Dolomitization Facilitated by Clay Minerals on Mixed Siliciclastic-Carbonate Shoals of Carboniferous Age in the Tarim Basin, China: Constraints on Element Mobility and Isotope Geochemistry
Abstract
:1. Introduction
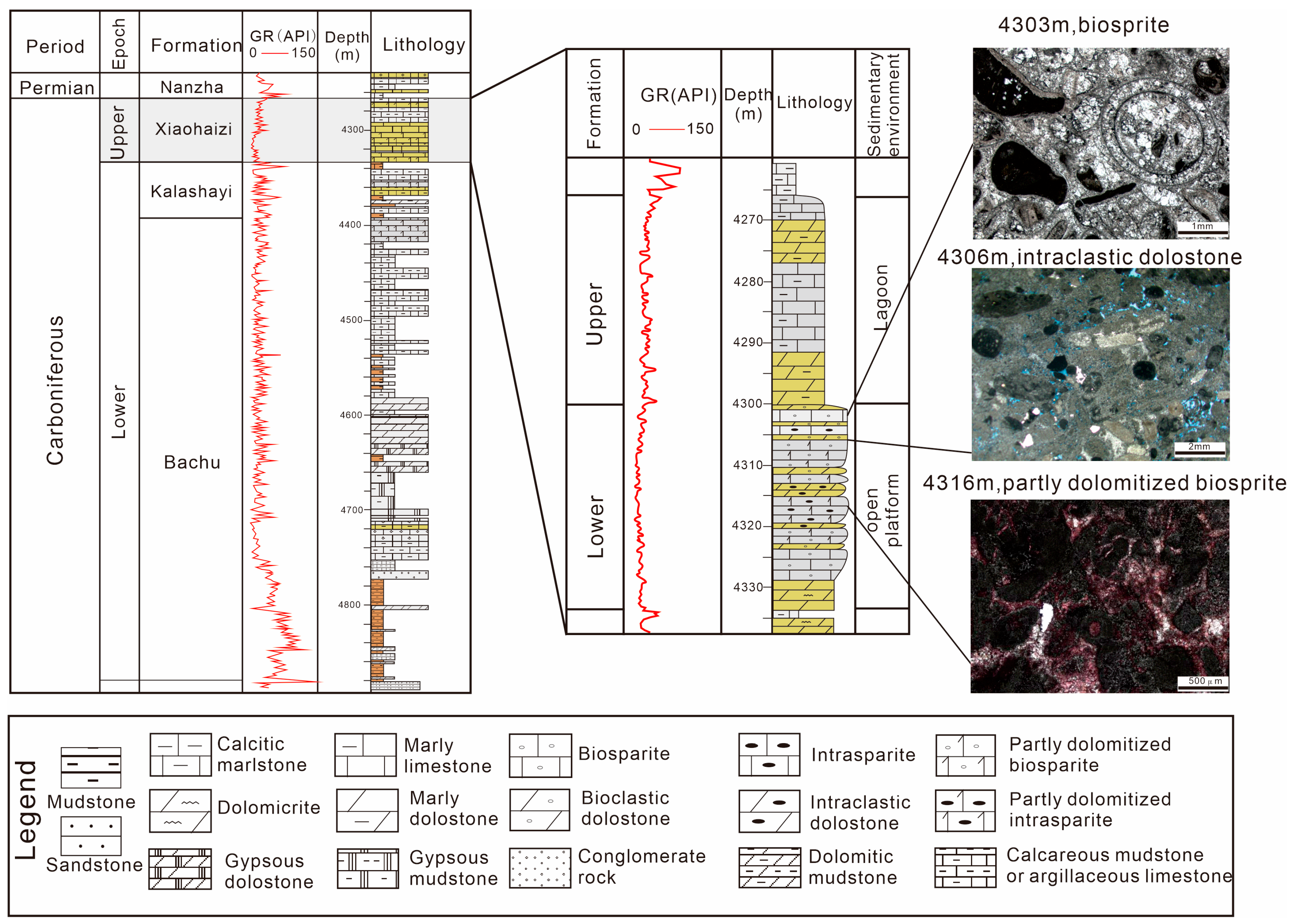
2. Geological Setting

3. Methods
4. Results
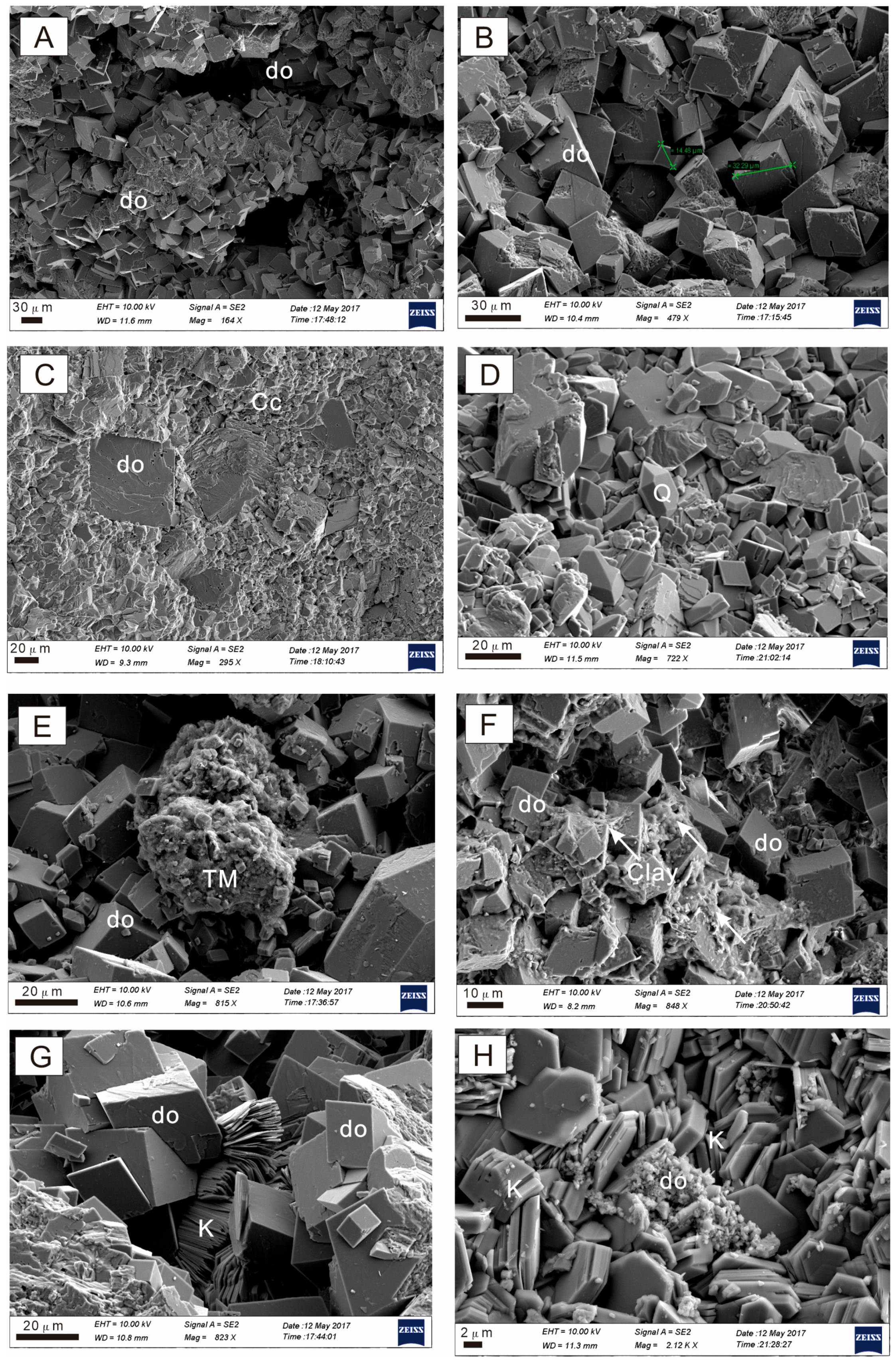
4.1. Lithology and Mineral Composition
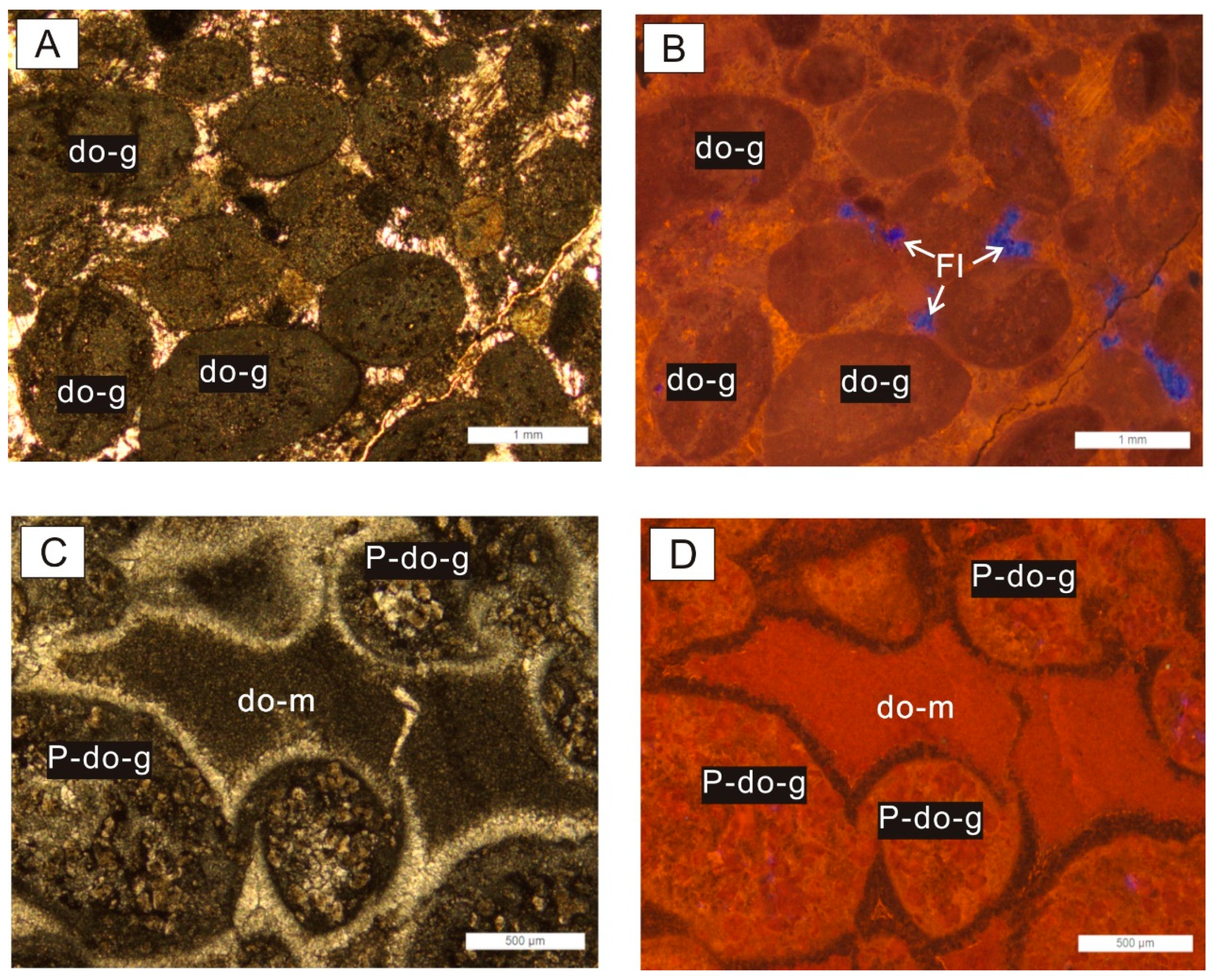
4.2. Types and Characteristics of Dolomites
4.2.1. Type A Dolomite
4.2.2. Type B Dolomite
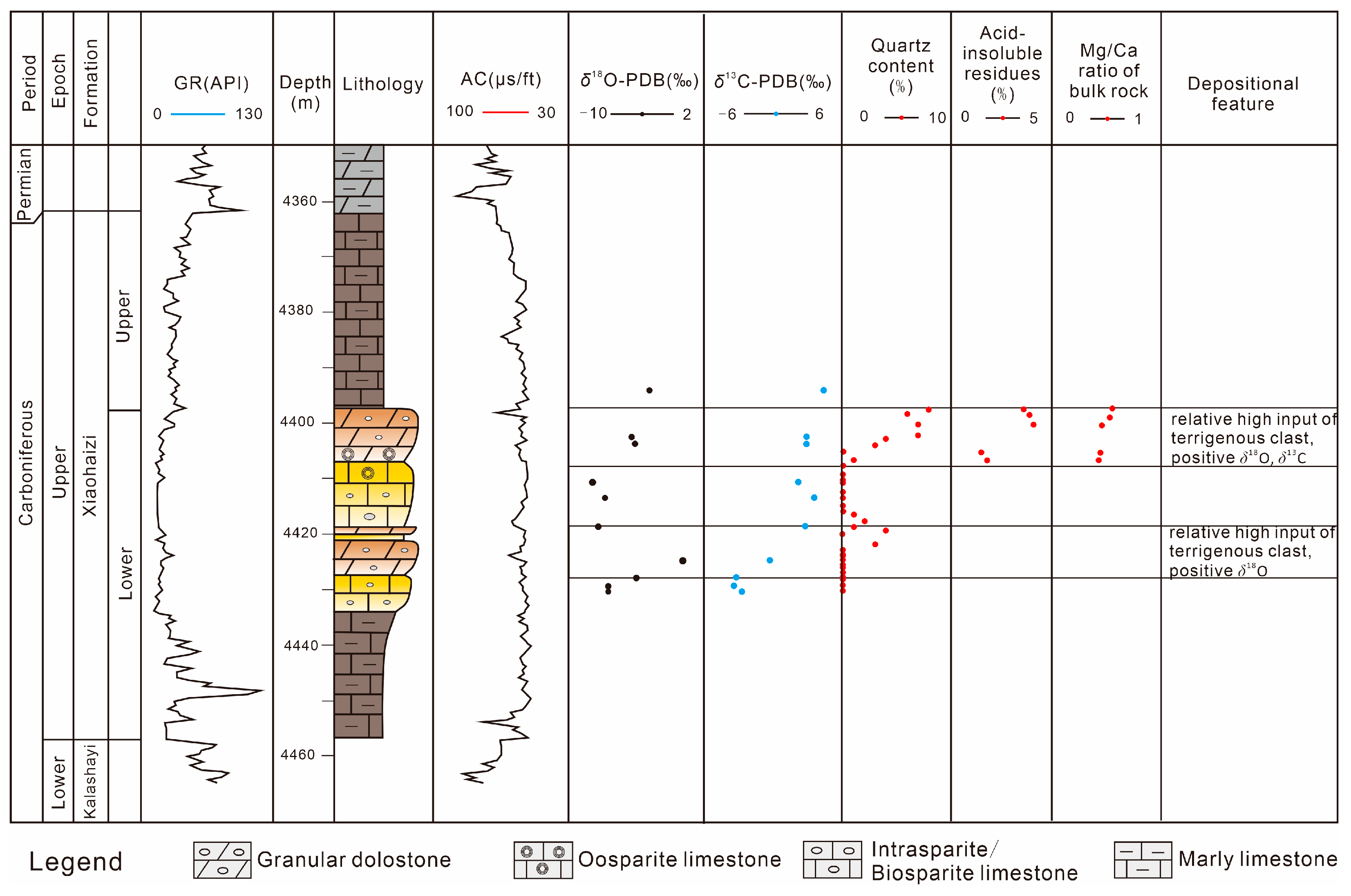
4.3. C, O Isotope
4.4. 87Sr/86Sr Isotope
4.5. Rare Earth Elements (REEs)
4.6. Major and Trace Elements
4.7. Fluid Inclusion
5. Discussion
5.1. The Implication of the Textural Characteristic of Dolomites
5.2. Timing of Dolomite Formation and Dolomitizing Fluid
5.3. The Depositional Environment of Dolomite Formation
5.4. The Catalysis Role of Clay Minerals on Dolomitization
5.5. The Density-Dependent Convection of Dolomitized Fluid and the Mechanism of Dolomitization
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chafetz, H.S.; Rush, P.F. Diagenetically altered sabkha-type Pleistocene dolomite from the Arabian Gulf. Sedimentology 1994, 41, 409–421. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Raub, T.D. Geochemical constraints on the origin of Marinoan cap dolostones from Nuccaleena Formation, South Australia. Chem. Geol. 2013, 351, 95–104. [Google Scholar] [CrossRef]
- Wang, R.; Jones, B.; Yu, K. Island dolostones: Genesis by time-transgressive or event dolomitization. Sediment. Geol. 2019, 390, 15–30. [Google Scholar] [CrossRef]
- Land, L.S. The origin of massive dolomite. J. Geol. Educ. 1985, 33, 112–125. [Google Scholar] [CrossRef]
- Purser, B.H.; Tucker, M.E.; Zenger, D.H. Dolomites-A Volume in Honour of Dolomieu; Special Publications; Blackwell Scientific Publications: Oxford, UK, 1994; p. 21. [Google Scholar]
- Qing, H.; Bosence, D.W.J.; Rose, P.F. Dolomitization by penesaline seawater in Early Jurassic pertidal platform carbonates, Gibraltar, western Mediterranean. Sedimentology 2001, 48, 153–163. [Google Scholar] [CrossRef]
- Machel, H.G. Concepts and models of dolomitization: A critical reappraisal. In The Geometry and Petrogenesis of Dolomite Hydrocarbon Reservoirs; Braithwaite, C.J.R., Rizzi, G., Darke, G., Eds.; Geological Society London, Special Publications: London, UK, 2004; Volume 235, pp. 7–63. [Google Scholar]
- Sena, C.M.; Cedric, M.J.; Anne-Lise, J.; Veerle, V.; Christina, M. Dolomitization of lower cretaceous peritidal carbonates by modified seawater: Constraints from clumped isotopic paleothermometry, elemental chemistry, and strontium isotopes. J. Sediment. Res. 2014, 84, 552–566. [Google Scholar] [CrossRef]
- Hollis, C.; Bastesen, E.; Boyce, A.; Corlett, H.; Gawthorpe, R.; Hirani, J.; Rotevatn, A.; Whitaker, F. Fault-controlled dolomitization in a rift basin. Geology 2017, 45, 219–222. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Y.; Papineau, D.; Yub, N.; Fan, Q.; Qiu, X.; Wang, H. Experimental evidence for abiotic formation of low-temperature proto-dolomite facilitated by clay minerals. Geochim. Cosmochim. Acta 2019, 247, 83–95. [Google Scholar] [CrossRef]
- Vandeginste, V.; Snell, O.; Hall, M.R.; Steer, E.; Vandeginste, A. Acceleration of dolomitization by zinc in saline waters. Nat. Commun. 2019, 10, 1851. [Google Scholar] [CrossRef]
- Jacquemyn, C.; Desouky, H.E.; Hunt, D.; Casini, G.; Swennen, R. Dolomitization of the Latemar platform: Fluid flow and dolomite evolution. Mar. Pet. Geol. 2014, 55, 43–67. [Google Scholar] [CrossRef]
- Mahboubi, A.; Nowrouzi, Z.; Al-Aasm, I.S.; Moussavi-Harami, R.; Mahmudy-Gharaei, M.H. Dolomitization of the Silurian Niur Formation, Tabas block, east central Iran: Fluid flow and dolomite evolution. Mar. Pet. Geol. 2016, 77, 791–805. [Google Scholar] [CrossRef]
