Jarosite versus Soluble Iron-Sulfate Formation and Their Role in Acid Mine Drainage Formation at the Pan de Azúcar Mine Tailings (Zn-Pb-Ag), NW Argentina
Abstract
:1. Introduction
1.1. Site Location and Description
1.2. Ore Geology, Mining and Tailings Impoundment History




2. Experimental Section
2.1. Sampling and Mineralogical Analysis of Tailings and Efflorescent Salts
2.2. Scanning Electron Microprobe with EDS Analysis
2.3. Sequential Extractions
2.4. Acid-Base Accounting
2.5. Acid Mine Drainage (AMD) and Acid Ponds
3. Results and Discussion
3.1. General Characteristics and Mineralogy of Tailings
3.1.1. Tailings Impoundment DC1


3.1.2. Tailings Impoundment DC2

Acid-Base Accounting

3.1.3. Tailings Impoundment CN

3.2. Secondary Minerals in the Tailings DC1, DC2, CN, and Metals Associations
3.2.1. Insoluble Iron Sulfates
| Sample | Fe | K-jt | Na-jt | Pb-jt | K-Na-Pb-jt | sh or H3O-jt |
| Step-3 | Step-3 | Step-3 | Step-3 | Step-3 | Step-3 | |
| Meas (wt %) | Calc (wt %) | Calc (wt %) | Calc (wt %) | Total Calc (wt %) | Cal (wt %) | |
| DC2-P2-1 | 0.79 | 0.43 | 0.04 | 0.06 | 0.52 | 0.27 |
| DC2-P2-2 | 1.70 | 0.73 | 0.15 | 0.02 | 0.90 | 0.80 |
| DC2-P2-3 | 1.08 | 0.56 | 0.07 | 0.09 | 0.73 | 0.35 |
| DC2-P2-5 | 0.96 | 0.64 | 0.04 | 0.07 | 0.75 | 0.21 |
| Sample | K-jt | Na-jt | Pb-jt | Total K-Na- Pb-jt | sh or H3O-jt | |
| Step-3 | Step-3 | Step-3 | Step-3 | Step-3 | ||
| Calc (%) | Calc (%) | Calc (%) | Calc (%) | Calc (%) | ||
| DC2-P2-1 | 54.23 | 4.61 | 7.53 | 66.38 | 33.61 | |
| DC2-P2-2 | 42.84 | 8.57 | 1.49 | 52.91 | 47.08 | |
| DC2-P2-3 | 51.57 | 6.74 | 8.82 | 67.14 | 32.85 | |
| DC2-P2-5 | 66.94 | 3.79 | 7.71 | 78.46 | 21.54 |
| Sample | S | K-jt | Na-jt | Pb-jt | K-Na-Pb-jt | sh H3O-jt | Fe*/S# in sh | S in sh (Fe*/(Fe*/S#)) |
|---|---|---|---|---|---|---|---|---|
| Step-3 | Step-3 | Step-3 | Step-3 | Step-3 | Step-3 | |||
| Meas | Calc | Calc | Calc | Total Calc | Cal | Meas | Calc | |
| (wt %) | (wt %) | (wt %) | (wt %) | (wt %) | (wt %) | (wt %) | ||
| DC2-P2-1 | 0.32 | 0.16 | 0.01 | 0.02 | 0.20 | 0.12 | 2.25 | 0.12 |
| DC2-P2-2 | 0.61 | 0.28 | 0.06 | 0.01 | 0.34 | 0.27 | 2.96 | 0.27 |
| DC2-P2-3 | 0.46 | 0.21 | 0.03 | 0.04 | 0.28 | 0.18 | 1.94 | 0.18 |
| DC2-P2-5 | 0.41 | 0.25 | 0.01 | 0.03 | 0.29 | 0.12 | 1.75 | 0.12 |
3.2.2. Soluble Sulfates

