Recovery of Cobalt as Cobalt Oxalate from Cobalt Tailings Using Moderately Thermophilic Bioleaching Technology and Selective Sequential Extraction
Abstract
:1. Introduction
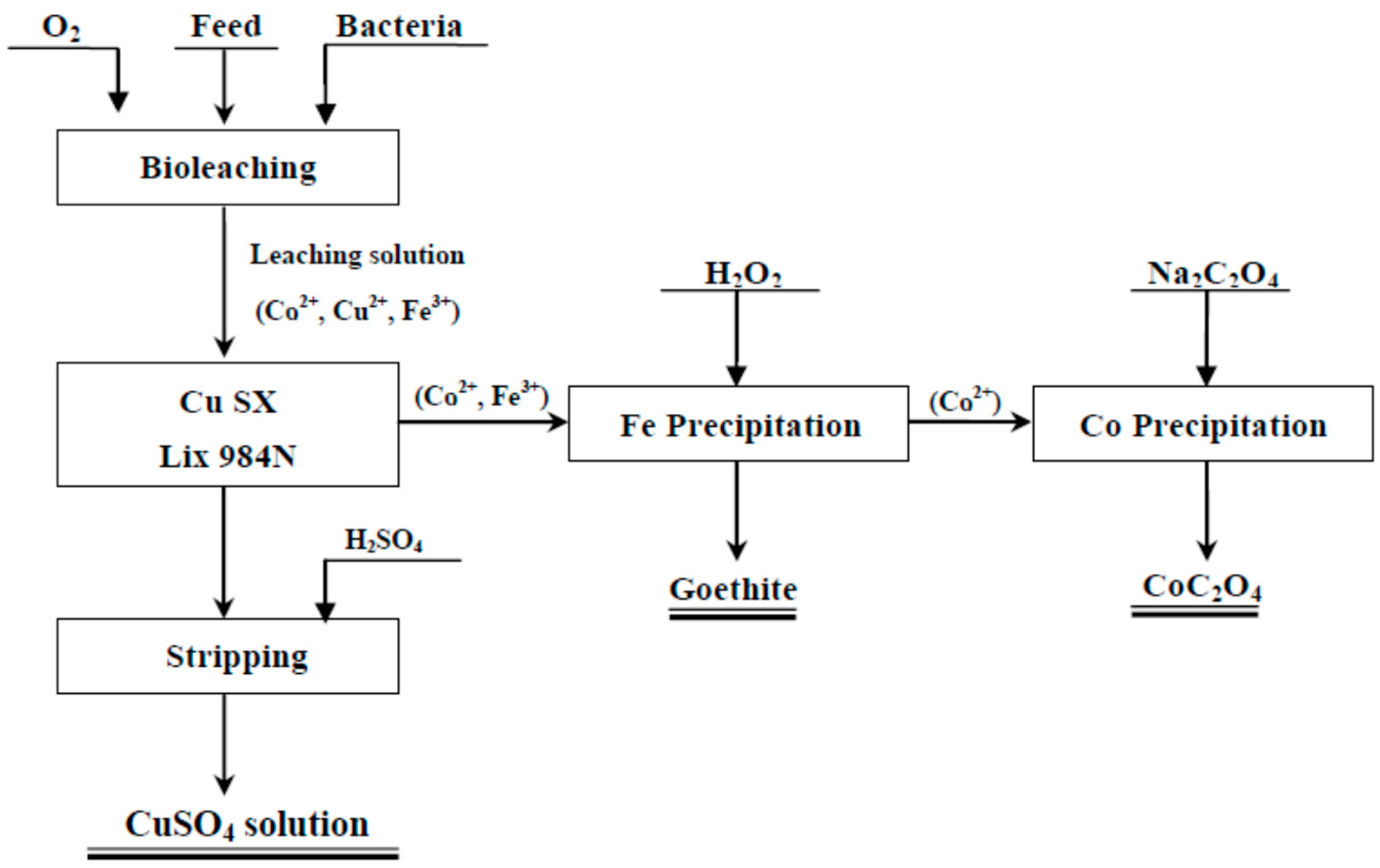
2. Experimental
2.1. Mineral Sample Preparation
2.2. Bacterial Strain and Growth Conditions
2.3. Bioleaching Process
2.4. Selective Sequential Extraction Procedure
- (1)
- Copper extraction
- (2)
- De-ironization
- (3)
- Preparation of CoC2O4 powder by the precipitation process
2.5. Analytical Method
3. Results and Discussion
3.1. Bioleaching Results of Cobalt Tailings
3.2. Copper Extraction Process from Bioleaching Solutions
3.2.1. Effect of Equilibrium pH
3.2.2. Effect of LIX 984N Concentration
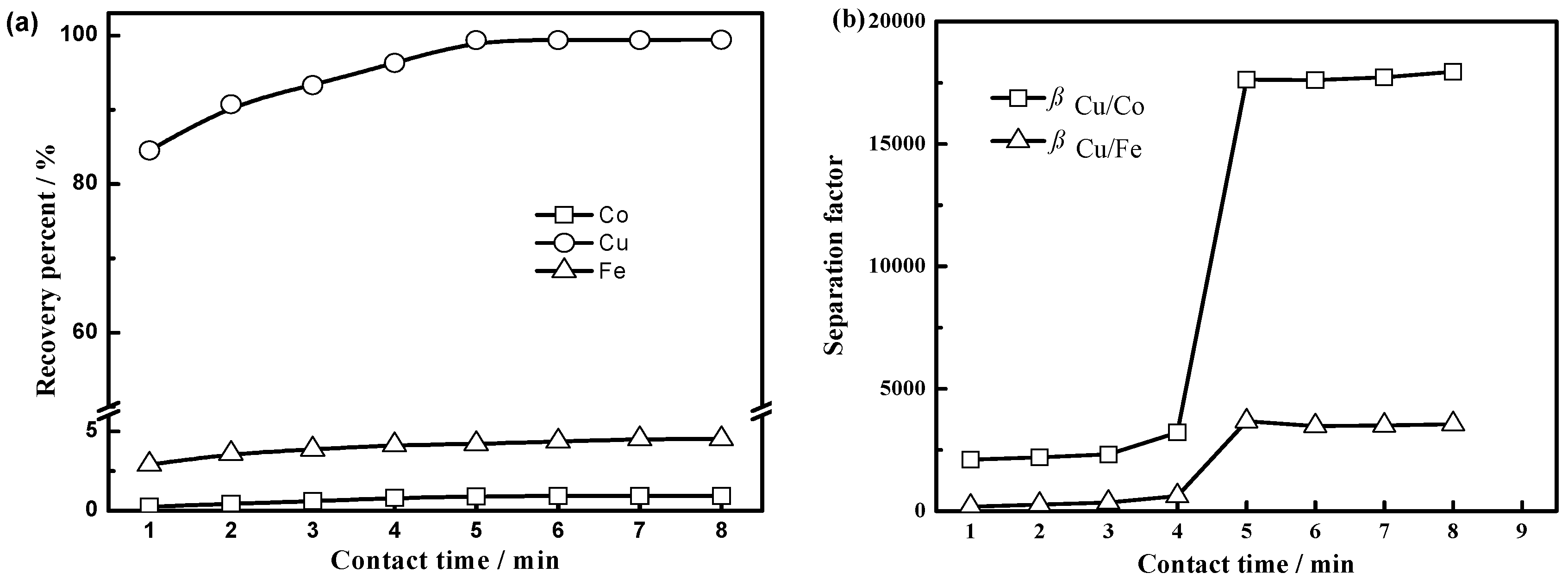
3.2.3. Effect of Contact Time
3.3. De-Ironization via a Precipitation Process
3.3.1. Effect of Equilibrium pH
3.3.2. Effect of Temperature
3.3.3. Effect of Soaking Time
3.4. Preparation of Cobalt Oxalate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, J.; He, L.; Liu, H.; Han, T.; Wang, Y.; Zhang, C. Controlled synthesis of porous anhydrous cobalt oxalate nanorods with high reversible capacity and excellent cycling stability. Electrochim. Acta 2015, 170, 85–91. [Google Scholar]
- Cheng, G.; Si, C.; Zhang, J.; Wang, Y.; Yang, W.; Dong, C.; Zhang, Z. Facile fabrication of cobalt oxalate nanostructures with superior specific capacitance and super-long cycling stability. J. Power Sources 2016, 312, 184–191. [Google Scholar] [CrossRef]
- Bahaa, M.A.; Salem, M.B.; Samia, A.K.; Wilhelm, S. Effect of microwave power on the thermal genesis of Co3O4 nanoparticles from cobalt oxalate micro-rods. Appl. Surf. Sci. 2015, 351, 600–609. [Google Scholar]
- Joël, P.T.K. Cobalt production and markets: A brief overview. JOM 2006, 58, 33–36. [Google Scholar]
- Zhao, S.; Chen, Y. The situation of supply and demand of cobalt resource in the world and China. Min. Metall. Eng. 2012, 32, 153–156. [Google Scholar]
