. Genetic Link between Podiform Chromitites in the Mantle and Stratiform Chromitites in the Crust: A Hypothesis. Minerals 2021, 11, 209. [Google Scholar] [CrossRef]
- Lago, B.L.; Rabinowicz, M.; Nicolas, A. Podiform chromite ore bodies: A genetic model. J. Petrol. 1982, 23, 103–125. [Google Scholar] [CrossRef]
- Boudier, F.; Al-Rajhi, A. Structural control on chromite deposits in ophiolites: The Oman case. In Tectonic Evolution of the Oman Mountain; Special Publications; Rollinson, H.R., Searle, M.P., Abbasi, I.A., Al-Lazki, A., Al Kindi, M.H., Eds.; The Geological Society of London: London, UK, 2014; Volume 392, pp. 259–273. [Google Scholar]
- Arai, S.; Yurimoto, H. Podiform chromitites of the Tari-Misaka ultramafic complex, southwestern Japan, as mantle-melt interaction products. Econ. Geol. 1994, 89, 1279–1288. [Google Scholar] [CrossRef]
- Zhou, M.F.; Robinson, P.T.; Malpas, J.; Li, Z. Podiform Chromitites in the Luobusa ophiolite (Southern Tibet): Implications for melt-rock interaction and chromite segregation in the upper mantle. J. Petrol. 1996, 37, 3–21. [Google Scholar] [CrossRef]
- Johan, Z.; Martin, R.F.; Ettler, V. Fluids are bound to be involved in the formation of ophiolitic chromite deposits. Eur. J. Mineral. 2017, 29, 543–555. [Google Scholar] [CrossRef]
- Arai, S.; Miura, M.; Tamura, A.; Akizawa, N.; Ishikawa, A. Hydrothermal Chromitites from the Oman Ophiolite: The Role of Water in Chromitite Genesis. Minerals 2020, 10, 217. [Google Scholar] [CrossRef]
- Melcher, F.; Grum, W.; Simon, G.; Thalhammer, T.V.; Stumpfl, E.F. Petrogenesis of the ophiolitic giant chromite deposits of Kempirsai, Kazakhstan: A study of solid and fluid inclusions in chromite. J. Petrol. 1997, 38, 1419–1458. [Google Scholar] [CrossRef]
- Matveev, S.; Ballhaus, C. Role of water in the origin of podiform chromitite deposits. Earth Planet. Sci. Lett. 2002, 203, 235–243. [Google Scholar] [CrossRef]
- Johan, Z.; Dunlop, H.; LeBel, L.; Robert, J.-L.; Volfinger, M. Origin of chromite deposits in ophiolitic complexes: Evidence for a volatile-and sodium-rich reducing fluid phase. Fortschr. Mineral. 1983, 61, 105–107. [Google Scholar]
- Etiope, G.; Ifandi, E.; Nazzari, M.; Procesi, M.; Tsikouras, B.; Ventura, G.; Steele, A.; Tardini, R.; Szatmari, P. Widespread abiotic methane in chromitites. Sci. Rep. 2018, 8, 8728. [Google Scholar] [CrossRef]
- Portella, Y.d.M.; Zaccarini, F.; Etiope, G. First Detection of Methane within Chromitites of an Archean-Paleoproterozoic Greenstone Belt in Brazil. Minerals 2019, 9, 256. [Google Scholar] [CrossRef]
- Etiope, G.; Ionescu, A. Low-temperature catalytic CO2 hydrogenation with geological quantities of ruthenium: A possible abiotic CH4 source in chromitite-rich serpentinized rocks. Geofluids 2015, 15, 438–452. [Google Scholar] [CrossRef]