- Newport, R.; Hollis, C.; Bodin, S.; Redfern, J. Examining the interplay of climate and low amplitude sea-level change on the distribution and volume of massive dolomitization: Zebbag Formation, Cretaceous, Southern Tunisia. Int. Assoc. Sedimentol. 2017, 3, 38–59. [Google Scholar] [CrossRef]
- McKenzie, J.A.; Hsü, K.J.; Schneider, J.F. Movement of subsurface waters under the sabkha, Abu Dhabi, UAE, and its relation to evaporative dolomite genesis. In Concepts and Models of Dolomitization; Zenger, D.H., Dunham, J.B., Ethington, R.L., Eds.; Special Publications; Society of Economic Paleontologists and Mineralogists: Tulsa, OK, USA, 1980; Volume 28, pp. 11–30. [Google Scholar]
- Bi, D.; Zhai, S.; Zhang, D.; Liu, X.; Liu, X.; Jiang, L.; Zhang, A. Constraints of fluid inclusions and C, O isotopic compositions on the origin of the dolomites in the Xisha Islands, South China Sea. Chem. Geol. 2018, 493, 504–517. [Google Scholar] [CrossRef]
- Aharon, P.; Socki, R.A.; Chan, L. Dolomitization of atolls by sea water convection flow: Test of a hypothesis at Niue, South Pacific. J. Geol. 1987, 95, 187–203. [Google Scholar] [CrossRef]
- Mazzullo, S.J.; Bischoff, W.D.; Teal, C.S. Holocene shallow-subtidal dolomitization by near-normal seawater, northern Belize. Geology 1995, 23, 341–344. [Google Scholar] [CrossRef]
- Whitaker, F.F.; Smart, P.L.; Vahrenkamp, V.C.; Nicholson, H.; Wogelius, R.A. Dolomitization by near-normal seawater? Field evidence from the Bahamas. In Dolomites-A Volume in Honour of Dolomieu; Purser, B.H., Tucker, M.E., Zenger, D.H., Eds.; Special Publications; Blackwell Scientific Publications: Oxford, UK, 1994; Volume 21, pp. 111–132. [Google Scholar]
- Hsü, K.J.; Siegenthaler, C. Preliminary experiments on hydrodynamic movements induced by evaporation and their bearing on the dolomite problem. Sedimentology 1969, 12, 11–25. [Google Scholar] [CrossRef]
- Sanford, W.E.; Whitaker, F.F.; Smart, P.L.; Jones, G. Numerical analysis of seawater circulation in carbonate platforms: I. Geothermal convection. Am. J. Sci. 1998, 298, 801–828. [Google Scholar] [CrossRef]
- Ngia, N.R.; Hu, M.; Gao, D. Tectonic and geothermal controls on dolomitization and dolomitizing fluid flows in the Cambrian-Lower Ordovician carbonate successions in the western and central Tarim Basin, NW China. J. Asian Earth Sci. 2019, 172, 359–382. [Google Scholar] [CrossRef]
- Rustichelli, A.; Iannace, A.; Tondi, E.; Celma, C.D.; Cilona, A.; Giorgioni, M.; Parente, M.; Girundo, M.; Invernizzi, C. Fault-controlled dolomite bodies as palaeotectonic indicators and geofluid reservoirs: New insights from Gargano Promontory outcrops. Sedimentology 2017, 64, 1871–1900. [Google Scholar] [CrossRef]
- Hirania, J.; Bastesen, E.; Boyce, A.; Corlett, H.; Gawthorpe, R.; Hollis, C.; John, C.M.; Robertson, H.; Rotevatn, A.; Whitaker, F. Controls on the formation of stratabound dolostone bodies, Hammam Faraun Fault block, Gulf of Suez. Sedimentology 2018, 65, 1973–2002. [Google Scholar] [CrossRef]
- Baldermann, A.; Deditius, A.P.; Dietzel, M.; Fichtner, V.; Fischer, C.; Hippler, D.; Leis, A.; Baldermann, C.; Mavromatis, V.; Stickler, C.P.; et al. The role of bacterial sulfate reduction during dolomite precipitation: Implications from Upper Jurassic platform carbonates. Chem. Geol. 2015, 412, 1–14. [Google Scholar] [CrossRef]
- Mountjoy, E.W.; Machel, H.G.; Green, D.; Duggan, J.; Williams-Jones, A.E. Devonian matrix dolomites and deep burial carbonate cements: A comparison between the Rimbey–Meadowbrook reef trend and the deep basin of west-central Alberta. Bull. Can. Pet. Geol. 1999, 47, 487–509. [Google Scholar]
- Qing, H.; Mountjoy, E.W. Large-scale fluid flow in the Middle Devonian Presqu’ile Barrier, Western Canada Sedimentary Basin. Geology 1992, 20, 903–906. [Google Scholar]
- Duggan, J.P.; Mountjoy, E.W.; Stasiuk, L.D. Fault-controlled dolomitization at Swan Hills Simonette oil field (Devonian), deep basin west-central Alberta, Canada. Sedimentology 2001, 48, 301–323. [Google Scholar] [CrossRef]
