One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures
Abstract
1. Introduction
2. Experiments and Methods
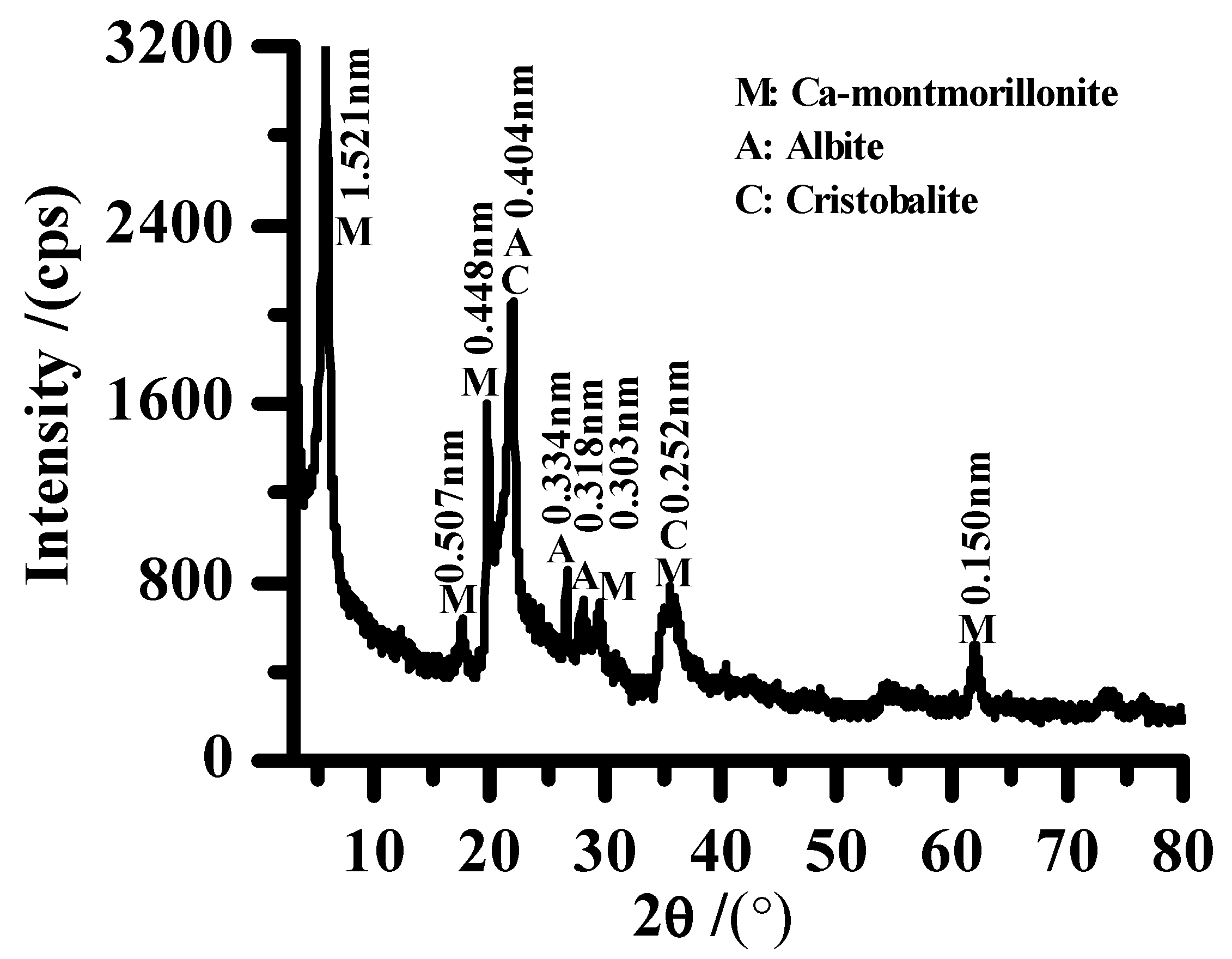
2.1. Materials
2.2. Synthesis
2.3. Characterization
3. Results and Discussion
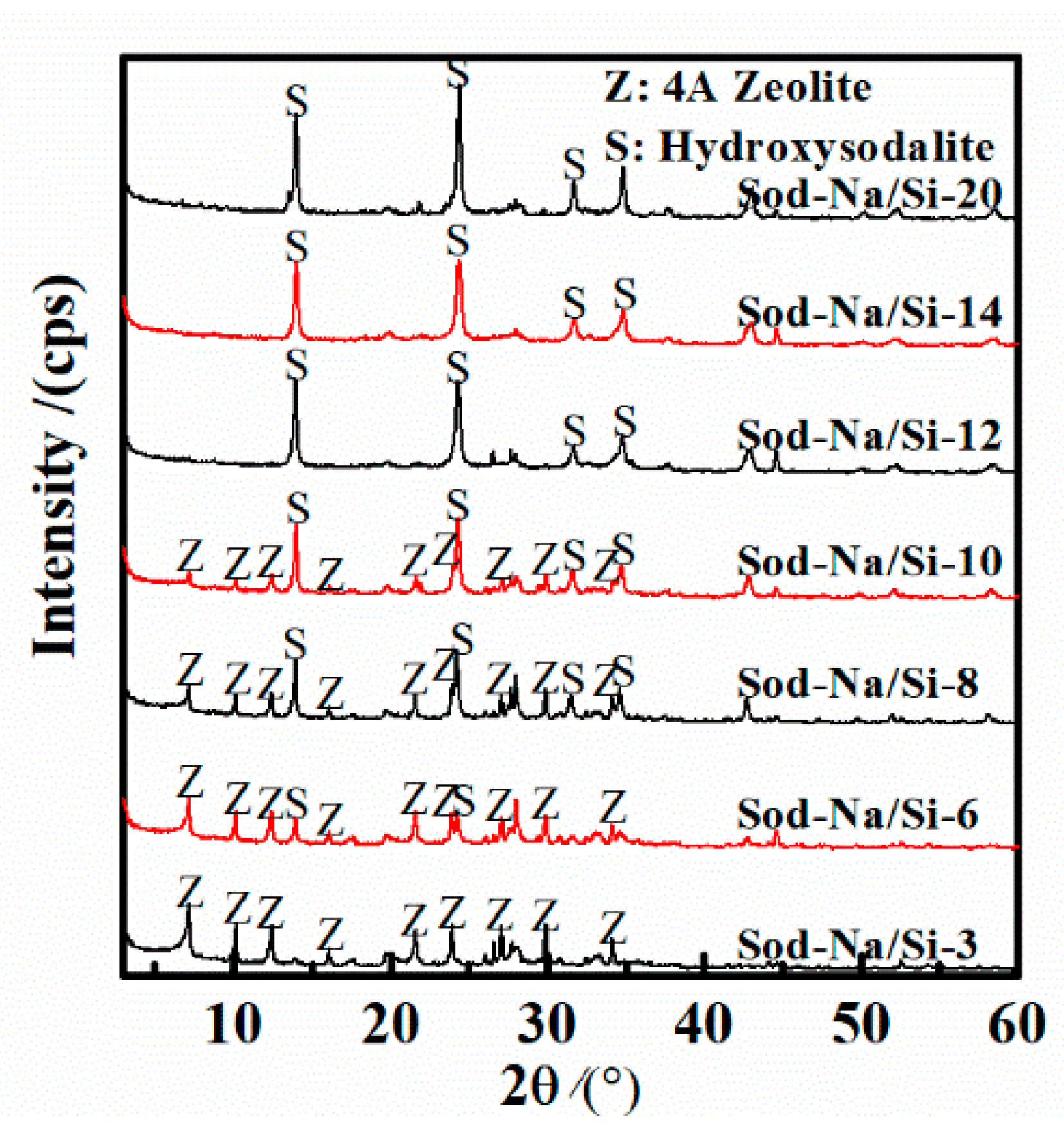
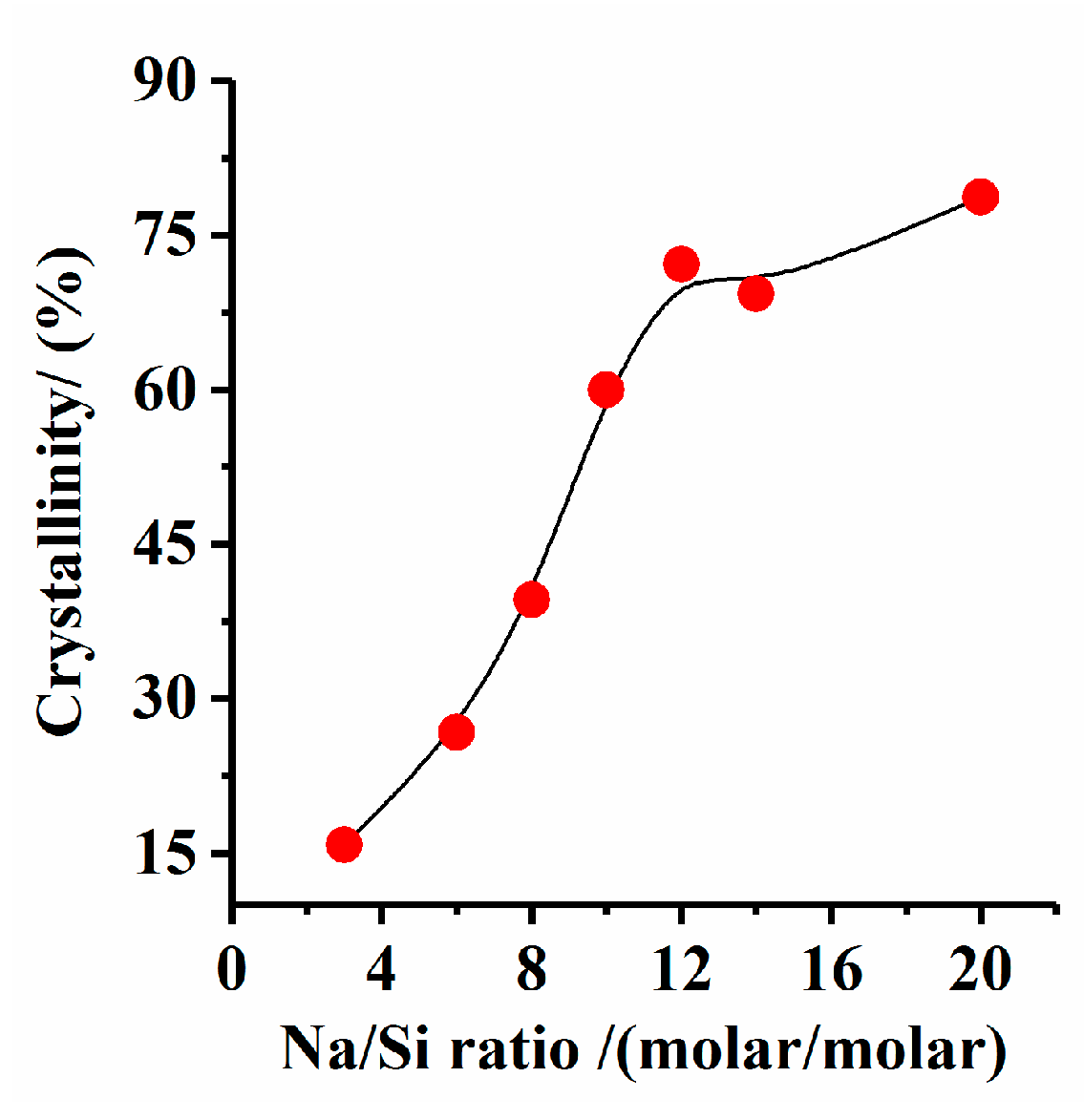
3.1. Effect of Na/Si Molar Ratio
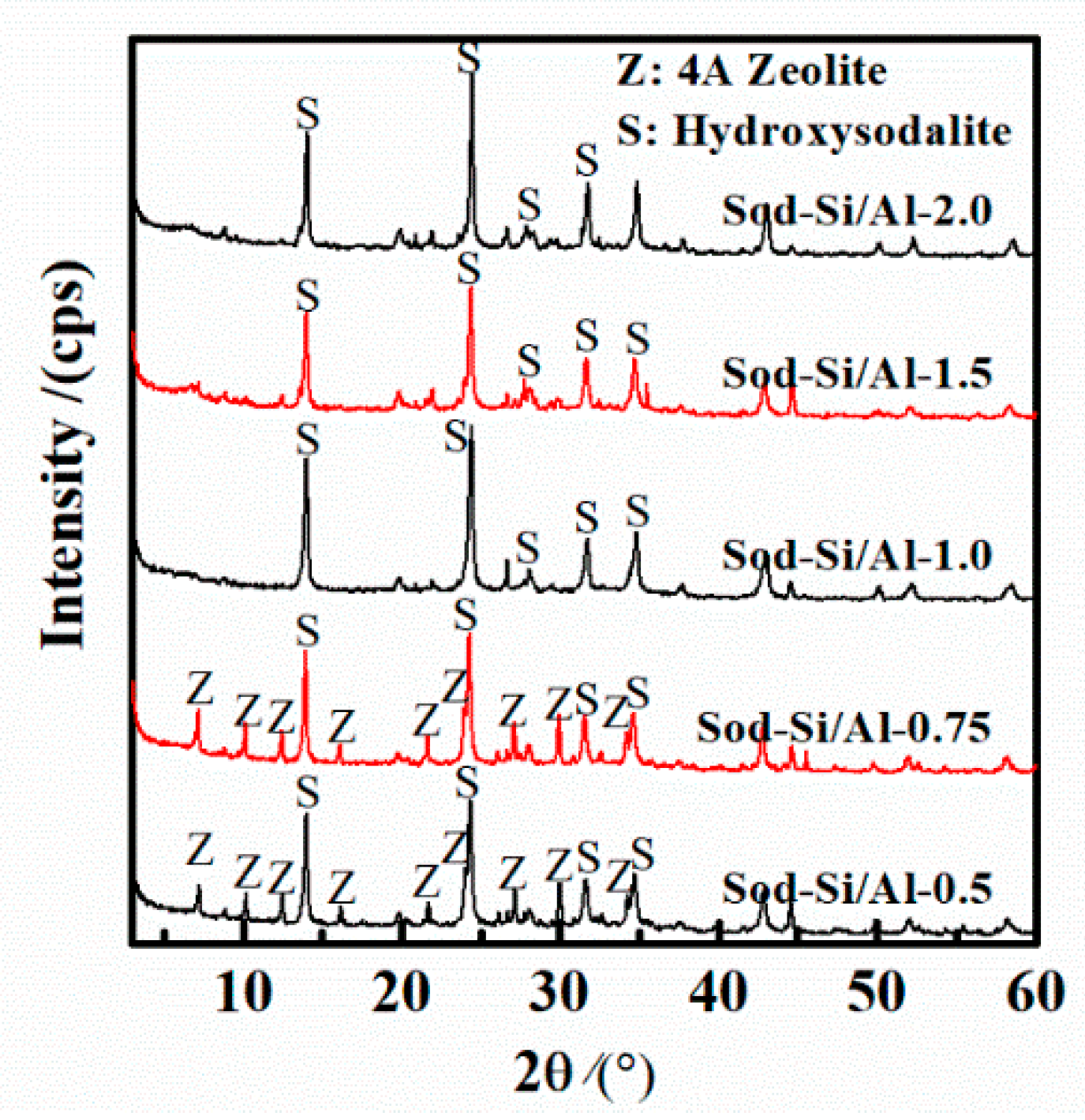
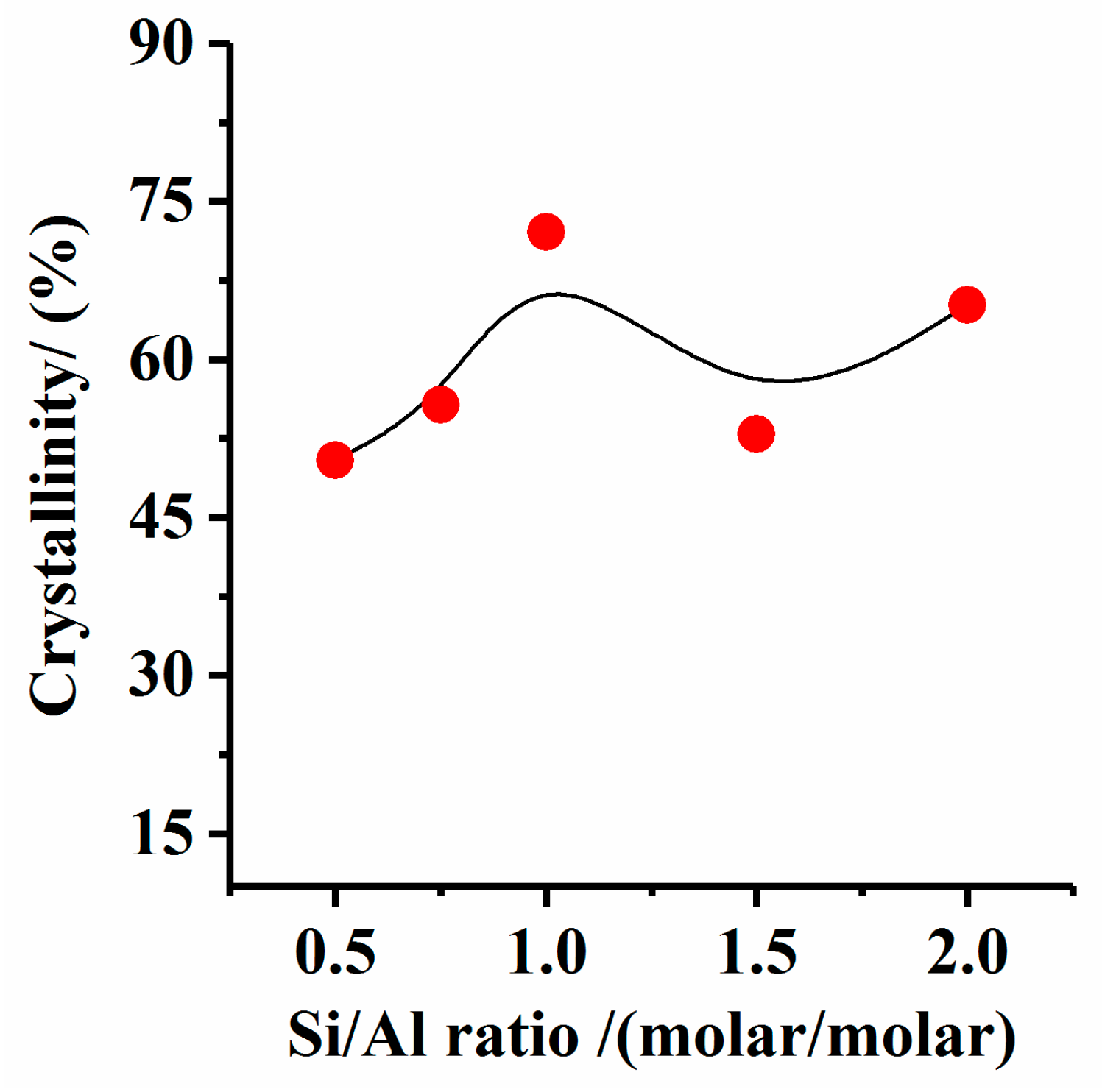
3.2. Effect of Si/Al Molar Ratio
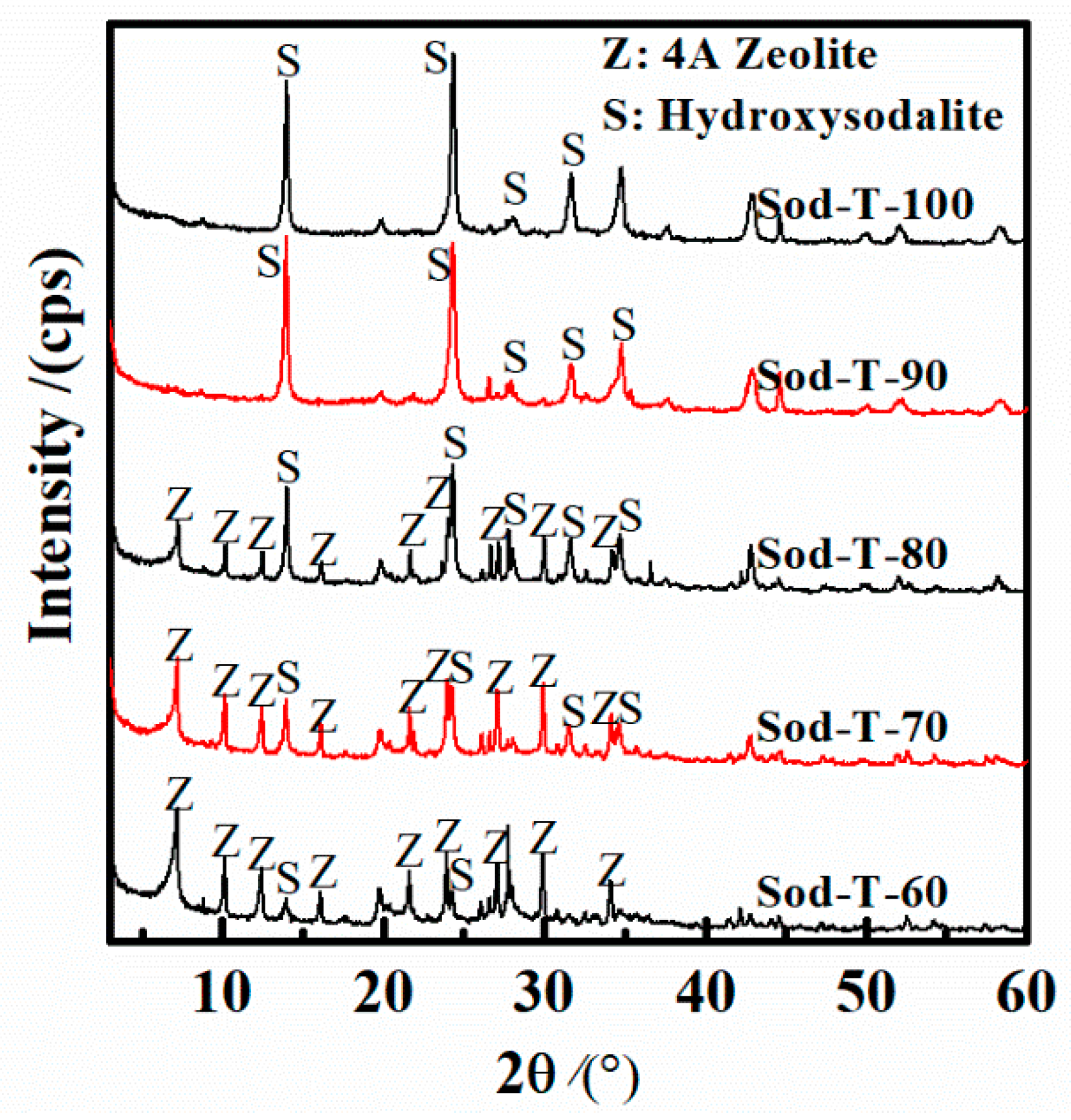
3.3. Effect of Reaction Temperature
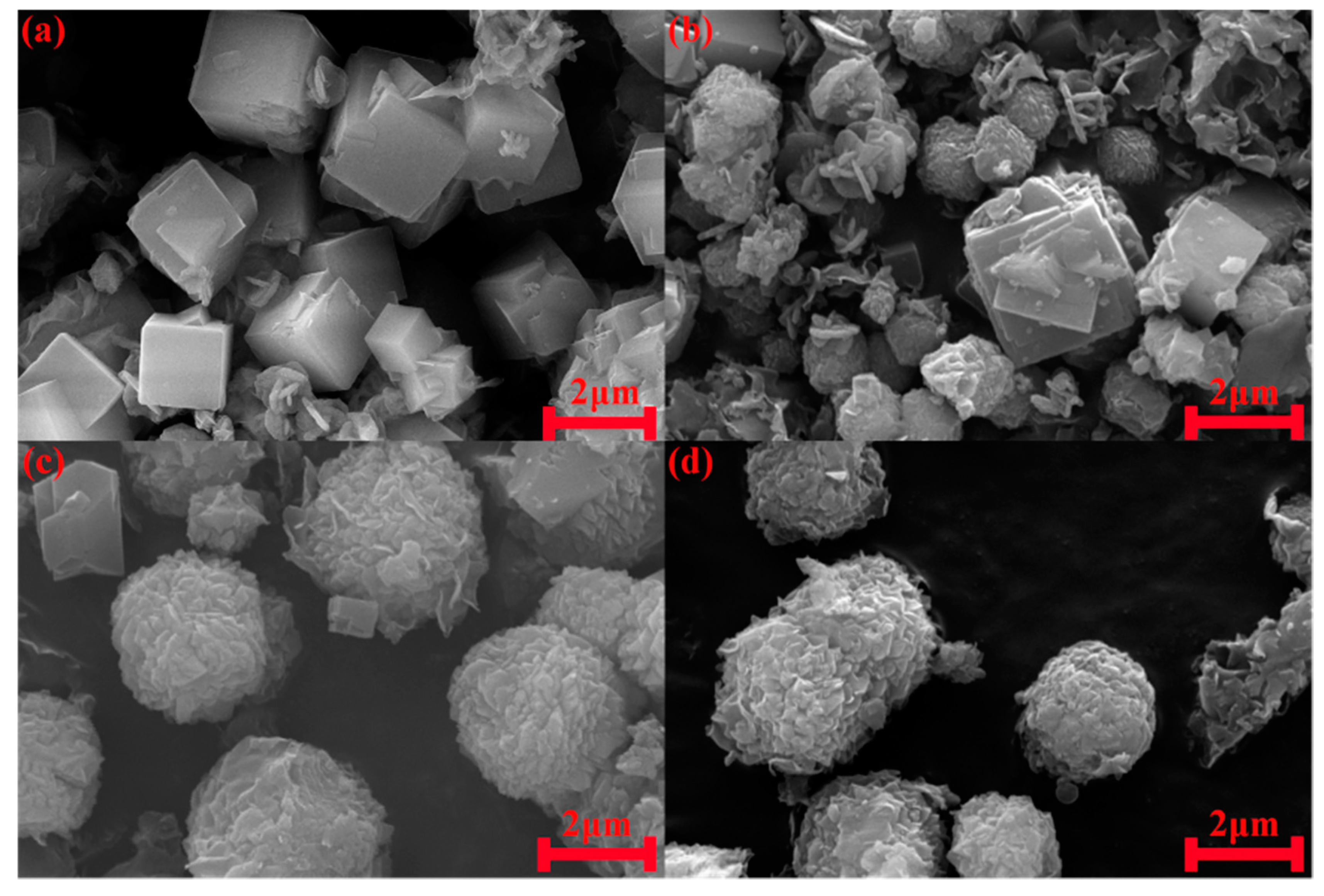
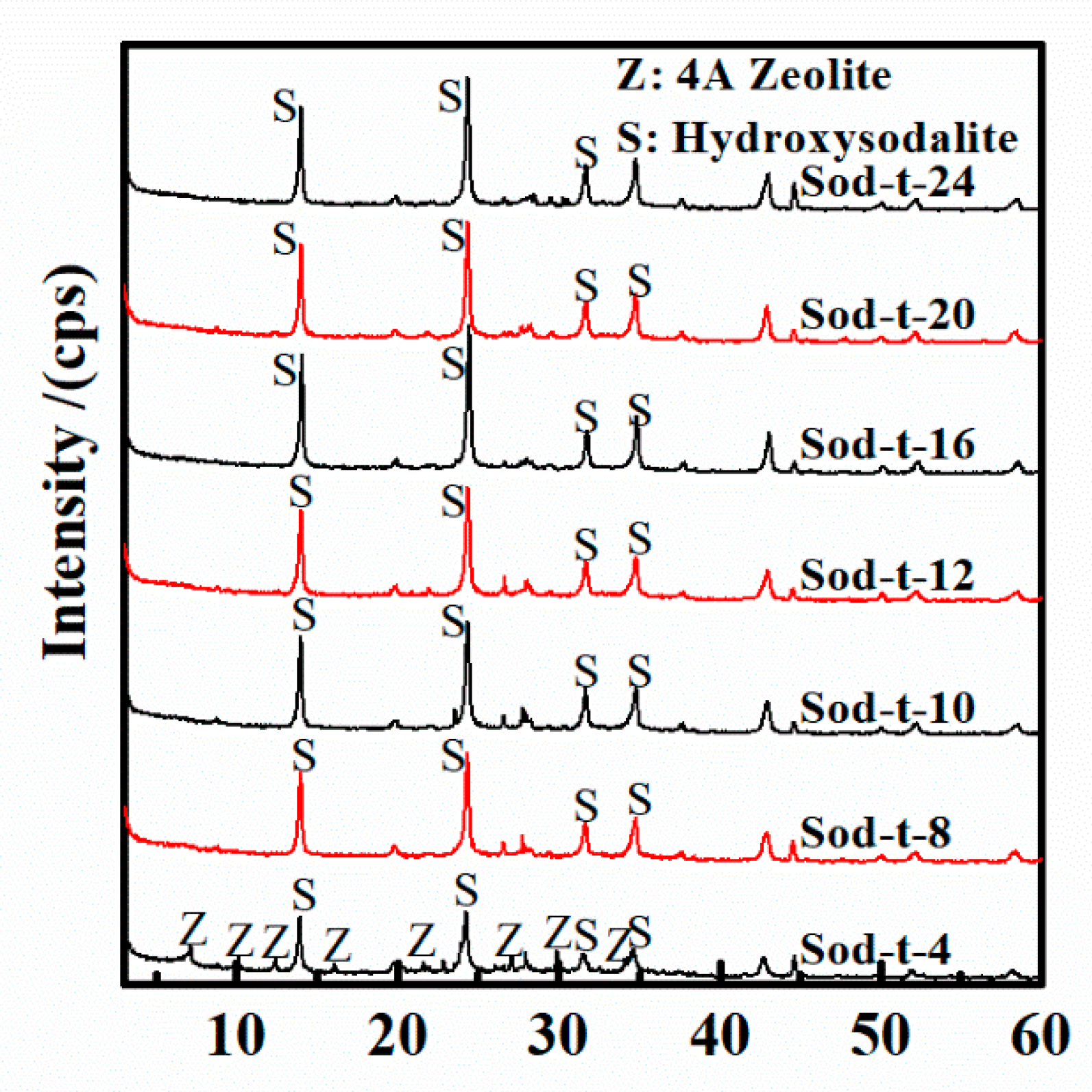
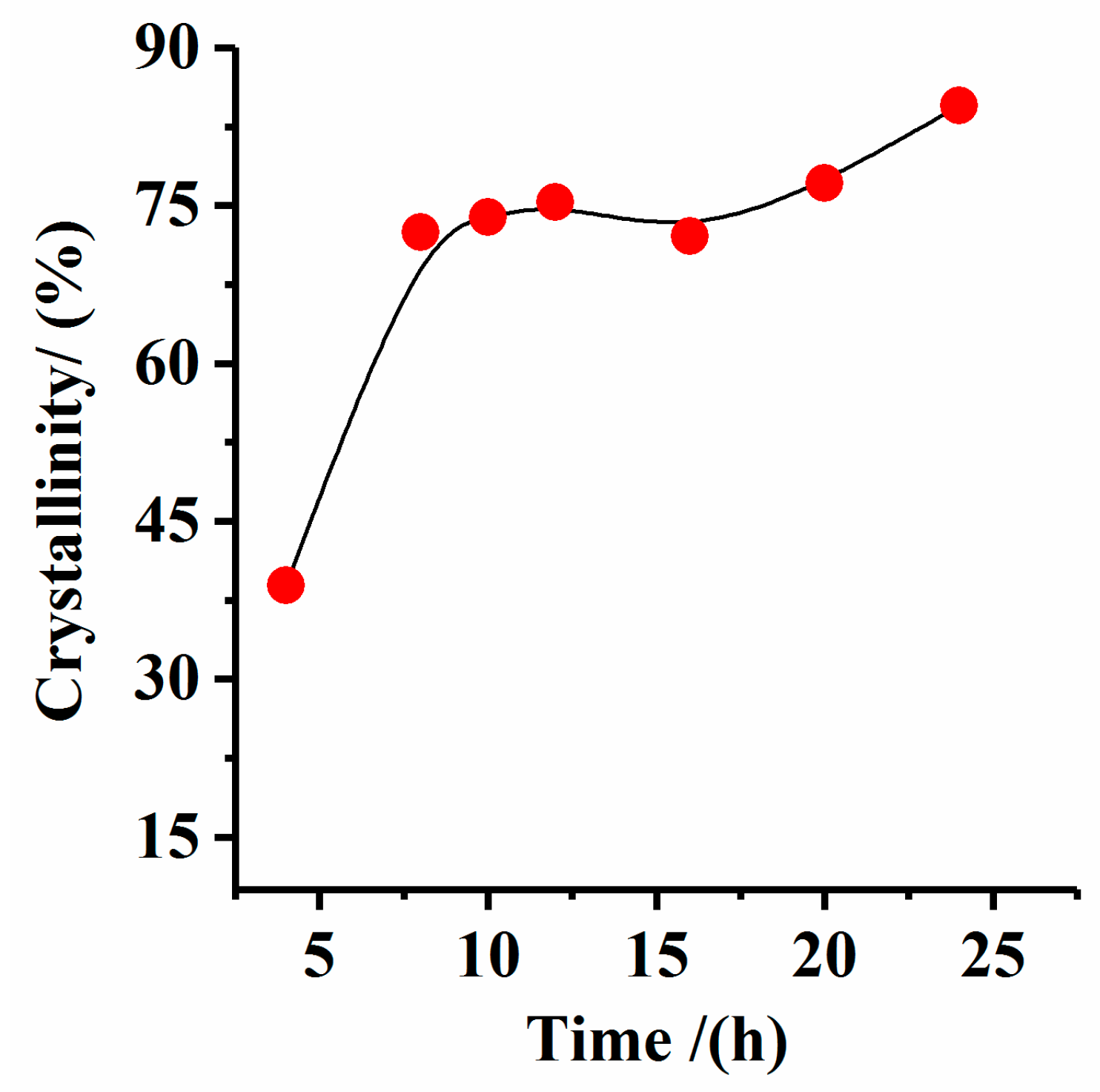
3.4. Effect of Reaction Time
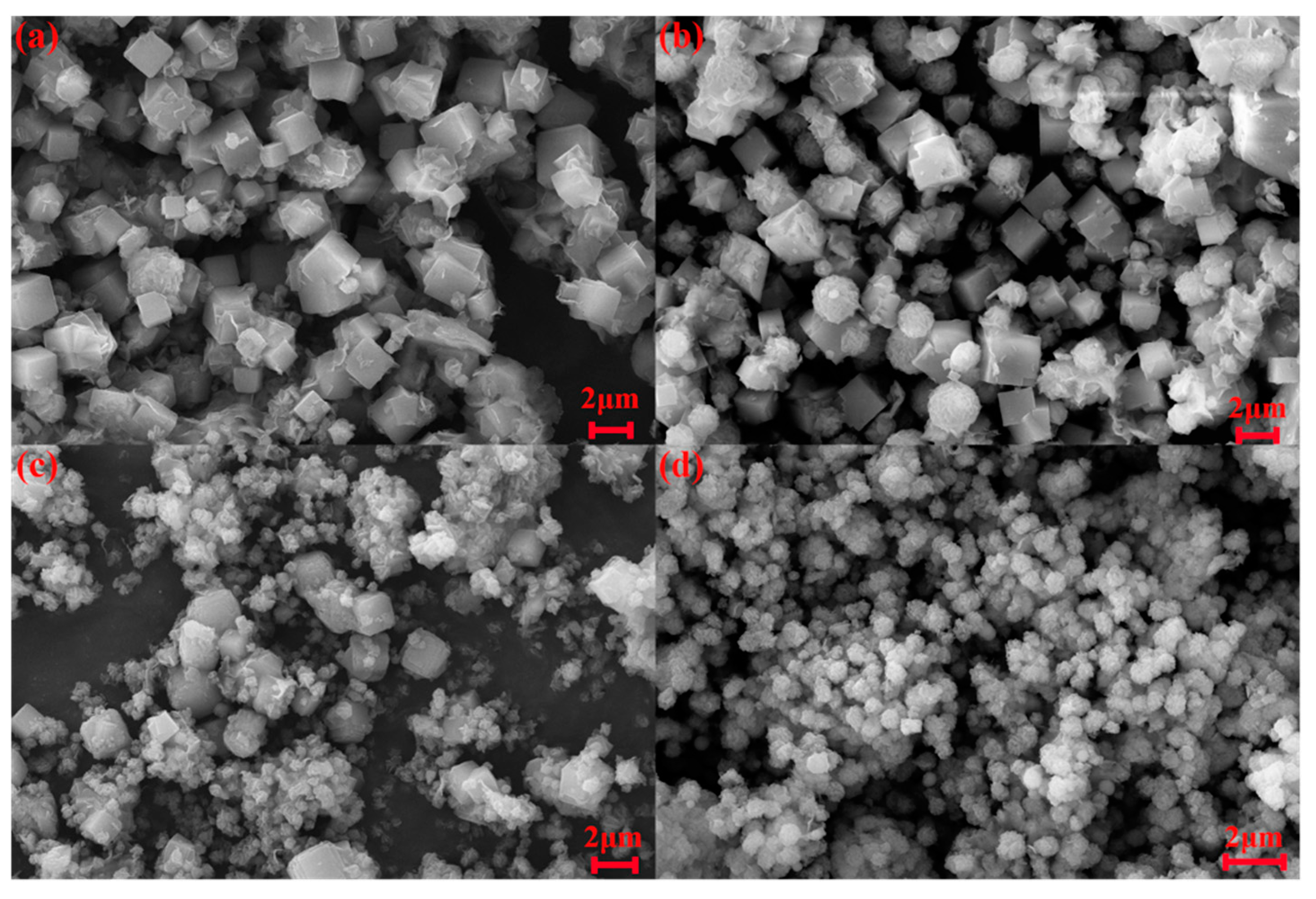
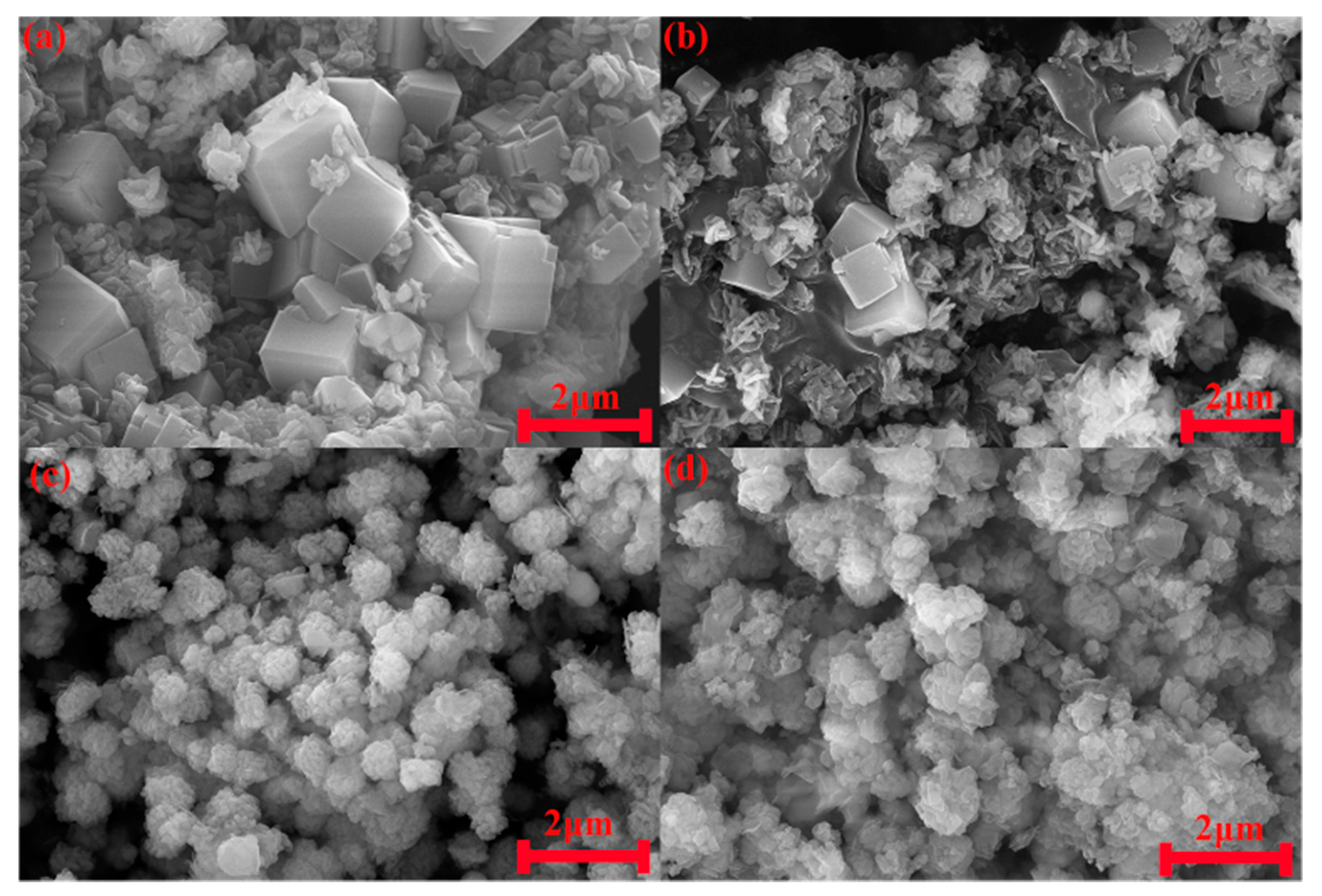
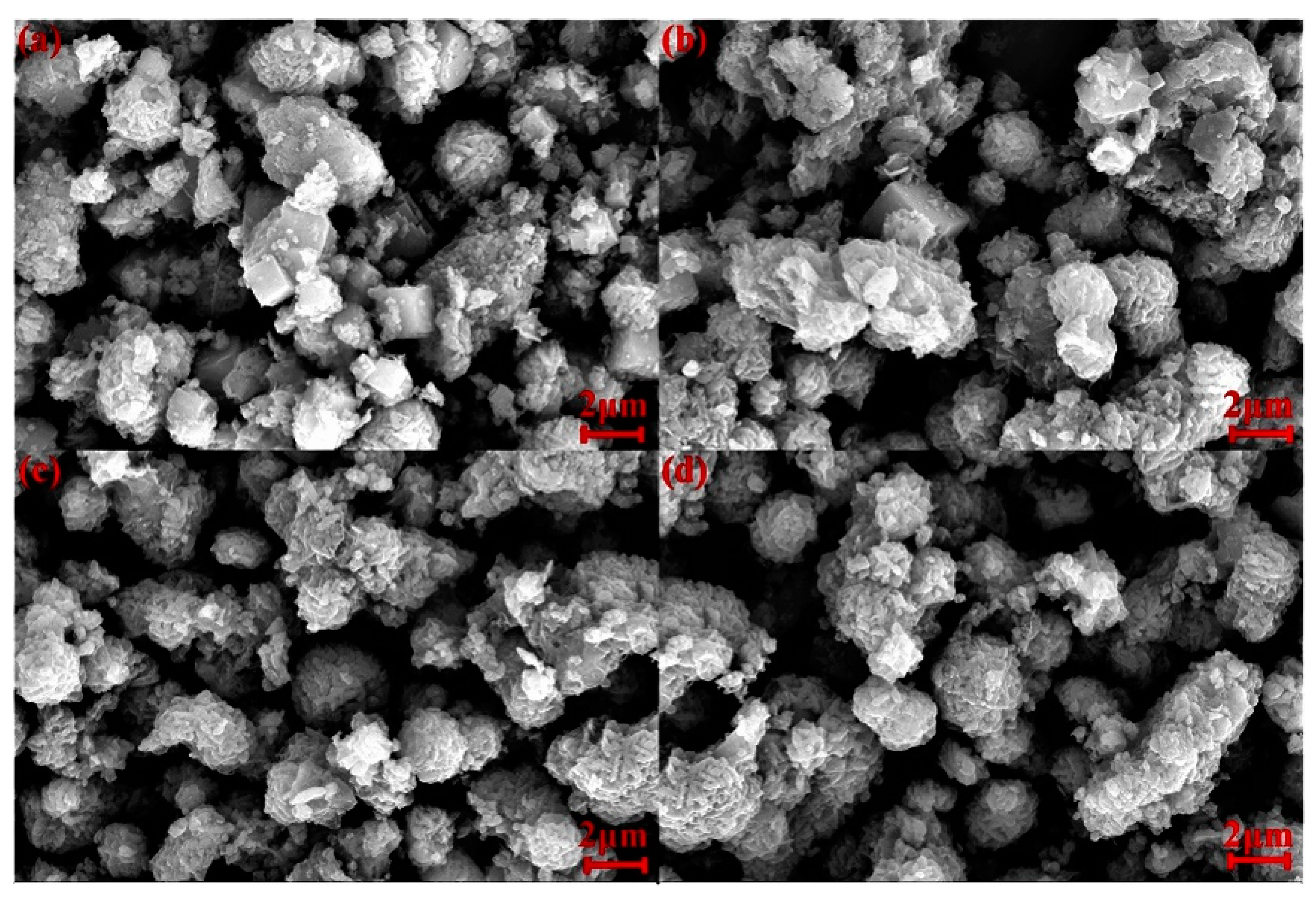
3.5. Characterization of Hydroxysodalite in Optimum Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bibby, D.M.; Dale, M.P. Synthesis of silica-sodalite from non-aqueous systems. Nature 1985, 317, 157. [Google Scholar] [CrossRef]
- Li, J.; Zeng, X.; Yang, X.; Wang, C.; Luo, X. Synthesis of pure sodalite with wool ball morphology from alkali fusion kaolin. Mater. Lett. 2015, 161, 157–159. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, L.; Wang, H. Synthesis of nanocrystalline sodalite with organic additives. Mater. Lett. 2008, 62, 4028–4030. [Google Scholar] [CrossRef]
