Silician Magnetite from the Copiapó Nordeste Prospect of Northern Chile and Its Implication for Ore-Forming Conditions of Iron Oxide–Copper–Gold Deposits
Abstract
:1. Introduction
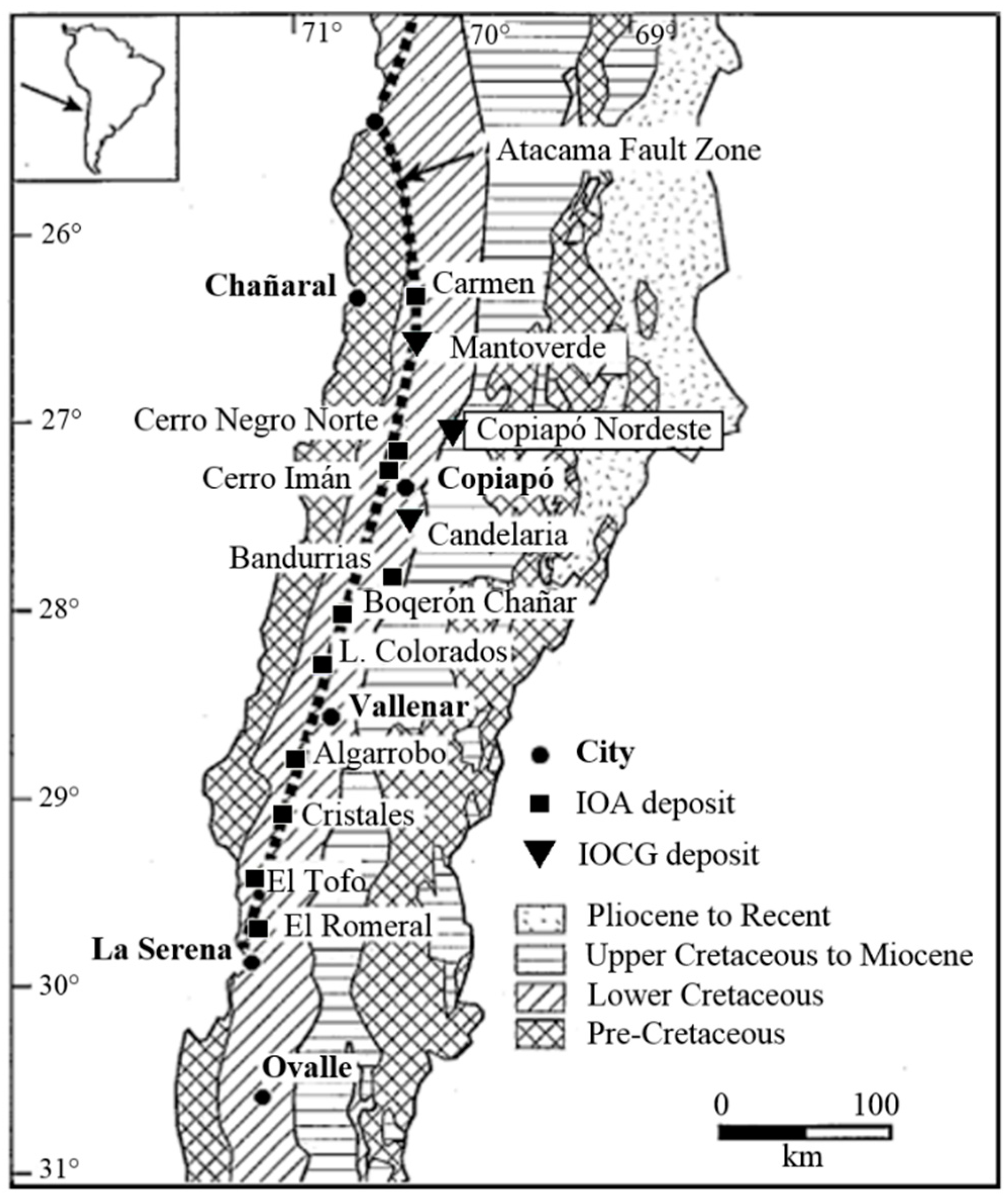
2. Geologic Framework
3. Analytical Methods
4. Analytical Results
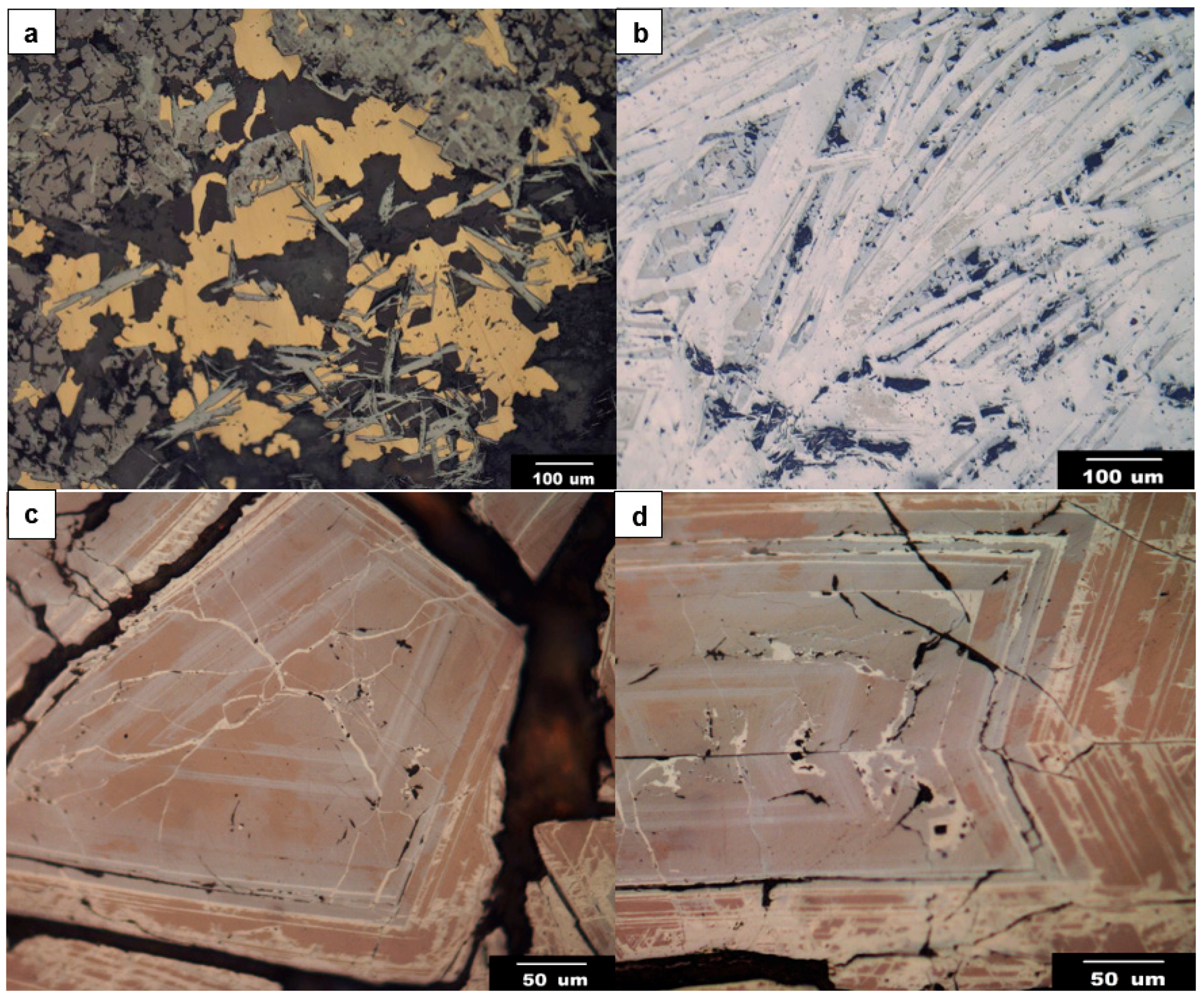
4.1. Microscopic Descriptions
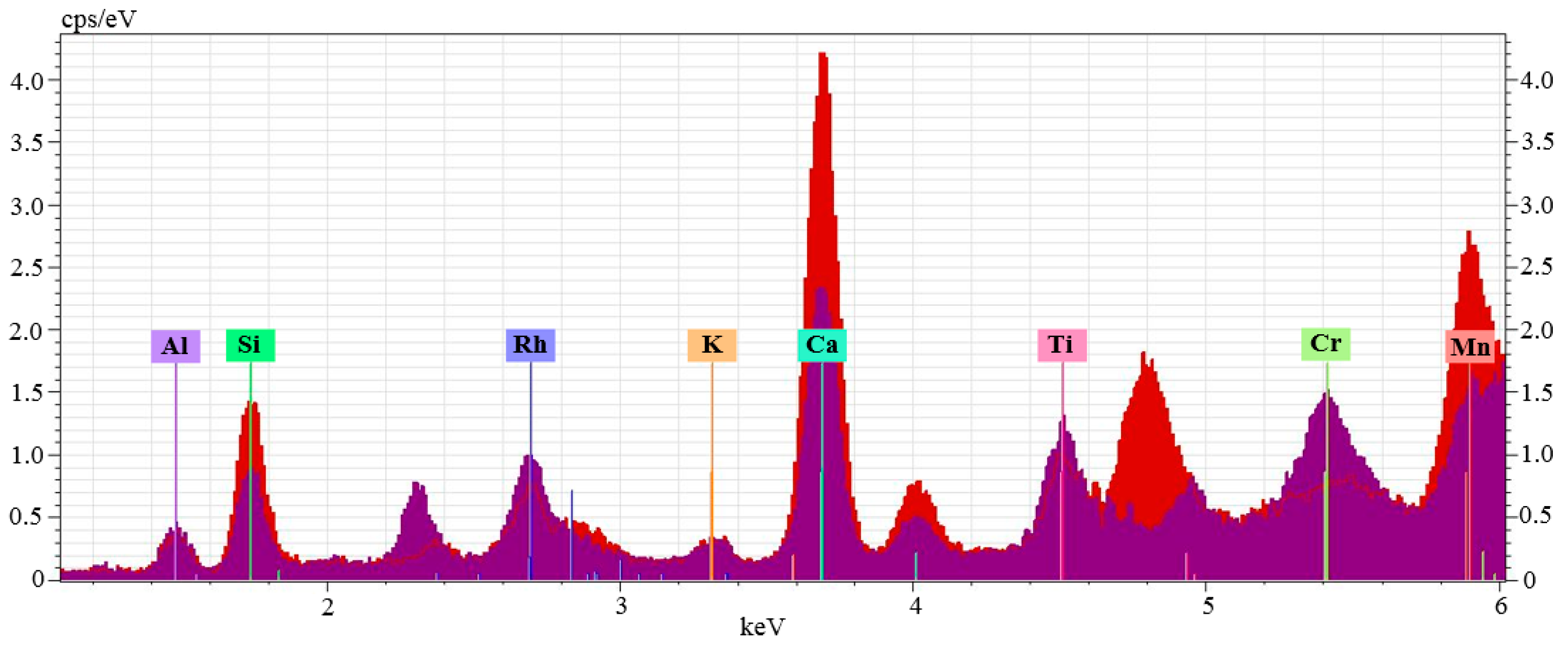
4.2. Micro-X-ray Fluorescence Analysis
4.3. EPMA
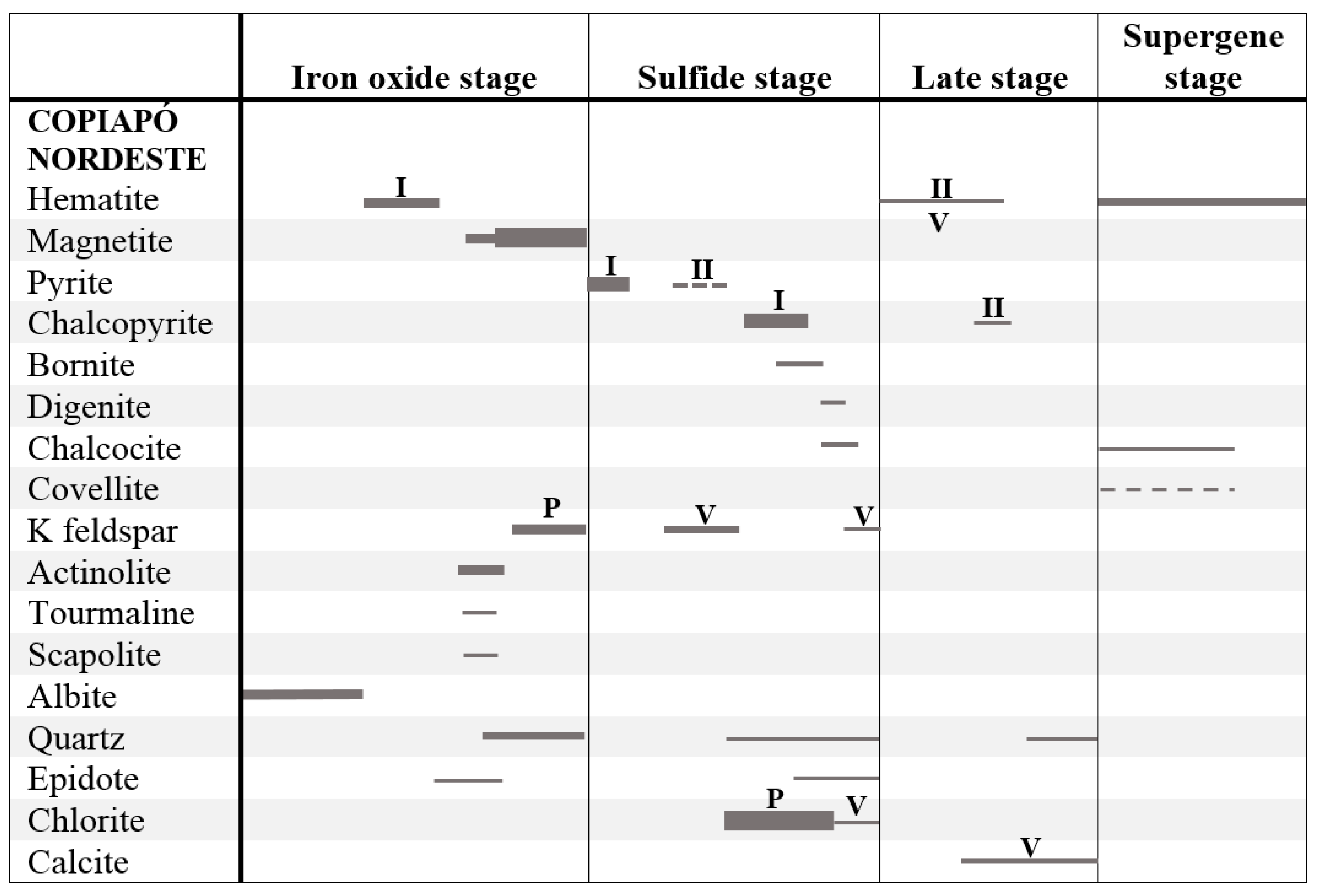