- van den Kerkhof, A.; Hein, U.F. Fluid inclusion petrography. Lithos 2001, 55, 27–47. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. Rev. Mineral. Mineral. Soc. Am. Wash. 1984, 12, 646. [Google Scholar]
- Anthonioz, P.M.; Correa, A.V. Inclusions fluides dans la chromite. Cas des chromites podiformes d’age Precambrien au Portugal du Nord-Est. Comp. Rend. Hebd. Sean. Acad. Sci. 1974, 278, 3271–3273. [Google Scholar]
- Dunlop, H.M.; Fouillac, A.M. Isotope geochemistry of Oman basic-ultrabasic rocks and chromite deposits. In Metallogeny of Basic and Ultrabasic Rocks; Gallagher, M.J., Ixer, R.A., Neary, C.R., Prichard, H.M., Eds.; IMM: London, UK, 1986; pp. 291–304. [Google Scholar]
- Johan, Z. Chromite deposits in the massif du Sud ophiolite, New Caledonia: Genetic considerations. In Chromite, UNESCO’s IGCP 197 Project, Metallogeny of Ophiolites; Petraschek, W., Karamata, S., Kravchenko, G., Johan, Z., Economou, M., Eds.; Theophrastus: Athens, Greece, 1986; pp. 311–339. [Google Scholar]
- McElduff, B. Inclusions in Chromite from Troodos (Cyprus) and Their Petrological Significance. Ph.D. Thesis, Montan-Universitat, Leoben, Austria, 1989; 143p. [Google Scholar]
- Al-Boghdady, A.; Economou-Eliopoulos, M. Fluid inclusions in chromite from a pyroxenite dike of the Pindos ophiolite complex. Geochemistry 2005, 65, 191–202. [Google Scholar] [CrossRef]
- Nayak, R.; Pal, D.; Chinnasamy, S.S.; Satyanarayanan, M. PGE geochemistry and platinum-group minerals in chromitites from Indus Suture Zone ophiolite, northwest Himalaya, India. J. Earth Syst. Sci. 2023, 132, 122. [Google Scholar] [CrossRef]
- Zaccarini, F.; Garuti, G.; Proenza, J.A.; Campos, L.; Thalhammer, O.A.R.; Aiglsperger, T.; Lewis, J.F. Chromite and platinum group elements mineralization in the Santa Elena Ultramafic Nappe (Costa Rica): Geodynamic implications. Geol. Acta 2011, 9, 407–423. [Google Scholar]
- Dilek, Y.; Furnes, H. Ophiolite genesis and global tectonics: Geochemical and tectonic fingerprinting of ancient oceanic lithosphere. Geol. Soc. Am. Bull. 2011, 123, 387–411. [Google Scholar] [CrossRef]
- Baumgartner, R.J.; Zaccarini, F.; Garuti, G.; Thalhammer, O.A.R. Mineralogical and geochemical investigation of layered chromitites from the Bracco-Gabbro complex, Ligurian ophiolite, Italy. Cont. Mineral. Petrol. 2013, 165, 477–493. [Google Scholar] [CrossRef]
- Barth, M.G.; Mason, P.R.D.; Davies, G.R.; Dijkstra, A.H.; Drury, M.R. Geochemistry of the Othris ophiolite, Greece: Evidence for refertilization? J. Petrol. 2003, 44, 1759–1785. [Google Scholar] [CrossRef]
- Koutsovitis, P.; Magganas, A. Boninitic and tholeiitic basaltic lavas and dikes from dispersed Jurassic East Othris ophiolitic units, Greece: Petrogenesis and geodynamic implications. Int. Geol. Rev. 2016, 58, 1983–2006. [Google Scholar] [CrossRef]