| Sample | Features | Efflorescent salts and ideal formula |
|---|---|---|
| DC2-P2-1-E | No salt formation observed | |
| DC2-P2-2-E | Red-brownish crust with scarce yellow salts | alunogen (Al2(SO4)3·17H2O) |
| DC2-P2-3-E | Predominance of yellow salts, less white | rhomboclase ((H3O)Fe3+(SO4)2·H2O) |
| DC2-P2-4-E | Mixture of yellow and white salts | rhomboclase ((H3O)Fe3+(SO4)2·H2O) |
| DC2-P2-5a-E | Predominance of yellow salts, less white | copiapite (Fe2+Fe43+(SO4)6(OH)2·20H2O) |
| DC2-P2-5b-E | Predominance of white salts, less yellow | rhomboclase ((H3O)Fe3+(SO4)2·H2O) |
| Parameter | DC2-Arr | DC2-P2-5 | DC3-L-1(T1) | DC3-L-2 (T3) | DC3-L-3 (T4) |
|---|---|---|---|---|---|
| pH | 2.1 | 2.08 | 2.42 | 3.75 | |
| Conductivity | ˃4,000 | ˃4,000 | 18,470 | 5,900 | |
| mg/L | mg/kg | mg/L | mg/L | mg/L | |
| SO4 | 139,000 | 122,000 | 5,130 | 9,690 | 3,870 |
| FeT | 47,900 | 1,500 | 1,280 | 2,270 | 843 |
| Zn | 8,960 | 88.5 | 338 | 686 | 264 |
| Al | 7,120 | 2,400 | 222 | 440 | 185 |
| Ca | 524 | 5,900 | 176 | 146 | 156 |
| Mg | 1,110 | 100 | 29 | 74.5 | 25.6 |
| Cu | 10.1 | 5.2 | 17.3 | 38.8 | 15.2 |
| As | 44.6 | <3 | 4.5 | 12.2 | 3.8 |
| Cd | 99.7 | <1 | 2.1 | 5.0 | 2.4 |
| Pb | 1.4 | 199 | 1.1 | 1.8 | 0.5 |
| Co | 9.5 | 320 | 0.3 | 0.8 | 0.3 |
| Ni | 15.2 | 2.0 | 0.3 | 0.7 | 0.4 |
| Sb | 4.7 | <5 | 0.1 | 0.4 | 0.1 |
| Cr | 1.7 | <1 | 0.2 | 0.4 | 0.2 |
| Sn | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 |

3.2.3. Jarosite and Soluble Fe3+ Salt Formation in the Erosion Channel at DC2 Tailings Impoundment

3.2.4. Jarosite and Soluble Fe3+ Salts Formation in the CN Tailings
3.3. Acid Pond Features and Variation in the Water Composition
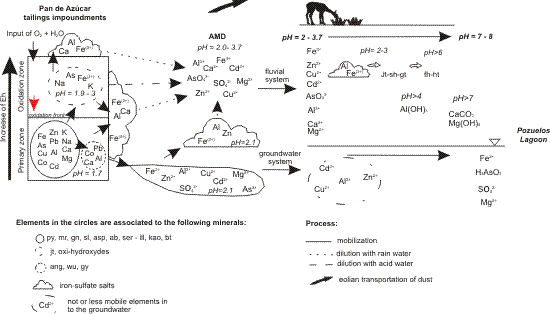
3.4. Conceptual Model for the Cycle of Sulfide Oxidation, Sulfate Formation and Metal Mobility at the Pan de Azúcar Mine; and Possible Pathway of Metals from the Tailings to the Fluvial and Groundwater Systems and on to Pozuelos Lagoon

