Strategizing Carbon-Neutral Mines: A Case for Pilot Projects
Abstract
:1. Introduction
2. Mine Sites: Untapped Potential to Sequester CO2
2.1. Comparative Studies of Ultramafic Mines

| Mine Site | Clinton Creek | Diavik | Mount Keith |
| Location | Yukon, Canada | Northwest Territories, Canada | Western Australia |
| Commodity | asbestos (abandoned) | diamonds (active) | nickel (active) |
| Environmental data [13,36,41] | |||
| Climate | subarctic | subarctic | desert |
| Mine site GHG emissions (kt CO2e/year) | n/a | 219 | 382 |
| Onsite power generation (CO2 point source) | n/a | yes | yes |
| Water usage (ML) | n/a | 376 directed to TSF | 9,534 in total |
| 72 treated sewage to TSF | 1,651 high quality | ||
| 12,491 discharged to Lac de Gras | 186 treated sewage effluent | ||
| Tailings production | ~11 Mt in total | ~2 Mt/year | ~11 Mt/year |
| Process water [Mg + Ca] (mg/L) | ~160 (pore water) | ~40 | ~3,500 |
| Mineralogical data [15,20] | |||
| Major minerals | chrysotile (~88 wt %) | lizardite (48 wt %), forsterite (25 wt %) | antigorite/lizardite (~81 wt %) |
| Minor minerals | dolomite, magnesite, quartz, magnetite, pyroaurite | vermiculite, phlogopite, calcite, muscovite, plagioclase, quartz, diopside, almandine-pyrope | iowaite, magnesite, woodallite, magnetite, chromite, dolomite, chrysotile |
| Highly reactive phases for carbon mineralization | trace brucite | n/a | brucite (~2.5 wt %) |
| MgO (%) | ~37% | ~33% | ~40% |
| Secondary Mg-carbonates from passive carbonation | nesquehonite, dypingite, hydromagnesite, lansfordite | nesquehonite | hydromagnesite |
| Carbon mineralization [11,17,18,19] | |||
| Passive carbonation rate (g CO2/m2/year) | ~6,200 | 374–418 | 2,400 |
| GHG emission offset from passive carbonation | ~82 kt CO2 total (1978 to 2004) | ~0.2% of total GHG emissions | ~11% of total GHG emissions |
| Potential GHG emissions offset based on full carbonation (kt CO2) to hydromagnesite | ~3,700 kt CO2 in total from chrysotile | ~670 kt/year from lizardite; ~230 kt/year from forsterite | 3,400 kt/year from antigorite/lizardite; 166 kt/year from brucite |
2.2. Passive Carbonation at Mines: Rates and Limitations

2.3. Evidence for Microbial Activity at Mine Sites
3. Strategies for Accelerating Carbon Mineralization

| Strategy | Rate-limitation targeted | Processes | Example technology | Key considerations |
|---|---|---|---|---|
| Bioleaching |
|
|
|
|
| Enhanced passive carbonation |
|
|
|
|
| CO2 injection |
|
|
|
|
| Oxidation of waste organics |
|
|
|
|
| Bioreactors |
|
|
|
|

3.1. Bioleaching of Ultramafic Mine Tailings

3.2. Increasing the Supply of CO2
3.2.1. Enhanced Passive Carbonation
3.2.2. CO2 Injection
3.2.3. Oxidation of Waste Organics
3.3. Bioreactors for Carbon Mineralization

4. A Case for Pilot Projects
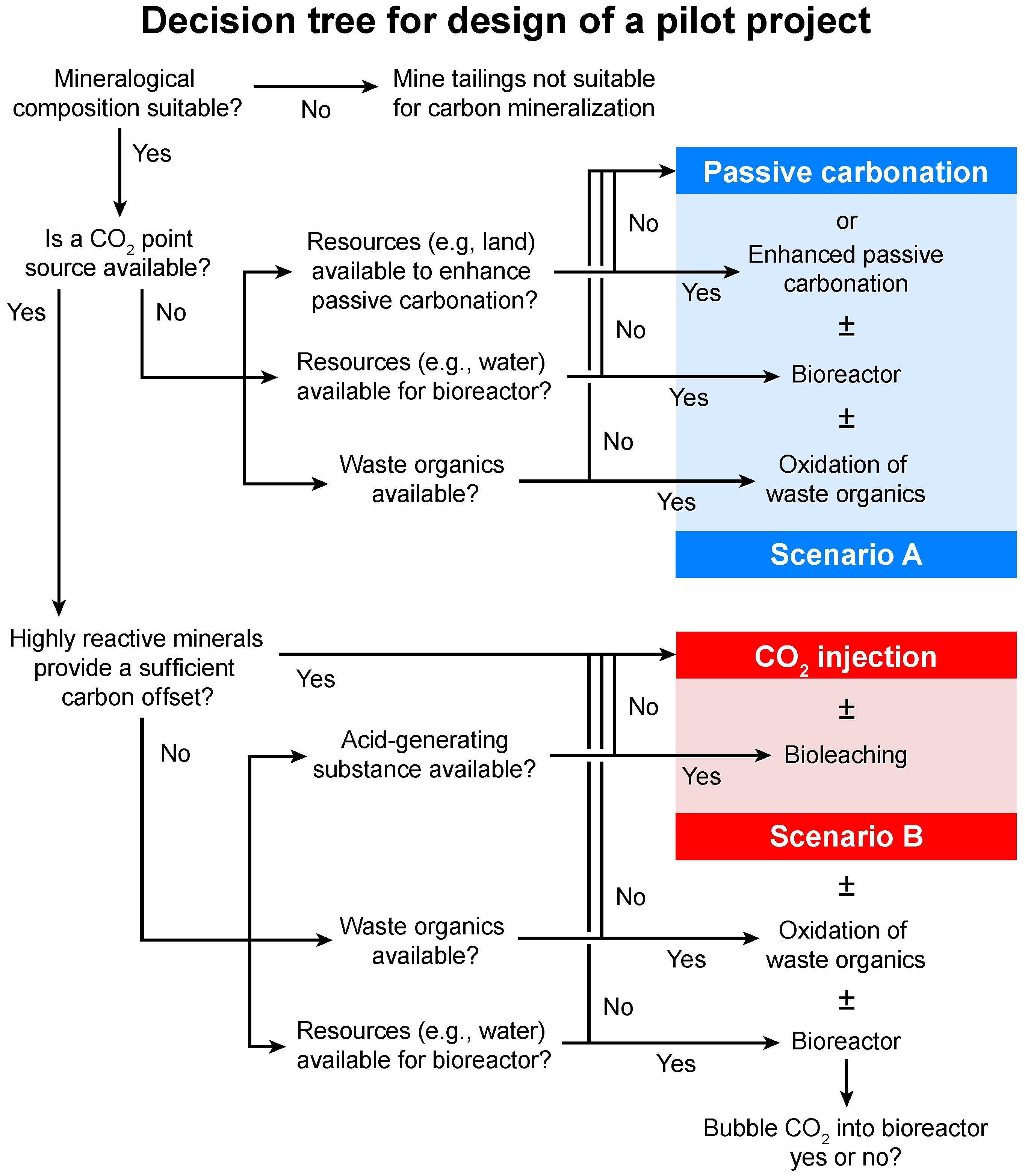
4.1. Scenario A
4.2. Scenario B
4.3. Monitoring and Verification
5. Estimating Operational Costs

6. Carbon Price
7. Valuation Model for Development of Carbon Mineralization in Mine Waste
| Investment Opportunity | Finite Life Resource | Net Present Value | Real Options Valuation | |
|---|---|---|---|---|
| Present value of the project’s underlying assets | Price of carbon (S) | Price of carbon (S) | Price of Carbon (S0) | |
| Expenditure required to acquire the project assets | Cost to sequester carbon (X) | Cost to sequester carbon (X) | Cost of sequestering carbon (X) | |
| Time value of money | Cost of capital (r) | Real risk free rate (rf) | ||
| Riskiness of the project assets | Incorporated into r | Volatility of carbon price (σ2) | ||
| Length of time the decision may be deferred | Time to get project to maturity (t) | |||
| Decision Making Criteria: Invest if Value > 0 | Value = S − X |  | Value is solution to the following partial differential equation:  | |