- Bahnan, A.E.; Carpentier, C.; Pironon, J.; Ford, M.; Ducoux, M.; Barré, G.; Mangenot, X.; Gaucher, E.C. Impact of geodynamics on fluid circulation and diagenesis of carbonate reservoirs in a foreland basin: Example of the Upper Lacq reservoir (Aquitaine basin, SW France). Mar. Pet. Geol. 2020, 111, 676–694. [Google Scholar] [CrossRef]
- Ren, M.; Jones, B. Genesis of island dolostones. Sedimentology 2018, 65, 2003–2033. [Google Scholar] [CrossRef]
- Katz, A.; Matthews, A. The dolomitization of CaCO3: An experimental study at 252–295 °C. Geochim. Cosmochim. Acta 1977, 41, 297–308. [Google Scholar] [CrossRef]
- Sibley, D.F.; Nordeng, S.H.; Borkowski, M.L. Dolomitization kinetics of hydrothermal bombs and natural settings. J. Sed. Res. 1994, 64, 630–637. [Google Scholar] [CrossRef]
- Land, L.S. Failure to precipitate dolomite at 25 °C from dilute solution despite 1000-fold oversaturation after 32 years. Aquat. Geochem. 1998, 4, 361–368. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Thornton, B. The effect of temperature on stoichiometry, cation ordering, and reaction rate in high-temperature dolomitization experiments. Chem. Geol. 2017, 468, 32–41. [Google Scholar] [CrossRef]
- Wright, D.T. The role of sulphate-reducing bacteria and cyanobacteria in dolomite formation in distal ephemeral lakes of the Coorong region, South Australia. Sediment. Geol. 1999, 126, 147–157. [Google Scholar] [CrossRef]
- Roberts, J.A.; Bennett, P.C.; Gonza’lez, L.A.; Macpherson, G.; Milliken, K.L. Microbial precipitation of dolomite methanogenic groundwater. Geology 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Krause, S.; Liebetrau, V.; Gorb, S.; Sa’nchez-Roma’n, M.; McKenzie, J.A.; Treude, T. Microbial nucleation of Mg-rich dolomite in exopolymeric substances under anoxic modern seawater salinity: New insight into an old enigma. Geology 2012, 40, 587–590. [Google Scholar] [CrossRef]
- Sibley, D.F.; Dedoes, R.E.; Bartlett, T.R. Kinetics of dolomitization. Geology 1987, 15, 1112–1114. [Google Scholar] [CrossRef]
- Kessels, L.A.; Sibley, D.F.; Nordeng, S.H. Nanotopography of synthetic and natural dolomite crystals. Sedimentology 2000, 47, 173–186. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Fan, T.; Yu, T.; Cao, Z.; He, H. Integrated Reservoir Characterization of Late Carboniferous Carbonate Inner Platform Shoals in Bamai Region, Tarim Basin. J. Jilin Univ. (Earth Sci. Ed.) 2013, 43, 371–381. [Google Scholar]
- Ma, Z.; Yang, S.; Xu, Q.; Zhang, L.; Zhang, J.; Huang, W. Main controlling factors of carbonate reservoirs of Xiaohaizi Formation in Xianbazha area, Tarim Basin. Pet. Geol. Exp. 2015, 37, 300–327. [Google Scholar]
- Wang, Q.; Shi, J.; Chen, G.; Xue, L. Characteristics of Diagenetic Environments of Carbonate Rocks in Western Tarim Basin and Their Controls on the Reservoir Property. Acta Sedimentol. Sin. 2001, 19, 548–555. [Google Scholar]
- Jia, C. Characteristics of Chinese Petroleum Geology: Geological Features and Exploration Cases of Stratigraphic, Foreland and Deep Formation Traps; Springer Science & Business Media: Berlin, Germany, 2013; p. 15. [Google Scholar]
- Zhu, R.X.; Yang, Z.Y.; Wu, H.N.; Ma, X.H.; Huang, B.C.; Meng, Z.F.; Fang, D.J. Paleomagnetic constraints on the tectonic history of the major blocks of China during the Phanerozoic. Sci. China 1998, 41, 1–19. [Google Scholar]
- Chen, Z. Devonian–Carboniferous brachiopod zonation of the Tarim Basin, Northwest China: Implications for biostratigraphy and biogeography. Geol. J. 2004, 39, 431–458. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, G.R. Late Paleozoic depositional history of the Tarim Basin, NW China: An integration of lithostratigraphic and biostratigraphic constraints. Am. Assoc. Pet. Geol. Bull. 2003, 87, 1323–1354. [Google Scholar]
