Thermal Decomposition and Phase Transformation of Chrysotile in Asbestos-Containing Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Heat Treatment
2.1.1. ACW and Reference Materials
2.1.2. Analytical Methods
3. Results and Discussion
3.1. Properties of the Reference Materials and the ACW
3.1.1. Chemical Composition of the ACW
3.1.2. Mineralogy
3.1.3. Thermal Characteristics of the Reference Materials and ACW
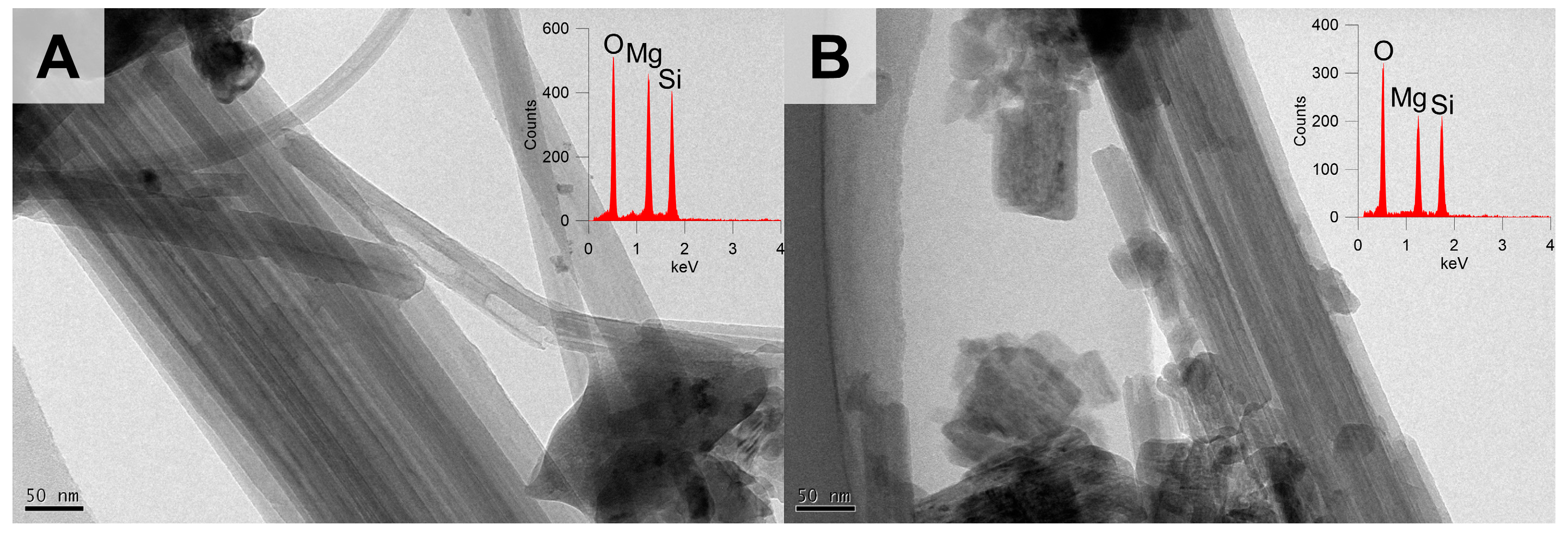
3.1.4. Optical, Morphological, and Structural Properties of the Chrysotile in ACW
3.2. Changes in the Characteristics of the ACW Following Heat Treatment
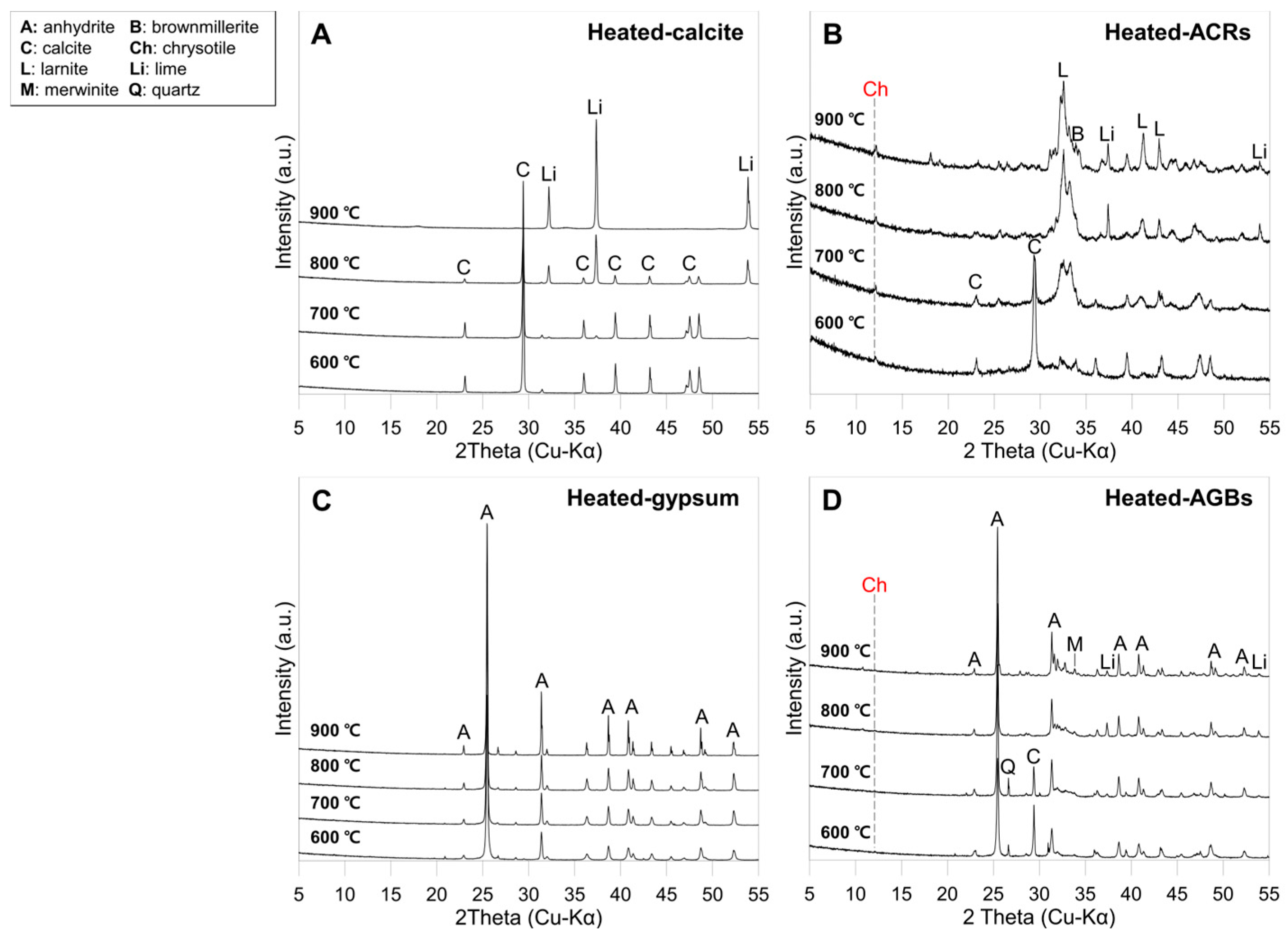
3.2.1. Mineralogical Changes in the ACW Following Heat Treatment
