Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions
Abstract
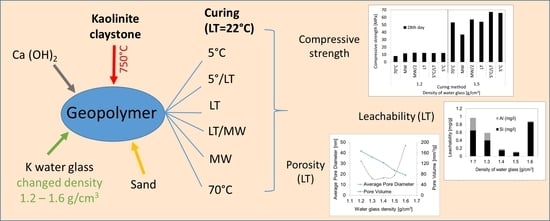
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Characterization
3. Results and Discussion
3.1. Compressive Strength
3.2. Leachability Tests
3.3. Porosity
4. Conclusions
- The correlation between pore volume and compressive strength was confirmed
- The low temperature (5 °C) during solidification has a positive effect on the GP—it increases compressive strength, decreases total pore volume and the leachability of Al.
- The higher density of the water glass used for the sample preparation resulted in lowering the total pore volume and increasing the compressive strength of the samples.
- Sudden increase of the Si and alkali metals leachability was proven in case of the GPs prepared from water glass of the highest density, i.e., 1.6 g·cm−3 compared to the GP made from water glass with lower densities.
- The most suitable GPs were prepared from water glass with density 1.5 g·cm−3 followed by solidification at a lowered temperature (5 °C). Such GPs then had the highest compressive strength, low total pore volume, and average pore diameter together with the lowest leachability of the structural elements and ions.
- Although the MW radiation shortened the solidification time to a minimum (26 min), it caused local overheating resulting in higher total pore volume (mainly in case of higher water glass density samples). This resulted in a lowered compressive strength of the MW treated samples.
Funding
Conflicts of Interest
References
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. Calorim. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Liew, Y.M.; Kamarudin, H.; Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Heah, C.Y. Optimization of solids-to-liquid and alkali activator ratios of calcined kaolin geopolymeric powder. Constr. Build. Mater. 2012, 37, 440–451. [Google Scholar] [CrossRef]
- Singh, N.B. Fly ash-based geopolymer binder: A future construction material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef]
- Rosas-Casarez, C.A.; Arredondo-Rea, S.P.; Cruz-Enríquez, A.; Corral-Higuera, R.; Gómez-Soberón, J.M.; Medina-Serna, T.D.J. Influence of size reduction of fly ash particles by grinding on the chemical properties of geopolymers. Appl. Sci. 2018, 8, 365. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S. The Physical and Chemical Characterisation of Fly Ash Based Geopolymers. Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, 2000. [Google Scholar]
- Temuujin, J.; Riessen, A.V.; MacKenzie, K.J.D. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Medina-Serna, T.D.J.; Arredondo-Rea, S.P.; Gómez-Soberón, J.M.; Rosas-Casarez, C.A.; Corral-Higuera, R. Effect of curing temperaturein the alkali-activated blast-furnace slag paste and their structural influence of porosity. Adv. Sci. Technol. Res. J. 2016, 10, 74–79. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Qianli, C.; Lin, C. Synthesis and heavy metal immobilization behaviours of slag based geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Zongjin, L. Composition design and microstructural characterization of calcined kaolin-based geopolymer cement. Appl. Clay Sci. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Granizo, M.L.; Varela, M.T.B.; Martinez-Ramirez, S. Alkali activation of metakaolins: Parameters affecting mechanical, structural and microstructural properties. J. Mater. Sci. 2007, 42, 2934–2943. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin based geopolymers. Constr. Build. Mater. 2012, 35, 912–992. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T.; Šupová, M. The identification of geopolymer affinity in specific cases of clay materials. Appl. Clay Sci. 2014, 102, 213–219. [Google Scholar] [CrossRef]
- Xie, J.; Kayali, O. Effect of water content on the development of fly ash-based geopolymers in heat and ambient curing conditions. In Proceedings of the Third International Conference on Sustainable Construction Materials and Technologies, Kyoto, Japan, 18–21 August 2013. [Google Scholar]
- Mu, S.; Liu, J.; Liu, J.; Wang, Y.; Shi, L.; Jiang, Q. Property and microstructure of waterborne self-setting geopolymer coating: Optimization effect of SiO2/Na2O molar ratio. Minerals 2018, 8, 162. [Google Scholar] [CrossRef]
- Subhash, V.P.; Yuwaraj, M.G.; Sanjay, S.J. Effect of concentration of sodium hydroxide and degree of heat curing on fly ash-based geopolymer mortar. Indian J. Mater. Sci. 2014, 2014, 938789. [Google Scholar] [CrossRef]
- Thakur, R.N.; Ghosh, S. Effect of mix composition on compressive strength and microstructure of fly ash based geopolymer composites. ARPN J. Eng. Appl. Sci. 2009, 4, 68–74. [Google Scholar]