- (1)
- Oxidation of sulfides (by oxygen, ferric iron, and microbial activity) and dilution of silicates in the oxidation front. Release of sulfate, acidity, iron, metals and alkalis precipitate as secondary minerals or mobilization through the tailings stratigraphy. Below the oxidation front, ferric iron could be reduced again by microbial reducing activity.
- (2)
- Precipitation of sulfates in the tailings:
- (2a)
- In the primary zone of tailings anglesite, wupatkite and gypsum precipitates retain Pb, Co, Ca, and Al. Section 3.2.2.
- (2b)
- Formation of insoluble sulfates in the oxidation zone (jarosite, Fe3+ oxy-hydroxyde sulfates like schwertmannite) sequestering mainly As, K and Na. Section 3.2.1.
- (2c)
- After oxidation of sulfide and dilution of silicates in the tailings, sulfate-rich pore water evaporates, allowing the formation of soluble iron sulfates in the wall of the erosion channel in DC2. Alunogen, copiapite, coquimbite, rhomboclase, römerite, gypsum and szomolokite retain mainly Fe3+, Fe2+, Ca, and Al, are the most common phases. Section 3.2.2 and Section 3.2.3.
- (2d)
- Precipitation of soluble iron sulfates in the surface of the tailings is produced by the capillarity ascent of metals (copiapite, coquimbite, halotrichite, rhomboclase), which retain mainly Fe3+ and Al. Section 3.2.4.
- (3)
- Ferrous iron, sulfate and metals rich AMD plume mobilizes downwards through the primary tailings zone. Section 3.2.2.
- (4)
- AMD plume seeps into the base of the tailings during the dry season, enabling the precipitation of soluble sulfate salts (melanterite, alunogen) and sequestering Fe2+, Zn, and Al. Section 3.2.2.
- (5)
- Dilution of soluble sulfate salts during the wet season, especially during the first rain events. AMD formation and release of SO42−, Fe2+, Zn2+, Al3+, Ca2+, Mg2+, Cu2+, Cd2+. Section 3.3.
- (6)
- Dilution of jarosite at pH = 2 by AMD, release of SO42−, AsO4. Section 3.3.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nordstrom, D.K.; Alpers, C.N. Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc. Natl. Acad. Sci. USA 1999, 96, 3455–3462. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metalsulphate salts from sulphide mineral oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Bea, S.A.; Ayora, C.; Carrera, J.; Saaltink, M.W.; Dold, B. Geochemical and environmental controls on the genesis of efflorescent salts on coastal mine tailings deposits: A discussion based on reactive transport modeling. J. Contam. Hydrol. 2010, 111, 65–82. [Google Scholar] [CrossRef]
- Dold, B. Element flows associated with marine shore mine tailings deposits. Environ. Sci. Technol. 2006, 40, 752–758. [Google Scholar] [CrossRef]
- lpers, C.N.; Nordstrom, D.K.; Thompson, M.J. Seasonal variations in Zn/Cu ratios in acid mine water from Iron Mountain, California. In Environmental Geochemistry of Sulphide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; ACS Publications: Washington, DC, USA, 1994; Volume 550, pp. 324–344. [Google Scholar]
- Hammarstrom, J.M.; Seal, R.R., II; Meier, A.L.; Kornfeld, J.M. Secondary sulphate minerals associated with acid drainage in the eastern US: Recucling of metals and acidity in surficial environments. Chem. Geol. 2005, 215, 407–431. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine waters. In The Environmental Geochemistry of Mineral Deposits; Plumlee, G.S., Logsdon, M.J., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 6A, pp. 133–160. [Google Scholar]
- Hudson, E. Sources, mineralogy, chemistry and fate of heavy metal-bearing particles in mining-affected river systems. Mineral. Mag. 2003, 2, 205–217. [Google Scholar] [CrossRef]
- Dold, B.; Fontbote, L. Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J. Geochem. Explor. 2001, 74, 3–55. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Robinson, C.; Alpers, C.N.; McCleskey, R.B.; Nordstrom, D.K.; Peterson, R.C. Major and trace element composition of copiapite-group minerals and coexisting water from the Richmond mine, Iron Mountain, California. Chem. Geol. 2005, 215, 387–405. [Google Scholar] [CrossRef]
- Bigham, J.M.; Carlson, L.; Murad, E. Schwertmannite, a new iron oxyhydroxy-sulphate from Pyhäsalmi, Finland, and other localities. Mineral. Mag. 1994, 58, 641–648. [Google Scholar]