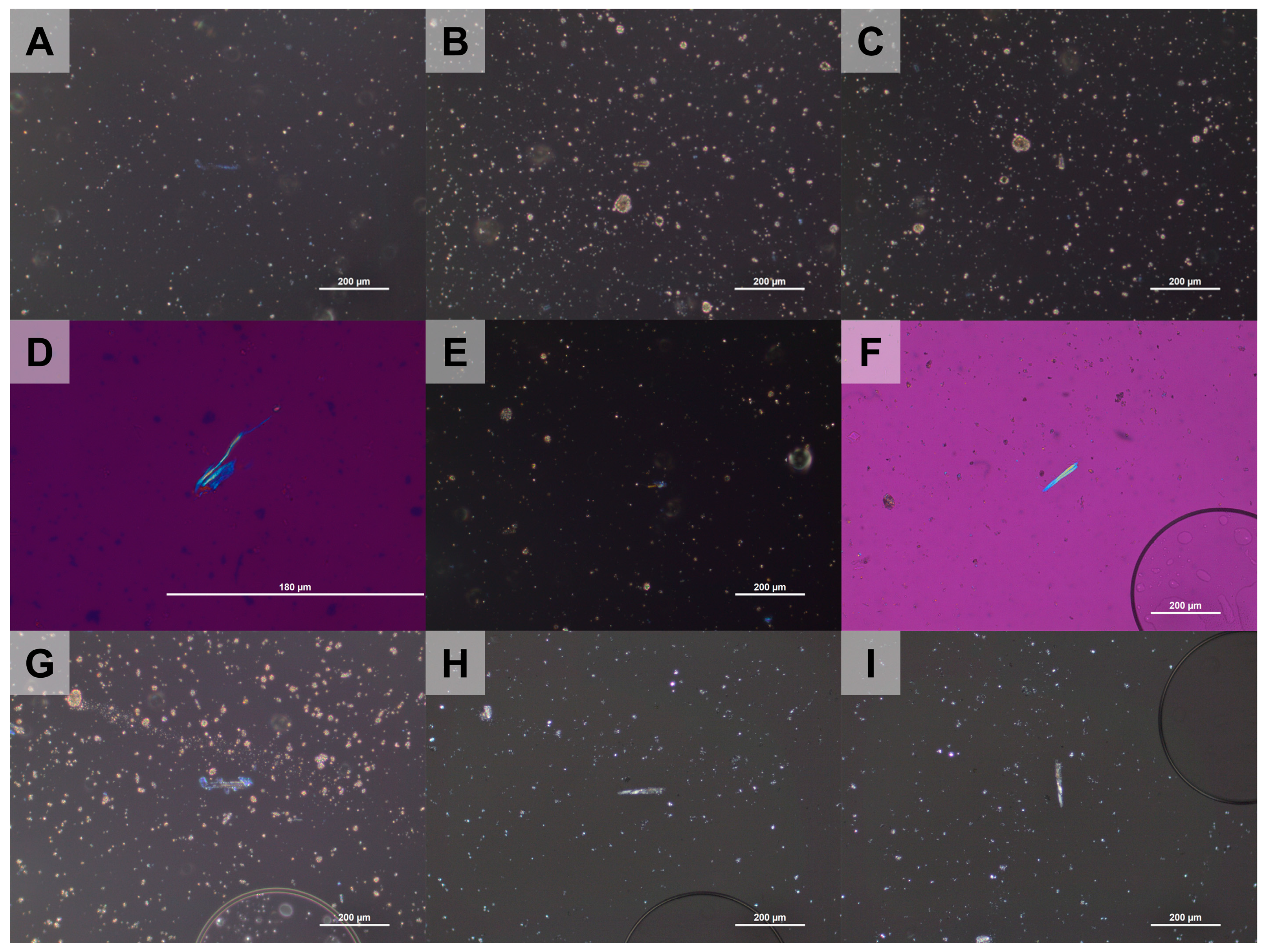
3.2.2. Changes in the Optical Properties of Chrysotile in the Heat-Treated ACW
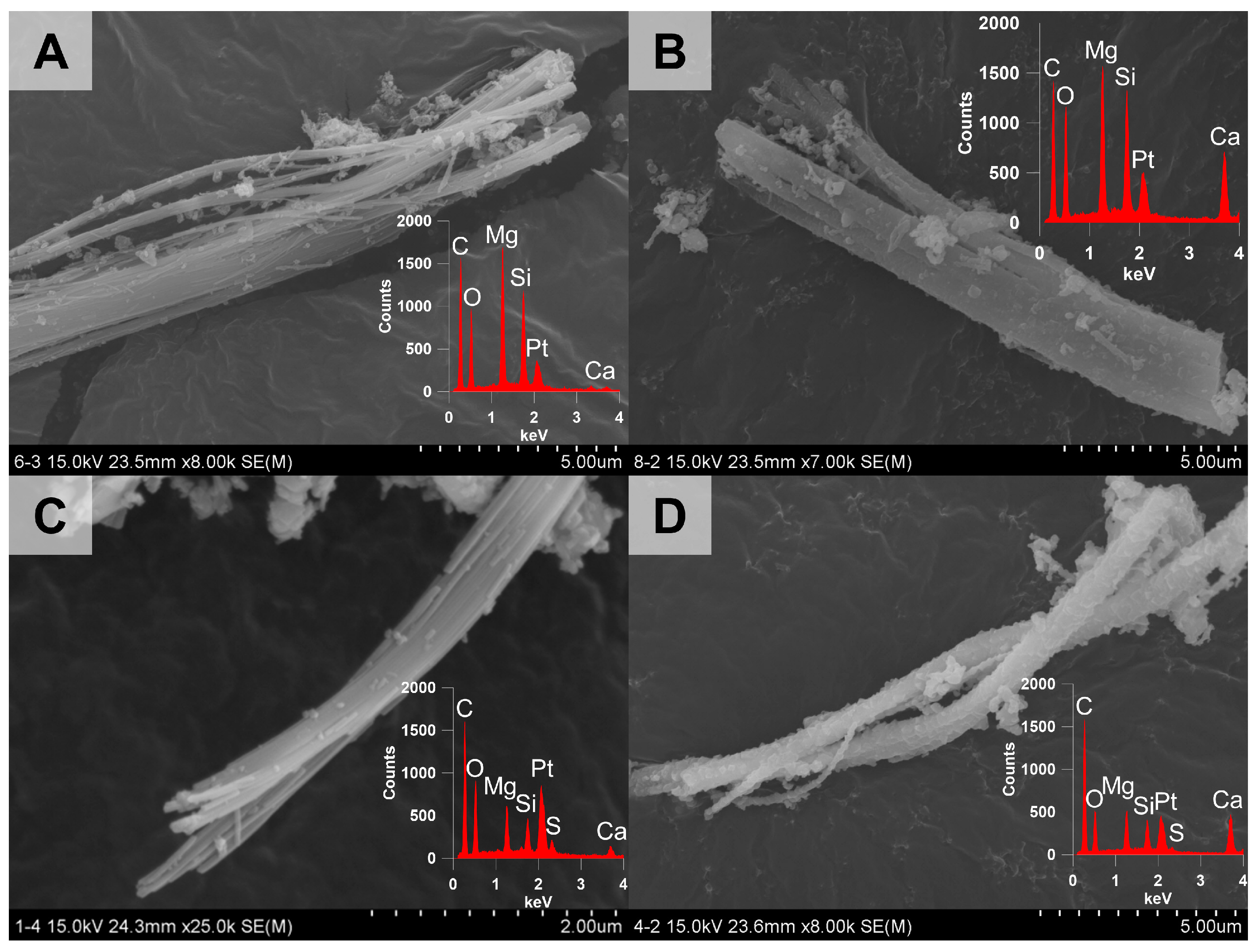
3.2.3. Morphological Changes of Chrysotile in the Heat-Treated ACW
3.2.4. Structural Changes of Chrysotile in the Heat-Treated ACW
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACW | Asbestos-containing waste |
| ACR | Asbestos cement roof |
| AGB | Asbestos gypsum board |
| N.D. | Not detected |
| a.u. | Arbitrary unit |
References
- Enforcement Rules of the Asbestos Safety Management Act; Act No.00503; Ministry of Environment: Sejong, Republic of Korea, 2022; Available online: https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=29423&type=part&key=39 (accessed on 2 December 2024).
- Asbestos Safety Management Act; Act No.18907; Ministry of Environment: Sejong, Republic of Korea, 2022; Available online: https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=60952&type=part&key=39 (accessed on 2 December 2024).
- Park, J.I.; Yoon, C.S.; Paik, N.W. A Study on Exposure among Asbestos Textile Workers and Estimation of Their Historical Exposures. J. Korean Soc. Occup. Environ. Hyg. 1995, 5, 16–39, (In Korean with English abstract). [Google Scholar]
- Virta, R.L. Worldwide Asbestos Supply and Consumption Trends from 1900 Through 2003; US Geological Survey: Reston, VA, USA, 2006; ISBN 1-4113-1167-1.