- Naskar, M.K.; Kundu, D.; Chatterjee, M. Effect of process parameters on surfactant-based synthesis of hydroxy sodalite particles. Mater. Lett. 2011, 65, 436–438. [Google Scholar] [CrossRef]
- Johnson, G.M.; Mead, P.J.; Weller, M.T. Synthesis of a range of anion-containing gallium and germanium sodalites. Microporous Mesoporous Mater. 2000, 38, 445–460. [Google Scholar] [CrossRef]
- Hu, T.; Qiu, J.; Wang, Y.; Wang, C.; Liu, R.; Meng, C. Synthesis of low Si/Al ratio hydroxysodalite from oil shale ash without pretreatment. J. Chem. Technol. Biotechnol. 2015, 90, 208–212. [Google Scholar] [CrossRef]
- Van den Berg, A.W.C.; Bromley, S.T.; Jansen, J.C. Thermodynamic limits on hydrogen storage in sodalite framework materials: A molecular mechanics investigation. Microporous Mesoporous Mater. 2005, 78, 63–71. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of hydroxy sodalite films as novel water selective membranes. J. Membr. Sci. 2009, 326, 153–160. [Google Scholar] [CrossRef]
- Khajavi, S.; Jansen, J.C.; Kapteijn, F. Application of a sodalite membrane reactor in esterification-coupling reaction and separation. Catal. Today 2010, 156, 132–139. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Song, C.; Yang, W.; Liu, J.; Lin, L. Microwave-assisted hydrothermal synthesis of hydroxy-sodalite zeolite membrane. Microporous Mesoporous Mater. 2004, 75, 173–181. [Google Scholar] [CrossRef]
- Nabavi, M.S.; Mohammadi, T.; Kazemimoghadam, M. Hydrothermal synthesis of hydroxy sodalite zeolite membrane: Separation of H2/CH4. Ceram. Int. 2014, 40, 5889–5896. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, X.; Feng, L.; Duanmu, C.; Jin, Y.; Hu, T.; Wu, J. Controllable synthesis of sodalite submicron crystals and microspheres from palygorskite clay using a two-step approach. Powder Tech. 2012, 217, 298–303. [Google Scholar] [CrossRef]
- Passos, F.A.C.M.; Castro, D.C.; Ferreira, K.K.; Simões, K.M.A.; Bertolino, L.C.; Barbato, C.N.; Garrido, F.M.S.; Felix, A.A.S.; Silva, F.A.N.G. Synthesis and characterization of sodalite and cancrinite from Kaolin. In Characterization of Minerals Metals and Materials; Springer: Berlin, Germany, 2017; pp. 279–288. [Google Scholar]
- Golbad, S.; Khoshnoud, P.; Abu-Zahra, N. Hydrothermal synthesis of hydroxy sodalite from fly ash for the removal of lead ions from water. Int. J. Environ. Sci. Technol. 2017, 14, 135–142. [Google Scholar] [CrossRef]