5. Discussion
5.1. Substitution Chemistry in Magnetite and Martitization
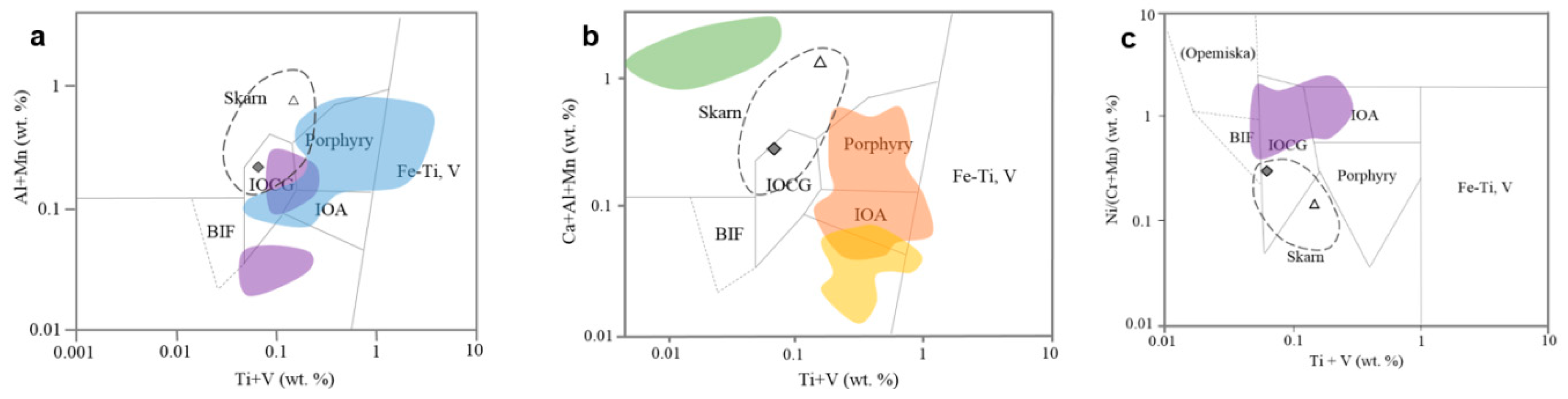
5.2. Comparison with Other Deposits
5.3. Magnetite Ore-Forming Condition
6. Summary and Conclusions
- Silician magnetite occurs in the calcic to potassic alteration stages as orderly oscillatory layers in polyhedral magnetite and isolated discrete grains. Silician magnetite is distinct in color and reflectance from normal magnetite, and can be optically distinguished with the reflecting microscope.
- Qualitative and quantitative microanalyses show that silician magnetite contains significant amounts of SiO2 with lesser contents of such “impure” components as Al2O3, CaO, MgO, TiO2, and MnO. In general, oscillatory-zoned magnetite is richer in SiO2 than the discrete grains, and the highest values of SiO2 contents reach up to 7.5 wt % in the oscillatory layers and to 2.8 wt % in the discrete grains. The SiO2 impurity prevents silician magnetite from undergoing martitization.
- The formation of silician magnetite is represented by the substitutional reactions of 2Fe3+ ⇌ Si4+ + Fe2+, with the incidental reactions of Fe3+ ⇌ Al3+ and 2Fe2+ ⇌ Ca2+ + Mg2+.
- Silician magnetite from the Copiapó Nordeste has no dissolution–reprecipitation texture, and displays an intermediate chemical characteristic between the skarn and porphyry–IOA deposits in the previously published discrimination diagrams.
