Abstract
The manufacture of barium sulfide or barium salts (BaS, BaCl2, Ba (OH)2, among others) requires high-purity barite ores (>90%). In this study, a new method to produce barium sulfide from low-grade barite ores (60% purity) is proposed. The method involves gravitational concentration of barite ore on a shaking table followed by mechanical activation of the barite concentrate with metallurgical coke in a ball mill. The mechanically activated mixture undergoes carbothermic reduction with an argon flow, resulting in the conversion of barite concentrate into barium sulfide. Gravitational concentration studies conducted using a shaking table demonstrated that, upon optimizing key operational parameters—namely, the wash-water flow rate, length, stroke frequency, the splitter positions of the concentrate, middlings, and tailings—a barite concentrate with a purity exceeding 95% BaSO4 was successfully achieved. Mechanical activation of the barite/coal mixture lowered the initial temperature of the carbothermic reduction from 1100 K to 990 K, enabling complete conversion of barite to BaS, as confirmed by thermogravimetric curves and XRD analysis. Furthermore, the activation energy during the carbothermic reduction ranged from 300 to 500 kJ/mol, suggesting a complex reduction process of barite with metallurgical coke that is difficult to represent by a single reaction.
1. Introduction
Barium compounds such as barium sulfate (BaSO4), barium hydroxide (Ba(OH)2), barium chloride (BaCl2), and barium carbonate (BaCO3) are significant chemical compounds generated from barite concentrates. These compounds are widely used in the paper and rubber industries as fillers or extenders in cloth, ink, and plastics products; in radiography (“barium milkshake”); as getter (scavenger) alloys in vacuum tubes; as deoxidizers for copper; as lubricants for anode rotors in X-ray tubes; and as spark-plug alloys, among others [1]. In the manufacturing of barium compounds, barite of purity higher than 90% is required [2]. Specifications for the various end usages of barite usually require a higher barite content then found in raw ore [3]. Therefore, ores need to be beneficiated to increase the barite content to match specifications. Chile has estimated reserves of barite ores on the order of 2,350,000 t; however, these deposits are unutilized, despite the internal and external demand for products derived from barium [4]. The main reason why these barium deposits in Chile are not utilized is that they are of low purity (<60% as BaSO4, which is below the required purity for conventional processing from barium ores) [5].
The main processes to increase the purity of barite ores are gravitational concentration and flotation. Gravity concentration processes (jigging, tabling, and spirals) are generally used on coarse-grained ores; they depend on the specific gravity difference between the barite and the gangue components contained in the ore. The direct or reverse flotation process is usually applied on finely disseminated ores; it depends on a combination of physical and chemical characteristics to separate the barite from the gangue components [6,7,8].
There are few studies in the literature about barite processing; Singh et al. [6], studied the concentration of a low-grade barite ore (72% of BaSO4) by gravity separation and froth flotation. They reported that when gravitational concentration (jigging–tabling) was applied to particles with size ranges between 0.6 and 1.7 mm, it was possible to obtain a concentrate with a grade between 86% and 91% of BaSO4 but with low barite recovery (66.3%–66.9% of BaSO4). Application of the flotation process to particles of 96.4% < 0.074 mm produced a concentrate with a grade of 91% BaSO4 and a recovery of 88.3% of BaSO4. Similar results were reported by Bhatti et al. [7] and Mgbemere et al. [8], who concluded that, when using gravity concentration, the low barite recovery could be attributed to the fact that the barite is not sufficiently liberated at coarser particle sizes, even though gravimetric techniques are effective at concentration.
The manufacturing process of barium compounds involves three steps [9]: carbothermic reduction, leaching–filtration, and precipitation. The carbothermic reduction is carried out in a fluidizing bed or rotating furnaces at a temperature between 1073 and 1623 K. In this step, the barite ore or concentrate (pure > 90 wt.%) is converted into water-soluble barium sulfide (BaS) using metallurgical coke or another suitable reduction agent. Subsequently, to separate the barium from the insoluble impurities (unreacted barite and metallurgical coke, silicates, iron minerals, among others), the product obtained from the carbothermic process is leached with hot water and filtered. Finally, the desired barium salt is precipitated using known precipitating chemical compounds. For example, to produce BaCl2, hydrochloric acid (HCl) is used.
Carbothermic reduction presents the following problems: high consumption of metallurgical coke, long residence time, low conversion efficiencies, high temperatures, and high production of greenhouse gases (CO2) [10]. A review of the literature shows that there are few studies addressed at solving the problems posed. Most of the studies conducted have focused on the use of catalysts, which have made it possible to increase the rate and decrease the temperature and activation energy of the carbothermic reduction [11,12,13]. Mechanical activation has been proposed to increase the reactivity between barite and metallurgical coke. Mechanical activation refers to friction, collision, impingement, shear, or other mechanical actions used to increase the substance reactivity. Guzmán et al. [2] studied the mechanical activation effect on the carbothermic reduction of barite. Their experimental results revealed that the mechanical activation process improves the carbothermic reduction kinetics of barite and decreases the final reaction temperature from 1573 K to 1223 K.
The carbothermic reduction of barite is a very intricate process. It involves several gas–solid and solid–solid reactions that can occur sequentially and simultaneously [14]. Until now, all the works that have addressed the kinetic study of this process have considered a single limiting step [2,10,13,15], which is not conceptually correct. This reason explains the great variety of results reported in the literature.
From a practical point of view, it is very convenient to know how the carbothermic reaction of barite progresses as a function of the temperature and heating rate. In this context, Suñol et al. [16] proposed a method to obtain the temperature–heating rate–transformation (T-HR-T) diagram for a particular reaction using non-isothermal thermogravimetric experiments. In this method, it is not necessary to know the limiting step controlling the process, which opens the possibility of modeling the kinetics of a complex reaction such as the carbothermic reduction of barite.
Considering the above-mentioned developments, the main objective of this work was to analyze the feasibility of producing barium sulfide from low-grade barite ores by a combination of gravimetric concentration, mechanical activation, and carbothermic reduction. Additionally, the possibility of obtaining the T-HR-T diagram for carbothermic barium sulfide production was evaluated.
2. Materials and Methods
A barite ore sample, weighing about 1600 kg, with particle sizes 100% < 203.2 mm, was collected from the Atacama region, Copiapó, Chile, and used in this study. The sample was subjected to successive stages of size reduction until 100% < 1 mm, first, using a 225 mm × 175 mm jaw crusher, then a 100 mm × 75 mm Braun jaw crusher, and finally a 150 mm × 225 mm Denver roller crusher.
2.1. Barite Ore Characterization
The barite ore sample with particle sizes of 100% < 1 mm was characterized by specific gravity determination, liberation degree, and chemical and mineralogical analyses. The specific gravity was determined in a Le Chatelier flask and the liberation degree through the technique of counting grains in images obtained from a scanning electron microscope (SEM), Zeiss Model EVO MA10.
The mineralogical composition was determined through X-ray diffraction (XRD) in a Shimadzu XRD6100 diffractometer (Cu Kα radiation) using an angular step of 0.02° (2θ) and a counting time per step of 3 s. The size, morphology, and elemental composition of the barite ore sample were analyzed using an SEM equipped with X-ray energy-dispersive spectroscopy (EDS). The magnetic material contained in the barite sample was determined by Davis tube tests (DTTs) and the sulfur content was determined using the combustion technique in LECO equipment (S-230SH).
2.2. Gravitational Concentration Studies
A shaking table was selected as the concentration equipment, taking into account the marked difference in densities between the barite and the minerals contained in the gangue of the barite ore: quartz, calcite, and anorthite. The concentration criterion was determined by applying the equation proposed by Taggart, who states that if the density difference is greater than 2.08, then gravity separation can be performed [17]. Specific samples of the ore varied between 2.5 and 1.75, suggesting that it should be possible to produce an effective separation of the barite from the gangue through gravimetric means.
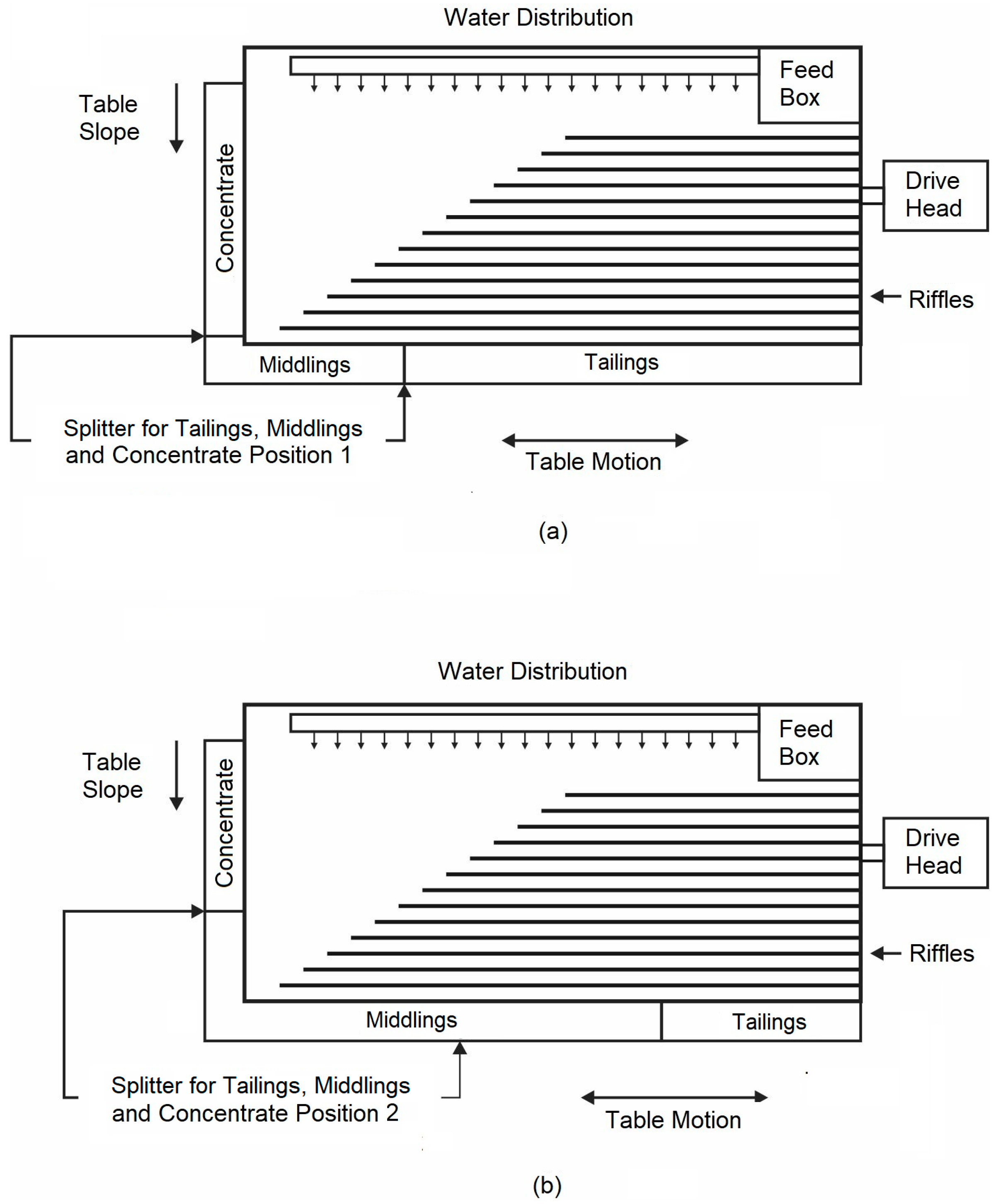
The laboratory Wilfley 13A table used in this work consists of a slightly inclined flat surface with a series of parallel riffles along the direction of motion, as schematically illustrated in Figure 1. The shaking table performance is influenced by many operating parameters, such as the deck inclination, the feed rate, the wash-water flow rate, the length, and the stroke frequency. Therefore, the quality and quantity of the different products obtained depend on the optimal combination of these variables in addition to the correct adjustment of the splitters. If the splitters are adjusted incorrectly, the concentrate could become contaminated with non-valuable mineral (gangue), or the valuable mineral (barite) could be rejected in the middlings or tailings.