- Bouberka, Z.; Kacha, S.; Kameche, M.; Elmaleh, S.; Derriche, Z. Sorption study of an acid dye from an aqueous solutions using modified clays. J. Hazard. Mater. 2005, 119, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Karnland, O.; Olsson, S.; Nilsson, U.; Sellin, P. Experimentally determined swelling pressures and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions. Phys. Chem. Earth Parts A/B/C 2007, 32, 275–286. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sonderby, C.; Sogaard, E.G. Synthesis and characterization of silicate polymers. J. Sol-Gel Sci. Technol. 2009, 50, 372. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sonderby, C.; Li, Z.; Søgaard, E.G. XPS and FT-IR investigation of silicate polymers. J. Mater. Sci. 2009, 44, 2079. [Google Scholar] [CrossRef]
- Tang, W.; Han, J.; Zhang, S.; Sun, J.; Li, H.; Gu, X. Synthesis of 4A zeolite containing La from kaolinite and its effect on the flammability of polypropylene. Polym. Compos. 2018, 39, 3461–3471. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, T.; Liu, H.; Wang, C.; Cheng, P.; Chen, D.; Xie, J. An insight into the comprehensive application of opal-palygorskite clay: Synthesis of 4A zeolite and uptake of Hg2+. Appl. Clay Sci. 2018, 165, 103–111. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, H.; Lin, J.; Lin, Z.; Sun, J. Zeolite A synthesized from alkaline assisted pre-activated halloysite for efficient heavy metal removal in polluted river water and industrial wastewater. J. Environ. Sci. 2017, 56, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Itani, L.; Liu, Y.; Zhang, W.; Bozhilov, K.N.; Delmotte, L.; Valtchev, V. Investigation of the physicochemical changes preceding zeolite nucleation in a sodium-rich aluminosilicate gel. J. Am. Chem. Soc. 2009, 131, 10127–10139. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.C.; Addai-Mensah, J.; Gerson, A.R. The mechanism of the sodalite-to-cancrinite phase transformation in synthetic spent Bayer liquor. Microporous Mesoporous Mater. 1999, 31, 287–302. [Google Scholar] [CrossRef]
- Liu, Q.; Navrotsky, A. Synthesis of nitrate sodalite: An in situ scanning calorimetric study. Geochim. Cosmochim. Acta 2007, 71, 2072–2078. [Google Scholar] [CrossRef]
- Fan, W.; Morozumi, K.; Kimura, R.; Yokoi, T.; Okubo, T. Synthesis of nanometer-sized sodalite without adding organic additives. Langmuir 2008, 24, 6952–6958. [Google Scholar] [CrossRef] [PubMed]