- Silician magnetite occurs in a wide variety of types of ore deposits from high-temperature porphyry- and skarn-type to low-temperature BIF. The higher contents of SiO2 (3.8–8.9 wt %) are noted in the intrusion-related magmatic–hydrothermal deposits, including the IOCG-type. These types of deposits are considered to have been derived from relatively high-temperature and hypersaline fluids, which are responsible for the formation of silician magnetite with high SiO2 contents.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Knipping, J.; Bilenker, J.; Simon, A.; Reich, M.; Barra, F.; Deditius, A.; Wälle, M.; Heinrich, C.; Holtz, F.; Munizaga, R. Trace elements in magnetite from massive iron oxide-apatite deposits indicate a combined formation by igneous and magmatic-hydrothermal processes. Geochim. Cosmochim. Acta 2015, 171, 15–38. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.J.; Barton, M.; Johnson, D.A.; Fontboté, L.; de Haller, A.; Mark, G.; Oliver, N.H.S.; Marschik, R. Iron-oxide copper-gold deposits: Geology, space-time distribution, and possible models of origin. In Economic Geology; 100th Anniversary Volume; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2005; pp. 371–405. [Google Scholar]
- Shiga, Y. Silician magnetite from the Kamaishi mine, Japan. Min. Geol. 1988, 38, 437–440. [Google Scholar]
- Shiga, Y. Further study of silician magnetite. Min. Geol. 1989, 39, 305–309. [Google Scholar]
- Shimazaki, H. On the occurrence of silician magnetites. Resour. Geol. 1998, 48, 23–29. [Google Scholar] [CrossRef]
- Espinoza, S. The Atacama-Coquimbo ferrifereous belt, northern Chile. In Stratabound Ore Deposits in the Andes; Society for Geology Applied to Mineral Deposits Special Publication 8; Springer: Berlin/Heidelberg, Germany, 1990; pp. 395–412. [Google Scholar]
- Lara, L.; Godoy, E. Hoja Quebrada Salitrosa, Región de Atacama. Servicio Nacional de Geología y Minería. Mapas Geol. 1998, 76, 30. (In Spanish) [Google Scholar]
- Arévalo, C. Carta Copiapó, Región de Atacama. Servicio Nacional de Geología y Minería. Carta Geol. Chile 2005, 91, 54. (In Spanish) [Google Scholar]
- Marschik, R.; Fontboté, L. The Candelaria-Punta del Cobre iron oxide Cu-Au (-Zn-Ag) deposits, Chile. Econ. Geol. 2001, 96, 1799–1826. [Google Scholar]
- Sillitoe, R.H. Iron oxide-copper-gold deposits: An Andean view. Miner. Depos. 2003, 38, 787–812. [Google Scholar] [CrossRef]
- Haschke, M.; Boehm, S. Micro-XRF in Scanning Electron Microscopes. In Advances in Imaging and Electron Physics; Peter, W., Hawkes, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–60. [Google Scholar]