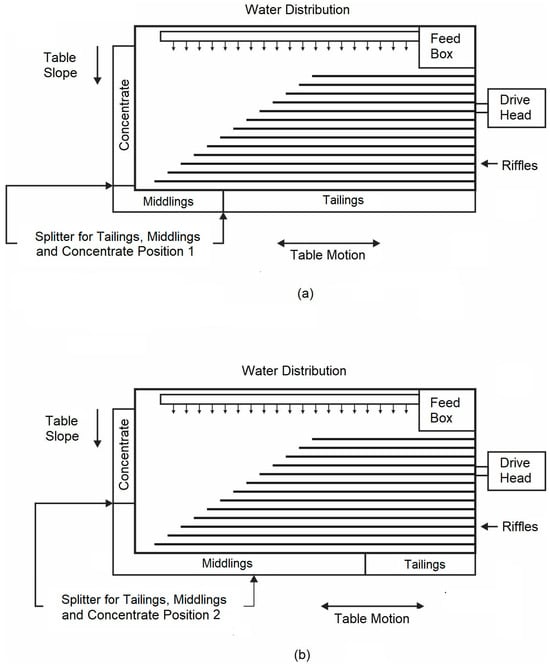
Figure 1.
Schematic illustration of a shaking table, showing positions (a) 1 and (b) 2 of the splitters for the tailings, middlings, and concentrate.
In this work, the flow effect of the washing water (5 and 7 L/min), the stroke length (8 and 14 mm), the stroke frequency (240 and 292 strokes/min), and the splitter setting (position 1 and 2, shown in Figure 1a,b, respectively) were assessed to explore the possibility of increasing the purity of the barite ore to produce barium sulfide. All tests were carried out with a granulometry of feeding 100% < 1 mm, with a feed flow of 25 kg/h and a deck inclination angle of 4°.
2.3. Mechanical Activation
The mechanical activation of the barite/coke mixture was carried out in a 169 mm × 233 mm (D × L) stainless-steel laboratory ball mill, applying a rotation speed of 78 rpm (76% of critical speed of the mill) and a grinding time of 1 h. Table 1 summarizes the key properties of the metallurgical coke employed in this study.

Table 1.
Technical specifications of the metallurgical coke used in this study.
The mill load consisted of 2018 g of barite concentrate (97.7% purity) and 524 g of metallurgical coke, corresponding to a BaSO4-to-coke mass ratio of approximately 4:1. This ratio represents a 150% excess of carbon relative to the stoichiometric requirement, as defined by reaction (1). The grinding media consisted of approximately 4.5 kg of 1-inch diameter balls and 4.5 kg of 1/2-inch diameter balls. All milling operations were carried out under vacuum and in dry conditions to prevent oxidation and ensure process consistency.
2.4. Carbothermic Reduction
The carbothermic reduction was executed in a Shimadzu thermobalance, model DTG 60H. Heating rates of 3, 5, 10, and 20 K/min were used until reaching a temperature of 1623 K. All tests were carried out under an argon atmosphere with a flow of 50 mL/min. The carbothermic reduction products were characterized by scanning electron microscopy on a Zeiss EVO MA-10, manufactured in Oberkochen, Germany and X-ray diffraction on a Shimadzu XRD 6100 diffractometer (Kα Cu), manufactured in Kyoto, Japan.
3. Results and Discussion
3.1. Ore Characterization
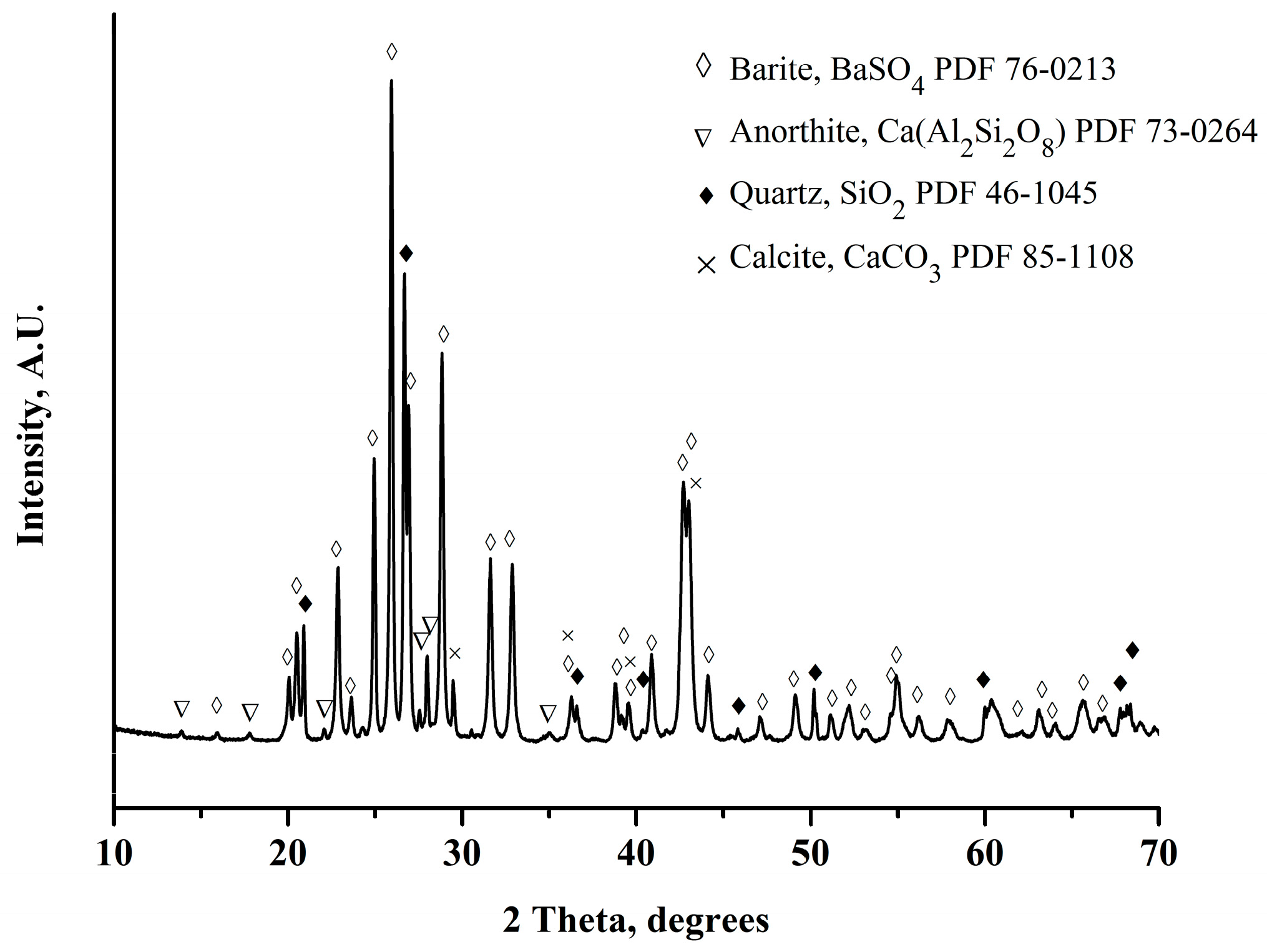
Figure 2 shows the XRD pattern of the barite ore. It can be observed that the ore is composed mainly of barite (BaSO4), with a small amount of quartz (SiO2), calcite (CaCO3), and anorthite (CaAl2Si2O8). Figure 3 shows the image obtained by backscattered electrons in the SEM of the barite ore. In this mode of operation, the particles with the highest atomic mass are observed with a white tonality, while the particles with the lowest atomic mass are perceived with dark coloration. By energy-dispersive X-ray spectroscopy (EDS) applied to one white particle (green circle, Figure 3), the constituent elements of the barite, mainly sulfur, oxygen, and barium, were identified, as presented in Table 2. On the other hand, the EDS analysis performed on one dark particle (red circle, Figure 3) revealed the presence of constituent elements of quartz, silicon, and oxygen principally, in addition to small amounts of aluminum, calcium, iron, sodium, potassium, and sulfur. A mapping was executed that included a considerable amount of white and dark particles to corroborate the EDS analyses performed on one white and one dark particle. It was confirmed that the white particles were composed of barium, sulfur, and oxygen (elements present in the barite), and the dark particles were principally composed of silica, aluminum, calcium, and oxygen (elements present in quartz, anorthite, and calcite). Moreover, the recovery of magnetic iron in the Davis tube was 0.24 ± 0.02%, which confirmed that the amount of iron in the barite ore was low.