8. Challenges and Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gerdemann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Penner, L.R.; Rush, H. Ex situ aqueous mineral carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L., Jr.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Fagerlund, J.; Songok, J.K. CO2 mineral sequestration: Developments toward large-scale application. Greenh. Gas Sci. Technol. 2011, 1, 48–57. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). CO2 Capture and Storage: A Key Carbon Abatement Option; IEA: Paris, France, 2008. [Google Scholar]
- Pellegrino, C.; Lodhia, S. Climate change accounting and the Australian mining industry: Exploring the links between corporate disclosure and the generation of legitimacy. J. Clean Prod. 2012, 36, 68–82. [Google Scholar] [CrossRef]
- Köhler, P.; Hartmann, J.; Wolf-Gladrow, D.A. Geoengineering potential of artificially enhanced silicate weathering of olivine. Proc. Natl. Acad. Sci. USA 2010, 107, 20228–20233. [Google Scholar] [CrossRef] [Green Version]
- Schuiling, R.D.; Krijgsman, P. Enhanced weathering: An effective and cheap tool to sequester CO2. Clim. Chang. 2006, 74, 349–354. [Google Scholar] [CrossRef]
- Washbourne, C.L.; Renforth, P.; Manning, D.A.C. Investigating carbonate formation in urban soils as a method for capture and storage of atmospheric carbon. Sci. Total Environ. 2012, 431, 166–175. [Google Scholar] [CrossRef]
- Renforth, P. The potential of enhanced weathering in the UK. Int. J. Greenh. Gas Control 2012, 10, 229–243. [Google Scholar] [CrossRef]
- Renforth, P.; Manning, D.A.C. Laboratory carbonation of artificial silicate gels enhanced by citrate: Implications for engineered pedogenic carbonate formation. Int. J. Greenh. Gas Control 2011, 5, 1578–1586. [Google Scholar] [CrossRef]
- Bea, S.A.; Wilson, S.A.; Mayer, K.U.; Dipple, G.M.; Power, I.M.; Gamazo, P. Reactive transport modeling of natural carbon sequestration in ultramafic mine tailings. Vadose Zone J. 2012, 11. [Google Scholar] [CrossRef]
- Mills, S.J.; Wilson, S.A.; Dipple, G.M.; Raudsepp, M. The decomposition of konyaite: importance in CO2 fixation in mine tailings. Mineral. Mag. 2010, 74, 903–917. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Southam, G. Bioleaching of ultramafic tailings by Acidithiobacillus spp. for CO2 Sequestration. Environ. Sci. Technol. 2010, 44, 456–462. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Dipple, G.M. Serpentinite carbonation for CO2 sequestration. Elements 2013, 9, 115–121. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Small, D.P.; Dipple, G.M.; Wan, W.K.; Southam, G. Microbially mediated mineral carbonation: Roles of phototrophy and heterotrophy. Environ. Sci. Technol. 2011, 45, 9061–9068. [Google Scholar] [CrossRef]
- Wilson, S.A.; Barker, S.L.L.; Dipple, G.M.; Atudorei, V. Isotopic disequilibrium during uptake of atmospheric CO2 into mine process waters: Implications for CO2 sequestration. Environ. Sci. Technol. 2010, 44, 9522–9529. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Barker, S.L.L.; Fallon, S.J.; Southam, G. Subarctic weathering of mineral wastes provides a sink for atmospheric CO2. Environ. Sci. Technol. 2011, 45, 7727–7736. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Gabites, J.E.; Southam, G. Carbon dioxide fixation within mine wastes of ultramafic-hosted ore deposits: Examples from the Clinton Creek and Cassiar chrysotile deposits, Canada. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Wilson, S.A.; Harrison, A.L.; Dipple, G.M.; Power, I.M.; Barker, S.L.L.; Mayer, K.U.; Fallon, S.J.; Raudsepp, M.; Southam, G. Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. Int. J. Greenh. Gas Control 2014, in press. [Google Scholar]