- Geon, J.K.; Wha, S.A. Synthesis and characterization of iron-modified ZSM-5. Appl. Catal. 1991, 71, 55–68. [Google Scholar] [CrossRef]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal synthesis of mixtures of NaA zeolite and sodalite from Ti-bearing electric arc furnace slag. RSC Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Tounsi, H.; Mseddi, S.; Djemel, S. Preparation and characterization of Na-LTA zeolite from Tunisian sand and aluminum scrap. Phys. Procedia 2009, 2, 1065–1074. [Google Scholar] [CrossRef]
- Mofrad, A.M.; Peixoto, C.; Blumeyer, J.; Liu, J.; Hunt, H.K.; Hammond, K.D. Vibrational spectroscopy of sodalite: Theory and experiments. J. Phys. Chem. C 2018, 122, 24765–24779. [Google Scholar] [CrossRef]
- Khajavi, S.; Kapteijn, F.; Jansen, J.C. Synthesis of thin defect-free hydroxy sodalite membranes: New candidate for activated water permeation. J. Membr. Sci. 2007, 299, 63–72. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Helffrich, G.R.; Bohlen, S.R.; Essene, E.J. The stability of sodalite in the system NaAlSiO4-NaCl. Geochim. Cosmochim. Acta 1989, 53, 1943–1954. [Google Scholar] [CrossRef]