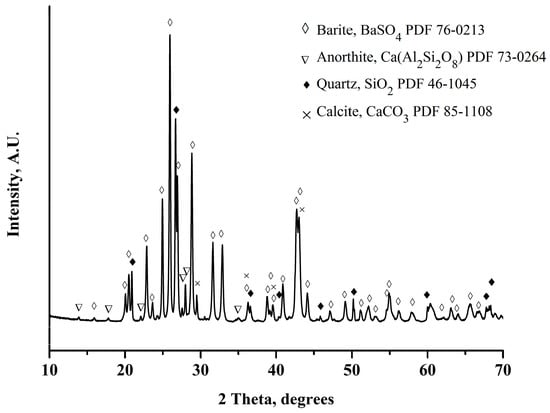
Figure 2.
XRD pattern of barite ores.

Figure 3.
SEM image of barite ore. The green and red circles show the particles with the highest and lowest atomic mass, respectively.

Table 2.
Chemical analysis by EDS of white and dark particles.
The XRD and SEM-EDS analyses results established that barite was the only mineralogical species in the ore that contained sulfur. Therefore, the barite grade was determined indirectly from the sulfur content in the barite ore and the shaking table products. Measurements using the LECO equipment showed that the barite ore contained 8.4 ± 0.2% of sulfur, which corresponds to 60.8 ± 1.8% of the barite. On the other hand, the results of determinations in the Le Chatelier flask showed that the specific gravity of the barite ore was 3.5 ± 0.5, and the results of the liberation studies showed that 76% of the barite particles were liberated under 0.21 mm.
3.2. Concentration on the Shaking Table
Table 3 presents the effect of the wash-water flow rate and stroke length and frequency on the barite grade and recovery when the splitters were adjusted in position 1. As can be seen in the table, the highest barite grade in the concentrate, 91.8%, was obtained in test 3, with a wash-water flow rate and length and frequency of stroke equal to 7 L/min, 14 mm, and 292 strokes/min, respectively; the barite recovery reached a value of 54.8%. A reduction in the wash-water flow rate to 5 L/min, test 2, adjusting the stroke length and frequency to 14 mm and 292 strokes/min, respectively, reduced the grade to 90.7% and increased the barite recovery in the concentrate to 60.3%. A decrease in the stroke length to 8 mm (test 1), adjusting the wash-water flow rate to 5 L/min and the stroke frequency to 292 strokes/min, reduced the barite grade and recovery in the concentrate to 87.8% and 48.8%, respectively. The highest barite recovery in the concentrate, 62.35%, was obtained in test 4, when the stroke frequency was reduced to 240 strokes/min and the wash-water flow rate was set at 5 L/min and the stroke length at 14 mm. However, the barite grade in the concentrate only reached a value of 89.3%. The variations produced in the barite grade and recovery in the concentrate are consistent with results obtained by other researchers based on changes in the values of the operating parameters. When the wash-water flow rate increased, the transport force also rose, causing more light particles and some heavy particles to fall into the intermediate product or tailings, which led to a decrease in recovery and an increase in the grade of heavy particles in the concentrate [18,19]. On the other hand, the higher barite grade in the concentrate in test 3 is in agreement with what was stated by Gupta and Yan [20], who reported that an increase in stroke length requires a higher wash-water flow to more quickly transport heavy particles to the heavy particle discharge zone.

Table 3.
Effect of wash-water flow rate, stroke length, and stroke frequency on barite grade and recovery in the concentrate obtained on the shaking table (splitters adjusted in position 1).
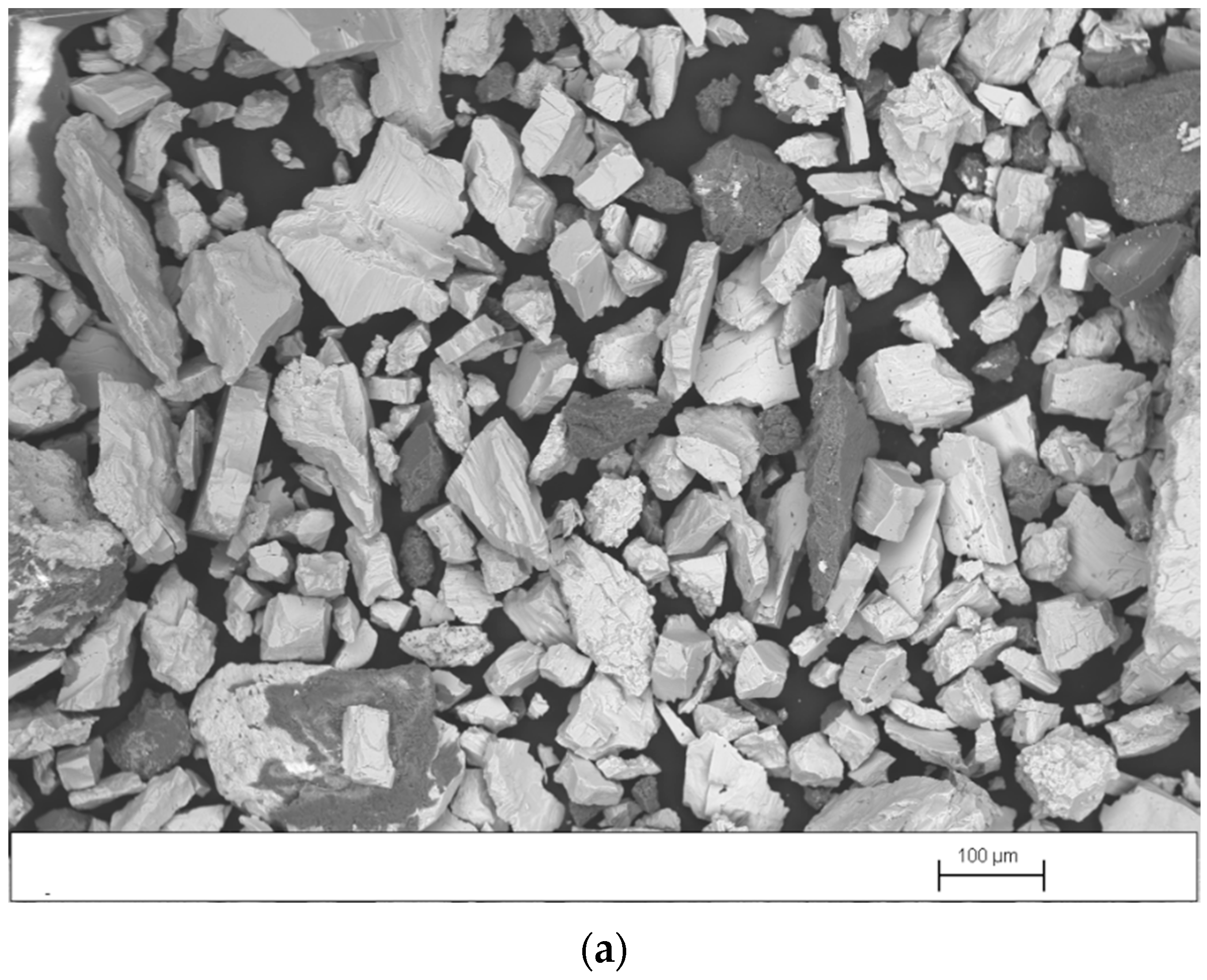
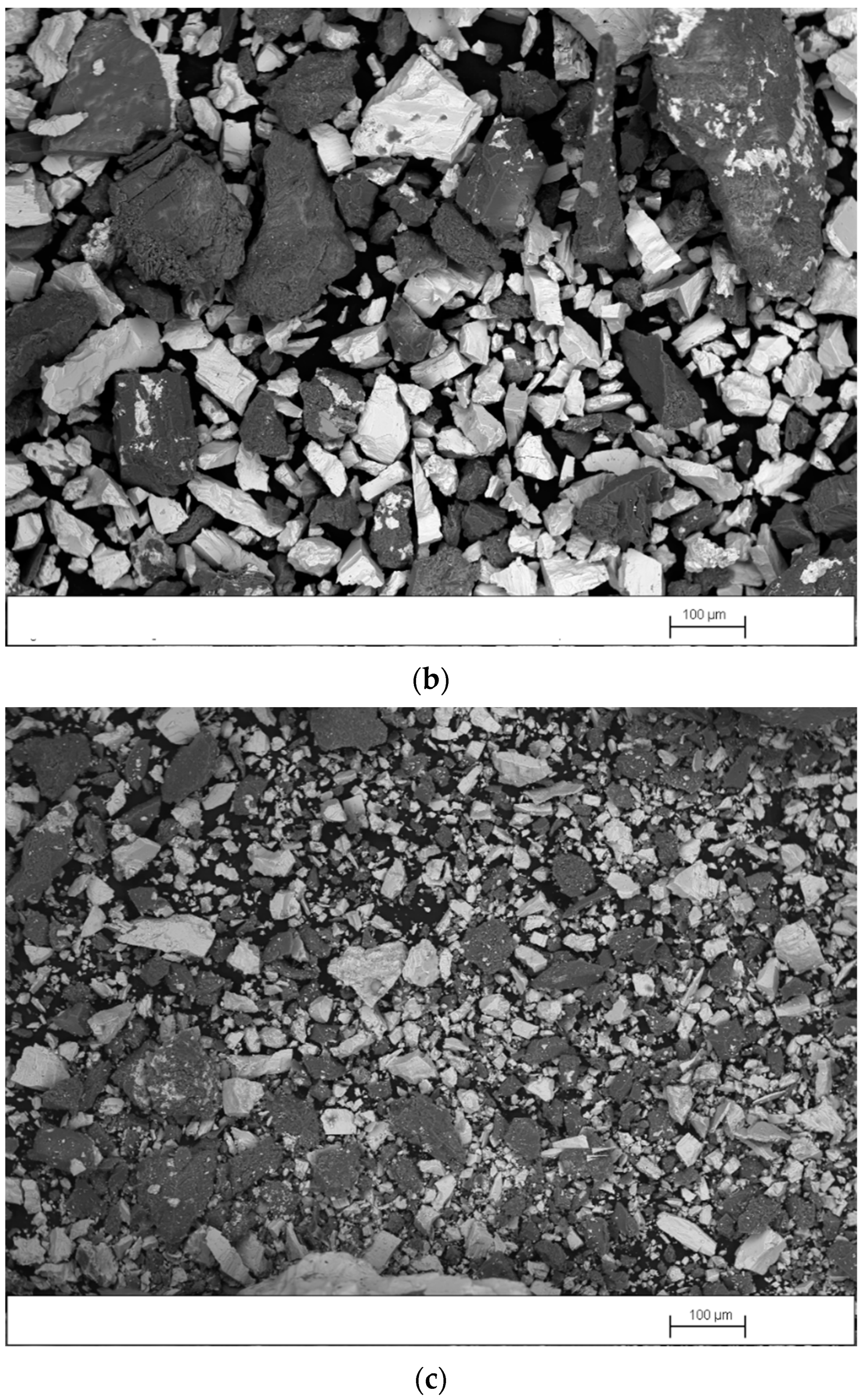
Figure 4a–c shows SEM images, obtained in backscattered-electron mode (BSE), of the concentrate, middlings, and tailings produced in test 3, performed with the splitters set at position 1. As expected, in Figure 4a it is observed that the concentrate contains a high proportion of barite particles (white particles) with a wide size range and a smaller quantity of gangue particles (dark particles) with a size >0.1 mm. Figure 4b shows that the middlings contain similar amounts of barite and gangue particles. However, most barite particles have a size <0.1 mm and most gangue particles have a size >0.1 mm. It is observed in Figure 4c that the tailings also contain similar amounts of barite and gangue particles. However, both types of particles have sizes <0.1 mm. Barite particles with a size <0.1 mm tend to travel in a perpendicular direction to the table movement. For this reason, they are recovered in the middlings or tailings.