- Wilson, S.A.; Raudsepp, M.; Dipple, G.M. Verifying and quantifying carbon fixation in minerals from serpentine-rich mine tailings using the Rietveld method with X-ray powder diffraction data. Am. Miner. 2006, 91, 1331–1341. [Google Scholar] [CrossRef]
- Wilson, S.A.; Raudsepp, M.; Dipple, G.M. Quantifying carbon fixation in trace minerals from processed kimberlite: A comparative study of quantitative methods using X-ray powder diffraction data with applications to the Diavik Diamond Mine, Northwest Territories, Canada. Appl. Geochem. 2009, 24, 2312–2331. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. CO2 Sequestration in chrysotile mining residues-implication of watering and passivation under environmental conditions. Ind. Eng. Chem. Res. 2012, 51, 8726–8734. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Beaudoin, G.; Molson, J. Dynamics of carbon dioxide uptake in chrysotile mining residues—Effect of mineralogy and liquid saturation. Int. J. Greenh. Gas Control 2013, 12, 124–135. [Google Scholar] [CrossRef]
- Pronost, J.; Beaudoin, G.; Lemieux, J.M.; Hebert, R.; Constantin, M.; Marcouiller, S.; Klein, M.; Duchesne, J.; Molson, J.W.; Larachi, F.; Maldague, X. CO2-depleted warm air venting from chrysotile milling waste (Thetford Mines, Canada): Evidence for in-situ carbon capture from the atmosphere. Geology 2012, 40, 275–278. [Google Scholar] [CrossRef]
- Pronost, J.; Beaudoin, G.; Tremblay, J.; Larachi, F.; Duchesne, J.; Hebert, R.; Constantin, M. Carbon sequestration kinetic and storage capacity of ultramafic mining waste. Environ. Sci. Technol. 2011, 45, 9413–9420. [Google Scholar] [CrossRef]
- Beinlich, A.; Austrheim, H. In situ sequestration of atmospheric CO2 at low temperature and surface cracking of serpentinized peridotite in mine shafts. Chem. Geol. 2012, 332, 32–44. [Google Scholar] [CrossRef]
- Goff, F.; Lackner, K.S. Carbon dioxide sequestering using ultramafic rocks. Environ. Geosci. 1998, 5, 89–101. [Google Scholar] [CrossRef]
- Harrison, A.L.; Power, I.M.; Dipple, G.M. Accelerated carbonation of brucite in mine tailings for carbon sequestration. Environ. Sci. Technol. 2013, 47, 126–134. [Google Scholar] [CrossRef]
- Assima, G.P.; Larachi, F.; Molson, J.; Beaudoin, G. Impact of temperature and oxygen availability on the dynamics of ambient CO2 mineral sequestration by nickel mining residues. Chem. Eng. J. 2014, 240, 394–403. [Google Scholar] [CrossRef]
- Levitan, D.M.; Hammarstrom, J.M.; Gunter, M.E.; Seal, R.R.; Chou, I.M.; Piatak, N.M. Mineralogy of mine waste at the Vermont Asbestos Group mine, Belvidere Mountain, Vermont. Am. Miner. 2009, 94, 1063–1066. [Google Scholar] [CrossRef]
- Price, W.A. Challenges Posed by Metal Leaching and Acid Rock Drainage, and Approaches to Addressing Them. In Environmental Aspects of Mine Wastes; Jambor, J.L., Blowes, D.W., Ritchie, A.I.M., Eds.; Mineralogical Association of Canada: Quebec City, QC, Canada, 2003; pp. 1–10. [Google Scholar]
- Salek, S.S.; Kleerebezem, R.; Jonkers, H.M.; Witkamp, G.J.; van Loosdrecht, M.C.M. Mineral CO2 sequestration by environmental biotechnological processes. Trends Biotechnol. 2013, 31, 139–146. [Google Scholar] [CrossRef]
- McCutcheon, J.; Power, I.M.; Harrison, A.; Small, D.; Pirani, A.; Dipple, G.; Southam, G. Microbially-Accelerated Carbon Sequestration: Magnesium Carbonate Mineralization in Mine Waste. In Proceedings of 22nd V.M. Goldschmidt Conference, Montréal, QC, Canada, 24–29 June 2012.
- U.S. Environmental Protection Agency (USEPA). Quantifying Greenhouse Gas Emissions from Key Industrial Sectors in the United States; USEPA: Washington, DC, USA, 2008. Available online: http://www.epa.gov/sectors/pdf/greenhouse-report.pdf (acessed on 1 July 2013).