- Ramdohr, P. The Ore Minerals and Their Intergrowths; Pergamon Press: Oxford, UK, 1980; p. 1207. [Google Scholar]
- Ohkawa, M.; Miyahara, M.; Ohta, E.; Hoshino, K. Silicon-substituted magnetite an accompanying iron oxides and hydroxides from the Kumano mine, Yamaguchi Prefecture, Japan: Reexamination of the so-called maghemite (γ-Fe2O3). J. Mineral. Petrol. Sci. 2007, 102, 182–193. [Google Scholar] [CrossRef]
- Hu, H.; Li, J.W.; Lentz, D.; Ren, Z.; Zhao, X.F.; Deng, X.D.; Hall, D. Dissolution-reprecipitation process of magnetite from the Chengchao iron deposit: Insight into ore genesis and implication for in-situ chemical analysis of magnetite. Ore Geol. Rev. 2014, 57, 393–405. [Google Scholar] [CrossRef]
- Hu, H.; Lentz, D.; Li, J.W.; McCarron, T.; Zhao, X.F.; Hall, D. Reequilibration processes in magnetite from iron skarn deposits. Econ. Geol. 2015, 110, 1–8. [Google Scholar] [CrossRef]
- Broughm, S.G.; Hanchar, J.M.; Tornos, F.; Westhues, A.; Attersley, S. Mineral chemistry of magnetite from magnetite-apatite mineralization and their rocks: Examples from Kiruna, Sweden, and El Laco, Chile. Miner. Depos. 2017, 52, 1223–1244. [Google Scholar] [CrossRef]
- Heidarian, H.; Lentz, D.; Alirezaei, S.; Peighambari, S. Using the chemical analysis of magnetite to constrain various stages in the formation and genesis of the Kiruna-type Chadormalu magnetite-apatite deposit, Bafq district, Central Iran. Mineral. Petrol. 2016, 110, 927–942. [Google Scholar] [CrossRef]
- Lund, C.R.F.; Dumesic, J.A. Strong oxide-oxide interactions in silica-supported magnetite catalysis.1. X-ray diffraction and Mössbauer spectroscopy evidence for interaction. J. Phys. Chem. 1981, 85, 3175–3180. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Crstallogr. 1969, B25, 925–945. [Google Scholar] [CrossRef]
- Huberty, J.M.; Konishi, H.; Heck, P.R.; Fournelle, J.H.; Valley, J.W.; Xu, H. Silician magnetite from the Dales Gorge member of the Brockman iron formation, Hamersley Group, Western Australia. Am. Mineral. 2012, 97, 26–37. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Simon, A.; Suvorova, A.; Knipping, J.; Roberts, M.P.; Rubanov, A.; Saunders, M. Nanogeochemistry of hydrothermal magnetite. Contrib. Mineral. Petrol. 2018, 173, 46. [Google Scholar] [CrossRef]
- Xu, H.; Shen, Z.; Konishi, H. Si-magnetite nano-precipitates in silician magnetite from banded iron formation: Z-contrast imaging and ab initio study. Am. Mineral. 2014, 99, 2196–2202. [Google Scholar] [CrossRef]