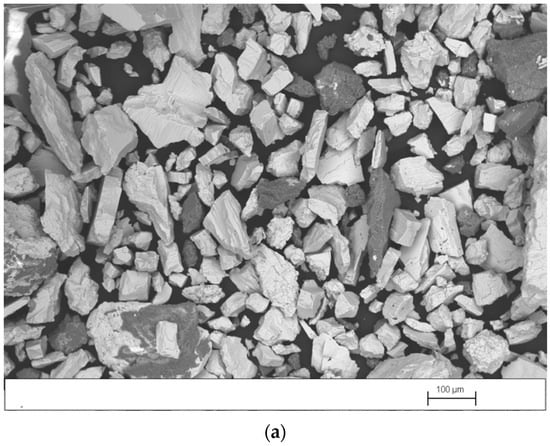
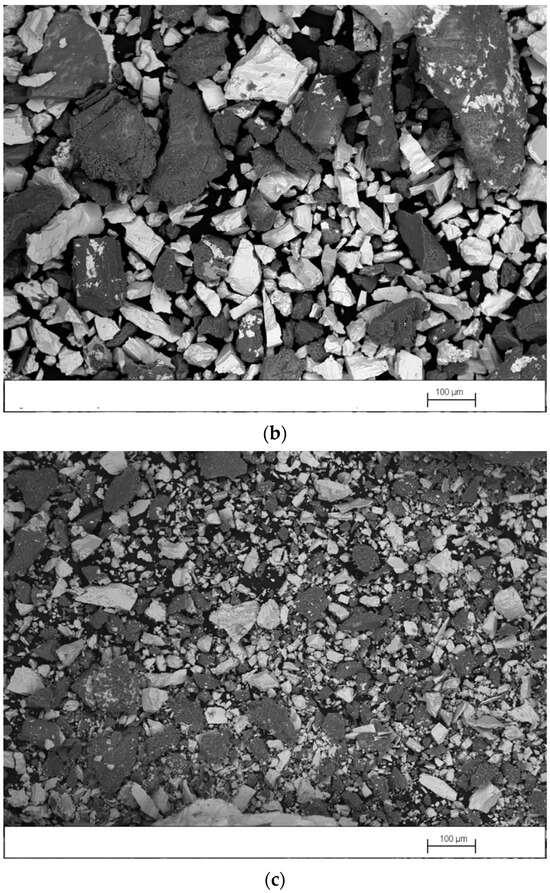
Figure 4.
SEM image, obtained in BSE mode, of (a) concentrate, (b) middlings, and (c) tailings produced in test 3.
The splitters were set at position 2 to increase the barite grade in the concentrate, maintaining the same values of the wash-water flow rate, and the length and frequency of the stroke as in test 3, thereby recovering the gangue particles > 0.1 mm, that contaminate the concentrate, in the middlings or tailings. Table 4 presents the average values of the barite grades and recoveries obtained in these tests. In this table, it is observed that the barite grade in the concentrate increased from 91.8 to 97.7%, and the recovery decreased by 3.4 percentage points, while the barite grade in the middlings decreased from 52.2 to 41.9% and the recovery increased by five percentage points. Whereas the barite grade in the tailings increased from 58.4 to 61.5%, and the recovery decreased by 1.6 percentage points. The highest barite grades being obtained in the concentrate and tailings is because the recovery of gangue particles was favored in this product by expanding the cutting range of the middlings.

Table 4.
Effect of wash-water flow rate, stroke length, and stroke frequency on barite grade and recovery in the concentrate obtained on the shaking table (splitters adjusted in position 2).
3.3. Barium Sulfide Production
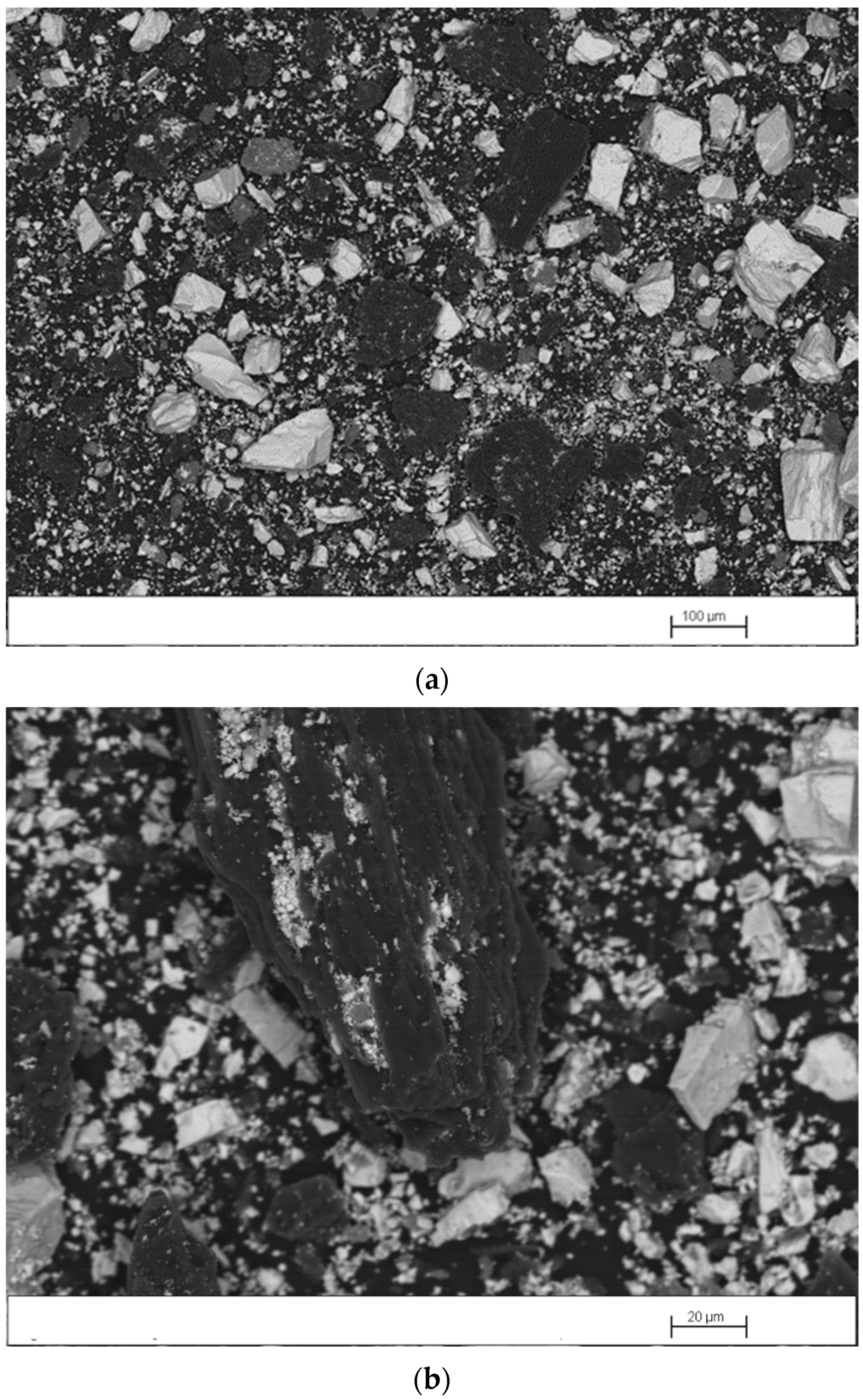
Figure 5 shows scanning electron microscopy images of the mechanically activated mixture. The formation of barite concentrate/coking coal agglomerates can be seen, products of the milling, which increases the contact between these two reactants. The d50 of the mixture particles decreased from 420 to 75 µm because of milling.