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon Mineralization: From Natural Analogues to Engineered Systems. In Geochemistry of Geologic CO2 Sequestration; DePaolo, D.J., Cole, D.R., Navrotsky, A., Bourg, I.C., Eds.; The Mineralogical Society of America: Chantilly, VA, USA, 2013; Volume 77, pp. 305–360. [Google Scholar]
- WMC Resources Ltd. Annual Sustainability Report 2004; WMC Resources Ltd.: Melbourne, Australia, 2005. [Google Scholar]
- Power, I.M.; Wilson, S.A.; Dipple, G.M.; Southam, G. Modern carbonate microbialites from an asbestos open pit pond, Yukon, Canada. Geobiology 2011, 9, 180–195. [Google Scholar]
- Thom, J.M.; Dipple, G.M.; Power, I.M.; Harrison, A.L. Chrysotile dissolution rates: Implications for carbon sequestration. Appl. Geochem. 2013, 35, 244–254. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon sequestration via carbonic anhydrase facilitated magnesium carbonate precipitation. Int. J. Greenh. Gas Control 2013, 16, 145–155. [Google Scholar] [CrossRef]
- Harrison, A.L.; Power, I.M.; Dipple, G.M. Strategies for Enhancing Carbon Sequestration in Mg-Rich Mine Tailings. In Proceedings of International Mine Water Association 2013 Annual Conference, Denver, CO, USA, 6–9 August 2013; Brown, A., Figueroa, L., Wolkersdorfer, C., Eds.; pp. 593–599.
- Diavik Diamond Mine. 2011 Sustainable Development Report; Diavik Diamond Mine: Yellowknife, NT, Canada, 2011. [Google Scholar]
- Oskierski, H.C.; Dlugogorski, B.Z.; Jacobsen, G. Sequestration of atmospheric CO2 in chrysotile mine tailings of the Woodsreef Asbestos Mine, Australia: Quantitative mineralogy, isotopic fingerprinting and carbonation rates. Chem. Geol. 2013, 358, 156–169. [Google Scholar] [CrossRef]
- Hostetler, P.B.; Coleman, R.G.; Mumpton, F.A.; Evan, B.W. Brucite in alpine serpentinites. Am. Miner. 1966, 51, 75–98. [Google Scholar]
- O’Neil, J.R.; Barnes, I. C13 and O18 compositions in some fresh-water carbonates associated with ultramafic rocks and serpentinites: Western United States. Geochim. Cosmochim. Acta 1971, 35, 687–697. [Google Scholar] [CrossRef]
- Königsberger, E.; Königsberger, L.; Gamsjager, H. Low-temperature thermodynamic model for the system Na2CO3-MgCO3-CaCO3-H2O. Geochim. Cosmochim. Acta 1999, 63, 3105–3119. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mg-carbonate system—Effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Schuiling, R.D.; Wilson, S.A.; Power, I.M. Enhanced silicate weathering is not limited by silicic acid saturation. Proc. Natl. Acad. Sci. USA 2011, 108, E41–E41. [Google Scholar] [CrossRef]
- Huh, Y.S. Chemical weathering and climate—A global experiment: A review. Geosci. J. 2003, 7, 277–288. [Google Scholar] [CrossRef]
- Amiotte Suchet, P.; Probst, J.L.; Ludwig, W. Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Torsvik, V.; Sørheim, R.; Goksøyr, J. Total bacterial diversity in soil and sediment communities—A review. J. Indust. Microbiol. 1996, 17, 170–178. [Google Scholar]
- Power, I.M.; Wilson, S.A.; Thom, J.M.; Dipple, G.M.; Southam, G. Biologically induced mineralization of dypingite by cyanobacteria from an alkaline wetland near Atlin, British Columbia, Canada. Geochem. Trans. 2007, 8. [Google Scholar] [CrossRef]
- Bales, R.C.; Morgan, J.J. Dissolution kinetics of chrysotile at pH 7 to 10. Geochim. Cosmochim. Acta 1985, 49, 2281–2288. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Kinetics and mechanism of forsterite dissolution at 25 °C and pH from 1 to 12. Geochim. Cosmochim. Acta 2000, 64, 3313–3325. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J. Experimental study of brucite dissolution and precipitation in aqueous solutions: Surface speciation and chemical affinity control. Geochim. Cosmochim. Acta 2004, 68, 31–45. [Google Scholar] [CrossRef]
- Daval, D.; Hellmann, R.; Martinez, I.; S., G.; Guyot, F. Lizardite serpentine dissolution kinetics as a function of pH and temperature, including effects of elevated pCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Yao, M.J.; Lian, B.; Teng, H.H.; Tian, Y.C.; Yang, X.Q. Serpentine dissolution in the presence of bacteria Bacillus mucilaginosus. Geomicrobiol. J. 2013, 30, 72–80. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Santos, R.M.; Monballiu, A.; Ghyselbrecht, K.; Martens, J.A.; Mattos, M.L.T.; Van Gerven, T.; Meesschaert, B. Effects of bioleaching on the chemical, mineralogical and morphological properties of natural and waste-derived alkaline materials. Miner. Eng. 2013, 48, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Burford, E.P.; Fomina, M.; Gadd, G.M. Fungal involvement in bioweathering and biotransformation of rocks and minerals. Mineral. Mag. 2003, 67, 1127–1155. [Google Scholar] [CrossRef]
- Padilla, G.A.; Cisternas, L.A.; Cueto, J.Y. On the optimization of heap leaching. Miner. Eng. 2008, 21, 673–678. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Johnson, D.B.; Grail, B.M.; Hallberg, K.B. A new direction for biomining: Extraction of metals by reductive dissolution of oxidized ores. Minerals 2013, 3, 49–58. [Google Scholar] [CrossRef]
- Fortin, D.; Davis, B.; Beveridge, T.J. Role of Thiobacillus and sulfate-reducing bacteria in iron biocycling in oxic and acidic mine tailings. FEMS Microbiol. Ecol. 1996, 21, 11–24. [Google Scholar] [CrossRef]
