Exploring High PT Experimental Charges Through the Lens of Phase Maps
Abstract
1. Introduction
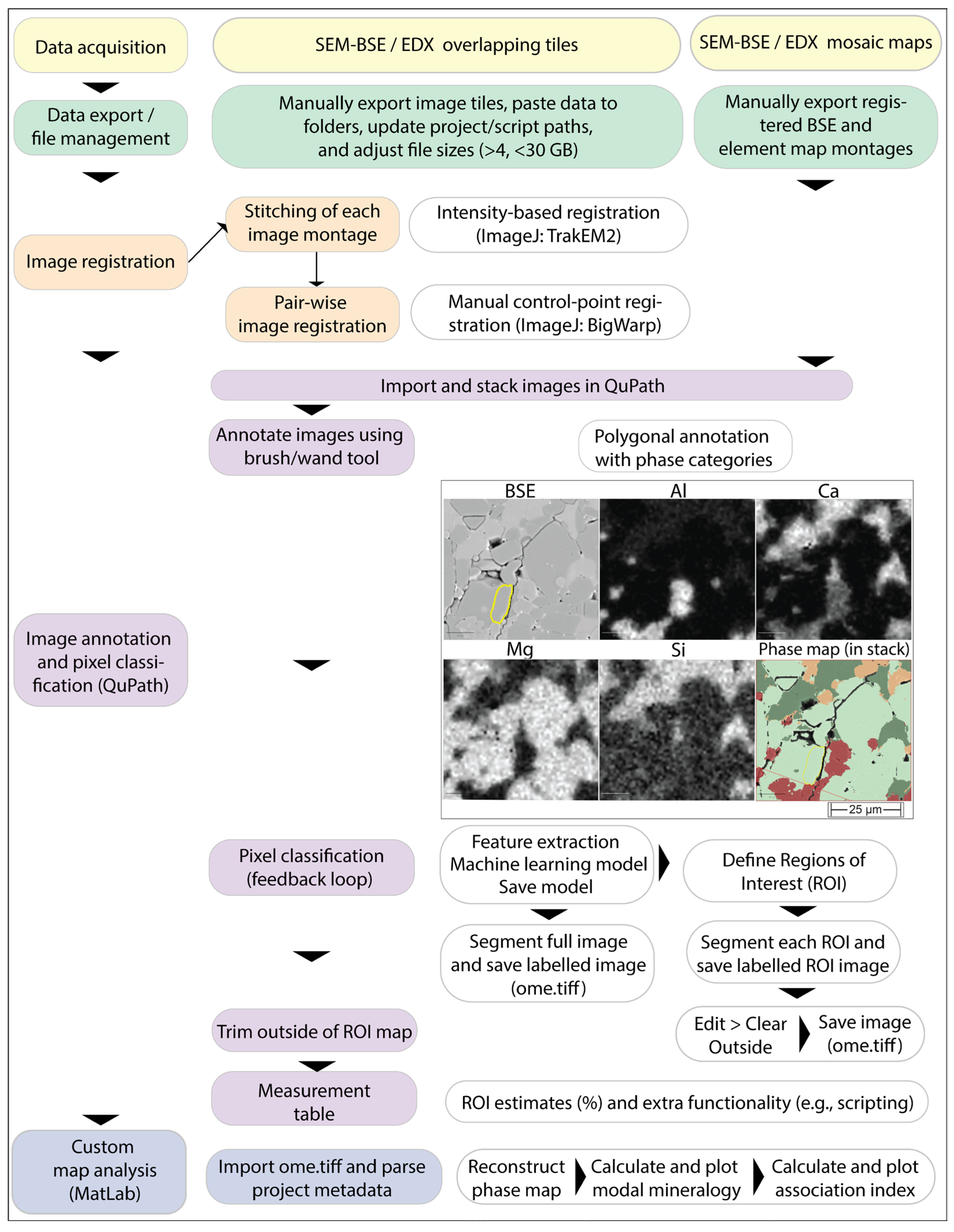
2. Materials and Methods
3. Results
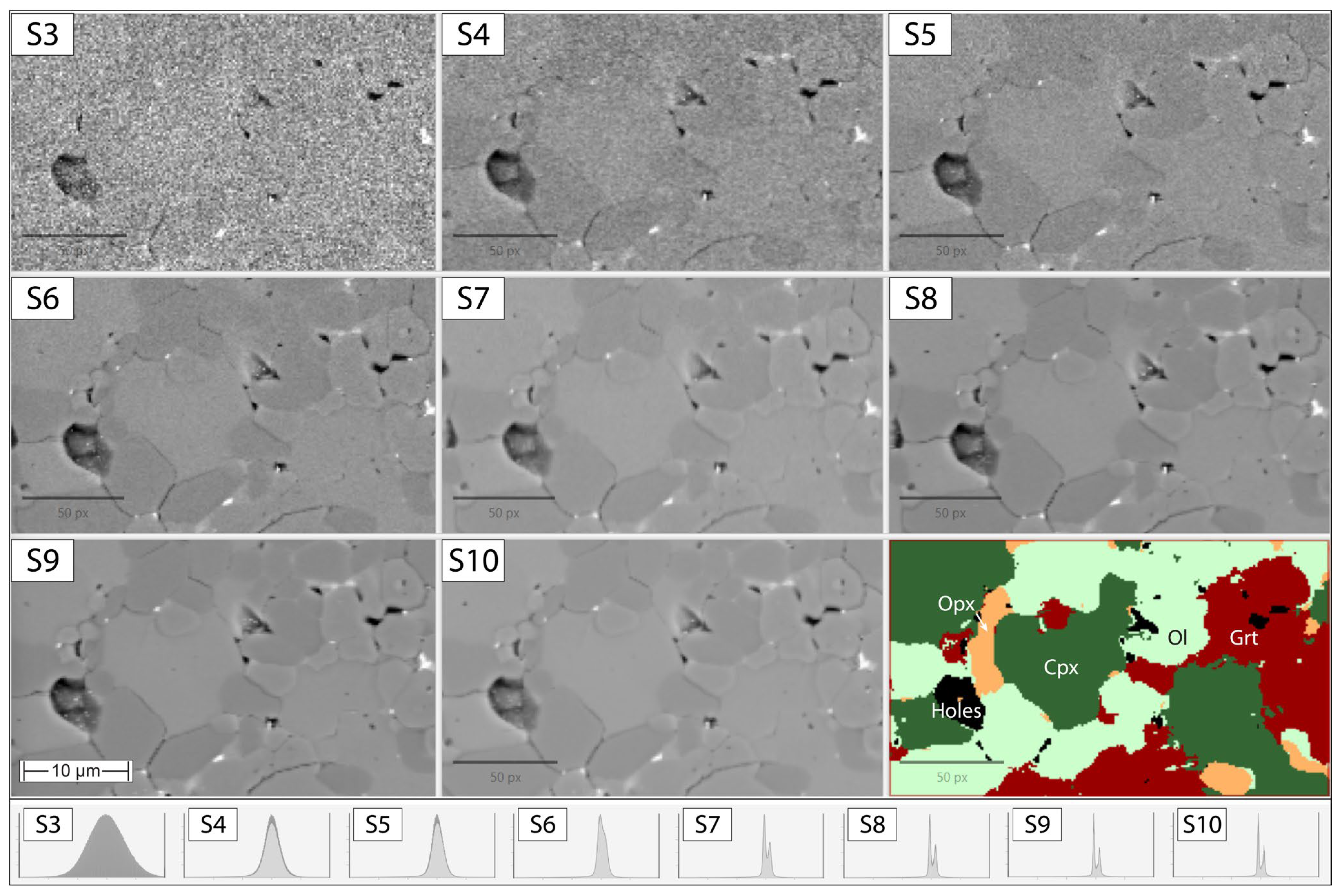
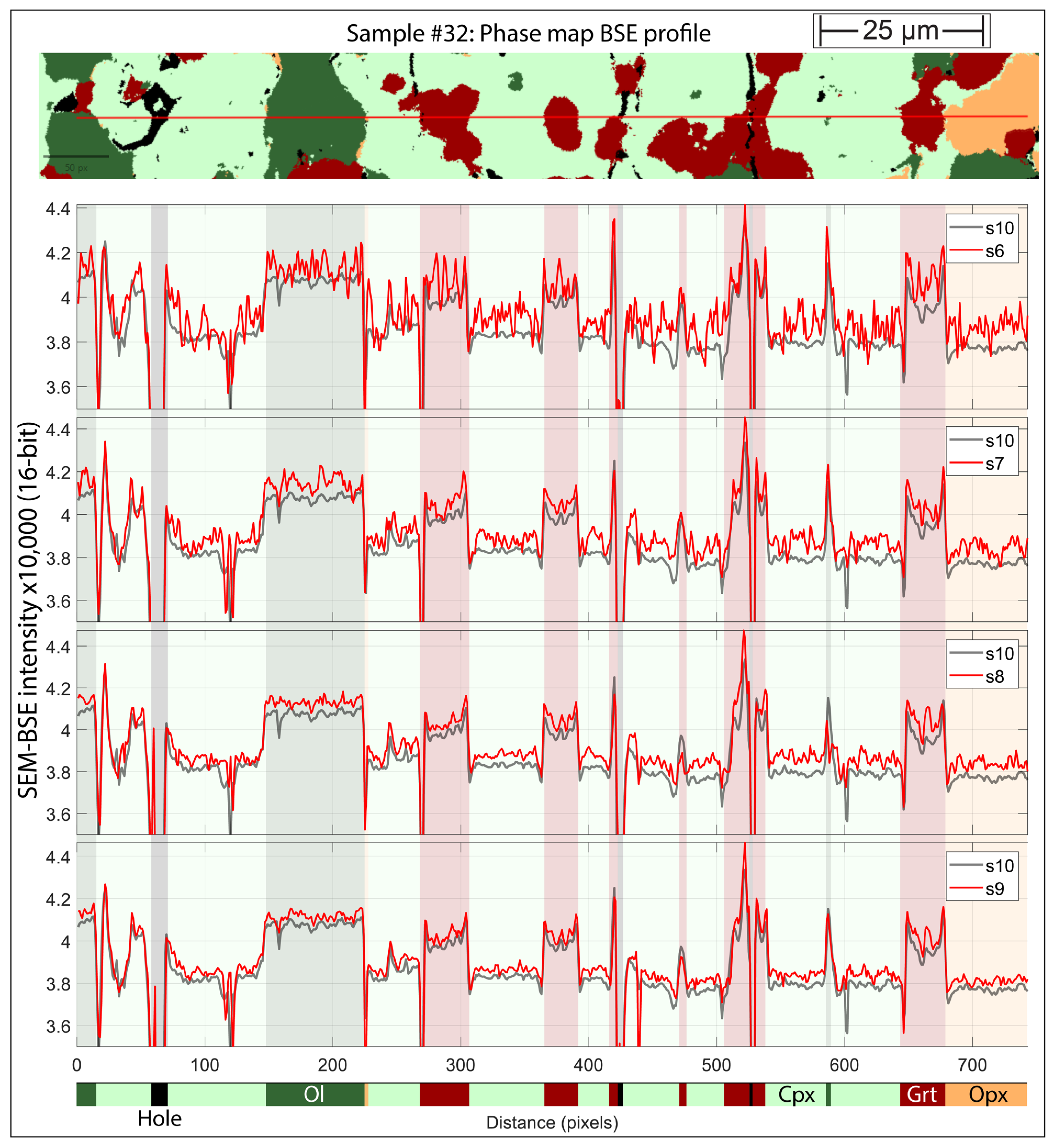
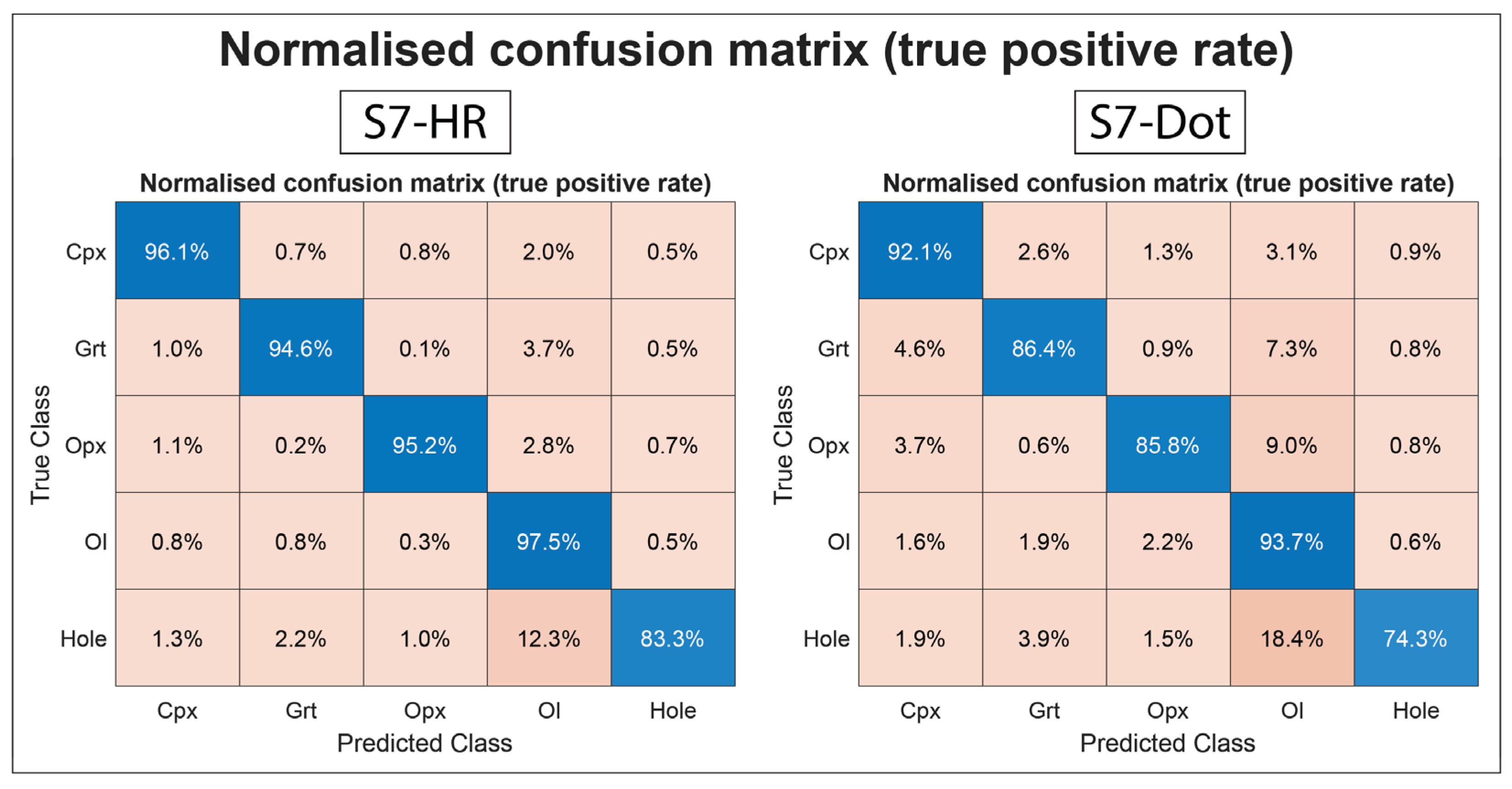
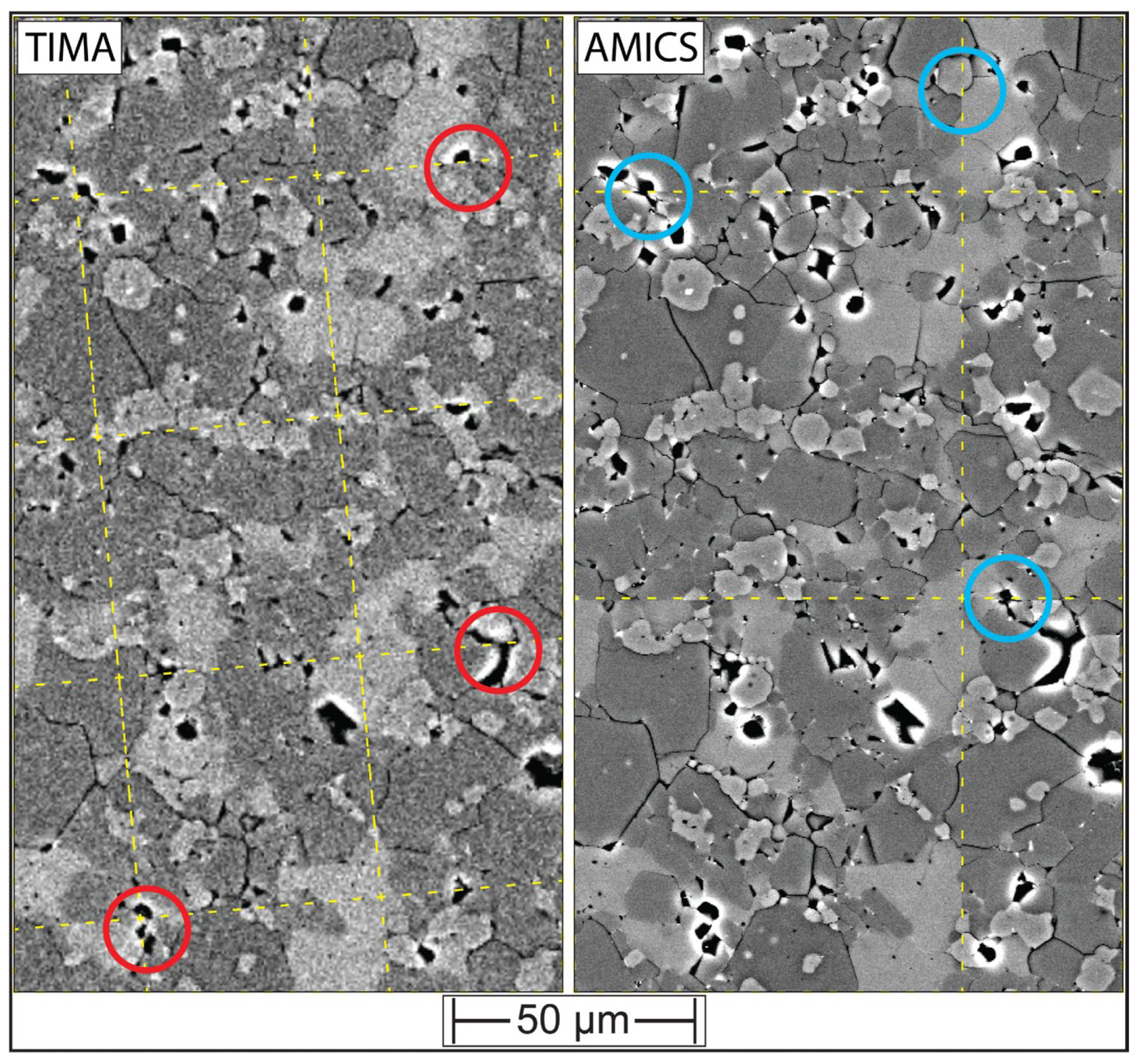
3.1. Evaluating BSE Scan Speeds and EDS Acquisition Settings