- Rassios, A.; Moores, E.; Green, H. Magmatic structure and stratigraphy of the Vourinos ophiolite cumulate zone, northern Greece. Ofioliti 1983, 8, 377–410. [Google Scholar]
- Robertson, A.H.F. Overview of the genesis and emplacment of Mesozoic ophiolites in the Eastern Mediterranean Tethyan region. Lithos 2002, 65, 1–67. [Google Scholar] [CrossRef]
- Panayiotou, A.; Michaelides, A.E.; Georgiou, E. The chromite deposits of the Troodos ophiolite complex, Cyprus. In Cromite; Petrascheck, W., Karamata, S., Kravchenko, G.G., Johan, J., Economou, M., Engin, T., Eds.; Theophrastus Publication: Athens, Greece, 1986; pp. 161–198. [Google Scholar]
- Bosi, F.; Biagioni, C.; Pasero, M. Nomenclature and classification of the spinel supergroup. Eur. J. Mineral. 2019, 31, 183–192. [Google Scholar] [CrossRef]
- Chi, G.; Diamond, L.W.; Lu, H.; Lai, J.; Chu, H. Common Problems and Pitfalls in Fluid Inclusion Study: A Review and Discussion. Minerals 2021, 11, 7. [Google Scholar] [CrossRef]
- Simmons, G.; Richter, D. Micro-cracks in rock. In The Physics and Chemistry of Minerals and Rocks; Strens, R.G.J., Ed.; Wiley: New York, NY, USA, 1976; pp. 105–137. [Google Scholar]
- Kranz, R.L. Micro-cracks in rocks: A review. Tectonophysics 1983, 100, 449–480. [Google Scholar] [CrossRef]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Klein, F.; Grozevab, N.G.; Seewalda, J.S. Abiotic methane synthesis and serpentinization in olivine-hosted fluid inclusions. Proc. Natl. Acad. Sci. USA 2019, 116, 17666–17672. [Google Scholar] [CrossRef]
- Etiope, G.; Whiticar, M.J. Abiotic methane in continental ultramafic rock systems: Towards a genetic model. Appl. Geochem. 2019, 102, 139–152. [Google Scholar] [CrossRef]
- Harper, G.D. Tectonics of slow spreading mid-ocean ridges and consequences of avariable depth to the brittle/ductile transition. Tectonics 1985, 4, 395–409. [Google Scholar] [CrossRef]
- Garuti, G.; Pushkarev, E.V.; Gottman, I.A.; Zaccarini, F. Chromite-PGM Mineralization in the Lherzolite Mantle Tectonite of the Kraka Ophiolite Complex (Southern Urals, Russia). Minerals 2021, 11, 1287. [Google Scholar] [CrossRef]
- Bussolesi, M.; Grieco, G.; Cavallo, A.; Zaccarini, F. Different Tectonic Evolution of Fast Cooling Ophiolite Mantles Recorded by Olivine-Spinel Geothermometry: Case Studies from Iballe (Albania) and Nea Roda (Greece). Minerals 2022, 12, 64. [Google Scholar] [CrossRef]
- Pappalardo, L.; Buono, G.; Procesi, M.; Etiope, G. The link between ophiolitic chromitites, natural hydrogen and methane: Insights from 3D microtomography. Chem. Geol. 2025, 676, 122575. [Google Scholar] [CrossRef]
- Arai, S.; Ishimaru, S.; Mizukami, T. Methane and propane micro-inclusions in olivine in titanoclinohumite-bearing dunites from the Sanbagawa high-P metamorphic belt, Japan: Hydrocarbon activity in a subduction zone and Ti mobility. Earth Planet. Sci. Lett. 2012, 353–354, 1–11. [Google Scholar] [CrossRef]