- Becklake, M.R. Asbestos-Related Diseases of the Lung and Other Organs: Their Epidemiology and Implications for Clinical Practice. Am. Rev. Respir. Dis. 1976, 114, 187–227. [Google Scholar] [PubMed]
- Choi, J.K.; Paek, D.M.; Paik, N.W. The Production, the Use, the Number of Workers and Exposure Level of Asbestos in Korea. J. Korean Soc. Occup. Environ. Hyg. 1998, 8, 242–253, (In Korean with English abstract). [Google Scholar]
- Roh, Y.; Jeong, H.Y.; Park, B.N.; Kim, C.W.; Kim, Y.M.; Seo, M.A.; Shin, H.S.; Kim, H.W.; Sung, Y.J. Asbestos Trend in Korea from 1918 to 2027 Using Text Mining Techniques in a Big Data Environment. Econ. Environ. Geol. 2023, 56, 457–473, (In Korean with English abstract). [Google Scholar] [CrossRef]
- Enforcement Decree of the Occupational Safety and Health Act; Act No.34603; Ministry of Employment and Labor: Sejong, Republic of Korea, 2009. Available online: https://elaw.klri.re.kr/eng_service/lawView.do?hseq=42061&lang=kor (accessed on 2 December 2024).
- 5th School Asbestos Management Manual; Ministry of Education: Sejong, Republic of Korea, 2019. (In Korean)
- 3rd Basic Plan For Asbestos Safety Management (2023–2027); Ministry of Environment: Sejong, Republic of Korea, 2022. (In Korean)
- Advanced Management and Administration Standard of Designated Waste Materials; National Institute of Environmental Research: Incheon, Republic of Korea, 2007; Volume 144. (In Korean)
- Promentilla, M.A.B.; Peralta, G.L. An Evaluation of Landfill Disposal of Asbestos-Containing Waste and Geothermal Residues within a Risk-Assessment Framework. J. Mater. Cycles Waste Manag. 2003, 5, 0013–0021. [Google Scholar] [CrossRef]
- Yoon, S.; Jeong, H.; Park, B.; Kim, Y.; Kim, H.; Park, J.; Son, B.; Kim, T.; Mun, Y.; Lee, S. Inactivation of Asbestos-Containing Slate Using High-Temperature Plasma Reactor. Korean J. Mineral. Petrol. 2020, 33, 407–417, (In Korean with English abstract). [Google Scholar] [CrossRef]
- Jung, H.S.; Cha, J.S.; Kim, S.M.; Lee, W.S.; Lim, H.J.; Kim, H.W. Evaluating the Efficiency of an Asbestos Stabilizer on Ceiling Tiles and the Characteristics of the Released Asbestos Fibers. J. Hazard. Mater. 2015, 300, 378–386. [Google Scholar] [CrossRef]
- Plescia, P.; Gizzi, D.; Benedetti, S.; Camilucci, L.; Fanizza, C.; De Simone, P.; Paglietti, F. Mechanochemical Treatment to Recycling Asbestos-Containing Waste. Waste Manag. 2003, 23, 209–218. [Google Scholar] [CrossRef]
- Bloise, A.; Catalano, M.; Gualtieri, A.F. Effect of Grinding on Chrysotile, Amosite and Crocidolite and Implications for Thermal Treatment. Minerals 2018, 8, 135. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Lavorgna, M.; Verdolotti, L.; De Stefano, L. Treatment and Recycling of Asbestos-Cement Containing Waste. J. Hazard. Mater. 2011, 195, 391–397. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Kozawa, T.; Onda, A.; Kanazawa, M.; Shinohara, J.; Takanami, T.; Shiraishi, M. A Novel Decomposition Technique of Friable Asbestos by CHClF2-Decomposed Acidic Gas. J. Hazard. Mater. 2009, 163, 593–599. [Google Scholar] [CrossRef]
- Nam, S.-N.; Jeong, S.K.; Lim, H.J. Thermochemical Destruction of Asbestos-Containing Roofing Slate and the Feasibility of Using Recycled Waste Sulfuric Acid. J. Hazard. Mater. 2014, 265, 151–157. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F.; Pollastri, S.; Rinaudo, C.; Croce, A.; Urso, G. Crystal Chemistry of the High Temperature Product of Transformation of Cement-Asbestos. J. Hazard. Mater. 2013, 248, 69–80. [Google Scholar] [CrossRef]
- Gualtieri, A.; Cavenati, C.; Zanatto, I.; Meloni, M.; Elmi, G.; Gualtieri, M.L. The Transformation Sequence of Cement–Asbestos Slates up to 1200 °C and Safe Recycling of the Reaction Product in Stoneware Tile Mixtures. J. Hazard. Mater. 2008, 152, 563–570. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Gerle, A. Thermal Decomposition of Asbestos-Containing Materials. J. Therm. Anal. Calorim. 2013, 113, 179–188. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Boccaccini, D.N.; Rivasi, M.R.; Barbieri, L.; Andreola, F.; Lancellotti, I.; Rabitti, D.; Pellacani, G.C. Microwave Thermal Inertisation of Asbestos Containing Waste and Its Recycling in Traditional Ceramics. J. Hazard. Mater. 2006, 135, 149–155. [Google Scholar] [CrossRef]