- Mielke, R.E.; Pace, D.L.; Porter, T.; Southam, G. A critical stage in the formation of acid mine drainage: Colonization of pyrite by Acidithiobacillus ferrooxidans under pH-neutral conditions. Geobiology 2003, 1, 81–90. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Southam, G. Geomicrobiology of sulfide mineral oxidation. In Geomicrobiology: Interactions between Microbes and Minerals; Banfield, J.F., Nealson, K.H., Eds.; The Mineralogical Society of America: Chantilly, VA, USA, 1997; Volume 35, pp. 361–390. [Google Scholar]
- Gould, W.D.; Kapoor, A. Chapter 10. The Microbiology of Acid Mine Drainage. In Environmental Aspects of Mine Wastes; Jambor, J.L., Blowes, D.W., Ritchie, A.I.M., Eds.; Mineralogical Association of Canada: Quebec City, QC, Canada, 2003; Volume 31, pp. 203–226. [Google Scholar]
- Edwards, K.J.; Goebel, B.M.; Rodgers, T.M.; Schrenk, M.O.; Gihring, T.M.; Cardona, M.M.; Hu, B.; McGuire, M.M.; Hamers, R.J.; Pace, N.R.; Banfield, J.F. Geomicrobiology of pyrite (FeS2) dissolution: Case study at Iron Mountain, California. Geomicrobiol. J. 1999, 16, 155–179. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic mine drainage: The rate determining step. Science 1970, 167, 1121–1123. [Google Scholar]
- Kirby, C.S.; Thomas, H.M.; Southam, G.; Donald, R. Relative contributions of abiotic and biological factors in Fe(II) oxidation in mine drainage. Appl. Geochem. 1999, 14, 511–530. [Google Scholar] [CrossRef]
- Cameron, R.A.; Lastra, R.; Mortazavi, S.; Bedard, P.L.; Morin, L.; Gould, W.D.; Kennedy, K.J. Bioleaching of a low-grade ultramafic nickel sulphide ore in stirred-tank reactors at elevated pH. Hydrometallurgy 2009, 97, 213–220. [Google Scholar] [CrossRef]
- Cameron, R.A.; Lastra, R.; Mortazavi, S.; Gould, W.D.; Thibault, Y.; Bedard, P.L.; Morin, L.; Kennedy, K.J. Elevated-pH bioleaching of a low-grade ultramafic nickel sulphide ore in stirred-tank reactors at 5 to 45 degrees C. Hydrometallurgy 2009, 99, 77–83. [Google Scholar] [CrossRef]
- Cameron, R.A.; Yeung, C.W.; Greer, C.W.; Gould, W.D.; Mortazavi, S.; Bedard, P.L.; Morin, L.; Lortie, L.; Dinardo, O.; Kennedy, K.J. The bacterial community structure during bioleaching of a low-grade nickel sulphide ore in stirred-tank reactors at different combinations of temperature and pH. Hydrometallurgy 2010, 104, 207–215. [Google Scholar]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review. Part II. Chloride and bio-technologies. Hydrometallurgy 2008, 91, 56–69. [Google Scholar] [CrossRef]
- Qin, W.Q.; Zhen, S.J.; Yan, Z.Q.; Campbell, M.; Wang, J.; Liu, K.; Zhang, Y.S. Heap bioleaching of a low-grade nickel-bearing sulfide ore containing high levels of magnesium as olivine, chlorite and antigorite. Hydrometallurgy 2009, 98, 58–65. [Google Scholar] [CrossRef]
- Zhen, S.J.; Yan, Z.Q.; Zhang, Y.S.; Wang, J.; Campbell, M.; Qin, W.Q. Column bioleaching of a low grade nickel-bearing sulfide ore containing high magnesium as olivine, chlorite and antigorite. Hydrometallurgy 2009, 96, 337–341. [Google Scholar] [CrossRef]
- Yang, C.R.; Qin, W.Q.; Lai, S.S.; Wang, J.; Zhang, Y.S.; Jiao, F.; Ren, L.Y.; Zhuang, T.A.; Chang, Z.Y. Bioleaching of a low grade nickel-copper-cobalt sulfide ore. Hydrometallurgy 2011, 106, 32–37. [Google Scholar] [CrossRef]
- Jambor, J.L. Mine-Waste Mineralogy and Mineralogical Perspectives of Acid-Base Acounting. In Environmental Aspects of Mine Wastes; Jambor, J.L., Blowes, D.W., Ritchie, A.I.M., Eds.; Mineralogical Association of Canada: Quebec City, QC, Canada, 2003; pp. 117–146. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry—Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Bass, D.H.; Hastings, N.A.; Brown, R.A. Performance of air sparging systems: A review of case studies. J. Hazard. Mater. 2000, 72, 101–119. [Google Scholar] [CrossRef]
- Kabelitz, N.; Machackova, J.; Imfeld, G.; Brennerova, M.; Pieper, D.H.; Heipieper, H.J.; Junca, H. Enhancement of the microbial community biomass and diversity during air sparging bioremediation of a soil highly contaminated with kerosene and BTEX. Appl. Microbiol. Biotechnol. 2009, 82, 565–577. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Schott, J.; Castillo, A. Kinetics of brucite dissolution at 25 degrees C in the presence of organic and inorganic ligands and divalent metals. Geochim. Cosmochim. Acta 2005, 69, 905–918. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.X.; Xu, Z.H.; Zeng, H.B. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Michael, K.; Allinson, G.; Golab, A.; Sharma, S.; Shulakova, V. CO2 Storage in Saline Aquifers II—Experience from Existing Storage Operations. In Greenhouse Gas Control Technologies 9; Gale, J., Herzog, H., Braitsch, J., Eds.; Elsevier Science BV: Amsterdam, The Netherlands, 2009; Volume 1, pp. 1973–1980. [Google Scholar]
- Bickle, M.; Chadwick, A.; Huppert, H.E.; Hallworth, M.; Lyle, S. Modelling carbon dioxide accumulation at Sleipner: Implications for underground carbon storage. Earth Planet. Sci. Lett. 2007, 255, 164–176. [Google Scholar] [CrossRef]