- Bigham, J.M.; Nordstrom, D.K. Iron and aluminum hydroxysulphates from acid sulphate water. In Sulphate Minerals: Crystallography, Geochemistry, and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Reviews in Mineralogy and Geochemistry Volume 40; Mineralogical Society of America: Chantilly, VA, USA, 2000; Volume 40, pp. 351–403. [Google Scholar]
- Dutrizac, J.E.; Jambor, J.L. Jarosites and their application in hydrometallurgy. In Sulphate Minerals: Crystallography, Geochemistry, and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Reviews in Mineralogy and Geochemistry Volume 40; Mineralogical Society of America: Chantilly, VA, USA, 2000; pp. 405–443. [Google Scholar]
- Stoffregen, R.E.; Alpers, C.; Jambor, J.L. Alunite-jarosite cristalography, thermodynamics and geochonolology. In Sulphate Minerals: Crystallography, Geochemistry, and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Reviews in Mineralogy and Geochemistry Volume 40; Mineralogical Society of America: Chantilly, VA, USA, 2000; pp. 454–475. [Google Scholar]
- Hudson-Edwards, K.A.; Wright, K. Computer simulations of the interactions of the (0 1 2) and (0 0 1) surfaces of jarosite with Al, Cd, Cu2+ and Zn. Geochim. Cosmochim. Acta 2011, 75, 52–62. [Google Scholar] [CrossRef]
- Kirschbaum, A.; Murray, J.; Arnosio, M.; Tonda, R.; Cacciabue, L. Pasivos ambientales mineros en el noroeste de Argentina: Aspectos mineralógicos. Geoquímicos y consecuencias ambientales. Rev. Mex. Cienc. Geol. 2012, 29, 248–264. (In Spanish) [Google Scholar]
- McGlue, M.M.; Ellis, G.S.; Cohen, A.S.; Swarzenski, P.W. Palya-lake sedimentation and organic matter accumulation in an Andean piggyback basin: The recent record from the Cuenca de Pozuelos, North-West Argentina. Sedimentology 2012, 59, 1237–1256. [Google Scholar] [CrossRef]
- Bianchi, A.R.; Yañez, C.E.; Acuña, L.R. Bases de Datos Mensuales de Precipitaciones del Noroeste Argentino. Available online: http://inta.gob.ar/documentos/las-precipitaciones-del-noroeste-argentino-periodo-1934-1990/ (accessed on 28 May 2014).
- Legates, D.R.; Willmott, C.J. Mean seasonal and spatial variability in gauge-corrected, global precipitation. Int. J. Climatol. 1990, 10, 111–127. [Google Scholar] [CrossRef]
- Legates, D.R.; Willmott, C.J. Mean seasonal and spatial variability in global surface air temperature. Theor. Appl. Climatol. 1990, 41, 11–21. [Google Scholar]
- Segal, S.J. Estudio mineralógico y consideraciones genéticas del distrito minero “Pan de Azúcar”, Departamento Rinconada, provincia de Jujuy. Rev. Asoc. Geol. Argent. 1980, 35, 375–400. (In Spanish) [Google Scholar]
- Caffe, P.; Coira, B.L. Complejos de domos volcánicos del mioceno medio de Puna Norte. Un modelo geológico y metalogenético para yacimientos de metales de base ricos en plata (estaño). In Recursos Minerales de la República Argentina; Zappettini, E.O., Ed.; Instituto de Geología y Recursos Minerales, Servicio Geológico Minero Argentino: Buenos Aires, Argentina, 1999; Volume 35, pp. 1569–1578. (In Spanish) [Google Scholar]
- Segal, S.J.; Caffe, P.J. El grupo minero Pan de Azúcar, Jujuy. In Recursos Minerales de la República Argentina; Zappettini, E.O., Ed.; Instituto de Geología y Recursos Minerales, Servicio Geológico Minero Argentino: Buenos Aires, Argentina, 1999; Volume 35, pp. 1579–1592. (In Spanish) [Google Scholar]
- Jackson, M.L. Análisis Químico de Suelos; Omega: Barcelona, Spain, 1976. (In Spanish) [Google Scholar]
- Jambor, J.L. Mineralogy of sulphide-rich tailings and their oxidation products. In The EnvironmentalGeochemistry of Sulphide Mine-Waste; Blowes, D.W., Jambor, J.L., Alpers, C.N., Eds.; Mineralogical Association of Canada: Quebec, QC, Canada, 1994; pp. 59–102. [Google Scholar]
- Dold, B. Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulphide mine waste. J. Geochem. Explor. 2003, 80, 55–68. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Robinson, C.; Alpers, C.N.; Nordstrom, D.K.; Poustovetov, A.; Lowers, H.A. The composition of coexisting jarosite-group minerals and water from the richmond mine, iron mountain, California. Can. Mineral. 2005, 43, 1225–1242. [Google Scholar] [CrossRef]
- Burton, E.; Bush, R.; Johnston, S.; Watling, K.; Hocking, R.; Sullivan, L.; Parker, G. Sorption of Arsenic(V) and Arsenic(III) to Schwertmannite. Environ. Sci. Technol. 2009, 43, 9202–9207. [Google Scholar] [CrossRef]