- Imai, A. Generation and evolution of ore fluids for porphyry Cu-Au mineralization of the Santo Tomas II (Philex) deposit, Philippines. Resour. Geol. 2001, 51, 71–96. [Google Scholar] [CrossRef]
- Shcheka, S.A.; Romanenko, I.M.; Chubarov, V.M. Silica bearing magnetite. Contrib. Mineral. Petrol. 1977, 63, 103–111. [Google Scholar] [CrossRef]
- Wang, L.J.; Shimazaki, H.; Shiga, Y. Skarns and genesis of the Huanggang Fe-Sn deposit, Inner Mongolia, China. Resour. Geol. 2008, 51, 359–376. [Google Scholar] [CrossRef]
- Westendorp, R.W.; Watkinson, D.H.; Jonasson, I.R. Silicon-bearing zoned magnetite crystals and the evolution of hydrothermal fluids at the Ansil Cu-Zn mine, Rouyn-Noranda, Quebec. Econ. Geol. 1991, 86, 1110–1114. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Rieger, A.A.; Marschik, R.; Díaz, M. The evolution of the hydrothermal IOCG system in the Mantoverde district, northern Chile: New evidence from microthermometry and stable isotope geochemistry. Miner. Depos. 2012, 47, 359–369. [Google Scholar] [CrossRef]
- Fournier, R.O. A method of calculating quartz solubilities in aqueous sodium chloride solutions. Geochim. Cosmochim. Acta 1983, 47, 579–586. [Google Scholar] [CrossRef]
- Fournier, R.O. The behavior of silica in hydrothermal solutions. Rev. Econ. Geol. 1985, 2, 45–61. [Google Scholar]
- Monecke, J.; Reynolds, T.J.; Tsuruoka, S.; Bennett, M.M.; Skews, W.B. Quartz solubility in the H2O-NaCl systems: A framework for understanding vein formation in porphyry copper deposits. Econ. Geol. 2018, 113, 1007–1046. [Google Scholar] [CrossRef]
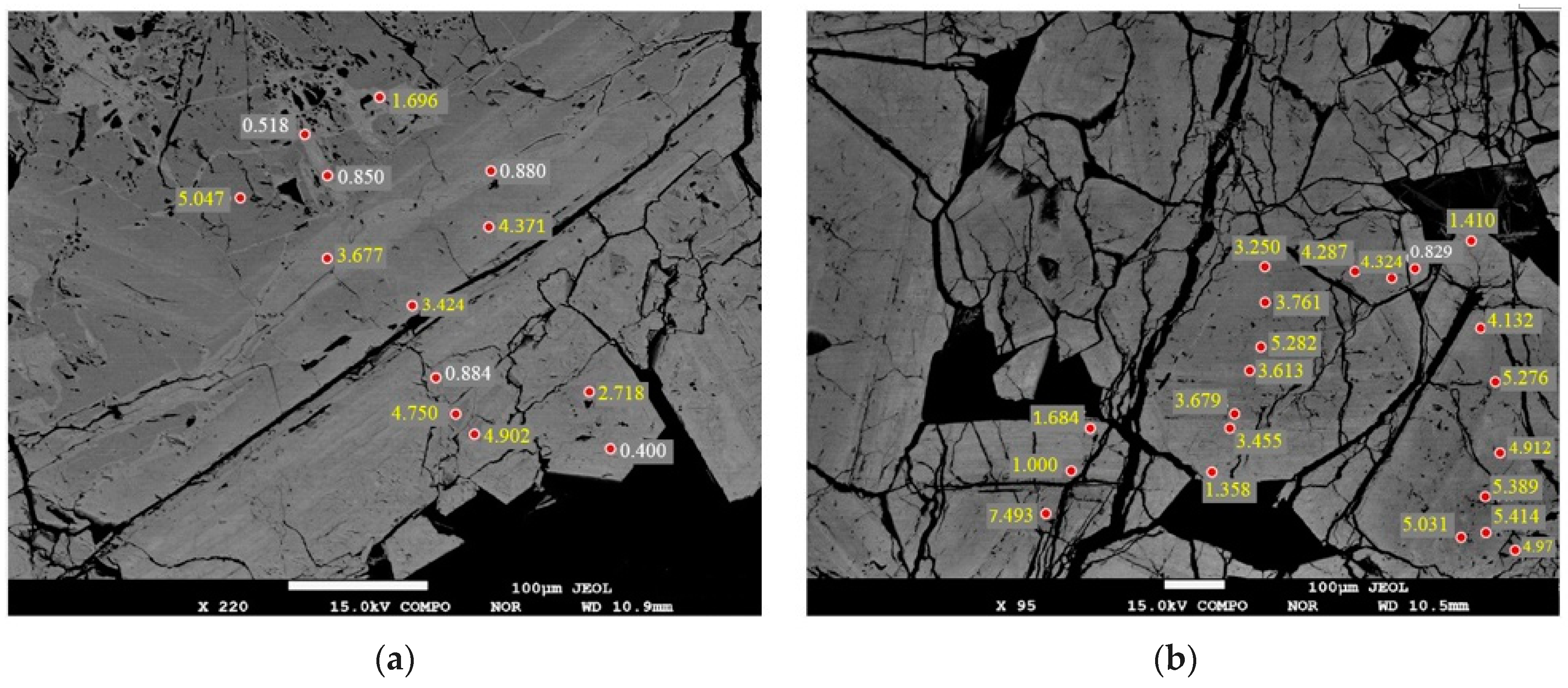
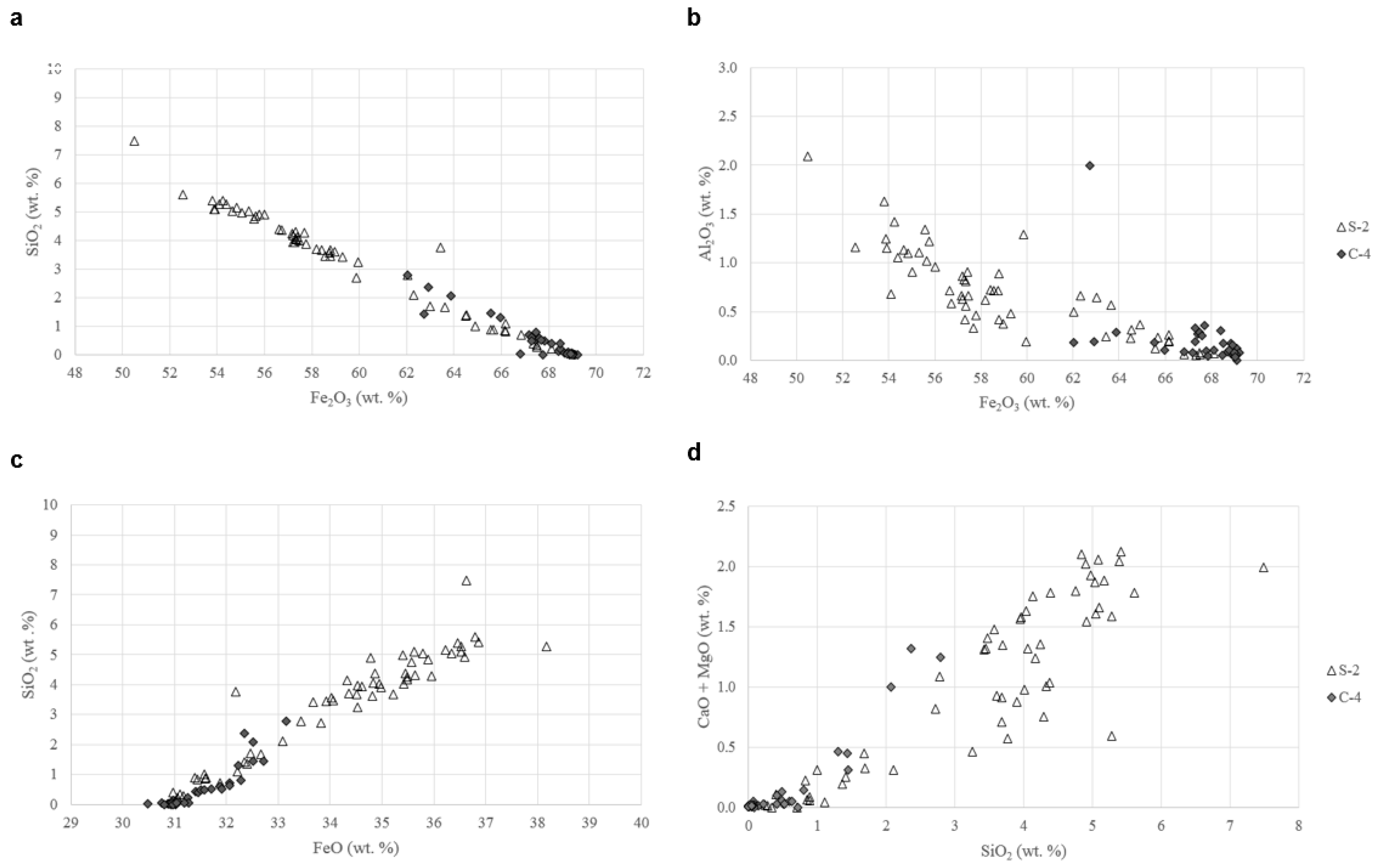
| No. | S2-1 | S2-2 | S2-3 | S2-4 | S2-5 | S2-6 | S2-7 | S2-8 | S2-9 | S2-10 | C4-1 | C4-2 | C4-3 | C4-4 | C4-5 | C4-6 | C4-7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 0.23 | 0.40 | 0.71 | 1.45 | 4.75 | 5.05 | 5.17 | 5.28 | 5.41 | 7.49 | 0.08 | 0.09 | 0.59 | 1.31 | 2.07 | 2.36 | 2.79 |
| TiO2 | 0.00 | 0.03 | 0.05 | 0.11 | 0.19 | 0.08 | 0.22 | 0.00 | 0.24 | 0.06 | 0.03 | 0.02 | 0.03 | 0.01 | 0.05 | 0.08 | 0.10 |
| Al2O3 | 0.07 | 0.05 | 0.06 | 0.69 | 1.34 | 1.11 | 1.10 | 0.68 | 1.63 | 2.10 | 0.17 | 0.11 | 0.25 | 0.10 | 0.29 | 0.19 | 0.19 |
| Cr2O3 | 0.01 | 0.02 | 0.06 | 0.00 | 0.07 | 0.00 | 0.02 | 0.01 | 0.00 | 0.29 | 0.02 | 0.00 | 0.02 | 0.02 | 0.05 | 0.00 | 0.06 |
| V2O3 | 0.01 | 0.00 | 0.01 | 0.49 | 0.12 | 0.00 | 0.00 | 0.03 | 0.00 | 0.03 | 0.00 | 0.05 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 |
| FeOt * | 92.35 | 91.58 | 92.02 | 89.70 | 85.57 | 86.13 | 85.55 | 86.85 | 85.26 | 82.06 | 92.99 | 93.01 | 92.70 | 91.57 | 89.99 | 88.96 | 88.97 |