- Ragnheidardottir, E.; Sigurdardottir, H.; Kristjansdottir, H.; Harvey, W. Opportunities and challenges for CarbFix: An evaluation of capacities and costs for the pilot scale mineralization sequestration project at Hellisheidi, Iceland and beyond. Int. J. Greenh. Gas Control 2011, 5, 1065–1072. [Google Scholar]
- Aradottir, E.S.P.; Sigurdardottir, H.; Sigfusson, B.; Gunnlaugsson, E. CarbFix: A CCS pilot project imitating and accelerating natural CO2 sequestration. Greenh. Gases 2011, 1, 105–118. [Google Scholar] [CrossRef]
- Steefel, C.I.; Molins, S.; Trebotich, D. Pore Scale Processes Associated with Subsurface CO2 Injection and Sequestration. In Geochemistry of Geologic CO2 Sequestration; DePaolo, D.J., Cole, D.R., Navrotsky, A., Bourg, I.C., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 2013; Volume 77, pp. 259–303. [Google Scholar]
- Warthmann, R.; van Lith, Y.; Vasconcelos, C.; McKenzie, J.A.; Karpoff, A.M. Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 2000, 28, 1091–1094. [Google Scholar] [CrossRef]
- López-García, P.; Kazmierczak, J.; Benzerara, K.; Kempe, S.; Guyot, F.; Moreira, D. Bacterial diversity and carbonate precipitation in the giant microbialites from the highly alkaline Lake Van, Turkey. Extremophiles 2005, 9, 263–274. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Von Knorre, H.; Krumbein, W.E. Bacterial Calcification. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Springer: Berlin, Germany, 2000; pp. 25–31. [Google Scholar]
- Mitchell, A.C.; Dideriksen, K.; Spangler, L.H.; Cunningham, A.B.; Gerlach, R. Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ. Sci. Technol. 2010, 44, 5270–5276. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, M.B.J.; Blowes, D.W.; Condon, P.D.; Ptacek, C.J. Organic carbon amendments for passive in situ treatment of mine drainage: Field experiments. Appl. Geochem. 2011, 26, 1169–1183. [Google Scholar] [CrossRef]
- Lindsay, M.B.J.; Wakeman, K.D.; Rowe, O.F.; Grail, B.M.; Ptacek, C.J.; Blowes, D.W.; Johnson, D.B. Microbiology and geochemistry of mine tailings amended with organic carbon for passive treatment of pore water. Geomicrobiol. J. 2011, 28, 229–241. [Google Scholar] [CrossRef]
- Pepper, I.L.; Zerzghi, H.G.; Bengson, S.A.; Iker, B.C.; Banerjee, M.J.; Brooks, J.P. Bacterial populations within copper mine tailings: Long-term effects of amendment with Class A biosolids. J. Appl. Microbiol. 2012, 113, 569–577. [Google Scholar] [CrossRef]
- Madejon, E.; Doronila, A.I.; Madejon, P.; Baker, A.J.M.; Woodrow, I.E. Biosolids, mycorrhizal fungi and eucalypts for phytostabilization of arsenical sulphidic mine tailings. Agrofor. Syst. 2012, 84, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Gardner, W.C.; Naeth, M.A.; Broersma, K.; Chanasyk, D.S.; Jobson, A.M. Influence of biosolids and fertilizer amendments on element concentrations and revegetation of copper mine tailings. Can. J. Soil Sci. 2012, 92, 89–102. [Google Scholar] [CrossRef]
- Salek, S.S.; Kleerebezem, R.; Jonkers, H.M.; Voncken, J.H.L.; van Loosdrecht, M.C.M. Determining the impacts of fermentative bacteria on wollastonite dissolution kinetics. Appl. Microbiol. Biotechnol. 2013, 97, 2743–2752. [Google Scholar]
- Ferris, F.G.; Wiese, R.G.; Fyfe, W.S. Precipitation of carbonate minerals by microorganisms: Implications for silicate weathering and the global carbon dioxide budget. Geomicrobiol. J. 1994, 12, 1–13. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Roberts, J.A.; Bennett, P.C.; Gonzalez, L.A.; Macpherson, G.L.; Milliken, K.L. Microbial precipitation of dolomite in methanogenic groundwater. Geology 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Riding, R. Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic–Cambrian changes in atmospheric composition. Geobiology 2006, 4, 299–316. [Google Scholar] [CrossRef]
- Jansson, C.; Northen, T. Calcifying cyanobacteria-the potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 2010, 21, 365–371. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Ajo-Franklin, C.M.; Northen, T.; Jansson, C. Cyanobacteria as Biocatalysts for Carbonate Mineralization. Minerals 2012, 2, 338–364. [Google Scholar] [CrossRef]
- Kaplan, A.; Reinhold, L. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Physiol. Plant Molec. Biol. 1999, 50, 539–570. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J. Exp. Bot. 2003, 54, 609–622. [Google Scholar]
- Thompson, J.B.; Ferris, F.G. Cyanobacterial precipitation of gypsum, calcite, and magnesite from natural alkaline lake water. Geology 1990, 18, 995–998. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Beveridge, T.J. Physicochemical characteristics of the mineral-forming S-layer from the cyanobacterium Synechococcus strain Gl24. Can. J. Microbiol. 1994, 40, 216–223. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Fortin, D.; Davis, B.S.; Beveridge, T.J. Mineralization of bacterial surfaces. Chem. Geol. 1996, 132, 171–181. [Google Scholar] [CrossRef]
- Obst, M.; Dittrich, M.; Kuehn, H. Calcium adsorption and changes of the surface microtopography of cyanobacteria studied by AFM, CFM, and TEM with respect to biogenic calcite nucleation. Geochem. Geophys. Geosyst. 2006, 7, Q06011. [Google Scholar] [CrossRef]