- Jiang, L.; Gu, J. Analysis on the sedimentary environment of the carboniferous carbonate and detrital rock alternating sedimentation in the Maigaiti slope of the Tarim basin. Pet. Geol. Exp. 2002, 24, 41–47, (Chinese, Abstract in English). [Google Scholar]
- Chen, Z. A Late Carboniferous algal mound from the Tarim Basin, NW China: Internal structure and palaeoecology. Geol. J. 2012, 47, 477–494. [Google Scholar] [CrossRef]
- Zhang, Y.; He, D.; Liu, C. Three-dimensional geological structure and genetic mechanism of the Bachu uplift in the Tarim Basin. Earth Sci. Front. 2019, 26, 134–148. [Google Scholar]
- Zhu, R.; Luo, P.; Luo, Z. Lithofacies palaeogeography of the late Devonian and carboniferous in Tarim Basin. J. Palaeogeogr. 2002, 4, 13–24. [Google Scholar]
- Yan, J.; Zhao, X.; He, Y.; Zhong, X.; Wei, S.; Zhou, G. Sedimentary facies of Carboniferous carbonate rock in Bashitop Oil Field, Tarim Basin. Pet. Geol. Exp. 2011, 33, 353–358. [Google Scholar]
- Fu, M.; Song, R.; Gluyas, J.; Zhang, S.; Huang, Q. Diagenesis and reservoir quality of carbonates rocks and mixed siliciclastic as response of the Late Carboniferous glacio-eustatic fluctuation: A case study of Xiaohaizi Formation in western Tarim Basin. J. Pet. Sci. Eng. 2019, 177, 1024–1041. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Guo, F. Sedimentary Features of Carboniferous in Bashituo Block in Tarim Basin. Xinjiang Pet. Geol. 2011, 32, 42–44, (Chinese, Abstract in English). [Google Scholar]
- Vasconcelos, C.; McKenzie, J.A.; Warthmann, R.; Bernasconi, S.M. Calibration of the δ18O paleothermometer for dolomite precipitated in microbial cultures and natural environments. Geology 2005, 33, 317–320. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare earth elements in the Penge and Kuruman ironformations, Transvaal Supergroup, SouthAfrica. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Mclennan, S.M. Rare Earth Elements in Sedimentary Rocks: Influence of Provenance and Sedimentary Processes. Mineral. Soc. Am. Rev. Mineral. 1989, 21, 169–200. [Google Scholar]
- Biscaye, P.E. Mineralogy and sedimentation of recent deep-sea clay in the Atlantic Ocean and adjacent seas and oceans. Geol. Soc. Am. Bull. 1965, 76, 803–832. [Google Scholar] [CrossRef]
- Gregg, J.M.; Sibley, D.F. Epigenetic dolomitization and the origin of xenotopic dolomite texture. J. Sediment. Petrol. 1984, 54, 908–931. [Google Scholar]
- Sibley, D.F. The origin of common dolomite fabrics: Clues from the Pliocene. J. Sediment. Petrol. 1982, 52, 1087–1100. [Google Scholar]
- Whitaker, F.F.; Smart, P.L.; Jones, G.D. Dolomitization: From conceptual to numerical models. Geol. Soc. Lond. Spec. Publ. 2004, 235, 99–139. [Google Scholar] [CrossRef]
- Jones, G.D.; Xiao, Y. Dolomitization, anhydrite cementation, and porosity evolution in a reflux system: Insights from reactive transport models. AAPG Bull. 2005, 89, 577–601. [Google Scholar] [CrossRef]
- Ueno, K.; Hayakawa, N.; Nakazawa, T.; Wang, Y.; Wang, X. Pennsylvanian–Early Permian cyclothemic succession on the Yangtze Carbonate Platform, South China. Geol. Soc. Lond. Spec. Publ. 2013, 376, 235–267. [Google Scholar] [CrossRef]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.; Buhl, D.; Bruhn, F.; Carden, G.A.F.; Diener, A.; Ebneth, S.; Godderis, Y.; et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 59–88. [Google Scholar] [CrossRef]
- Major, R.P.; Lloyd, R.M.; Lucia, F.J. Oxygen isotope composition of Holocene dolomite formed in a humid hypersaline setting. Geology 1992, 20, 586–588. [Google Scholar] [CrossRef]
- Qing, H.; Mountjoy, E.W. Rare earth element geochemistry of dolomites in the Middle Devonian Presqu’ile barrier, Western Canada Sedimentary Basin: Implications for fluid-rock ratios during dolomitization. Sedimentology 1994, 41, 787–804. [Google Scholar] [CrossRef]