- Gualtieri, A.; Tartaglia, A. Thermal Decomposition of Asbestos and Recycling in Traditional Ceramics. J. Eur. Ceram. Soc. 2000, 20, 1409–1418. [Google Scholar] [CrossRef]
- Zaremba, T.; Peszko, M. Investigation of the Thermal Modification of Asbestos Wastes for Potential Use in Ceramic Formulation. J. Therm. Anal. Calorim. 2008, 92, 873–877. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Podwórny, J. Utilisation of Cement-Asbestos Wastes by Thermal Treatment and the Potential Possibility Use of Obtained Product for the Clinker Bricks Manufacture. J. Mater. Sci. 2015, 50, 6757–6767. [Google Scholar] [CrossRef]
- Durczak, K.; Pyzalski, M.; Brylewski, T.; Juszczyk, M.; Leśniak, A.; Libura, M.; Ustinovičius, L.; Vaišnoras, M. Modern Methods of Asbestos Waste Management as Innovative Solutions for Recycling and Sustainable Cement Production. Sustainability 2024, 16, 8798. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J. The Potential Use of Cement–Asbestos Waste in the Ceramic Masses Destined for Sintered Wall Clay Brick Manufacture. Ceram. Int. 2014, 40, 11995–12002. [Google Scholar] [CrossRef]
- Gualtieri, A.; Boccaletti, M. Recycling of the Product of Thermal Inertization of Cement–Asbestos for the Production of Concrete. Constr. Build. Mater. 2011, 25, 3561–3569. [Google Scholar] [CrossRef]
- Jeong, H.; Moon, W.; Roh, Y. Characterization of Mineralogical Changes of Chrysotile and Its Thermal Decomposition by Heat Treatment. Econ. Environ. Geol. 2016, 49, 77–88, (In Korean with English abstract). [Google Scholar] [CrossRef]
- Martin, C. The Thermal Decomposition of Chrysotile. Mineral. Mag. 1977, 41, 453–459. [Google Scholar] [CrossRef]
- Jolicoeur, C.; Duchesne, D. Infrared and Thermogravimetric Studies of the Thermal Degradation of Chrysotile Asbestos Fibers: Evidence for Matrix Effects. Can. J. Chem. 1981, 59, 1521–1526. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Bartholomew Press: London, UK, 1974; Volume 4. [Google Scholar]
- Antao, S.M.; Duane, M.J.; Hassan, I. DTA, TG, and XRD Studies of Sturmanite and Ettringite. Can. Mineral. 2002, 40, 1403–1409. [Google Scholar] [CrossRef]
- Hass, M.; Sutherland, G.B.B.M. The Infra-Red Spectrum and Crystal Structure of Gypsum. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1956, 236, 427–445. [Google Scholar] [CrossRef]
- Bloise, A.; Kusiorowski, R.; Gualtieri, M.L.; Gualtieri, A.F. Thermal Behaviour of Mineral Fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; European Mineralogical Union: London, UK, 2017; ISBN 978-0-903056-65-6. [Google Scholar]
- Karunadasa, K.S.; Manoratne, C.; Pitawala, H.; Rajapakse, R. Thermal Decomposition of Calcium Carbonate (Calcite Polymorph) as Examined by in-Situ High-Temperature X-Ray Powder Diffraction. J. Phys. Chem. Solids 2013, 134, 21–28. [Google Scholar] [CrossRef]
- Dellisanti, F.; Minguzzi, V.; Morandi, N. Experimental Results from Thermal Treatment of Asbestos Containing Materials. GeoActa 2002, 1, 2001–2002. [Google Scholar]
- Dias, C.M.R.; Cincotto, M.A.; Savastano Jr, H.; John, V.M. Long-Term Aging of Fiber-Cement Corrugated Sheets–The Effect of Carbonation, Leaching and Acid Rain. Cem. Concr. Compos. 2008, 30, 255–265. [Google Scholar] [CrossRef]
- Miriello, D.; Bloise, A.; Crisci, G.M.; Barrese, E.; Apollaro, C. Effects of Milling: A Possible Factor Influencing the Durability of Historical Mortars. Archaeometry 2010, 52, 668–679. [Google Scholar] [CrossRef]
- Kwon, J. Asbestos Analysis of Bulk Samples Using a Polarized Light Microscope; Occupational Safety and Health Research Institute: Incheon, Republic of Korea, 2009; p. 80. (In Korean) [Google Scholar]
- U.S. Environmental Protection Agency. Interim Method for the Determination of Asbestos in Bulk Insulation Sample; EPA-600/M4-82-020; BiblioGov: Cincinanati, OH, USA, 1982. [Google Scholar]
- Perkins, R.L.; Harvey, B.W. Test Method: Method for the Determination of Asbestos in Bulk Building Materials. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=9100TKSO (accessed on 12 February 2025).