- Dudev, T.; Cowan, J.A.; Lim, C. Competitive binding in magnesium coordination chemistry: Water versus ligands of biological interest. J. Am. Chem. Soc. 1999, 121, 7665–7673. [Google Scholar] [CrossRef]
- Zhang, F.F.; Xu, H.F.; Konishi, H.; Shelobolina, E.S.; Roden, E.E. Polysaccharide-catalyzed nucleation and growth of disordered dolomite: A potential precursor of sedimentary dolomite. Am. Miner. 2012, 97, 556–567. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kenward, P.A.; Fowle, D.A.; Goldstein, R.H.; Gonzalez, L.A.; Moore, D.S. Surface chemistry allows for abiotic precipitation of dolomite at low temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 14540–14545. [Google Scholar]
- Slaughter, M.; Hill, R.J. The influence of organic matter in organogenic dolomitization. J. Sediment. Petrol. 1991, 61, 296–303. [Google Scholar] [CrossRef]
- Wright, D.T.; Wacey, D. Precipitation of dolomite using sulphate-reducing bacteria from the Coorong Region, South Australia: Significance and implications. Sedimentology 2005, 52, 987–1008. [Google Scholar] [CrossRef]
- Kluge, S.; Weston, J. Can a hydroxide ligand trigger a change in the coordination number of magnesium ions in biological systems. Biochemistry 2005, 44, 4877–4885. [Google Scholar] [CrossRef]
- Fenter, P.; Zhang, Z.; Park, C.; Sturchio, N.C.; Hu, X.M.; Higgins, S.R. Structure and reactivity of the dolomite (104)-water interface: New insights into the dolomite problem. Geochim. Cosmochim. Acta 2007, 71, 566–579. [Google Scholar] [CrossRef]
- Couradeau, E.; Benzerara, K.; Gerard, E.; Moreira, D.; Bernard, S.; Brown, G.E.; Lopez-Garcia, P. An early-branching microbialite cyanobacterium forms intracellular carbonates. Science 2012, 336, 459–462. [Google Scholar] [CrossRef]
- Shirokova, L.S.; Mavromatis, V.; Bundeleva, I.A.; Pokrovsky, O.S.; Benezeth, P.; Gerard, E.; Pearce, C.R.; Oelkers, E.H. Using Mg isotopes to trace cyanobacterially mediated magnesium carbonate precipitation in alkaline lakes. Aquat. Geochem. 2013, 19, 1–24. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, K.S. Effects of CO2 concentrations on the freshwater microalgae, Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). J. Appl. Phycol. 2003, 15, 379–389. [Google Scholar]
- Beardall, J.; Raven, J.A. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 2004, 43, 26–40. [Google Scholar] [CrossRef]
- Ramos, J.B.E.; Biswas, H.; Schulz, K.G.; LaRoche, J.; Riebesell, U. Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Glob. Biogeochem. Cycle 2007, 21, GB2028. [Google Scholar] [CrossRef]
- Levitan, O.; Rosenberg, G.; Setlik, I.; Setlikova, E.; Grigel, J.; Klepetar, J.; Prasil, O.; Berman-Frank, I. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Chang. Biol. 2007, 13, 531–538. [Google Scholar] [CrossRef]
- Riebesell, U.; Schulz, K.G.; Bellerby, R.G.J.; Botros, M.; Fritsche, P.; Meyerhofer, M.; Neill, C.; Nondal, G.; Oschlies, A.; Wohlers, J.; Zollner, E. Enhanced biological carbon consumption in a high CO2 ocean. Nature 2007, 450, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.X.; Warner, M.E.; Zhang, Y.H.; Feng, Y.Y.; Hutchins, D.A. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria). J. Phycol. 2007, 43, 485–496. [Google Scholar] [CrossRef]
- Ramanan, R.; Kannan, K.; Deshkar, A.; Yadav, R.; Chakrabarti, T. Enhanced algal CO2 sequestration through calcite deposition by Chlorella sp. and Spirulina platensis in a mini-raceway pond. Bioresour. Technol. 2010, 101, 2616–2622. [Google Scholar]
- Lee, S.W.; Park, S.B.; Jeong, S.K.; Lim, K.S.; Lee, S.H.; Trachtenberg, M.C. On carbon dioxide storage based on biomineralization strategies. Micron 2010, 41, 273–282. [Google Scholar] [CrossRef]
- Ramanan, R.; Kannan, K.; Sivanesan, S.D.; Mudliar, S.; Kaur, S.; Tripathi, A.K.; Chakrabarti, T. Bio-sequestration of carbon dioxide using carbonic anhydrase enzyme purified from Citrobacter freundii. World J. Microb. Biot. 2009, 25, 981–987. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, A. Enhanced biomimetic sequestration of CO2 into CaCO3 using purified carbonic anhydrase from indigenous bacterial strains. J. Mol. Catal. B Enzym. 2010, 67, 122–128. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, A.; Shrivastava, A. Biomimetic CO2 sequestration using purified carbonic anhydrase from indigenous bacterial strains immobilized on biopolymeric materials. Enzyme Microb. Technol. 2011, 48, 416–426. [Google Scholar] [CrossRef]
- Barbero, R.; Carnelli, L.; Simon, A.; Kao, A.; Monforte, A.D.; Ricco, M.; Bianchi, D.; Belcher, A. Engineered yeast for enhanced CO2 mineralization. Energy Environ. Sci. 2013, 6, 660–674. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: a review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sust. Energ. Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second Generation biofuels: High-efficiency microalgae for biodiesel production. BioEnergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J. Appl. Phycol. 2006, 18, 703–712. [Google Scholar] [CrossRef]
- Environment Canada (2004) Metal Mining—Guidance Manual for Estimating Greenhouse Gas Emissions. Available online: http://publications.gc.ca/collections/Collection/En49-2-9-2E.pdf (accessed on 24 July 2013).