- Brierley, J.A. A perspective on developments in biohydrometallurgy. Hydrometallurgy 2008, 94, 2–7. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Gahan, C.S. Biohydrometallurgy of secondary metal resources: A potential alternative approach for metal recovery. J. Chem. Technol. Biotechnol. 2013, 88, 2115–2132. [Google Scholar] [CrossRef]
- Brierley, J.A.; Brierley, C.L. Present and future commercial applications of biohydrometallurgy. Hydrometallurgy 2001, 59, 233–239. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Liu, W.; Chen, G.-B.; Liu, Y.-Y.; Tong, L.-L.; Jin, Z.-N.; Liu, Z.-L. Function of microorganism and reaction pathway for carrollite dissolution during bioleaching. Trans. Nonferrous Met. Soc. China 2015, 25, 2718–2724. [Google Scholar] [CrossRef]
- Liu, W.; Yang, H.-Y.; Song, Y.; Tong, L.-L. Catalytic effects of activated carbon and surfactants on bioleaching of cobalt ore. Hydrometallurgy 2015, 152, 69–75. [Google Scholar] [CrossRef]
- Muchez, P.H.; Brems, D.; Clara, E. Evolution of Cu-Co mineralizing fluids at Nkana Mine, Central African Copperbelt, Zambia. J. Afr. Earth Sci. 2010, 58, 457–474. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Tong, L-L; Liu, W.; Chen, G.-B. A Breeding Method of Strain for Cobalt Bioleaching. Chinese Patent CN 102965303B, 3 June 2015. [Google Scholar]
- Buckley, A.N.; Skinner, W.M.; Harmer, S.L.; Pring, A.; Fan, L.-J. Electronic environments in carrollite, CuCo2S4, determined by soft X-ray photoelectron and absorption spectroscopy. Geochim. Cosmochim. Acta 2009, 73, 4452–4467. [Google Scholar] [CrossRef]
- Chang, Y.; Zhai, X.; Li, B.; Fu, Y. Removal of iron from acidic leach liquor of lateritic nickel ore by goethite precipitate. Hydrometallurgy 2010, 101, 84–87. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The physical chemistry of iron precipitation in the zinc industry, Lead-Zinc-Tin’80. In Proceedings of the World Symposium on Metallurgy and Envirnmental Control, Las Vegas, NV, USA, 24–28 February 1980; pp. 532–564.