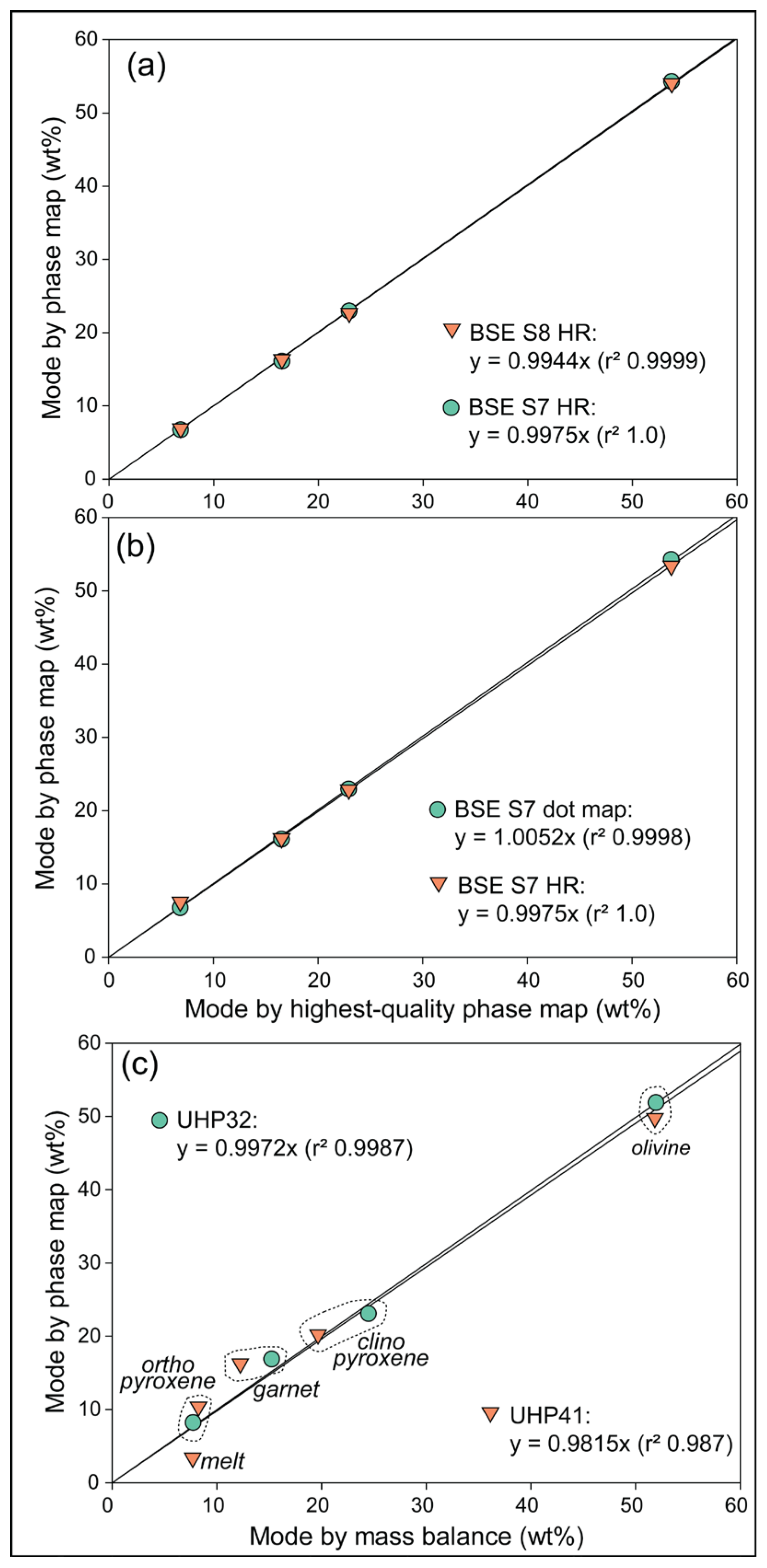
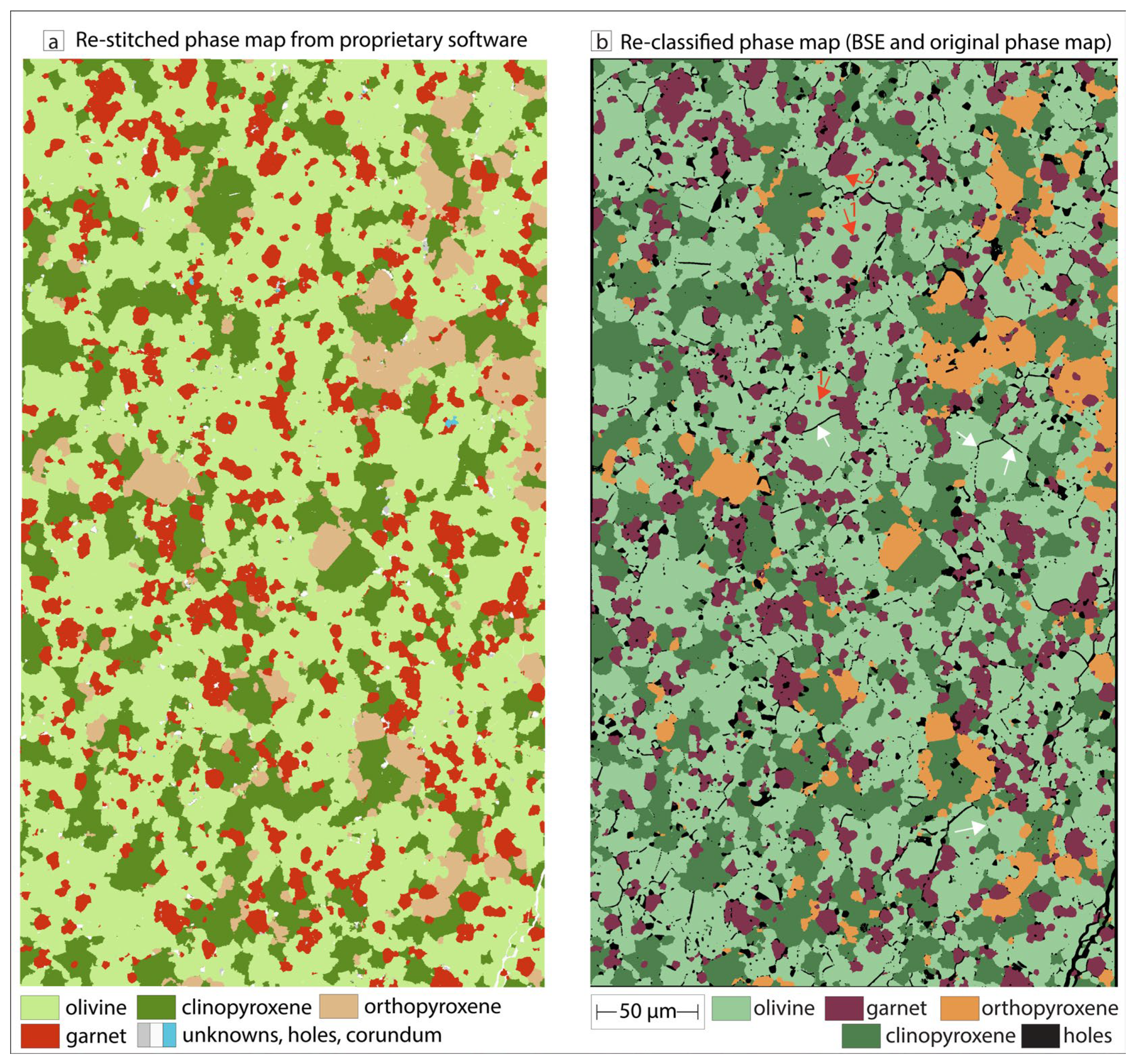
3.2. Phase Maps and Modal Mineralogy for Thermally Equilibrated Experiments
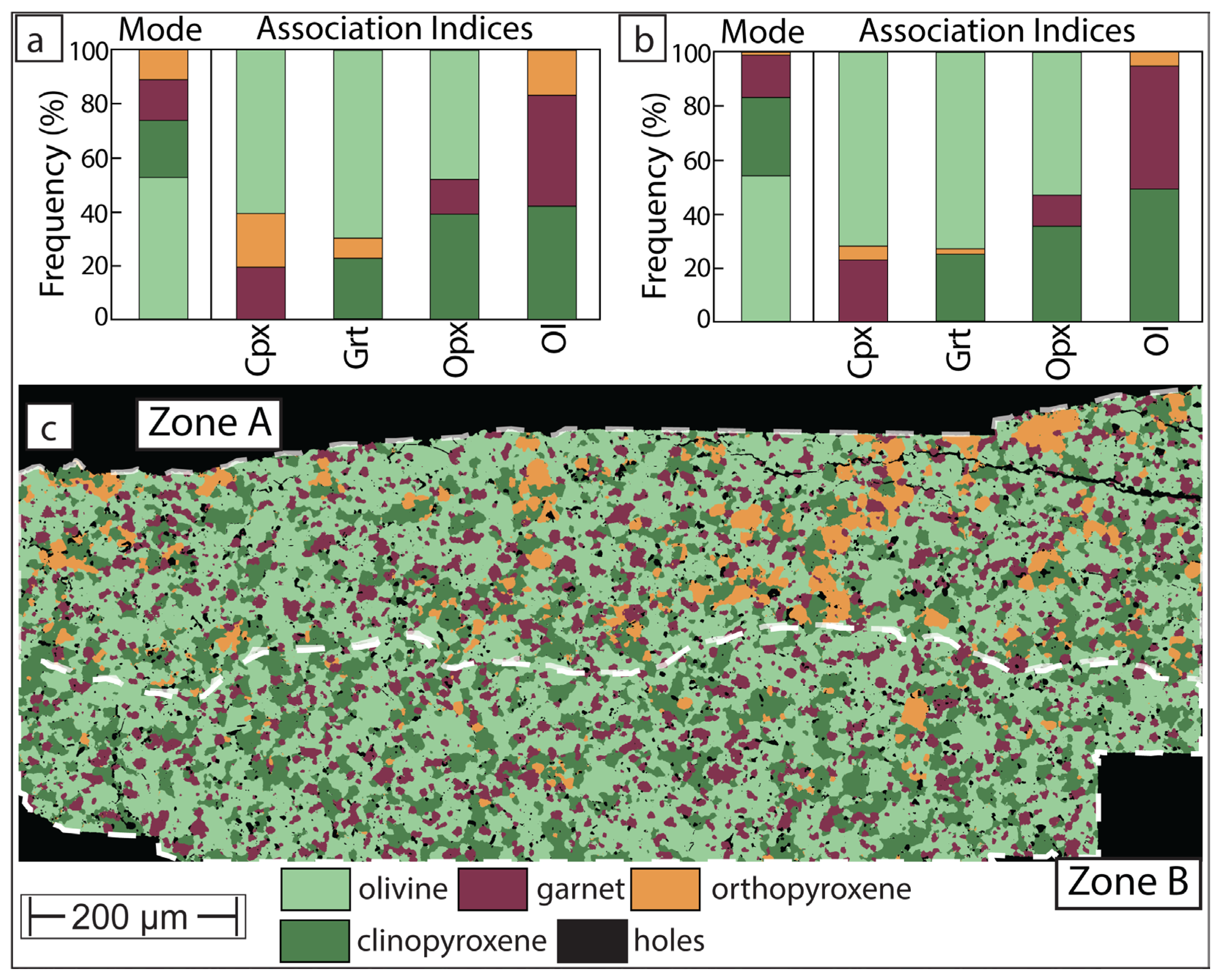
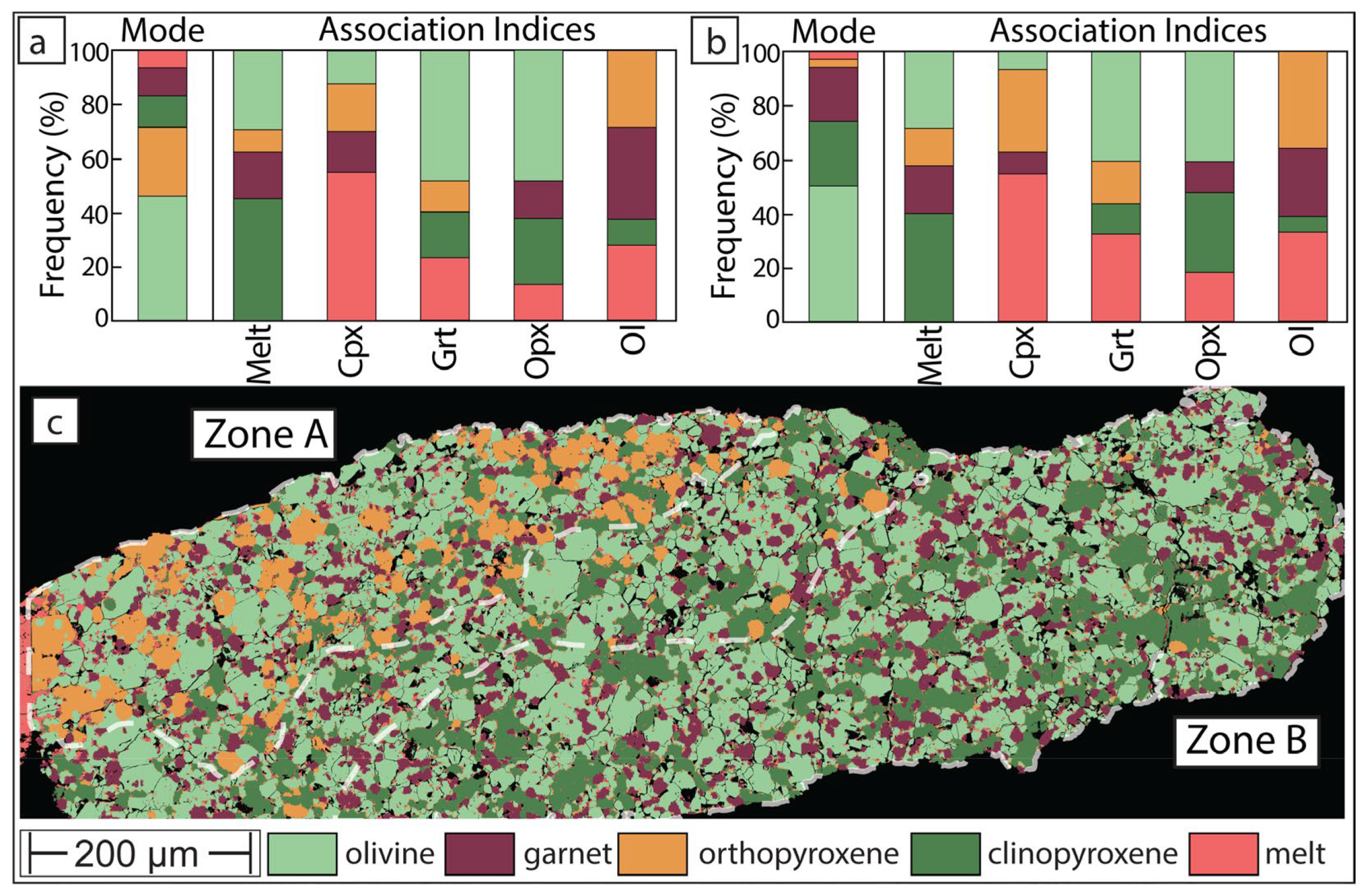
3.3. Phase Map for Experiment Which Did Not Experience Thermal Equilibrium
4. Discussion
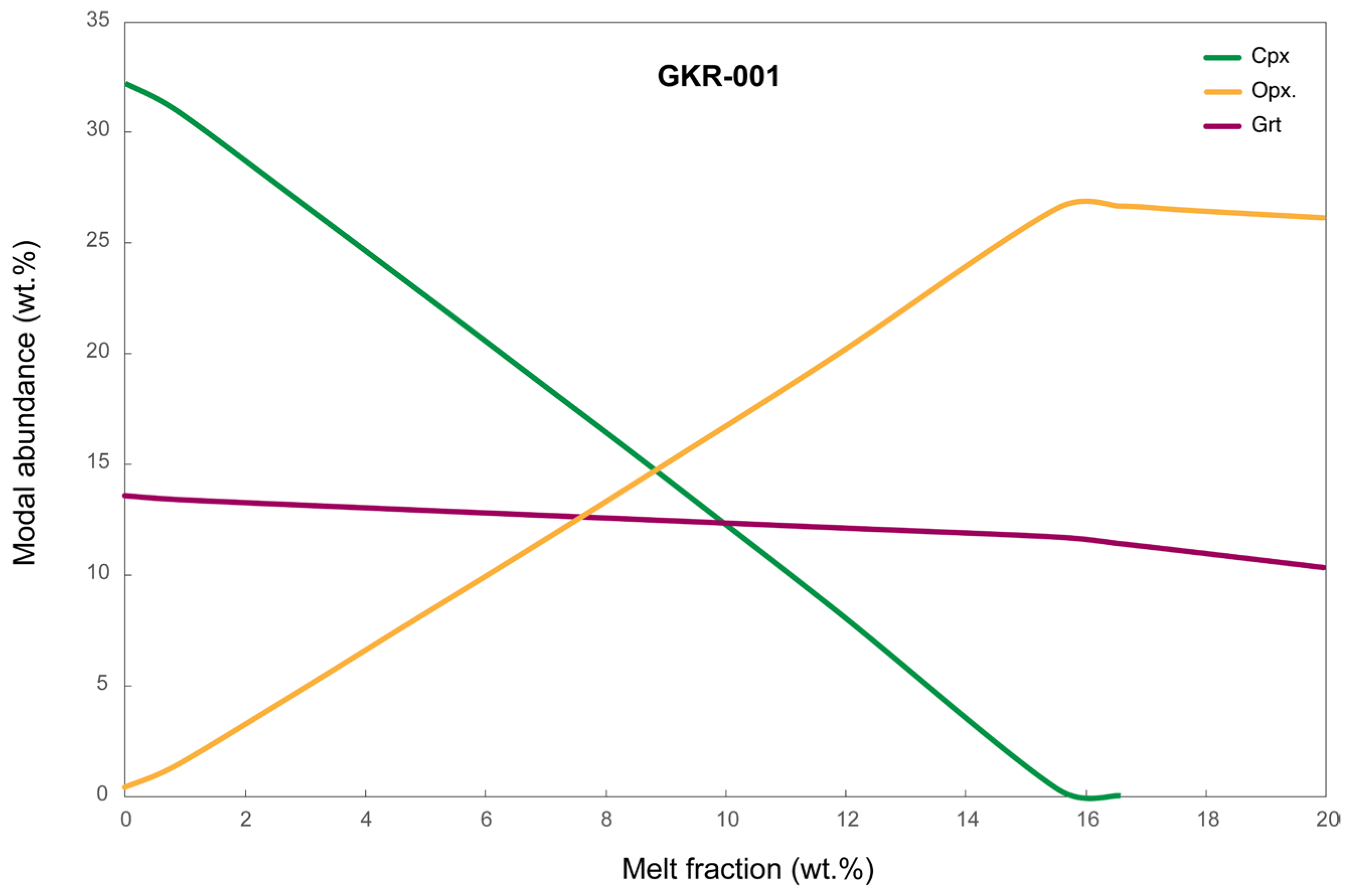
4.1. Insights from Phase Maps of High PT Charges