- WMC Resources Ltd. 2002 Sustainability Report; WMC Resources Ltd.: London, UK, 2002. Available online: http://www.bhpbilliton.com/home/investors/reports/Documents/wmcsustainability2002.pdf (accessed on 21 January 2014).
- Joyce, D.; Potulski, B.C. Mt Keith Nickel Mine Centralised Tailings Disposal System. In Proceedings of 1996 National Engineering Conference: Engineering Tomorrow Today, Darwin, Australia, 21–24 April 1996; pp. 341–346.
- Stolberg, D.J. Rehabilitation Studies on Tailings Storage Facilities in an Arid Hypersaline Region. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2005. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Price, W.A. Guidelines and Recommended Methods for the Prediction of Metal Leaching and Acid Rock Drainage at Minesites in British Columbia; British Columbia Ministry of Employment and Investment, Energy and Minerals Division: Simthers, BC, Canada, 1997. [Google Scholar]
- Hitch, M.; Dipple, G.M. Economic feasibility and sensitivity analysis of integrating industrial-scale mineral carbonation into mining operations. Miner. Eng. 2012, 39, 268–275. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L.A. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Ribeiro, L.A.; da Silva, P.P. Surveying techno-economic indicators of microalgae biofuel technologies. Renew. Sust. Energ. Rev. 2013, 25, 89–96. [Google Scholar] [CrossRef]
- Stolaroff, J.K.; Lowry, G.V.; Keith, D.W. Using CaO- and MgO-rich industrial waste streams for carbon sequestration. Energy Conv. Manag. 2005, 46, 687–699. [Google Scholar] [CrossRef]
- Pindyck, R.S. Pricing carbon when we don’t know the right price. Regulation 2013, 36, 43–46. [Google Scholar]
- Burgess, J.; Jeffery, L.; Lowe, A.; Schuck, S.; Flentje, W. The KPMG Green Tax Index 2013: An Exploration of Green Tax Incentives and Penalties; KPMG: Amstelveen, The Netherlands, 2013. [Google Scholar]
- Stern, N. The Economics of Climate Change: The Stern Review; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Brennan, M.J.; Schwartz, E.S. Evaluating natural resource investments. J. Bus. 1985, 58, 135–157. [Google Scholar]
- Brennan, M.J.; Schwartz, E.S. A new approach to evaluating natural resource investments. 1985, 3, 37–47. [Google Scholar]
- Paddock, J.D.; Siegel, R.D.; Smith, J. Option valuation of claims on real assets: The case of offshore petroleum leases. Q. J. Econ. 1998, 103, 479–509. [Google Scholar] [CrossRef]
- Kelly, S. A binomial lattice approach for valuing a mining property IPO. Q. Rev. Econ. Financ. 1998, 38, 693–709. [Google Scholar] [CrossRef]
- Dixit, A.K.; Pindyck, R.S. Investment under Uncertainty; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Black, F.; Scholes, M. The pricing of options and corporate liabilities. J. Polit. Econ. 1973, 81, 637–654. [Google Scholar]
- Chung, C.C.; Lee, S.H.; Beamish, P.W.; Southam, C.; Nam, D. Pitting real options theory against risk diversification theory: International diversification and joint ownership control in economic crisis. J. World Bus. 2013, 48, 122–136. [Google Scholar] [CrossRef]
- Baker, H.K.; Dutta, S.; Saadi, S. Management views on real options in capital budgeting. J. Appl. Financ. 2011, 21, 18–29. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Power, I.M.; McCutcheon, J.; Harrison, A.L.; Wilson, S.; Dipple, G.M.; Kelly, S.; Southam, C.; Southam, G. Strategizing Carbon-Neutral Mines: A Case for Pilot Projects. Minerals 2014, 4, 399-436. https://doi.org/10.3390/min4020399
Power IM, McCutcheon J, Harrison AL, Wilson S, Dipple GM, Kelly S, Southam C, Southam G. Strategizing Carbon-Neutral Mines: A Case for Pilot Projects. Minerals. 2014; 4(2):399-436. https://doi.org/10.3390/min4020399
Chicago/Turabian StylePower, Ian M., Jenine McCutcheon, Anna L. Harrison, Sasha Wilson, Gregory M. Dipple, Simone Kelly, Colette Southam, and Gordon Southam. 2014. "Strategizing Carbon-Neutral Mines: A Case for Pilot Projects" Minerals 4, no. 2: 399-436. https://doi.org/10.3390/min4020399
APA StylePower, I. M., McCutcheon, J., Harrison, A. L., Wilson, S., Dipple, G. M., Kelly, S., Southam, C., & Southam, G. (2014). Strategizing Carbon-Neutral Mines: A Case for Pilot Projects. Minerals, 4(2), 399-436. https://doi.org/10.3390/min4020399