- Bortnovsky, O.; Sobalik, S.; Tvaruzkova, Z.; Dedecek, J.; Roubicek, P.; Prudkova, Z.; Svoboda, M. Structure and stability of geopolymer synthesized from kaolinitic and shale clay residues. In Geopolymer, Green Chemistry and Sustainable Development Solutions: Proceedings of the World Congress Geopolymer; Davidovits, J., Ed.; Geopolymer Institute: Saint-Quentin, France, 2005; pp. 81–84. [Google Scholar]
- Somaratna, J.; Ravikumar, D.; Neithalath, N. Response of alkali activated fly ash mortars to microwave curing. Cem. Concr. Res. 2010, 40, 1688–1696. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Taebuanhuad, S. Role of microwave radiation in curing the fly ash geopolymer. Adv. Powder Technol. 2013, 24, 703–707. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the developmentof hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Patil, S.G.; ManojKumar. Factors Influencing compressive strength of geopolymer concrete. Int. J. Res. Eng. Technol. 2013, 372–375. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Aspects 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Aspects 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Xu, H. Geopolymerisation of Aluminosilicate Minerals. Ph.D. Thesis, University of Melbourne, Melbourne, Australia, 2001. [Google Scholar]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Shen, Y.J.; Zhang, Y.L.; Gao, F.; Yang, G.S.; Lai, X.P. Influence of Temperature on the Microstructure Deterioration of Sandstone. Energies 2018, 11, 1753. [Google Scholar] [CrossRef]
- Joseph, B.; Mathew, G. Influence of aggregate content on the behavior of fly ash based geopolymer concrete. Sci. Iranica 2012, 19, 1188–1194. [Google Scholar] [CrossRef]
- Kuenzel, C.; Li, L.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Influence of sand on the mechanical properties of metakaolin geopolymers. Constr. Build. Mater. 2014, 66, 442–446. [Google Scholar] [CrossRef]
- Latella, B.A.; Perera, D.S.; Durce, D.; Mehrtens, E.G.; Davis, J. Mechanical properties of metakaolin-based geopolymers with molar ratios of Si/Al ≈ 2 and Na/Al ≈ 1. J. Mater. Sci. 2008, 43, 2693–2699. [Google Scholar] [CrossRef]
- Granizo, N.; Palomo, A.; Fernandez-Jiménez, A. Effect of temperature and alkaline concentration on metakaolin leaching kinetics. Ceram. Int. 2014, 40, 8975–8985. [Google Scholar] [CrossRef] [Green Version]
- Panagiotopoulou, C.; Kontori, E.; Perraki, T.; Kakali, G. Dissolution of aluminosilicate minerals and by-products in alkaline media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Rouyer, J.; Benavent, V.; Frizon, F.; Poulesquen, A. Influence of geopolymer formulation parameters on the elastic and porous properties over a one-year monitoring. Mater. Lett. 2017, 207, 121–124. [Google Scholar] [CrossRef]








| Chemical Composition (wt %) | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | Na2O | CaO | TiO2 | Fe2O3 | LOI H2O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcined kaolinite claystone | 0.13 | 41.45 | 52.03 | 0.06 | 0.20 | 0.79 | 0.15 | 1.62 | 1.05 | 2.52 | |
| Water glass | 27.75 | 26.17 | 1.11 | 44.97 |
| Geopolymer | Water Glass Density (g·cm−3) | Total Molar Ratio | ||||
|---|---|---|---|---|---|---|
| Si:Al | M:Al | H2O:Al | K:Na | Ca:Al | ||
| GP1.2 | 1.2 | 1.40 | 0.48 | 8.61 | 16.12 | 0.12 |
| GP1.3 | 1.3 | 1.54 | 0.66 | 7.71 | 15.95 | 0.12 |
| GP1.4 | 1.4 | 1.65 | 0.82 | 6.93 | 15.87 | 0.12 |
| GP1.5 | 1.5 | 1.76 | 0.95 | 6.25 | 15.82 | 0.12 |
| GP1.6 | 1.6 | 1.85 | 1.06 | 5.66 | 15.78 | 0.12 |
| Label | Curing Methods | Time to Hardening |
|---|---|---|
| LT | solidify at ambient temperature in laboratory (22 °C) | 12 h |
| 70 °C | solidify at 70 °C during 3 h | 3 h |
| MW/2 | solidify at ambient temperature and 2nd day 26 min at microwave radiation | 12 h |
| MW | solidify 26 min at microwave radiation | 26 min |
| 5 °C/LT | solidify at 5 °C during 12 h and then at ambient temperature in laboratory (22 °C) | 18 h |
| 5 °C | solidify at 5 °C | 24 h |
| Water Glass Density (g·cm−3) | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 |
|---|---|---|---|---|---|
| Pore Volume (mm3·g−1) | 166.9 | 143.5 | 122.7 | 94.2 | 75.8 |
| Average Pore Diameter (nm) | 32.4 | 16 | 16.5 | 19 | 47.2 |
| Curing Method | 70 °C | MW | MW/2 | LT | 5 °C/LT | 5 °C |
|---|---|---|---|---|---|---|
| Pore Volume (mm3·g−1) | 104.1 | 110.7 | 100.5 | 94.2 | 81.4 | 73.3 |
| Average Pore Diameter (nm) | 15.4 | 16.9 | 14.5 | 19.0 | 17.6 | 19.2 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hájková, P. Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals 2018, 8, 444. https://doi.org/10.3390/min8100444
Hájková P. Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals. 2018; 8(10):444. https://doi.org/10.3390/min8100444
Chicago/Turabian StyleHájková, Pavlína. 2018. "Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions" Minerals 8, no. 10: 444. https://doi.org/10.3390/min8100444
APA StyleHájková, P. (2018). Kaolinite Claystone-Based Geopolymer Materials: Effect of Chemical Composition and Curing Conditions. Minerals, 8(10), 444. https://doi.org/10.3390/min8100444