- Dold, B.; Wade, C.; Fontboté, L. Water management for acid mine drainage control at the polymetallic Zn-Pb-(Ag-Bi-Cu) deposit of Cerro de Pasco, Peru. J. Geochem. Explor. 2009, 100, 133–141. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-ray Difraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Dold, B.; Blowes, D.W.; Dickhout, R.; Spangenberg, J.E.; Pfeifer, H.R. Low molecular weight carboxylic acids in oxidizing sulphide mine tailings. Environ. Sci. Technol. 2005, 39, 2515–2521. [Google Scholar] [CrossRef]
- Dold, B.; Gonzalez-Toril, E.; Aguilera, A.; Lopez-Pamo, E.; Cisternas, M.-E.; Amils, R. Acid rock drainage and rock weathering in Antarctica—Important sources for iron cycling in the Southern Ocean. Environ. Sci. Technol. 2013, 47, 6129–6136. [Google Scholar]
- Merwin, H.E.; Posnjak, E. Sulphate incrustations in the Copper Queen Mine, Bisbee, Arizona. Am. Mineral. 1937, 22, 567–571. [Google Scholar]
- Smuda, J.; Dold, B.; Spangenberg, J.E.; Bustos, C.; Kobek, M.; Pfeifer, H.-R. Element cycling during the transition from alkaline to acidic environment in an active porphyry copper tailings impoundment, Chuquicamata, Chile. J. Geochem. Explor. 2014, 140, 23–40. [Google Scholar] [CrossRef]
- Smith, A.M.; Dubbin, W.E.; Wright, K.; Hudson-Edwards, K.A. Dissolution of lead- and lead-arsenic-jarosites at pH 2 and 8 and 20 °C: Insights from batch experiments. Chem. Geol. 2006, 229, 344–361. [Google Scholar] [CrossRef]
- Kendall, M.; Madden, A.S.; Elwood Madden, E.M.; Hu, Q. Effects of arsenic incorporation on jarosite dissolution rates and reaction products. Geochim. Cosmochim. Acta 2013, 112, 192–207. [Google Scholar]
- Hudson-Edwards, K.A.; Schell, C.; Macklin, M.G. Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Appl. Geochem. 1999, 14, 55–70. [Google Scholar]
- Taylor, M.P.; Hudson-Edwards, K.A. The dispersal and storage of sediment-associated metals in an arid river system: The Leichhardt River, Mount Isa, Queensland, Australia. Environ. Pollut. 2008, 152, 193–204. [Google Scholar] [CrossRef]
- Kirschbaum, A.; Murray, J.; López, E.; Equiza, A.; Arnosio, M.; Boaventura, G. The environmental impact of Aguilar mine on the heavy metalconcentrations of the Yacoraite River, Jujuy Province, NW Argentina. Environ. Earth Sci. 2012, 65, 493–504. [Google Scholar] [CrossRef]
- Murray, J.; Kirschbaum, A.; Dold, B. Acid mine drainage from Pan de Azúcar Mine (Zn-Pb-Ag) and possible Arsenic input on the water quality of Pozuelos Lagoon Basin, Northwest Argentina. In Proceedings of 1st International Conference on Mine Water Solution in Extreme Environments, Lima, Peru, 15–17 April 2013; pp. 342–350.
- Hudson-Edwards, K.A.; Archer, J. Geochemistry of arsenic (As) in spring and stream waters from San Antonio de los Cobres, NW Argentina. Mineral. Mag. 2008, 72, 425–427. [Google Scholar] [CrossRef]
- Plaza Cazón, J.; Benítez, L.; Murray, J.; Kirschbaum, A.; Kirschbaum, P.; Donati, E. Environmental impact on soil, water and plants from the abandoned Pan de Azúcar Mine. Adv. Mater. Res. 2013, 825, 88–91. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murray, J.; Kirschbaum, A.; Dold, B.; Guimaraes, E.M.; Miner, E.P. Jarosite versus Soluble Iron-Sulfate Formation and Their Role in Acid Mine Drainage Formation at the Pan de Azúcar Mine Tailings (Zn-Pb-Ag), NW Argentina. Minerals 2014, 4, 477-502. https://doi.org/10.3390/min4020477
Murray J, Kirschbaum A, Dold B, Guimaraes EM, Miner EP. Jarosite versus Soluble Iron-Sulfate Formation and Their Role in Acid Mine Drainage Formation at the Pan de Azúcar Mine Tailings (Zn-Pb-Ag), NW Argentina. Minerals. 2014; 4(2):477-502. https://doi.org/10.3390/min4020477
Chicago/Turabian StyleMurray, Jesica, Alicia Kirschbaum, Bernhard Dold, Edi Mendes Guimaraes, and Elisa Pannunzio Miner. 2014. "Jarosite versus Soluble Iron-Sulfate Formation and Their Role in Acid Mine Drainage Formation at the Pan de Azúcar Mine Tailings (Zn-Pb-Ag), NW Argentina" Minerals 4, no. 2: 477-502. https://doi.org/10.3390/min4020477
APA StyleMurray, J., Kirschbaum, A., Dold, B., Guimaraes, E. M., & Miner, E. P. (2014). Jarosite versus Soluble Iron-Sulfate Formation and Their Role in Acid Mine Drainage Formation at the Pan de Azúcar Mine Tailings (Zn-Pb-Ag), NW Argentina. Minerals, 4(2), 477-502. https://doi.org/10.3390/min4020477