- Taylor, H. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Vijayakumar, N.; Venkatraman, S.K.; Choudhary, R.; Indurkar, A.; Chatterjee, A.; Abraham, J.; Ostrovskiy, S.; Senatov, F.; Locs, J.; Swamiappan, S. Conversion of Biowaste into Larnite by Sol-gel Combustion Route for Biomedical Applications. ChemistrySelect 2022, 7, e202103783. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, A.; Freemen, J. Raman, MIR, and NIR Spectroscopic Study of Calcium Sulfates: Gypsum, Bassanite, and Anhydrite. In Proceedings of the 40th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 23–27 March 2009; p. 2128. [Google Scholar]
- Belardi, G.; Piga, L. Influence of Calcium Carbonate on the Decomposition of Asbestos Contained in End-of-Life Products. Thermochim. Acta 2013, 573, 220–228. [Google Scholar] [CrossRef]
- Ham, S.H.; Hwang, S.H.; Yoon, C.S.; Park, D.U. Review on asbestos analysis. J. Korean Soc. Occup. Environ. Hyg. 2009, 19, 213–232, (In Korean with English abstract). [Google Scholar]
- Dodson, R.F.; Hammar, S.P. Asbestos: Risk Assessment, Epidemiology, and Health Effects, 1st ed.; CRC press: Boca Raton, FL, USA, 2005; ISBN 0-429-12242-X. [Google Scholar]
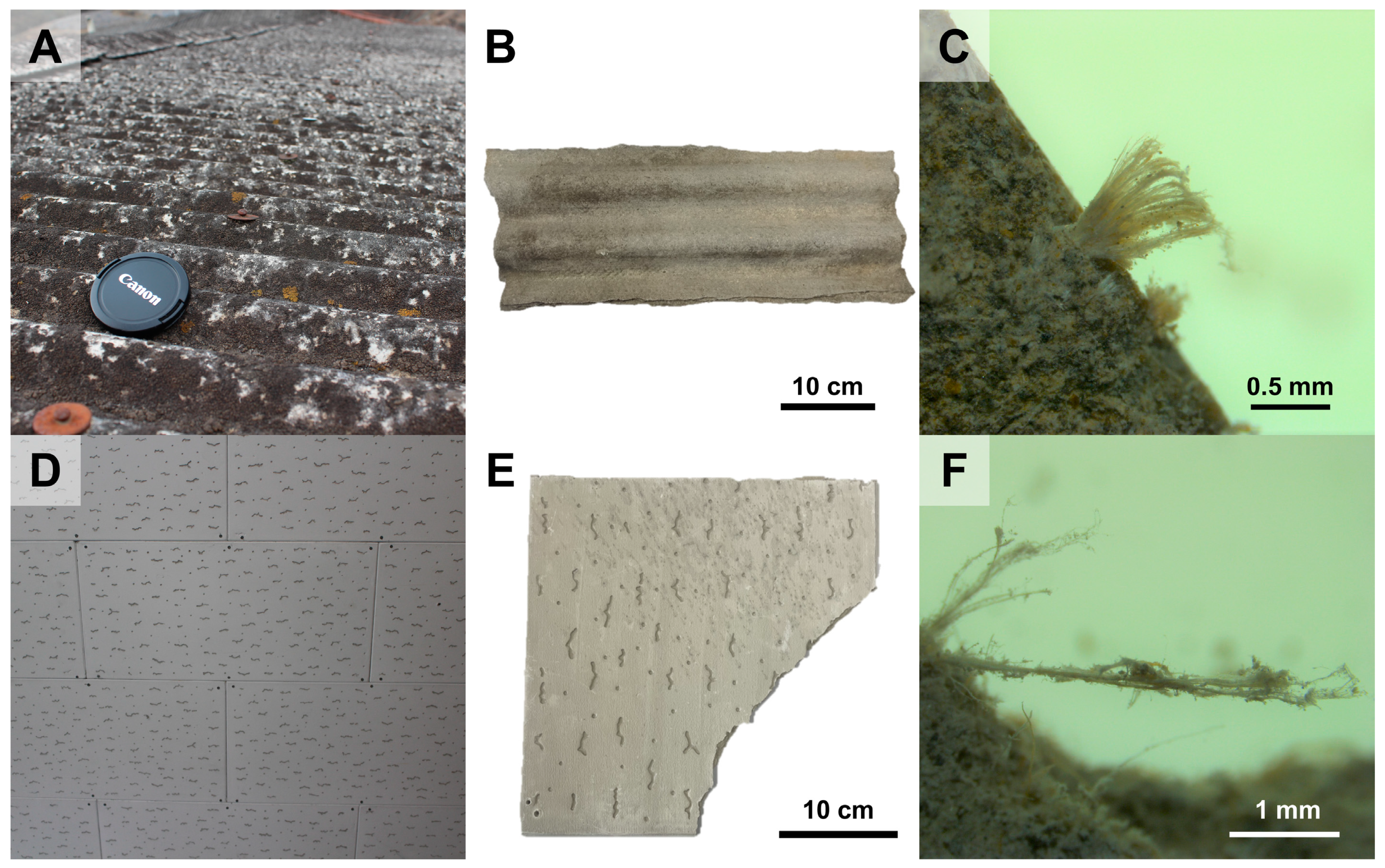
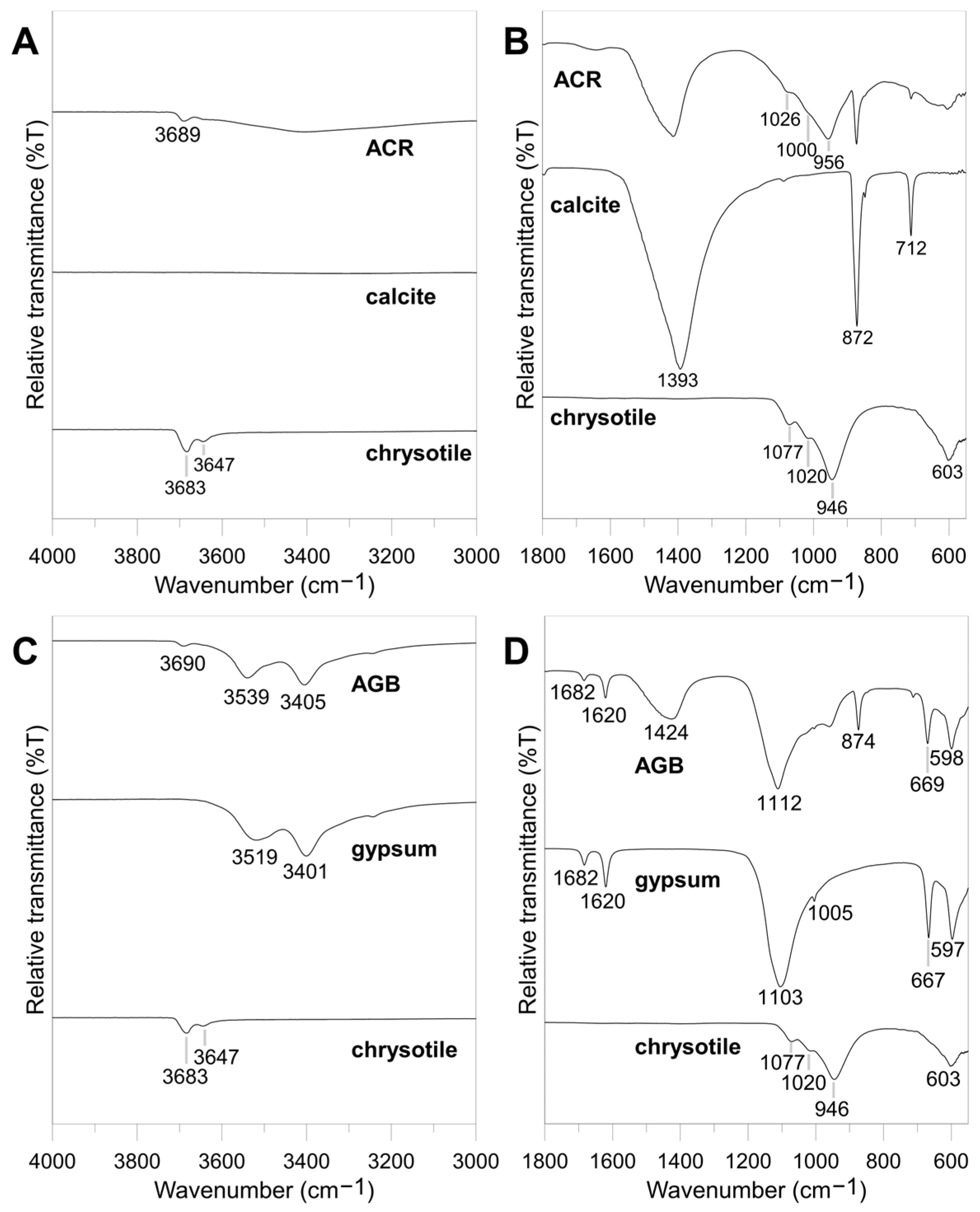

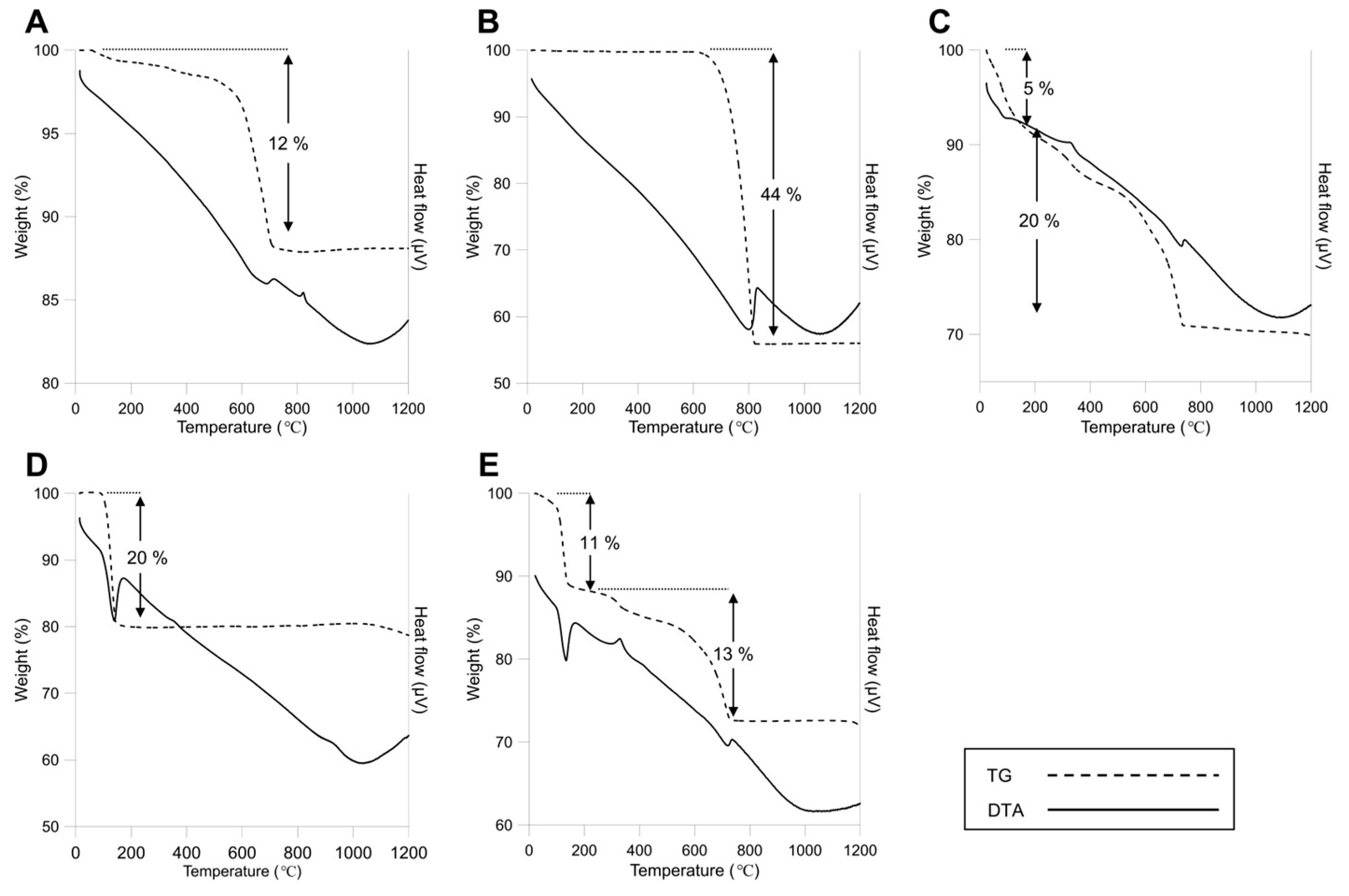

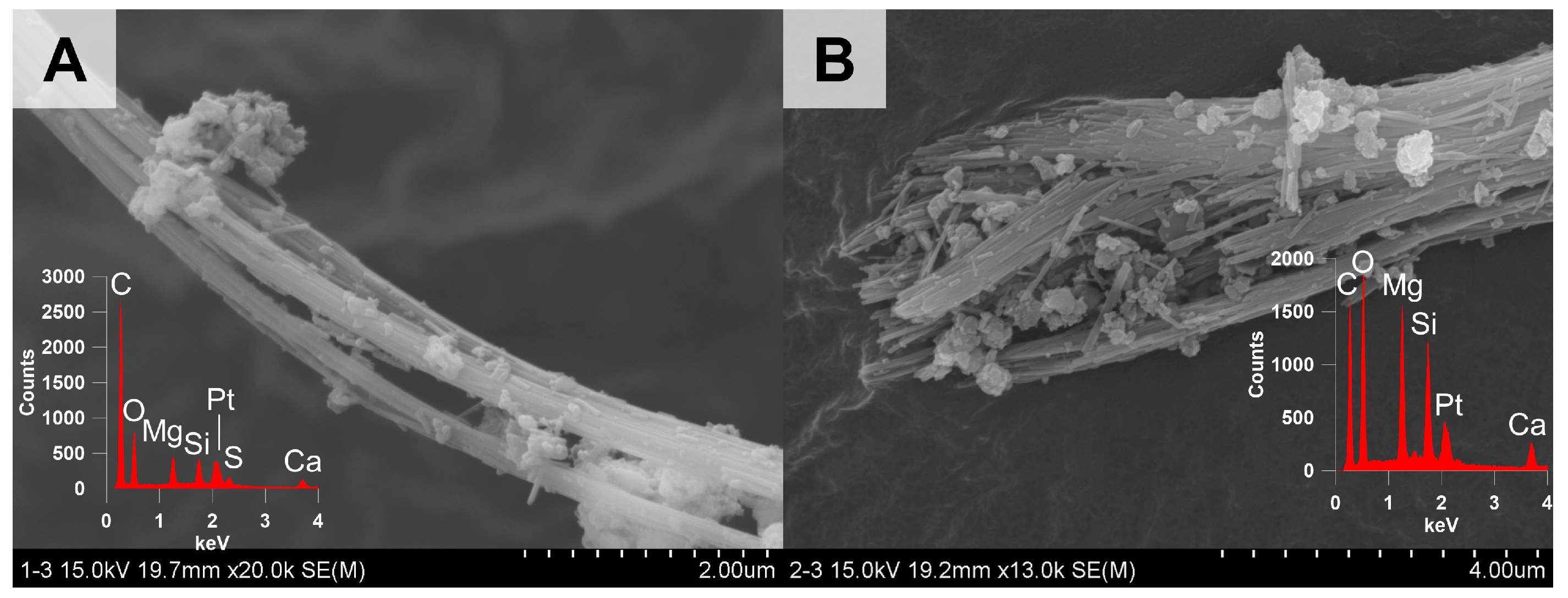


| ACW Type | Asbestos Content (%) | Constituents | Use |
|---|---|---|---|
| ACR | 8–14 | Cement and asbestos | Roofing |
| AGB | 4–6 | Gypsum, asbestos, cement, and paper | Ceilings |
| ACW | CaO | SiO2 | SO3 | MgO | Al2O3 | Fe2O3 | TiO2 | K2O | Cl | P2O5 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACRs | 57.9 | 21.9 | 3.0 | 7.7 | 4.5 | 3.9 | 0.4 | 0.2 | 0.2 | N.D. | 99.7 |
| AGBs | 49.3 | 8.4 | 33.5 | 4.7 | 1.1 | 1.7 | 0.4 | 0.2 | N.D. | 0.5 | 99.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Kim, Y.; Roh, Y. Thermal Decomposition and Phase Transformation of Chrysotile in Asbestos-Containing Waste. Minerals 2025, 15, 344. https://doi.org/10.3390/min15040344
Kim C, Kim Y, Roh Y. Thermal Decomposition and Phase Transformation of Chrysotile in Asbestos-Containing Waste. Minerals. 2025; 15(4):344. https://doi.org/10.3390/min15040344
Chicago/Turabian StyleKim, Chaewon, Yumi Kim, and Yul Roh. 2025. "Thermal Decomposition and Phase Transformation of Chrysotile in Asbestos-Containing Waste" Minerals 15, no. 4: 344. https://doi.org/10.3390/min15040344
APA StyleKim, C., Kim, Y., & Roh, Y. (2025). Thermal Decomposition and Phase Transformation of Chrysotile in Asbestos-Containing Waste. Minerals, 15(4), 344. https://doi.org/10.3390/min15040344