4.2. Working Towards Optimized Operating Procedures for High-Quality Phase Maps of Experimental Charges
5. Summary
- Due to their small sizes, it is feasible to obtain high-quality BSE and EDS imagery for high PT experimental charges, requiring reasonable SEM instrument time (1 to 3 h). This effort is modest in relation to the production of the experiment and analysis of phase chemistry on the EMPA.
- Phase maps of experimental charges give readers more tangible and complete evidence of phase equilibrium and phase relations than microphotographs of representative areas alone.
- Phase maps generated with commercial automated mineralogy software are suitable to document phase relationships within charges, constrain modes, test for equilibrium, and identify EMPA analysis targets.
- For charges with phases in low abundance or similar atomic number, open-source user-assisted phase mapping with high-resolution BSE images holds greater promise for obtaining accurate modal abundance estimates than those generated with current proprietary software.
- In the studied sub-solidus charge, the system chemistry calculated from the phase map corresponded very well with the nominal chemistry and demonstrated a closed system.
- Phase maps are particularly suitable for charges exposing reactions, for example, caused by chemical potential in layered experiments.
- In charges with reactions, mutual pixel neighborhood relationships (quantified as association indices) can be used to verify the plausibility of mass balance-derived stoichiometries.
- Phase maps have the potential to add retrospective additional value to historic experimental charges.