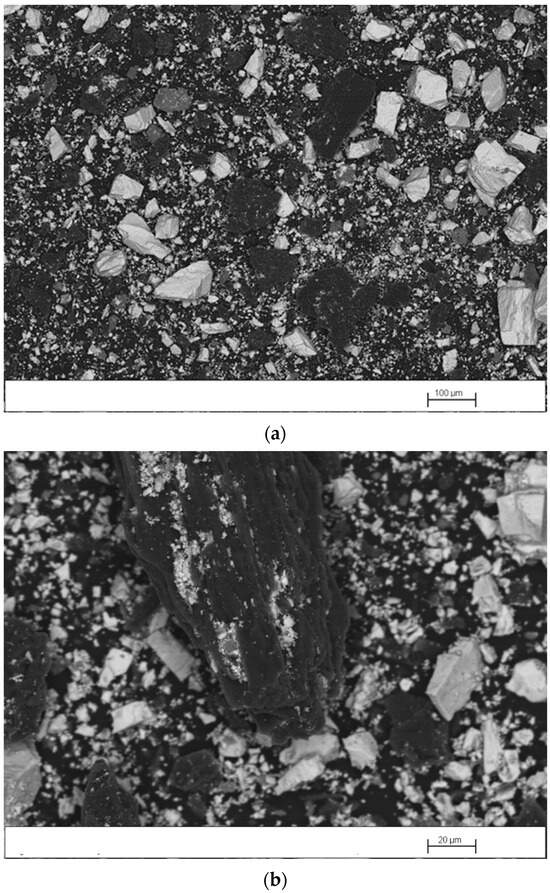
Figure 5.
Scanning electron microscopy images of the mixture mechanically activated for 1 h: (a) general view; (b) enlarged view of the agglomerate.
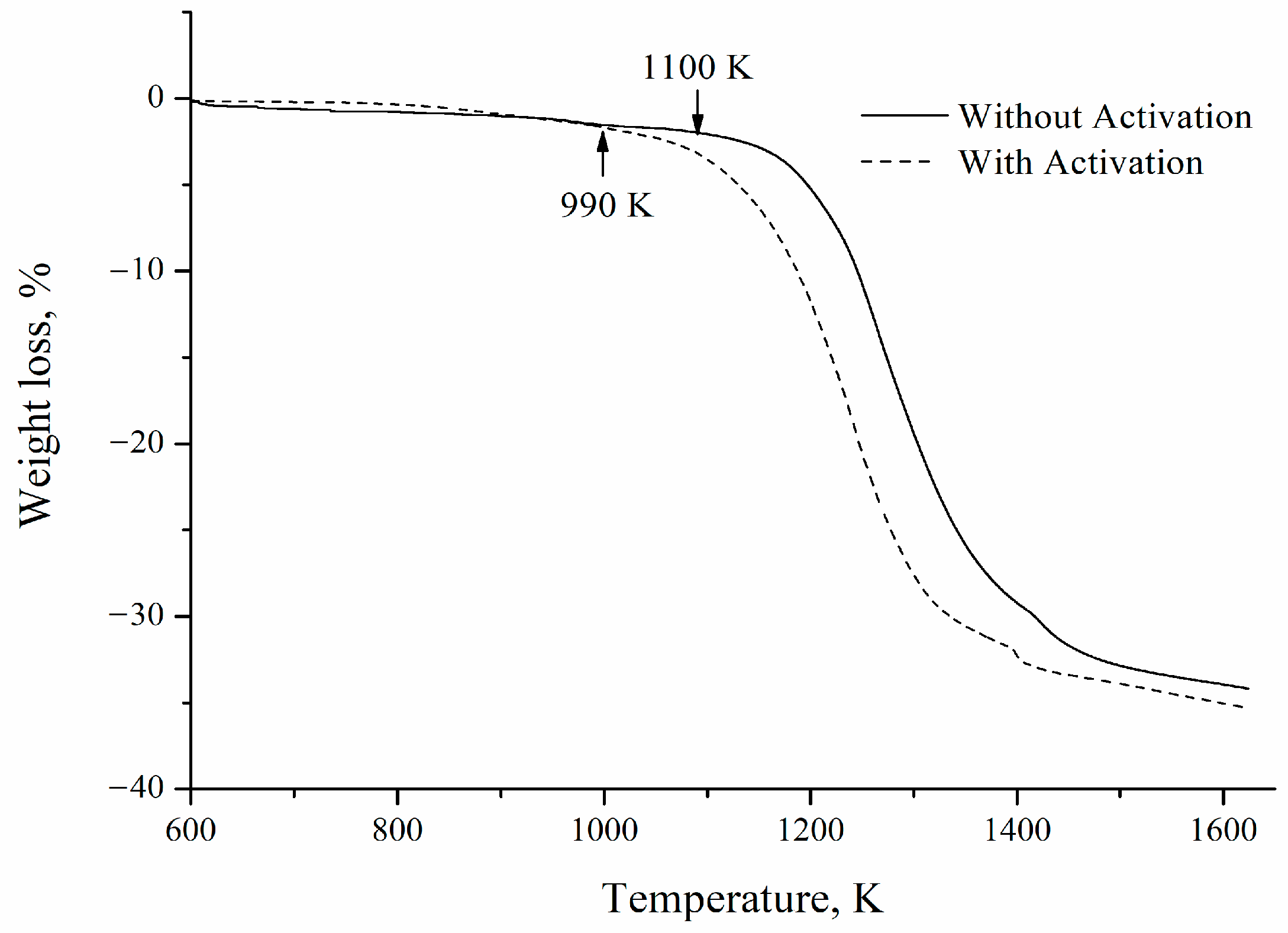
Figure 6 shows a comparison between the mass loss curves for a sample with and without mechanical activation. It can be seen that the mechanical activation process improves the kinetics of the carbothermic reduction of barite. The initial reaction temperature decreases from 1100 to 990 K. As previously reported by our research group, this behavior can be explained by considering the increase in the reactants’ enthalpies due to the energy stored by the powders and the intimate contact between fine barite and coking coal particles promoted by the milling process [2].
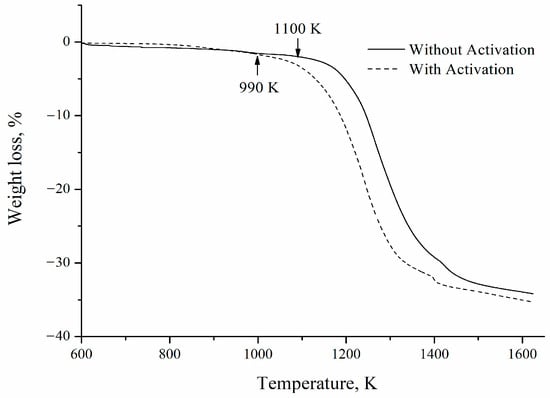
Figure 6.
Thermogravimetric curves obtained at a heating rate of 10 K min−1 for samples with and without mechanical activation.
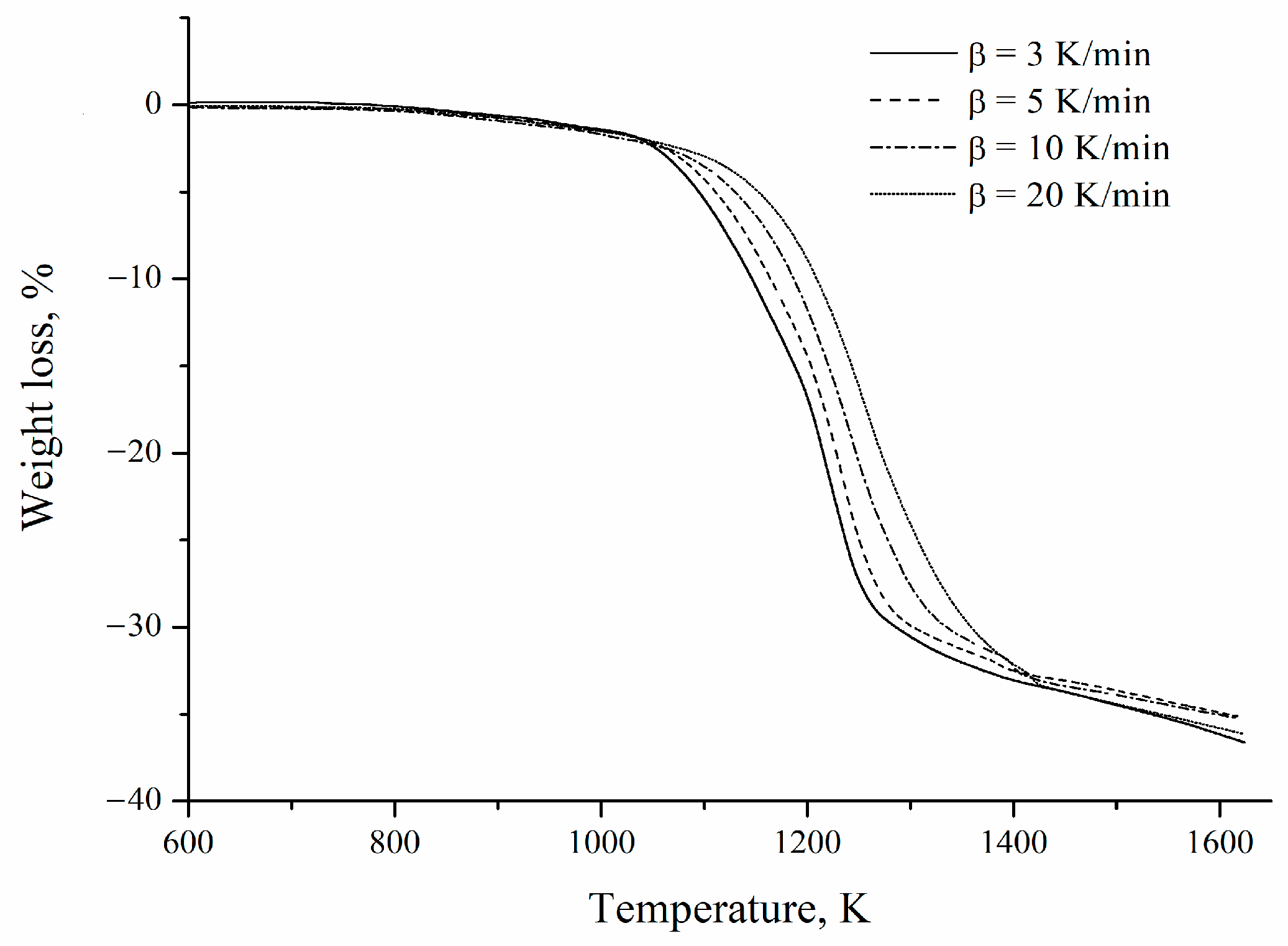
Figure 7 shows the mass-loss curves obtained at different heating rates for the mechanically activated mixture. An average mass reduction of 35.82 ± 0.68% is observed, close to the 33% of the theoretical mass loss of reaction 1 considering a concentrate with a barite grade of 97.6%. The most significant mass loss detected may be related to a possible carbon reaction with oxygen trapped in the coking coal pores. Similar results were reported by Guzmán et al. [2].