- Nothdurft, L.D.; Webb, G.E.; Kamber, B.S. Rare earth element geochemistry of Late Devonian reefal carbonates, Canning Basin, Western Australia: Confirmation of a seawater REE proxy in ancient limestones. Geochim. Cosmochim. Acta 2004, 68, 263–283. [Google Scholar] [CrossRef]
- Zhang, J.; Nozaki, Y. Rare earth elements and yttrium in seawater: ICP-MS determinations in the east Caroline, Coral Sea, and South Fiji basins of the western South Pacific Ocean. Geochim. Cosmochim. Acta 1996, 60, 4631–4644. [Google Scholar] [CrossRef]
- Frank, J.R.; Carpener, A.B.; Oglesby, T.W. Cathodo-luminescence and composition of calcite cement in the Taum Sauk limestone (upper Cambrian), southeast Missouri. J. Sediment. Res. 1982, 52, 631–638. [Google Scholar]
- Irwin, H.; Curtis, C.; Coleman, M. Isotopic evidence for source of diagenetic carbonates formed during burial of organic-rich sediments. Nature 1977, 269, 209–213. [Google Scholar] [CrossRef]
- Wang, L.C.; Hu, W.X.; Wang, X.L.; Cao, J.; Wu, H.G.; Liao, Z.W.; Wan, Y. Changes in Strontium Content and Strontium Isotope Fractionation during Dolomitization: Implications and Characteristics. Oil Gas Geol. 2016, 37, 464–472. [Google Scholar]
- Huang, S. Relationship between cathodoluminescence and concentration of iron and manganese in Carbonate Minerals. Mineral. Petrol. 1992, 12, 74–79. [Google Scholar]
- Lumsden, D.N.; Lloyd, R.V. Mn (II) partitioning between calcium and magnesium sites in studies of dolomite origin. Geochim. Cosmochim. Acta 1984, 48, 1861–1865. [Google Scholar] [CrossRef]
- Lumsden, D.N.; Shipe, L.G.; Lloyd, R.V. Mineralogy and Mn geochemistry of laboratorysynthesized dolomite. Geochim. Cosmochim. Acta 1989, 53, 2325–2329. [Google Scholar] [CrossRef]
- Rygel, M.C.; Fielding, C.R.; Frank, T.D.; Birgenheier, L.P. The magnitude of Late Paleozoic glacioeustatic fluctuations: A synthesis. J. Sediment. Res. 2008, 78, 500–511. [Google Scholar] [CrossRef]
- Buggisch, W.; Krainer, K.; Schaffhauser, M.; Joachimski, M.M.; Korte, C. Late Carboniferous to Late Permian carbon isotope stratigraphy: A new record from post-Variscan carbonates from the Southern Alps (Austria and Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 433, 174–190. [Google Scholar] [CrossRef]
- Guo, C.; Chen, D.; Qing, H.; Zhou, X.; Ding, Y. Early dolomitization and recrystallization of the Lower-Middle Ordovician carbonates in western Tarim Basin (NW China). Mar. Pet. Geol. 2019, 111, 332–349. [Google Scholar] [CrossRef]
- Sibley, D.F. Unstable to stable transformations during dolomitization. J. Geol. 1990, 98, 739–748. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Sibley, D.F. Direct physical evidence of dolomite recrystallization. Sedimentology 2014, 61, 1862–1882. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Sibley, D.F. On the evolution of dolomite stoichiometry and cation order during high-temperature synthesis experiments: An alternative model for the geochemical evolution of natural dolomites. Sed. Geol. 2011, 240, 30–40. [Google Scholar] [CrossRef]
- Tucker, M.E. Mixed clastic–carbonate cycles and sequences: Quaternary of Egypt and Carboniferous of England. Geologia Croatica 2003, 56, 19–37. [Google Scholar] [CrossRef]
- Burns, S.J.; McKenzie, J.A.; Vasconcelos, C. Dolomite formation and biogeochemical cycles in the Phanerozoic. Sedimentology 2000, 47, 49–61. [Google Scholar] [CrossRef]
- Maliva, R.G.; Missimer, T.M.; Guo, W. Paleohydrological modeling of penesaline reflux dolomitization: Avon Park Formation (Middle Eocene), East Central Florida. Carbonates Evaporites 2019, 34, 941–954. [Google Scholar] [CrossRef]



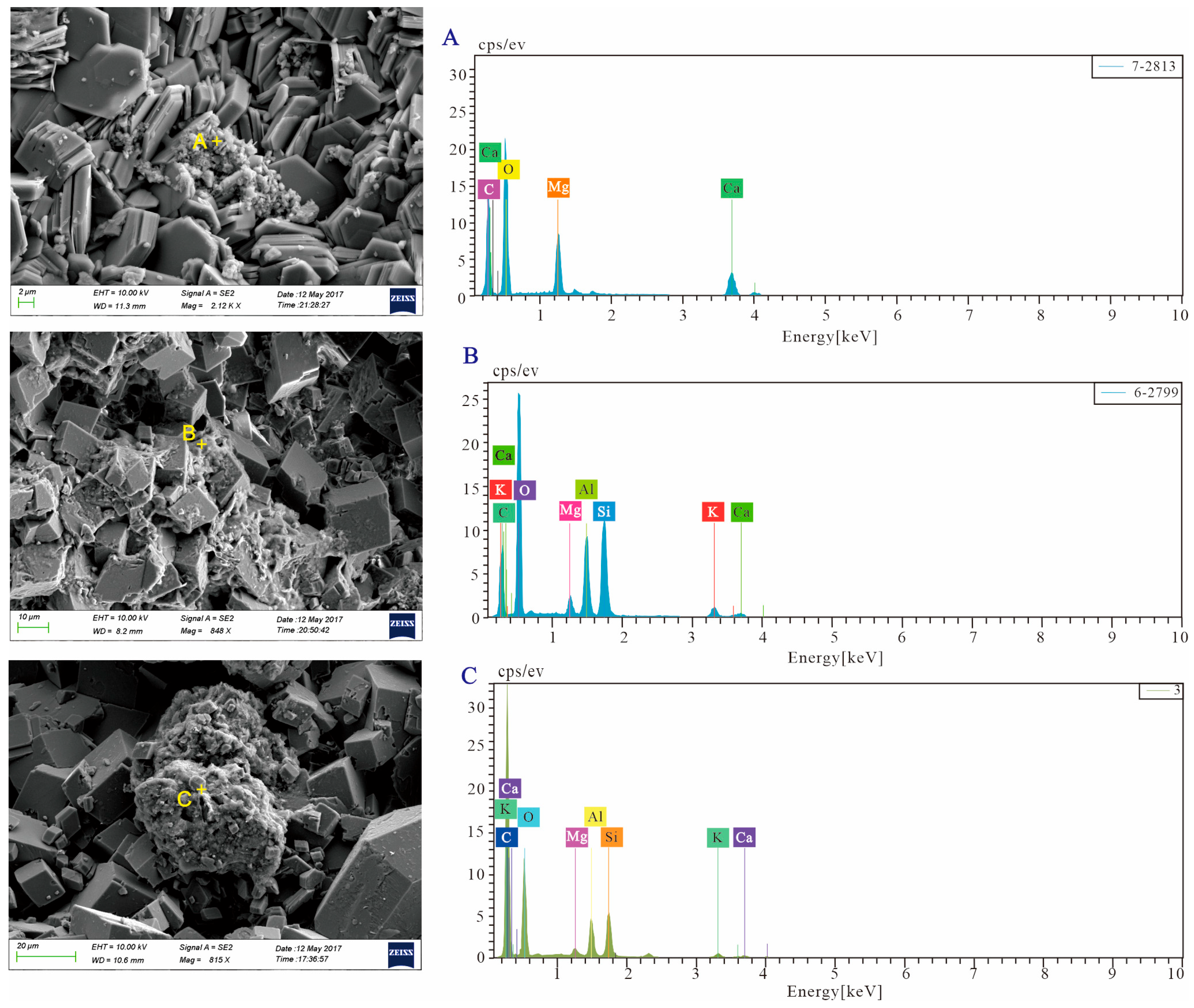
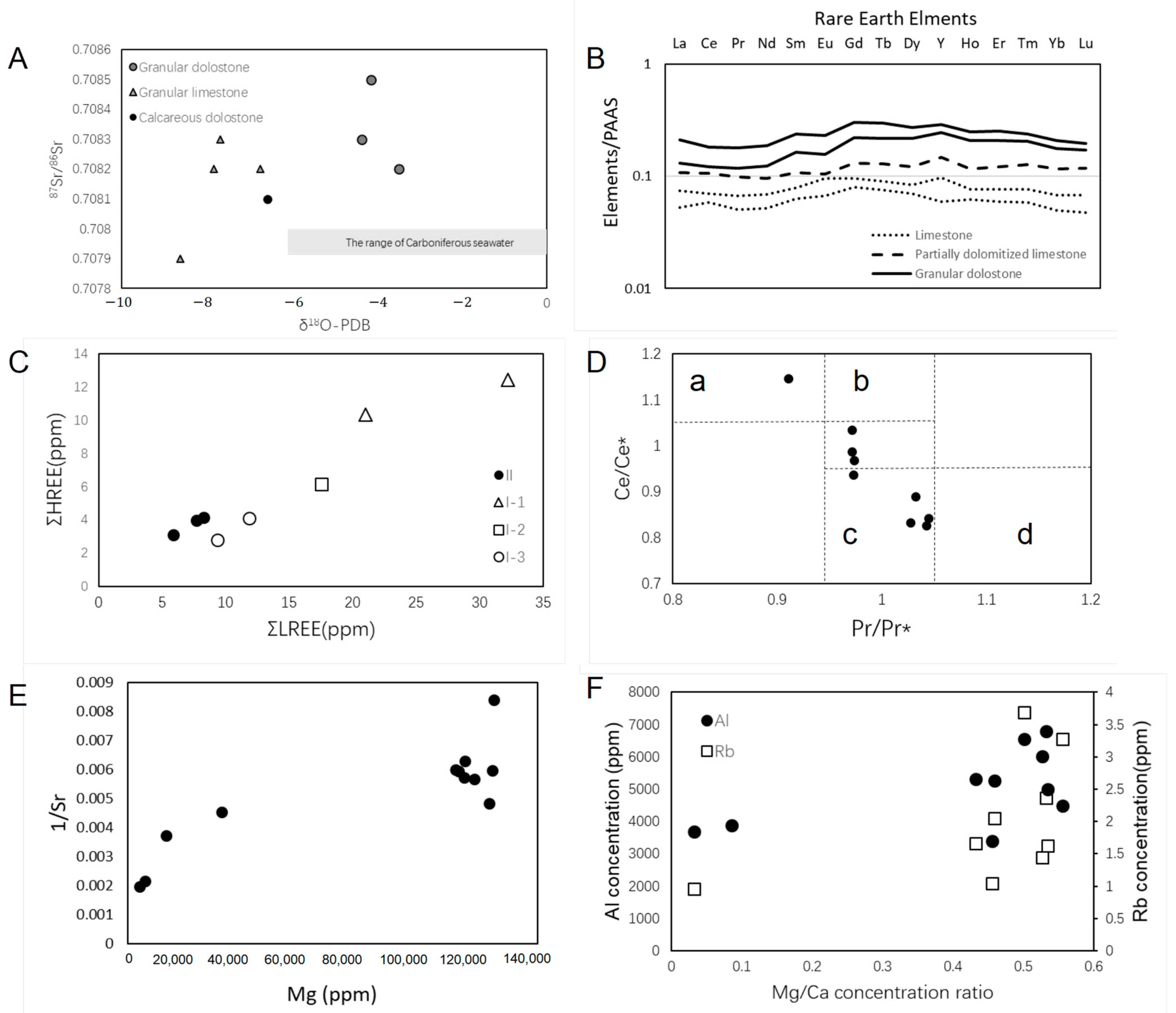

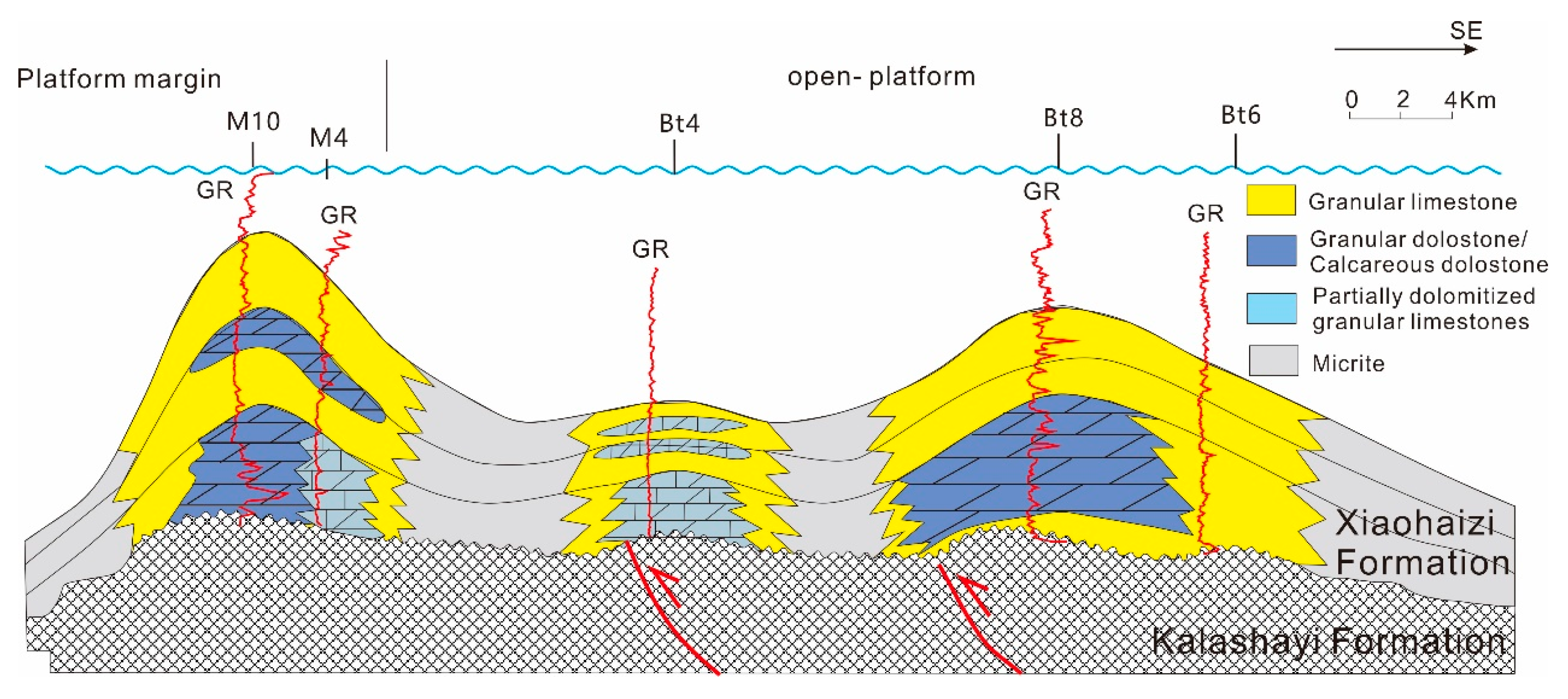

| Well | Thickness of the Cored Intervals (m) | Total Thickness of Tested (m) | The Number of Thin Sections | TH | Elements Composition | SEM Observation | δ13C | δ18O | 87Sr/86Sr | XRD |
|---|---|---|---|---|---|---|---|---|---|---|
| M4 | 10.8 | 8.25 | 7 | √ | √ | √ | √ | √ | ||