- In the future, when combined with high spatial resolution trace element geochemical maps, phase maps have the potential to improve counting statistics for low abundance trace elements.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, M.J. Melting of garnet peridotite and the origin of komatiite and depleted lithosphere. J. Petrol. 1998, 39, 29–60. [Google Scholar]
- Canil, D. Orthopyroxene stability along the peridotite solidus and the origin of cratonic lithosphere beneath southern Africa. Earth Planet. Sci. Lett. 1992, 111, 83–95. [Google Scholar]
- Herzberg, C.; Gasparik, T.; Sawamoto, H. Origin of mantle peridotite: Constraints from melting experiments to 16.5 GPa. J. Geophys. Res. Solid Earth 1990, 95, 15779–15803. [Google Scholar] [CrossRef]
- Daczko, N.R.; Kamber, B.S.; Gardner, R.L.; Piazolo, S.; Cathey, H.E. Signatures of komatiite reactive melt flow through the Archaean Kaapvaal cratonic mantle. Contrib. Mineral. Petrol. 2025, 180, 5. [Google Scholar] [CrossRef]
- Lissenberg, C.J.; MacLeod, C.J. A reactive porous flow control on mid-ocean ridge magmatic evolution. J. Petrol. 2016, 57, 2195–2220. [Google Scholar]
- Yaxley, G.M.; Green, D.H. Reactions between eclogite and peridotite: Mantle refertilisation by subduction of oceanic crust. Schweiz. Mineral. Und Petrogr. Mitteilungen 1998, 78, 243–255. [Google Scholar]
- Mallik, A.; Dasgupta, R. Reaction between MORB-eclogite derived melts and fertile peridotite and generation of ocean island basalts. Earth Planet. Sci. Lett. 2012, 329, 97–108. [Google Scholar]
- Ezad, I.S.; Blanks, D.E.; Foley, S.F.; Holwell, D.A.; Bennett, J.; Fiorentini, M.L. Lithospheric hydrous pyroxenites control localisation and Ni endowment of magmatic sulfide deposits. Miner. Depos. 2024, 59, 227–236. [Google Scholar]
- Song, W.; Xu, C.; Veksler, I.V.; Kynicky, J. Experimental study of REE, Ba, Sr, Mo and W partitioning between carbonatitic melt and aqueous fluid with implications for rare metal mineralization. Contrib. Mineral. Petrol. 2016, 171, 1. [Google Scholar]
- London, D. The application of experimental petrology to the genesis and crystallization of granitic pegmatites. Can. Mineral. 1992, 30, 499–540. [Google Scholar]
- Brey, G.P.; Köhler, T. Geothermobarometry in four-phase lherzolites II. New thermobarometers, and practical assessment of existing thermobarometers. J. Petrol. 1990, 31, 1353–1378. [Google Scholar] [CrossRef]
- Nickel, K.G.; Green, D.H. Empirical geothermobarometry for garnet peridotites and implications for the nature of the lithosphere, kimberlites and diamonds. Earth Planet. Sci. Lett. 1985, 73, 158–170. [Google Scholar]
- Sudholz, Z.J.; Yaxley, G.M.; Jaques, A.L.; Brey, G.P. Experimental recalibration of the Cr-in-clinopyroxene geobarometer: Improved precision and reliability above 4.5 GPa. Contrib. Mineral. Petrol. 2021, 176, 11. [Google Scholar]
- Albarede, F.; Provost, A. Petrological and geochemical mass-balance equations: An algorithm for least-square fitting and general error analysis. Comput. Geosci. 1977, 3, 309–326. [Google Scholar]
- Pertermann, M.; Hirschmann, M.M. Partial melting experiments on a MORB-like pyroxenite between 2 and 3 GPa: Constraints on the presence of pyroxenite in basalt source regions from solidus location and melting rate. J. Geophys. Res. Solid Earth 2003, 108, 2125. [Google Scholar]
- Schulz, B.; Sandmann, D.; Gilbricht, S. SEM-based automated mineralogy and its application in geo-and material sciences. Minerals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Hrstka, T.; Gottlieb, P.; Skala, R.; Breiter, K.; Motl, D. Automated mineralogy and petrology-applications of TESCAN Integrated Mineral Analyzer (TIMA). J. Geosci. 2018, 63, 47–63. [Google Scholar]
- Acevedo Zamora, M.A.; Kamber, B.S. Petrographic microscopy with ray tracing and segmentation from multi-angle polarisation whole-slide images. Minerals 2023, 13, 156. [Google Scholar] [CrossRef]
- Lesher, C.E.; Pickering-Witter, J.; Baxter, G.; Walter, M. Melting of garnet peridotite: Effects of capsules and thermocouples, and implications for the high-pressure mantle solidus. Am. Mineral. 2003, 88, 1181–1189. [Google Scholar]
- Holland, T.J.; Green, E.C.; Powell, R. Melting of peridotites through to granites: A simple thermodynamic model in the system KNCFMASHTOCr. J. Petrol. 2018, 59, 881–900. [Google Scholar]
- Tomlinson, E.L.; Holland, T.J. A thermodynamic model for the subsolidus evolution and melting of peridotite. J. Petrol. 2021, 62, egab012. [Google Scholar]
- Walsh, C.; Kamber, B.S.; Tomlinson, E.L. Deep, ultra-hot-melting residues as cradles of mantle diamond. Nature 2023, 615, 450–454. [Google Scholar]
- Farmer, N.; O’Neill, H.S.C. The use of MgO–ZnO ceramics to record pressure and temperature conditions in the piston–cylinder apparatus. Eur. J. Mineral. 2024, 36, 473–489. [Google Scholar]
- Riel, N.; Kaus, B.J.; Green, E.C.R.; Berlie, N. MAGEMin, an efficient Gibbs energy minimizer: Application to igneous systems. Geochem. Geophys. Geosyst. 2022, 23, e2022GC010427. [Google Scholar] [CrossRef]
- Cardona, A.; Saalfeld, S.; Schindelin, J.; Arganda-Carreras, I.; Preibisch, S.; Longair, M.; Tomancak, P.; Hartenstein, V.; Douglas, R.J. TrakEM2 software for neural circuit reconstruction. PLoS ONE 2012, 7, e38011. [Google Scholar] [CrossRef]
- Bogovic, J.A.; Hanslovsky, P.; Wong, A.; Saalfeld, S. Robust registration of calcium images by learned contrast synthesis. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 13–16 April 2016; IEEE: New York, NY, USA, 2016; pp. 1123–1126. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar]
- Chiaruttini, N.; Burri, O.; Haub, P.; Guiet, R.; Sordet-Dessimoz, J.; Seitz, A. An open-source whole slide image registration workflow at cellular precision using Fiji, QuPath and Elastix. Front. Comput. Sci. 2022, 3, 780026. [Google Scholar]
- Koch, P.H. Particle Generation for Geometallurgical Process Modeling. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2017. [Google Scholar]
- Kim, J.J.; Ling, F.T.; Plattenberger, D.A.; Clarens, A.F.; Peters, C.A. Quantification of mineral reactivity using machine learning interpretation of micro-XRF data. Appl. Geochem. 2022, 136, 105162. [Google Scholar]