2BaSO4 + 4C→2BaS + 4CO2
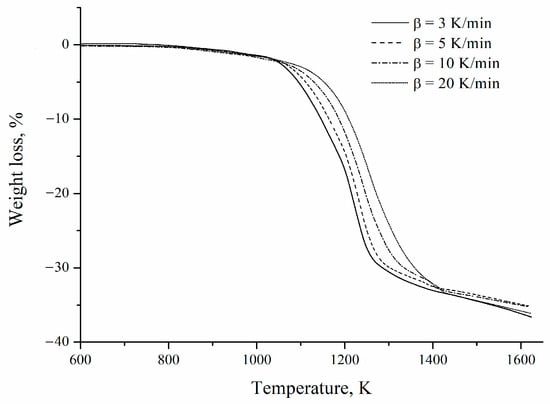
Figure 7.
Thermogravimetric curves of the barite concentrate/coking coal mixture obtained at different heating rates.
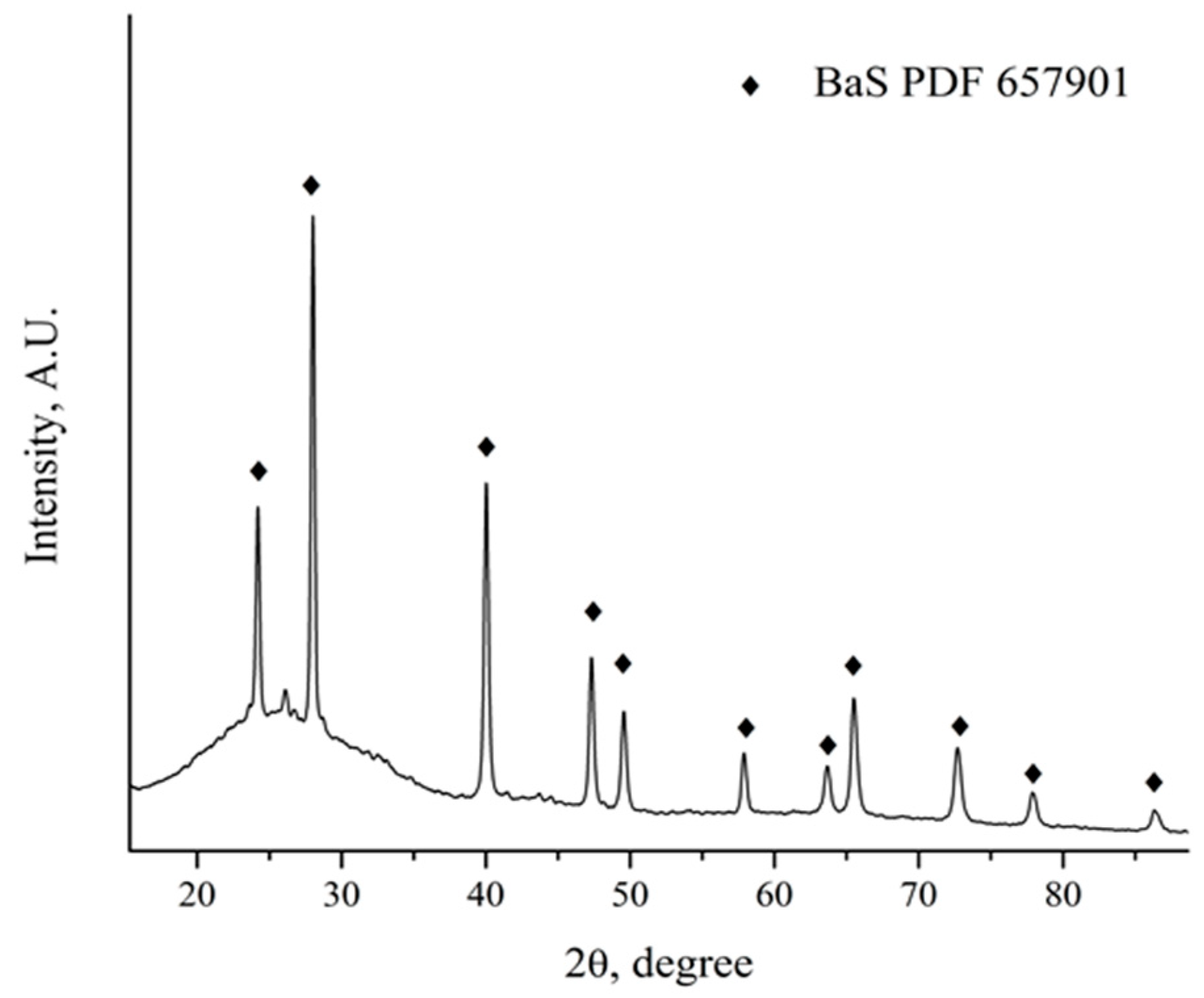
The mass losses obtained from the thermogravimetric results suggest a total transformation of barite to BaS. Heat-treated powders were analyzed by X-ray diffraction to confirm these results (Figure 8). The results obtained indicated that the only crystalline species present after the carbothermic reduction process was BaS. Additionally, the remaining carbon presence was detected, as visualized by a diffraction halo between 24 and 40 degrees 2θ. At this stage, further studies are recommended to evaluate the reducing of the use of metallurgical coke, with the aim of optimizing the carbothermic reduction process of barite.
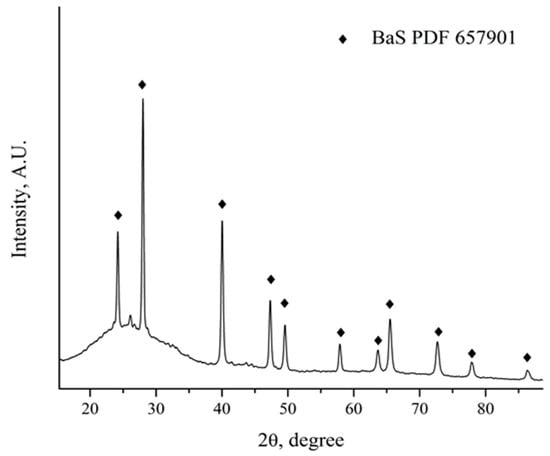
Figure 8.
XRD pattern of the mechanically active coal–barite mixture after the carbothermic reduction process.
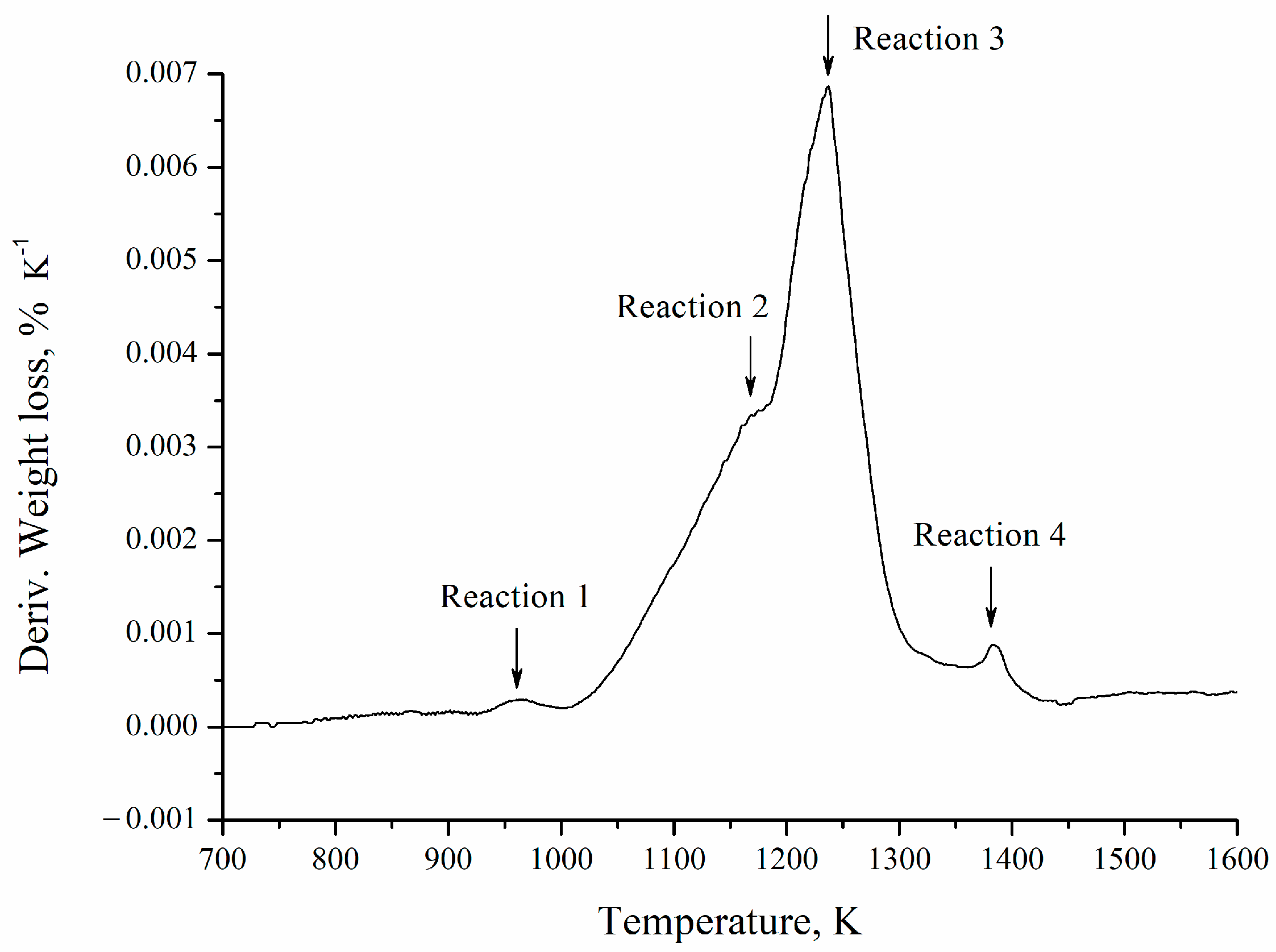
In the derivative of the thermogravimetric curve (Figure 9), four reactions are observed. This is related to the complexity of the barite carbothermic reduction process, which includes the solid-state reaction between BaSO4 and carbon, carbon oxidation, and barite reduction by CO (g), among other processes [14]. These reactions are competitive in nature since the preponderance (area under the curve) of each of them is affected by the heating rate, which hinders the global process analysis, making it impossible to establish a single model to explain the kinetics of the reaction [21]. It is worth noting that the individual identification and characterization of each of these reactions lies beyond the scope of the present study, given the complexity and concurrent nature of the mechanisms involved. Nevertheless, this aspect could be addressed in future research aimed at advancing the mechanistic understanding of the carbothermic reduction of barite.