- Gaidoumi, A.E.; Benabdallah, A.C.; Bali, B.E.; Kherbeche, A. Synthesis and characterization of zeolite HS using natural pyrophyllite as new clay source. Arabian J. Sci. Eng. 2018, 43, 191–197. [Google Scholar] [CrossRef]




| Sample | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | TiO2 | K2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Bentonite | 67.73 | 11.54 | 1.75 | 1.64 | 1.74 | 0.01 | 0.09 | 0.28 | 15.30 |
| NO. | Bentonite (g) | NaOH (g) | NaAlO2 (g) | T (°C) | t (h) | H2O (mL) |
|---|---|---|---|---|---|---|
| Sod-Na/Si-3 | 1 | 0.99 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-6 | 1 | 2.35 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-8 | 1 | 3.25 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-10 | 1 | 4.15 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-12 | 1 | 5.06 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-14 | 1 | 5.96 | 0.74 | 90 | 12 | 35 |
| Sod-Na/Si-20 | 1 | 8.67 | 0.74 | 90 | 12 | 35 |
| Sod-Si/Al-0.5 | 1 | 4.61 | 1.67 | 90 | 12 | 35 |
| Sod-Si/Al-0.75 | 1 | 4.91 | 1.05 | 90 | 12 | 35 |
| Sod-Si/Al-1.0 | 1 | 5.06 | 0.74 | 90 | 12 | 35 |
| Sod-Si/Al-1.5 | 1 | 5.21 | 0.43 | 90 | 12 | 35 |
| Sod-Si/Al-2.0 | 1 | 5.28 | 0.28 | 90 | 12 | 35 |
| Sod-T-60 | 1 | 5.06 | 0.74 | 60 | 12 | 35 |