- Tomlinson, E.L.; Kamber, B.S. Depth-dependent peridotite-melt interaction and the origin of variable silica in the cratonic mantle. Nat. Commun. 2021, 12, 1082. [Google Scholar]
- Walter, M.J.; Sisson, T.W.; Presnall, D.C. A mass proportion method for calculating melting reactions and application to melting of model upper mantle lherzolite. Earth Planet. Sci. Lett. 1995, 135, 77–90. [Google Scholar] [CrossRef]
- Pertermann, M.; Hirschmann, M.M. Anhydrous partial melting experiments on MORB-like eclogite: Phase relations, phase compositions and mineral–melt partitioning of major elements at 2–3 GPa. J. Petrol. 2003, 44, 2173–2201. [Google Scholar] [CrossRef]
- Lund, C.; Lamberg, P.; Lindberg, T. Development of a geometallurgical framework to quantify mineral textures for process prediction. Miner. Eng. 2015, 82, 61–77. [Google Scholar]
- Lanari, P.; Engi, M. Local bulk composition effects on metamorphic mineral assemblages. Rev. Mineral. Geochem. 2015, 83, 55–102. [Google Scholar]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral characterization using scanning electron microscopy (SEM): A review of the fundamentals, advancements, and research directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Otto, T.; Stevens, G.; Mayne, M.J.; Moyen, J.F. Phase equilibrium modelling of partial melting in the upper mantle: A comparison between different modelling methodologies and experimental results. Lithos 2023, 444, 107111. [Google Scholar] [CrossRef]
- Acevedo Zamora, M.A.A.; Caulfield, J.T.; Laupland, E.; Kamber, B.S.; Allen, C.M. Mineral Separate Microanalysis with Intelligent Spot Placement, Manual Edition, and Simulation: Two Correlative Microscopy Prototypes for Relating Zircon Texture, Age, and Geochemistry. In 13th Asia Pacific Microscopy Congress 2025 (APMC13); ScienceOpen: Lexington, MA, USA, 2025; p. 280. [Google Scholar]
- Ogliore, R.C. Acquisition and Online Display of High-Resolution Backscattered Electron and X-Ray Maps of Meteorite Sections. Earth Space Sci. 2021, 8, e2021EA001747. [Google Scholar] [CrossRef]
- Nowell, M.M.; Wright, S.I. Phase differentiation via combined EBSD and XEDS. J. Microsc. 2004, 213, 296–305. [Google Scholar]
- Acevedo Zamora, M.A.; Schrank, C.E.; Kamber, B.S. Using the traditional microscope for mineral grain orientation determination: A prototype image analysis pipeline for optic-axis mapping (POAM). J. Microsc. 2024, 295, 147–176. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Yu, T.; Rivers, M.; Wang, Y.; Zhao, S. Crystallographic evidence for simultaneous growth in graphic granite. Gondwana Res. 2015, 27, 1550–1559. [Google Scholar]
- Bussweiler, Y.; Gervasoni, F.; Rittner, M.; Berndt, J.; Klemme, S. Trace element mapping of high-pressure, high-temperature experimental samples with laser ablation ICP time-of-flight mass spectrometry–Illuminating melt-rock reactions in the lithospheric mantle. Lithos 2020, 352, 105282. [Google Scholar]
- Acevedo Zamora, M.A.A.; Kamber, B.S.; Jones, M.W.; Schrank, C.E.; Ryan, C.G.; Howard, D.L.; Paterson, D.J.; Ubide, T.; Murphy, D.T. Tracking element-mineral associations with unsupervised learning and dimensionality reduction in chemical and optical image stacks of thin sections. Chem. Geol. 2024, 650, 121997. [Google Scholar]



| GKR-001 | UHP32 (5 GPa, 1525 °C, 9 h) | ||||
| Ol (32) 2 | Opx (17) | Cpx (30) | Grt (23) | ||
| SiO2 | 45.52 | 40.83 (23) 3 | 56.36 (22) | 55.03 (23) | 42.37 (28) |
| TiO2 | 0.07 | 0.08 (06) | 0.04 (01) | 0.04 (01) | 0.21 (07) |
| Al2O3 | 4.41 | 0.17 (20) | 2.55 (17) | 2.67 (09) | 22.70 (60) |
| Cr2O3 | 0.32 | 0.09 (02) | 0.28 (02) | 0.30 (03) | 1.29 (23) |
| FeOt | 7.11 | 8.80 (16) | 5.18 (08) | 4.57 (15) | 5.66 (16) |
| MnO | 0.15 | 0.14 (01) | 0.13 (02) | 0.15 (02) | 0.21 (03) |
| MgO | 38.18 | 49.97 (37) | 33.15 (59) | 25.50 (31) | 22.48 (20) |
| NiO | 0.10 | 0.08 (03) | 0.06 (02) | 0.05 (01) | bdl |
| CaO | 3.96 | 0.30 (15) | 2.62 (68) | 11.53 (43) | 5.07 (26) |
| Na2O | 0.09 | 0.03 (00) | 0.07 (02) | 0.24 (01) | bdl |
| K2O | 0.01 | bdl 1 | bdl | bdl | bdl |
| Total | 99.90 | 100.39 | 100.40 | 100.05 | 99.99 |
| Mg# | 90.54 | 91.01 | 91.94 | 90.87 | 90.76 |
| Mode (wt%) | |||||
| Mass balance | 51.95 | 7.74 | 24.48 | 15.23 | |
| Phase map | 51.87 | 8.19 | 23.07 | 16.86 | |
| UHP40 (5 GPa, 1600 °C, 5 h) | |||||
| Ol (22) | Opx (29) | Cpx (29) | Grt (27) | Melt (07) 4 | |
| SiO2 | 41.10 (22) | 56.12 (43) | 54.74 (26) | 42.85 (23) | 46.74 (97) |
| TiO2 | bdl | 0.03 (01) | 0.03 (01) | 0.15 (03) | 0.24 (07) |
| Al2O3 | 0.15 (02) | 3.11 (34) | 3.12 (12) | 22.71 (36) | 8.90 (1.0) |
| Cr2O3 | 0.10 (01) | 0.32 (03) | 0.35 (02) | 1.36 (22) | 0.44 (04) |
| FeOt | 8.21 (13) | 4.93 (09) | 4.56 (14) | 5.22 (16) | 11.11 (02) |
| MnO | 0.13 (01) | 0.12 (01) | 0.15 (01) | 0.20 (02) | 0.17 (01) |
| MgO | 50.24 (28) | 32.98 (32) | 26.74 (56) | 22.71 (24) | 18.00 (1.2) |
| NiO | 0.02 (01) | 0.03 (01) | 0.02 (00) | bdl | bdl |
| CaO | 0.28 (01) | 2.56 (18) | 9.79 (69) | 4.95 (22) | 13.95 (07) |
| Na2O | 0.02 (01) | 0.07 (01) | 0.21 (02) | bdl | 0.45 (02) |
| K2O | bdl | bdl | bdl | bdl | 0.01 (00) |
| Total | 100.32 | 100.24 | 99.69 | 100.15 | 100.00 |
| Mg# | 91.50 | 92.26 | 91.27 | 90.27 | 74.27 |
| Mode (wt%) | |||||
| Mass balance | 51.84 | 8.24 | 19.66 | 12.23 | 7.69 |
| Phase map | 49.78 | 10.37 | 20.22 | 16.24 | 3.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamber, B.S.; Acevedo Zamora, M.A.; Rodrigues, R.F.; Li, M.; Yaxley, G.M.; Ng, M. Exploring High PT Experimental Charges Through the Lens of Phase Maps. Minerals 2025, 15, 355. https://doi.org/10.3390/min15040355
Kamber BS, Acevedo Zamora MA, Rodrigues RF, Li M, Yaxley GM, Ng M. Exploring High PT Experimental Charges Through the Lens of Phase Maps. Minerals. 2025; 15(4):355. https://doi.org/10.3390/min15040355
Chicago/Turabian StyleKamber, Balz S., Marco A. Acevedo Zamora, Rodrigo Freitas Rodrigues, Ming Li, Gregory M. Yaxley, and Matthew Ng. 2025. "Exploring High PT Experimental Charges Through the Lens of Phase Maps" Minerals 15, no. 4: 355. https://doi.org/10.3390/min15040355
APA StyleKamber, B. S., Acevedo Zamora, M. A., Rodrigues, R. F., Li, M., Yaxley, G. M., & Ng, M. (2025). Exploring High PT Experimental Charges Through the Lens of Phase Maps. Minerals, 15(4), 355. https://doi.org/10.3390/min15040355