| MnO | 0.04 | 0.06 | 0.00 | 0.61 | 0.19 | 0.09 | 0.11 | 0.16 | 0.06 | 0.21 | 0.02 | 0.07 | 0.07 | 0.00 | 0.00 | 0.00 | 0.07 |
| MgO | 0.02 | 0.11 | 0.00 | 0.15 | 0.84 | 0.56 | 0.73 | 0.12 | 0.83 | 1.24 | 0.02 | 0.00 | 0.03 | 0.18 | 0.53 | 0.60 | 0.49 |
| CaO | 0.00 | 0.00 | 0.00 | 0.26 | 0.95 | 1.05 | 1.16 | 0.47 | 1.29 | 0.76 | 0.00 | 0.00 | 0.02 | 0.28 | 0.47 | 0.72 | 0.76 |
| CuO | 0.07 | 0.07 | 0.01 | 0.00 | 0.00 | 0.06 | 0.11 | 0.00 | 0.00 | 0.44 | 0.01 | 0.00 | 0.01 | 0.02 | 0.03 | 0.02 | 0.12 |
| NiO | 0.00 | 0.02 | 0.06 | 0.00 | 0.01 | 0.07 | 0.02 | 0.00 | 0.00 | 0.20 | 0.06 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| CoO | 0.11 | 0.12 | 0.17 | 0.00 | 0.06 | 0.06 | 0.15 | 0.13 | 0.10 | 0.14 | 0.20 | 0.19 | 0.16 | 0.15 | 0.17 | 0.20 | 0.09 |
| Total | 92.90 | 92.46 | 93.14 | 93.46 | 92.56 | 94.26 | 94.33 | 93.74 | 94.82 | 95.00 | 93.59 | 93.54 | 93.95 | 93.64 | 93.64 | 93.14 | 93.63 |
| Fe2O3 | 68.11 | 67.35 | 66.83 | 64.26 | 55.56 | 55.33 | 54.83 | 54.10 | 53.79 | 50.49 | 68.85 | 68.83 | 67.61 | 65.96 | 63.88 | 62.93 | 62.03 |
| FeO | 31.06 | 30.98 | 31.88 | 32.44 | 35.58 | 36.35 | 36.22 | 38.17 | 36.85 | 36.63 | 31.04 | 31.08 | 31.87 | 32.23 | 32.51 | 32.34 | 33.16 |
| Total | 99.73 | 99.21 | 99.84 | 100.46 | 99.67 | 99.81 | 99.82 | 99.16 | 100.21 | 100.06 | 100.49 | 100.43 | 100.72 | 100.25 | 100.04 | 99.44 | 99.85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, E.; Kojima, S.; Ichii, Y.; Tanaka, T.; Fujimoto, Y.; Ogata, T. Silician Magnetite from the Copiapó Nordeste Prospect of Northern Chile and Its Implication for Ore-Forming Conditions of Iron Oxide–Copper–Gold Deposits. Minerals 2018, 8, 529. https://doi.org/10.3390/min8110529
González E, Kojima S, Ichii Y, Tanaka T, Fujimoto Y, Ogata T. Silician Magnetite from the Copiapó Nordeste Prospect of Northern Chile and Its Implication for Ore-Forming Conditions of Iron Oxide–Copper–Gold Deposits. Minerals. 2018; 8(11):529. https://doi.org/10.3390/min8110529
Chicago/Turabian StyleGonzález, Elías, Shoji Kojima, Yoshihiko Ichii, Takayuki Tanaka, Yoshikazu Fujimoto, and Takeyuki Ogata. 2018. "Silician Magnetite from the Copiapó Nordeste Prospect of Northern Chile and Its Implication for Ore-Forming Conditions of Iron Oxide–Copper–Gold Deposits" Minerals 8, no. 11: 529. https://doi.org/10.3390/min8110529
APA StyleGonzález, E., Kojima, S., Ichii, Y., Tanaka, T., Fujimoto, Y., & Ogata, T. (2018). Silician Magnetite from the Copiapó Nordeste Prospect of Northern Chile and Its Implication for Ore-Forming Conditions of Iron Oxide–Copper–Gold Deposits. Minerals, 8(11), 529. https://doi.org/10.3390/min8110529