| Sod-T-70 | 1 | 5.06 | 0.74 | 70 | 12 | 35 |
| Sod-T-80 | 1 | 5.06 | 0.74 | 80 | 12 | 35 |
| Sod-T-90 | 1 | 5.06 | 0.74 | 90 | 12 | 35 |
| Sod-T-100 | 1 | 5.06 | 0.74 | 100 | 12 | 35 |
| Sod-t-4 | 1 | 5.06 | 0.74 | 90 | 4 | 35 |
| Sod-t-8 | 1 | 5.06 | 0.74 | 90 | 8 | 35 |
| Sod-t-10 | 1 | 5.06 | 0.74 | 90 | 10 | 35 |
| Sod-t-12 | 1 | 5.06 | 0.74 | 90 | 12 | 35 |
| Sod-t-16 | 1 | 5.06 | 0.74 | 90 | 16 | 35 |
| Sod-t-20 | 1 | 5.06 | 0.74 | 90 | 20 | 35 |
| Sod-t-24 | 1 | 5.06 | 0.74 | 90 | 24 | 35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Sun, H.; Peng, T.; He, Q. One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures. Minerals 2018, 8, 521. https://doi.org/10.3390/min8110521
Liu B, Sun H, Peng T, He Q. One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures. Minerals. 2018; 8(11):521. https://doi.org/10.3390/min8110521
Chicago/Turabian StyleLiu, Bo, Hongjuan Sun, Tongjiang Peng, and Qian He. 2018. "One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures" Minerals 8, no. 11: 521. https://doi.org/10.3390/min8110521
APA StyleLiu, B., Sun, H., Peng, T., & He, Q. (2018). One-Step Synthesis of Hydroxysodalite Using Natural Bentonite at Moderate Temperatures. Minerals, 8(11), 521. https://doi.org/10.3390/min8110521