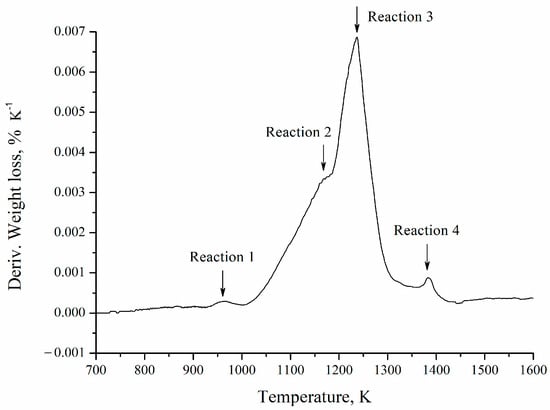
Figure 9.
Derivative of the thermogravimetric curve obtained from the test with a heating rate of 5 K min−1.
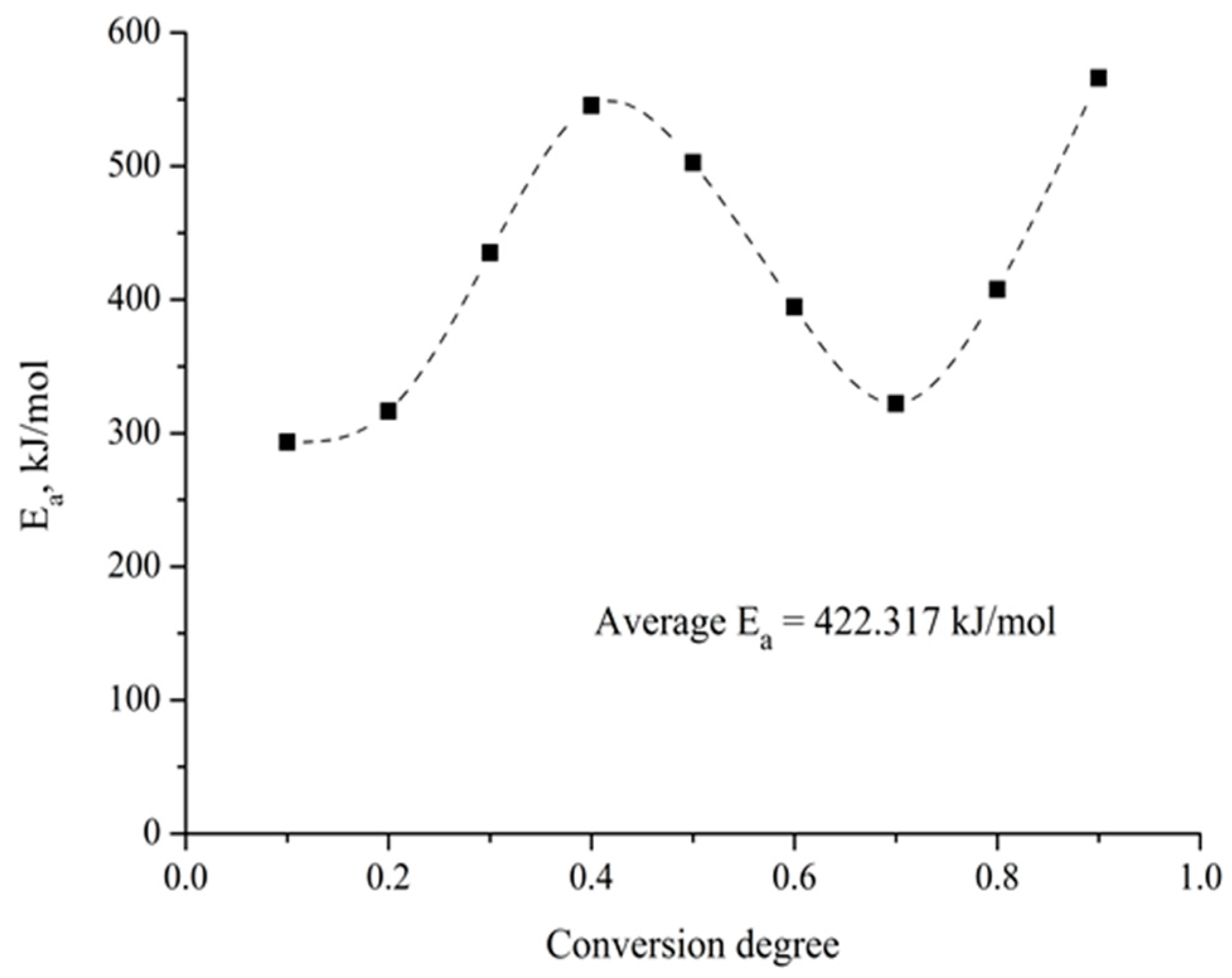
To determine the dependence of the activation energy on the degree of advancement of the carbothermic reduction process, and to corroborate the observations made in the previous paragraph, the thermogravimetric results were analyzed using the isoconversional method proposed by Friedman [22], which allows for the calculation of the activation energy without having prior knowledge of the kinetic model followed by the process. The results obtained are presented in Figure 10. As expected, it is observed that the activation energy varies during the carbothermic reduction process between 300 and 500 kJ/mol, which is evidence of the complexity of the process under study.
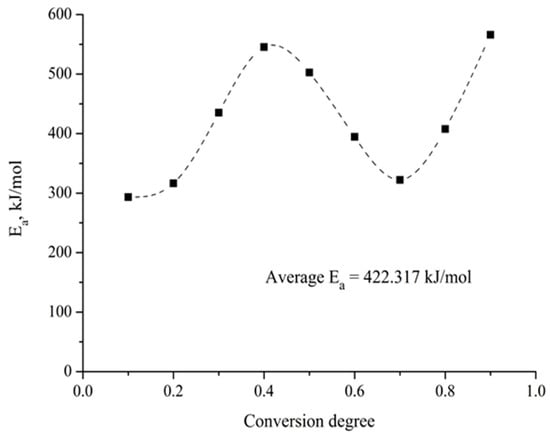
Figure 10.
Variation in the activation energy with the conversion degree in the barite carbothermic reduction.
Numerous studies have addressed the kinetic analysis of the barite carbothermic reduction process [1,7,10,12]. However, there is no agreement between the reported activation energy values, which fluctuate between 28 and 519 kJ/mol. This difference can be understood by considering that hitherto all the analyses carried out have proposed that the kinetics of the barite carbothermic reduction process is controlled by a single limiting step, which is not correct in light of the results presented in Figure 10.
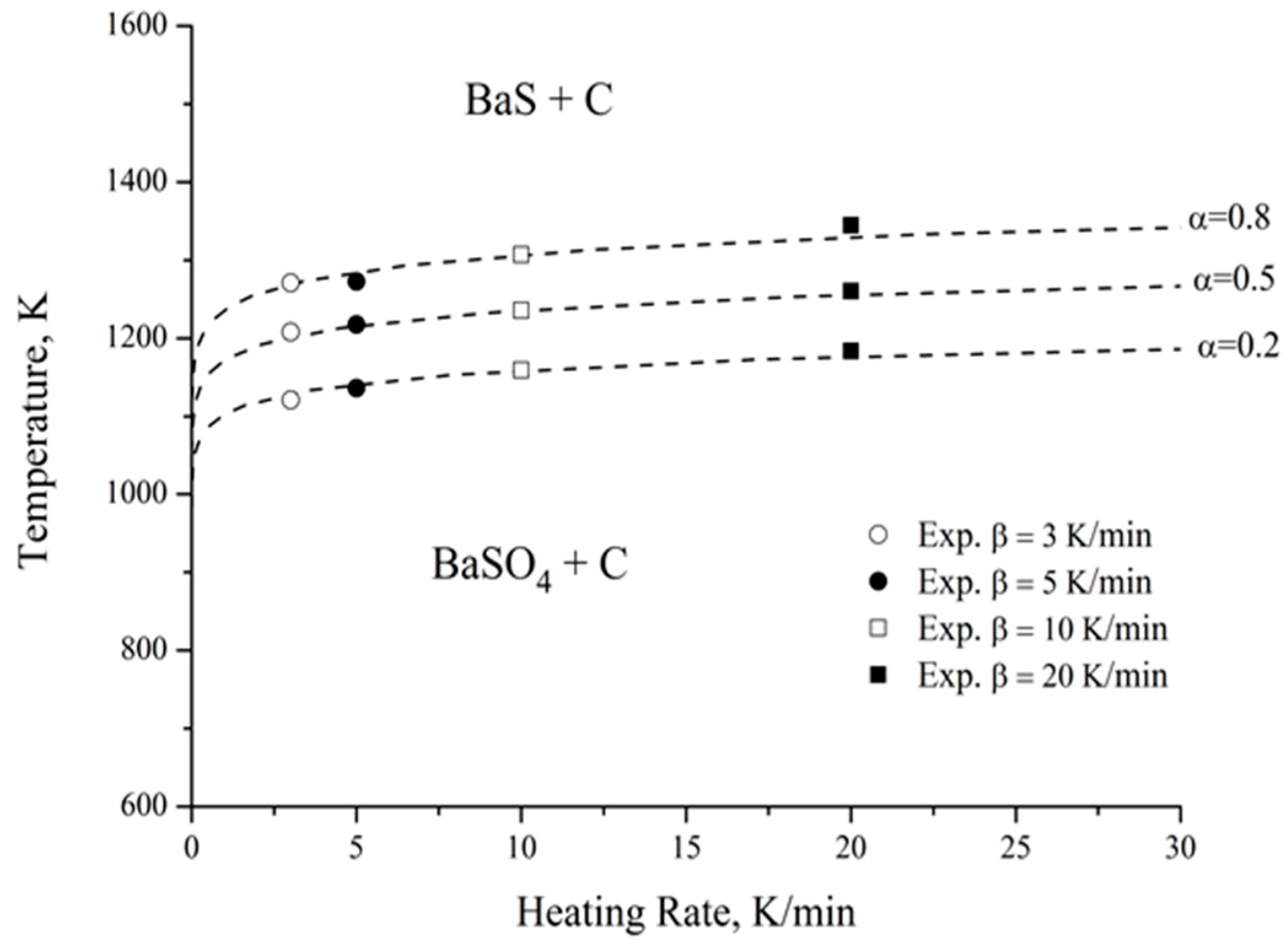
A temperature–heating rate–transformation (T-HR-T) diagram was drawn up to simulate the carbothermic reduction of barite, using the methodology proposed by Suñol et al. [13], considering the complexity of the process under study, which involves several simultaneous reactions. This methodology allows for modeling of a process without the need to know the kinetic equation that governs it. Figure 11 shows the results obtained for degrees of advancement (α) of 0.2, 0.5, and 0.8. Additionally, the experimental values for the heating rates of 3, 5, 10, and 20 K min−1 are presented. There is a very good fit between the experimental data and the proposed model, with an average error of less than 0.7%, thus reinforcing the strategy chosen to simulate the carbothermic reduction process. Nevertheless, future studies should address its applicability at an industrial scale in order to validate the methodology under real operating conditions.