| M10 | 51.62 | 36.22 | 36 | √ | √ | √ | √ | √ | ||
| BC1 | 11.65 | 8.76 | 8 | √ | √ | |||||
| BT2 | 9.54 | 8.4 | 5 | √ | √ | √ | √ | √ | ||
| BT3 | 15.89 | 14.79 | 9 | √ | √ | √ | √ | √ | ||
| BT4 | 18.92 | 16.23 | 18 | √ | √ | √ | √ | √ | √ | √ |
| BT5 | 21.5 | 12 | 15 | √ | √ | |||||
| BT6 | 16.2 | 15.42 | 29 | √ | √ | |||||
| BT7 | 7 | 6.26 | 3 | √ | √ | |||||
| BT8 | 37.23 | 28.75 | 31 | √ | √ |
| Items | Bachu Uplift | South of the Maigaiti Slope | North of the Maigaiti Slope | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Well | BC1 | BT2 | BT3 | BT5 | BT6 | BT8 | BT9 | M4 | M10 | BT4 |
| The interval of burial depth (m) | 1935–2033 | 1876–1971 | 1868–1944 | 1887.5–1970 | 4405–4486 | 4458–4544 | 4543–4655 | 4343–4417 | 4361.5–4457 | 4266–4333.5 |
| The total thickness of the Xiaohaizi Formation (m) | 98 | 95 | 76 | 82.5 | 81 | 86 | 112 | 74 | 95.5 | 67.5 |
| The thickness of shoal (m) | 30.35 | 36.5 | 38.5 | 33 | 36.37 | 54.44 | 49 | 45.5 | 57.5 | 31.81 |
| The thickness of dolomitized carbonate rock (m) | 19.75 | 35.5 | 17 | 33 | 9.4 | 28.12 | 24 | 11.9 | 38.7 | 21.58 |
| The proportion of the shoal to the total thickness of the Xiaohaizi Formation (%) | 30.97 | 38.42 | 50.66 | 40 | 44.9 | 63.3 | 43.75 | 61.49 | 60.21 | 47.13 |
| The proportion of dolomitized shoal to the total thickness of shoal (%) | 65.07 | 97.26 | 44.16 | 100 | 25.85 | 51.65 | 48.98 | 26.15 | 23.82 | 67.84 |
| Well | Depth (m) | Insoluble Residues Content # (wt.%) |
|---|---|---|
| M10 | 4397.30 | 3.63 |
| M10 | 4398.10 | 3.97 |
| M10 | 4400.00 | 4.17 |
| M10 | 4404.94 | 1.47 |
| M10 | 4406.54 | 1.69 |
| BT3 | 1918.02 | 3.15 |
| Well | Depth (m) | Lithology | Content of Minerals (wt.%) | Degree of Order of Dolomite | |||||
|---|---|---|---|---|---|---|---|---|---|
| Kaolinite | Quartz | Calcite | Dolomite | Illite | Pyrite | ||||
| M10 | 4397.30 | granular dolostone | T | 1 | 3 | 96 | T | 0 | 0.53 |
| M10 | 4398.10 | calcareous granular dolostone | 2 | 2 | 33 | 63 | T | 0 | 0.63 |
| M10 | 4400.00 | calcareous granular dolostone | 1 | 2 | 27 | 70 | T | 0 | 0.52 |
| M10 | 4406.54 | calcareous granular dolostone | T | T | 25 | 75 | T | 0 | 0.5 |
| M10 | 4404.94 | calcareous granular dolostone | T | T | 21 | 79 | T | 0 | 0.59 |
| BT4 | 4312.11 | microcrystalline dolostone | 6 | T | 9 | 84 | T | 1 | 0.41 |
| BT3 | 1918.02 | granular dolostone | T | 1 | 2 | 97 | T | 0 | 0.6 |
| Well | BT3 | BT3 | BT3 | BT4 |
|---|---|---|---|---|
| Depth (m) | 1913.62 | 1913.62 | 1911.72 | 4312.11 |
| Lithology | Granular Dolostone | Granular Dolostone | Granular Dolostone | Dolomicrite |
| Crystal size | 15 μm | 30 μm | 12 μm | 10 μm |
| Type of dolomite | A | A | A | A |
| F | 0.25 | 0.09 | 0.00 | 0.00 |
| SrO | 0.04 | 0.00 | 0.00 | 0.01 |
| K2O | 0.00 | 0.00 | 0.01 | 0.09 |
| SO3 | 0.28 | 0.03 | 0.07 | 0.16 |
| Na2O | 0.02 | 0.00 | 0.02 | 0.01 |
| BaO | 0.00 | 0.00 | 0.00 | 0.00 |
| MnO | 0.00 | 0.01 | 0.01 | 0.02 |
| CaO | 58.58 | 58.03 | 57.72 | 55.16 |
| MgO | 39.21 | 40.62 | 40.24 | 43.09 |
| TiO2 | 0.00 | 0.00 | 0.14 | 0.04 |
| FeO | 0.85 | 0.63 | 0.84 | 1.39 |
| SiO2 | 0.32 | 0.29 | 0.45 | 0.03 |
| Al2O3 | 0.46 | 0.29 | 0.49 | 0.00 |
| Well | Depth (m) | Lithology | δ18Odol-PDB | δ13Cdol-PDB | δ18Oseawater-PDB |
|---|---|---|---|---|---|
| BT8 | 4532.05 | Granular dolostone | 2.8 | −1.4 | 3.41 |
| BT5 | 1933.10 | Granular dolostone | −0.4 | 3.5 | 0.12 |
| BT5 | 1937.01 | Granular dolostone | 0.1 | 0.1 | 0.63 |
| BT5 | 1936.56 | Granular dolostone | 1.1 | 0.1 | 1.66 |
| BT8 | 4512.40 | Granular dolostone | −1.6 | 1.8 | −1.12 |
| BT8 | 4525.20 | Granular dolostone | −2.0 | −2.1 | −1.53 |
| BT8 | 4525.66 | Granular dolostone | −2.2 | −2.0 | −1.74 |
| BT8 | 4533.82 | Granular dolostone | −2.6 | −2.0 | −2.15 |
| BT8 | 4534.30 | Granular dolostone | −1.4 | −1.8 | −0.92 |
| BT8 | 4534.65 | Granular dolostone | −3.2 | −2.0 | −2.77 |