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.J. Recovery of cobalt as cobalt oxalate from spent lithium ion batteries by using glycine as leaching agent. J. Environ. Chem. Eng. 2016, 4, 2378–2383. [Google Scholar] [CrossRef]









| Fe | Cu | Co | S | SiO2 | CaO | MgO |
|---|---|---|---|---|---|---|
| 27.79 | 18.82 | 1.06 | 33.06 | 10.21 | 2.96 | 2.82 |
| Pulp Density/% | Cell Density/mL−1 | Time/Day | Recovery of Co/% | Recovery of Cu/% | Co Content in Residue/% | Cu Content in Residue/% |
|---|---|---|---|---|---|---|
| 5 | 109 | 2 | 97.23 | 30.21 | 0.078 | 24.58 |
| 10 | 109 | 4 | 96.51 | 26.32 | 0.087 | 21.26 |
| 15 | 109 | 4 | 92.59 | 35.12 | 0.11 | 22.17 |
| 18 | 109 | 5 | 90.86 | 30.29 | 0.12 | 23.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Yang, H.; Li, H.; Tong, L. Recovery of Cobalt as Cobalt Oxalate from Cobalt Tailings Using Moderately Thermophilic Bioleaching Technology and Selective Sequential Extraction. Minerals 2016, 6, 67. https://doi.org/10.3390/min6030067
Chen G, Yang H, Li H, Tong L. Recovery of Cobalt as Cobalt Oxalate from Cobalt Tailings Using Moderately Thermophilic Bioleaching Technology and Selective Sequential Extraction. Minerals. 2016; 6(3):67. https://doi.org/10.3390/min6030067
Chicago/Turabian StyleChen, Guobao, Hongying Yang, Haijun Li, and Linlin Tong. 2016. "Recovery of Cobalt as Cobalt Oxalate from Cobalt Tailings Using Moderately Thermophilic Bioleaching Technology and Selective Sequential Extraction" Minerals 6, no. 3: 67. https://doi.org/10.3390/min6030067
APA StyleChen, G., Yang, H., Li, H., & Tong, L. (2016). Recovery of Cobalt as Cobalt Oxalate from Cobalt Tailings Using Moderately Thermophilic Bioleaching Technology and Selective Sequential Extraction. Minerals, 6(3), 67. https://doi.org/10.3390/min6030067