- Liu, W.; Fei, P.X. Methane-rich fluid inclusions from ophiolitic dunite and post-collisional mafic-ultramafic intrusion: The mantle dynamics beneath the Paleo-Asian Ocean through to the post-collisional period. Earth Planet. Sci. Lett. 2006, 242, 286–301. [Google Scholar] [CrossRef]
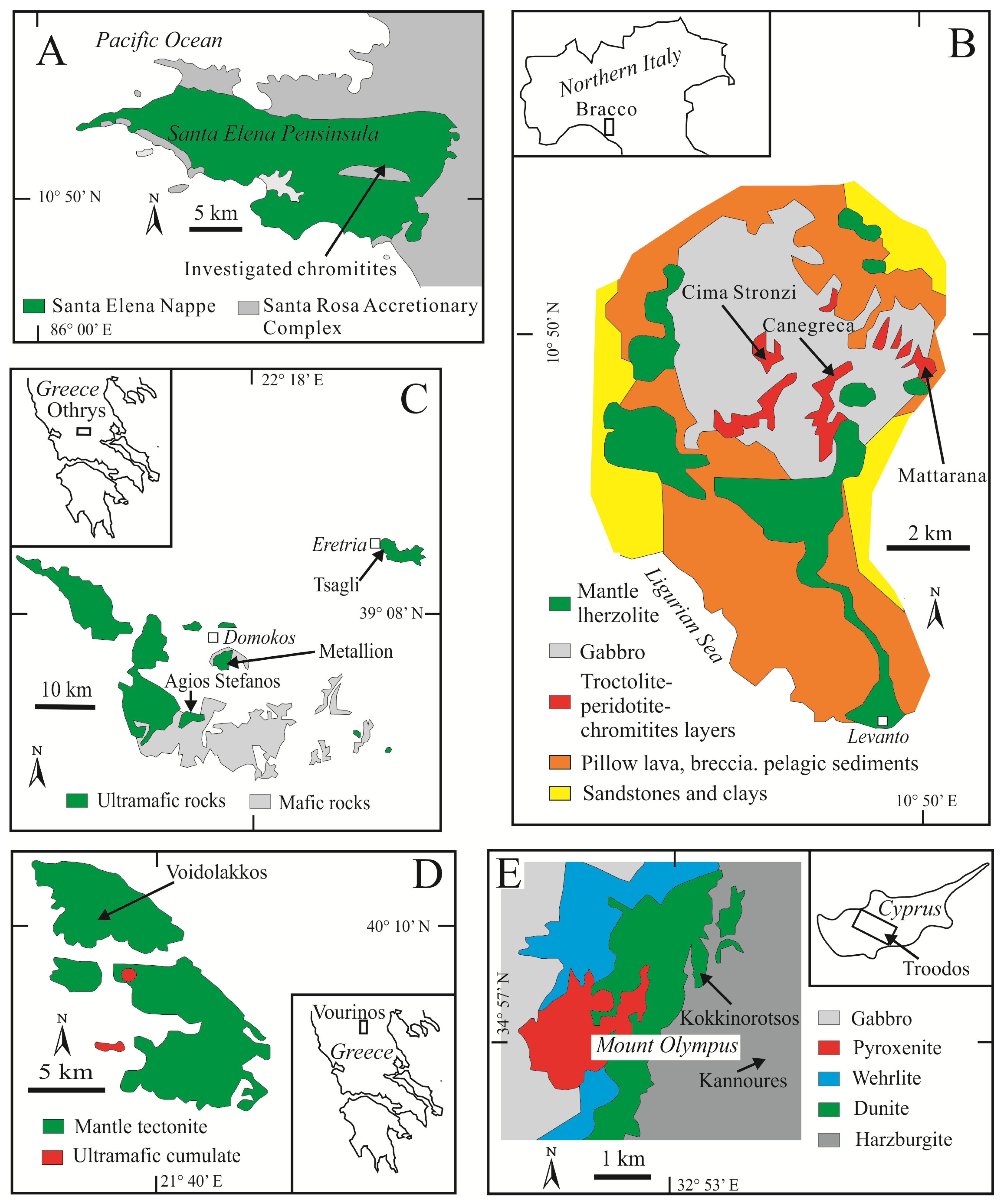
| Country | Ophiolite | Age | Geodynamic Setting | Name of the Deposit | Stratigraphy | Host Peridotite | Chromitite Type | Chromite Composition | References |
|---|---|---|---|---|---|---|---|---|---|
| COSTA RICA | Santa Elena | Cretaceous | SSZ | Mantle | Harzburgite | Small pod | High-Cr | [22] p.w. | |
| ITALY | Bracco | Jurassic | MOR | Canegreca, | Mantle-cumulate | Lherzolite | Layered | High-Al | [23,24] p.w. |
| Cima Stronzi, | Mantle-cumulate | Lherzolite | Layered | High-Al | |||||
| Mattarana | Mantle-cumulate | Lherzolite | Layered | High-Al | |||||
| GREECE | Othrys | Jurassic-Cretaceous | MOR-SSZ | Agios Stefanos, | Mantle | Harzburgite-Lherzolite | Massive pod | High-Al, High-Cr | [25,26] p.w. |
| Metallion, | Mantle | Harzburgite-Lherzolite | Massive pod | High-Al, High-Cr | |||||
| Tsangli | Mantle | Harzburgite-Lherzolite | Massive pod | High-Al | |||||
| Vourinos | Jurassic-Cretaceous | SSZ | Voidolakkos | Mantle | Harzburgite | Schlieren | High-Cr | [27,28] p.w. | |
| CYPRUS | Troodos | Cretaceous | SSZ | Kokkinorotsos, | Mantle | Harzburgite-Dunite | Massive pod | High-Cr | [16,29] p.w. |
| Kannoures | Mantle | Harzburgite | Massive pod | High-Cr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccarini, F.; Kiss, G.B.; Garuti, G.; Mauro, D.; Economou-Eliopoulos, M.; Hegedűs, M.; Biagioni, C. Methane in Fluid Inclusions in Ophiolitic Chromitites Revealed by Raman Spectroscopy: Preliminary Results. Minerals 2025, 15, 335. https://doi.org/10.3390/min15040335
Zaccarini F, Kiss GB, Garuti G, Mauro D, Economou-Eliopoulos M, Hegedűs M, Biagioni C. Methane in Fluid Inclusions in Ophiolitic Chromitites Revealed by Raman Spectroscopy: Preliminary Results. Minerals. 2025; 15(4):335. https://doi.org/10.3390/min15040335
Chicago/Turabian StyleZaccarini, Federica, Gabriella B. Kiss, Giorgio Garuti, Daniela Mauro, Maria Economou-Eliopoulos, Máté Hegedűs, and Cristian Biagioni. 2025. "Methane in Fluid Inclusions in Ophiolitic Chromitites Revealed by Raman Spectroscopy: Preliminary Results" Minerals 15, no. 4: 335. https://doi.org/10.3390/min15040335
APA StyleZaccarini, F., Kiss, G. B., Garuti, G., Mauro, D., Economou-Eliopoulos, M., Hegedűs, M., & Biagioni, C. (2025). Methane in Fluid Inclusions in Ophiolitic Chromitites Revealed by Raman Spectroscopy: Preliminary Results. Minerals, 15(4), 335. https://doi.org/10.3390/min15040335