| BT4 | 4317.55 | dolomicrite | 1.3 | 1.9 | 1.87 |
| BT5 | 1938.30 | dolomicrite | 1.7 | −0.1 | 2.28 |
| BT5 | 1944.46 | dolomicrite | 1.0 | −2.2 | 1.56 |
| BT5 | 1933.25 | dolomicrite | 0.5 | 0.4 | 1.04 |
| Well | Depth (m) | Lithology | δ18Ocal-PDB | δ13Ccal-PDB |
|---|---|---|---|---|
| M10 | 4410.75 | granular limestone | −7.90 | 2.30 |
| BC1 | 1976.76 | granular limestone | −6.90 | −1.20 |
| BT6 | 4450.60 | granular limestone | −7.60 | 1.70 |
| BT8 | 4515.35 | granular limestone | −6.80 | −1.30 |
| BT7 | 4434.51 | granular limestone | −6.50 | 1.60 |
| BT7 | 4432.07 | granular limestone | −7.40 | 1.50 |
| M10 | 4430.42 | granular limestone | −6.50 | −2.60 |
| Well | Depth (m) | Lithology | δ18O V-PDB | 87Sr/86Sr | Error (2σ) |
|---|---|---|---|---|---|
| BT2 | 1926.30 | Granular dolostone | −4.13 | 0.7085 | 4.63 × 10−5 |
| BT2 | 1923.60 | Granular dolostone | −4.36 | 0.7083 | 3.43 × 10−5 |
| BT2 | 1928.70 | Partially dolomitized limestone | −6.73 | 0.7082 | 7.05 × 10−5 |
| BT3 | 1920.27 | Granular limestone | none | 0.7081 | 4.28 × 10−5 |
| BT4 | 4303.37 | Granular limestone | −8.62 | 0.7079 | 9.49 × 10−6 |
| BT4 | 4311.52 | Granular limestone | none | 0.7080 | 2.34 × 10−5 |
| BT4 | 4316.40 | Granular limestone | −7.67 | 0.7083 | 7.23 × 10−5 |
| BT4 | 4319.60 | Granular limestone | −7.83 | 0.7082 | 2.41 × 10−5 |
| BT4 | 4309.34 | Granular limestone | none | 0.7079 | 3.23 × 10−5 |
| M4 | 4388.50 | Granular dolostone | −3.48 | 0.7082 | 4.05 × 10−5 |
| M4 | 4386.85 | Calcareous dolostone | −6.56 | 0.7081 | 2.03 × 10−5 |
| M4 | 4393.29 | Granular limestone | none | 0.7080 | 3.81 × 10−5 |
| Well | Depth (m) | Lithology | ΣLREE | ΣHREE | ΣLREE/ΣHREE | Y/Ho | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT2 | 1926.3 | Granular dolostone | 8.28 | 4.16 | 1.99 | 37.46 | 2.13 | 3.46 | 0.44 | 1.73 | 0.33 | 0.19 | 0.38 | 0.06 | 0.36 | 0.07 | 0.22 | 0.03 | 0.21 | 0.03 |
| BT2 | 1923.6 | Granular dolostone | 7.70 | 3.98 | 1.94 | 39.88 | 1.95 | 3.19 | 0.40 | 1.61 | 0.34 | 0.21 | 0.35 | 0.06 | 0.34 | 0.07 | 0.20 | 0.03 | 0.17 | 0.03 |
| BT4 | 4316.4 | Granular limestone | 5.89 | 3.12 | 1.89 | 40.90 | 1.48 | 2.49 | 0.31 | 1.25 | 0.25 | 0.11 | 0.27 | 0.04 | 0.25 | 0.05 | 0.16 | 0.02 | 0.15 | 0.02 |
| M4 | 4386.85 | Granular dolostone | 32.20 | 12.45 | 2.59 | 31.59 | 8.05 | 14.60 | 1.60 | 6.38 | 1.32 | 0.25 | 1.42 | 0.23 | 1.28 | 0.25 | 0.72 | 0.10 | 0.59 | 0.09 |
| M4 | 4388.5 | Granular dolostone | 20.99 | 10.35 | 2.03 | 32.43 | 5.02 | 9.67 | 1.05 | 4.18 | 0.91 | 0.17 | 1.03 | 0.17 | 1.02 | 0.21 | 0.59 | 0.08 | 0.50 | 0.07 |
| BT2 | 1928.7 | Partially dolomitizedlimestone | 17.52 | 6.17 | 2.84 | 34.50 | 4.16 | 8.54 | 0.87 | 3.23 | 0.60 | 0.11 | 0.61 | 0.10 | 0.57 | 0.12 | 0.35 | 0.05 | 0.33 | 0.05 |
| M4 | 4387.35 | Granular dolostone | 4.01 | 1.71 | 2.35 | 33.29 | 0.96 | 1.76 | 0.22 | 0.86 | 0.18 | 0.03 | 0.19 | 0.03 | 0.17 | 0.03 | 0.10 | 0.01 | 0.09 | 0.01 |
| BT4 | 4314.46 | Granular limestone | 11.86 | 4.10 | 2.89 | 34.91 | 2.83 | 5.56 | 0.59 | 2.33 | 0.44 | 0.10 | 0.45 | 0.07 | 0.39 | 0.08 | 0.22 | 0.03 | 0.19 | 0.03 |
| BT4 | 4303.37 | Granular limestone | 9.32 | 2.79 | 3.35 | 26.18 | 2.01 | 4.70 | 0.44 | 1.75 | 0.35 | 0.07 | 0.37 | 0.06 | 0.33 | 0.06 | 0.17 | 0.02 | 0.14 | 0.02 |
| Well | Depth (m) | Litholoy | Nb/YbSN | La/La*SN | (Ce/Ce*)SN | (Eu/Eu*)SN | (Pr/Pr*)SN | (Gd/Gd*)SN | (Y/Y*)SN | Ba (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|
| M4 | 4386.85 | Granular dolostone | 0.91 | 1.27 | 0.94 | 0.85 | 0.97 | 1.15 | 1.11 | 5.44 |
| M4 | 4388.5 | Granular dolostone | 0.70 | 1.19 | 0.97 | 0.81 | 0.97 | 1.17 | 1.16 | 14.23 |
| BT2 | 1928.70 | Partially dolomitized limestone | 0.82 | 1.04 | 1.04 | 0.87 | 0.97 | 1.12 | 1.24 | 4.65 |
| M4 | 4387.35 | Partially dolomitized limestone | 0.82 | 1.11 | 0.89 | 0.90 | 1.03 | 1.11 | 1.17 | 4.76 |
| BT4 | 4314.46 | Granular Limestone | 1.02 | 1.15 | 0.99 | 1.10 | 0.97 | 1.03 | 1.22 | 175.74 |