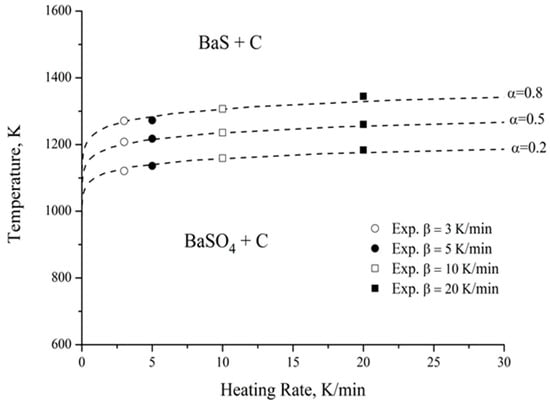
Figure 11.
T-HR-T diagram for the carbothermic reduction of barite.
4. Conclusions
Based on the results obtained from the shaking table tests, it can be concluded that it is possible to produce concentrates with a BaSO4 grade of 97.7% and a recovery of 51.4% from low-grade barite ores (<61% BaSO4) from the Atacama region, Chile. The correct adjustment of the splitters determines the grade and recovery of barite attained in the concentrate. When the position of the splitters was changed from position 1 to 2, and the wash-water flow rate, stroke length, and stroke frequency were fixed at 7 L/min, 14 mm, and 292 strokes/min, respectively, the barite grade increased from 91.8% to 97.7% and the recovery decreased from 54.8% to 51.4%.
Regarding the mechanical activation effect on the carbothermic reduction of barite concentrate, the results indicated that it is possible to reduce the initial reaction temperature from 1100 to 990 K when the barite and the metallurgical coke are milled for 1 h in a conventional ball mill. The improvement in reaction kinetics can be attributed mainly to the intimate contact between the fine barite and coal particles promoted by the milling process and to the energy stored by the powders due to particle size reduction. The thermogravimetric curves and XRD results showed a total transformation of barite to BaS during the reduction process.
A variation in the activation energy between 300 and 500 kJ/mol was observed during the carbothermic reduction process. These results suggest that barite reduction with metallurgical coke is a complex process, which cannot be described by considering a single reaction. A temperature–heating rate–transformation (T-HR-T) diagram was drawn up based on the process complexity to simulate the carbothermic reduction of barite. There was good agreement between the experimental data and the proposed model, with an average error of less than 0.7%.
Finally, the obtained results showed that it is possible to produce barium sulfide from low-grade barite ores by the combination of gravimetric concentration, mechanical activation, and carbothermic reduction. This new processing route could open the possibility of valorizing low-grade barite mineral resources that are not being utilized currently.
Author Contributions
Conceptualization, D.G. and M.S.; methodology, D.G., M.S., and L.V.; validation, D.G., M.S., and L.P.-M.; formal analysis, M.S., D.G., and M.N.; investigation, M.N.; resources, D.G.; data curation, M.S.; writing—original draft preparation, M.S. and D.G.; writing—review and editing, E.C.; visualization, M.N.; supervision, D.G.; project administration, D.G.; funding acquisition, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDEF [Project No. D13R20004].
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the Metallurgy Department of the Universidad de Atacama for the XRD, SEM, and Tg analyses [Projects EQM 130125, EQUV 003, EQM 210139, and EQUR 16002] and Marisela Navea thanks the Universidad de Atacama for the postgraduate scholarship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Penaloza, I.; Tita, A.; McNew, E.; Chu, P. Barite resources, production and recovery using flotation: A review. Miner. Eng. 2023, 203, 108327. [Google Scholar] [CrossRef]
- Guzmán, D.; Fernández, J.; Ordonez, S.; Aguilar, C.; Rojas, P.A.; Serafini, D. Effect of mechanical activation on the barite carbothermic reduction. Inter. J. Miner. Process. 2012, 102–103, 124–129. [Google Scholar] [CrossRef]
- Otoijamun, I.; Kigozi, M.; Adetunji, A.R.; Onwualu, P.A. Characterization and Suitability of Nigerian Barites for Different Industrial Applications. Minerals 2021, 11, 360. [Google Scholar] [CrossRef]
- Servicio Nacional de Geología y Minería (SERNAGEOMIN). Chilean Mining Yearbook 2023; SERNAGEOMIN: Santiago, Chile, 2023. [Google Scholar]
- Navea, M. Study of the Concentration and Effect of Mechanical Activation on the Carbothermic Reduction Process of Barite Ores. Master’s Thesis, University of Atacama, Copiapó, Atacama, Chile, 2018. [Google Scholar]
- Singh, R.; Banerjee, B.; Bhattacharyya, K.; Srivastava, J.P. Upgrading of barite waste to marketable grade concentrate. In Proceedings of the XXIII International Mineral Processing Congress, Istanbul, Turkey, 3–8 September 2006. [Google Scholar]
- Bhatia, M.A.; Kazmia, K.; Mehmooda, R.; Ahdb, A.; Tabassum, A.; Akrama, A. Beneficiation study on barite ore of Duddar Area, District Lasbela, Balochistan Province, Pakistan. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2017, 60, 9–22. [Google Scholar] [CrossRef]
- Mgbemere, H.E.; Obidiegwu, E.O.; Obareki, E. Beneficiation of Azara barite ore using a combination of jigging, froth flotation and leaching. Niger. J. Technol. 2018, 37, 957–962. [Google Scholar] [CrossRef]
- McKetta, J. Encyclopedia of Chemical Processing and Design; Marcel Dekker: New York, NY, USA, 1977. [Google Scholar]
- Jamshidi, S.; Salem, A. Role of extrusion process on kinetic of carbothermal reduction of barite. Thermochim. Acta 2010, 503–504, 108–114. [Google Scholar] [CrossRef]
- Jagtap, S.; Pande, A.; Gokarn, A. Effect of catalysts on the kinetics of reduction of barite by carbon. Ind. Eng. Chem. Res. 1990, 29, 795–799. [Google Scholar] [CrossRef]
- Gokarn, A.; Pradhan, S.; Pathak, G.; Kulkarni, S. Vanadium-catalyzed gasification of carbon and its application in the carbothermic reduction of barite. Fuel 2000, 79, 821–827. [Google Scholar] [CrossRef]
- Salem, A.; Tavakkoli-Osgouei, Y.; Jamshidi, S. Kinetic study of barite carbothermic reduction in presence of sodium carbonate as catalyst. Iran J. Chem. Eng. 2010, 7, 58–67. [Google Scholar]
- Murthy, J.S.N.; Reddy, P.V.V. Solid-state reaction between barium sulfate and carbon. Chem. Eng. Commun. 2012, 199, 966–990. [Google Scholar] [CrossRef]
- Bafghi, M.S.; Yarahmadi, A.; Ahmadi, A.; Mehrjoo, H. Effect of the type of carbon material on the reduction kinetics of barium sulfate. Iran J. Mater. Sci. Eng. 2011, 8, 1–7. [Google Scholar]
- Suñol, J.J.; Clavaguera, N.; Mora, M.T. Thermal Stability Study of Fe-Ni-Based Alloys Determination of T-HR-T and T-T-T diagrams. J. Therm. Anal. Calorim. 1988, 52, 853–862. [Google Scholar] [CrossRef]
- Escalante, P.; Oliva, J.; Anticoi, H.; Sampaio, C.; Mohanty, K. Characterization of mineralogical impurities in a carbonate-rich material using MLA. Miner. Eng. 2025, 230, 109409. [Google Scholar] [CrossRef]
- Jordão, H.; Sousa, A.J.; Carvalho, M.T. Optimization of wet shaking table process using response surface methodology applied to the separation of copper and aluminum from the fine fraction of shredder ELVs. Waste Manag. 2016, 48, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.K.; Ramamurthy, Y.; Singh, V. Recovery of chromite values from plant tailing by gravity concentration. J. Miner. Mater. Charact. Eng. 2011, 10, 13–25. [Google Scholar] [CrossRef]
- Gupta, A.; Yan, D.S. Mineral Processing Design and Operation: An Introduction; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Flynn, J.H. The effect of heating rate upon the coupling of complex reactions. I. Independent and competitive reactions. Thermochim. Acta 1980, 37, 225–238. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetic of thermal degradation of char-forming plastics from thermogravometry. Application to a phenolic plastic. J. Polym. Sci. A Polym. Chem. 1964, 6, 183–195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).