| BT4 | 4303.37 | Granular Limestone | 1.03 | 1.10 | 1.15 | 0.94 | 0.91 | 1.12 | 0.90 | 3.49 |
| BT2 | 1926.30 | Granular dolostone | 0.69 | 1.22 | 0.83 | 2.55 | 1.04 | 0.62 | 1.36 | 374.08 |
| BT2 | 1923.60 | Granular dolostone | 0.77 | 1.28 | 0.83 | 2.82 | 1.03 | 0.57 | 1.44 | 542.79 |
| BT4 | 4316.40 | Partially dolomitized limestone | 0.69 | 1.17 | 0.84 | 2.01 | 1.04 | 0.72 | 1.50 | 191.12 |
| Well | Depth (m) | Lithology | Mg/Ca | Sr (μg/g) | Ba (μg/g) | Ti (μg/g) | Mn (μg/g) | Fe (μg/g) | Al (μg/g) | Rb (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| BT3 | 1918.02 | Granular dolostone | 0.534 | 119 | 11 | 49 | 76 | 3581 | 4993 | 1.624 |
| BT3 | 1921.48 | Calcareous dolostone | 0.375 | 168 | 545 | 56 | 112 | 5923 | 6090 | / |
| BT4 | 4306.97 | Granular dolostone | 0.531 | 207 | 17 | 99 | 168 | 14,153 | 6792 | 2.363 |
| BT4 | 4316.09 | Micrite | 0.032 | 268 | 178 | 38 | 36 | 1369 | 3698 | 0.956 |
| BT4 | 4317.55 | Calcareous dolostone | 0.086 | 221 | 6 | 47 | 34 | 1500 | 3880 | / |
| M10 | 4397.30 | Granular dolostone | 0.555 | 674 | 12 | 95 | 119 | 3575 | 4480 | 3.271 |
| M10 | 4398.10 | Calcareous dolostone | 0.526 | 166 | 20 | 120 | 160 | 7102 | 6017 | 1.445 |
| M10 | 4400.00 | Calcareous dolostone | 0.459 | 176 | 9 | 107 | 152 | 8423 | 5260 | 2.056 |
| M10 | 4404.94 | Calcareous dolostone | 0.455 | 167 | 3 | 46 | 61 | 4138 | 3400 | 1.038 |
| M10 | 4406.54 | Calcareous dolostone | 0.432 | 159 | 29 | 48 | 71 | 5290 | 5315 | 1.664 |
| Well | Depth (m) | Host Mineral | Type | Gas/Liquid Ratio | Th (°C) |
|---|---|---|---|---|---|
| BT4 | 4312.11 | calcite cement | saline | 8 | 107.3 |
| BT4 | 4312.11 | calcite cement | saline | 6 | 106.7 |
| M10 | 4407.54 | calcite cement | saline | 7 | 103.4 |
| M10 | 4407.54 | calcite cement | saline | 6 | 96.3 |
| BT4 | 4316.09 | calcite vein | saline | 6 | 86.1 |
| BT4 | 4316.09 | calcite vein | saline | 7 | 81.8 |
| BT4 | 4316.09 | calcite vein | saline | 6 | 94.2 |
| BT4 | 4316.09 | calcite vein | saline | 5 | 88.1 |
| BT4 | 4316.09 | calcite vein | saline | 8 | 85.7 |
| M10 | 4400.00 | calcite cement | saline | 8 | 81.7 |
| M10 | 4400.00 | calcite cement | saline | 7 | 76.3 |
| M10 | 4400.00 | calcite cement | saline | 6 | 84.3 |
| M10 | 4400.00 | calcite cement | saline | 6 | 95.2 |
| M10 | 4400.00 | calcite cement | saline | 7 | 82.4 |
| BT4 | 4306.97 | calcite cement | saline | 10 | 73.6 |
| BT4 | 4306.97 | calcite cement | saline | 8 | 122.1 |
| BT4 | 4306.97 | calcite cement | saline | 7 | 86.3 |
| BT4 | 4306.97 | calcite cement | saline | 7 | 102.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Fu, M.; Gluyas, J.; Song, R.; Lan, H.; Fan, Y.; Wu, D. Dolomitization Facilitated by Clay Minerals on Mixed Siliciclastic-Carbonate Shoals of Carboniferous Age in the Tarim Basin, China: Constraints on Element Mobility and Isotope Geochemistry. Minerals 2025, 15, 419. https://doi.org/10.3390/min15040419
Liu X, Fu M, Gluyas J, Song R, Lan H, Fan Y, Wu D. Dolomitization Facilitated by Clay Minerals on Mixed Siliciclastic-Carbonate Shoals of Carboniferous Age in the Tarim Basin, China: Constraints on Element Mobility and Isotope Geochemistry. Minerals. 2025; 15(4):419. https://doi.org/10.3390/min15040419
Chicago/Turabian StyleLiu, Xuan, Meiyan Fu, Jon Gluyas, Rongcai Song, Haoxiang Lan, Yunjie Fan, and Dong Wu. 2025. "Dolomitization Facilitated by Clay Minerals on Mixed Siliciclastic-Carbonate Shoals of Carboniferous Age in the Tarim Basin, China: Constraints on Element Mobility and Isotope Geochemistry" Minerals 15, no. 4: 419. https://doi.org/10.3390/min15040419
APA StyleLiu, X., Fu, M., Gluyas, J., Song, R., Lan, H., Fan, Y., & Wu, D. (2025). Dolomitization Facilitated by Clay Minerals on Mixed Siliciclastic-Carbonate Shoals of Carboniferous Age in the Tarim Basin, China: Constraints on Element Mobility and Isotope Geochemistry. Minerals, 15(4), 419. https://doi.org/10.3390/min15040419





