1. Introduction
Pyrite has been a vital mineral source to produce sulfur acid, a considerable amount of pyrite is closely associated with arsenopyrite [
1]. Separation or selective depression of arsenopyrite from pyrite becomes imperative, especially extraction of the gold or silver associated with the pyrite matrix. In addition, when arsenic-bearing pyrite concentrate is smelted, hydrogen arsenide, arsenite and organic arsenide will be produced, posing a serious threat to the environment [
2,
3]. Given the strict requirements for arsenic content in pyrite concentrate, higher separation efficiency of pyrite and arsenopyrite is of more significance. Separation of arsenopyrite from pyrite is usually achieved by selective depression of arsenopyrite using depressants like cyanide or oxidizing agents (e.g., hydrogen peroxide and magnesia-ammonia mixture) to selectively oxidize arsenopyrite [
3,
4,
5]. As arsenopyrite is more sensitive to oxidation than pyrite, the floatability of arsenopyrite is reduced by controlling the concentrations of the depressants and collectors and the flotation parameters [
5]. The hydrophilic iron hydroxyl compounds and the arsenate preferentially precipitate on the arsenopyrite surface by drawing upon the difference in oxidation rate, while pyrite still maintains a high recovery [
6,
7].
However, as the physical and chemical properties of arsenopyrite are similar to those of pyrite, arsenopyrite presents a similar hydrophobicity with pyrite in the flotation process when xanthate serves as collector [
8,
9]. As their hydrophobic surface species are dixanthogen, xanthates are not cation-selective in the case of separation of arsenopyrite and pyrite, which makes it very difficult by flotation separation [
10,
11]. No matter what type of depressant is applied, it can also depress pyrite and arsenopyrite simultaneously [
12]. Moreover, the floatability of pyrite will also be significantly reduced when the flotation proceeds in a high alkali pulp [
13,
14]. Furthermore, the conventional arsenopyrite depressants such as cyanide and potassium permanganate are harmful to human health and environment, which limits the application of reagents in flotation separation. Since flotation selectivity and separation efficiency are dependent on the reagent concentrations, pulp redox potential and slurry pH in the conditioning stage, rigorously controlling of these parameters is also considered necessary for the prevention of depressing the pyrite [
3].
Low temperature plasma has been proven as an effective and powerful method in surface modification of organic and inorganic materials [
15,
16]. Low temperature plasma is formed by introducing electric energy to the gas, causing the breakdown of the gas into ions and electrons [
17]. It is a collection of positively and negatively charged particles and neutral species, consisting of electrons, ions, neutral atoms, molecules and large numbers of other species (e.g., excited species and various radicals) [
18]. The electrons and ions have abundant high energy to activate the gas and create reactive species by electron impact excitation, ionization, dissociation and attachment, which can break the chemical bonds on the material surfaces and form new bonds [
19]. When the materials are put into the low temperature plasma, energy exchange, charge transfer, molecular decomposition or recombination and electron adsorption will be resulted in by the reactive species collide with the material surfaces, causing physical or chemical reactions on the surface of the materials to achieve the surface modification of the materials [
18]. Compared with flotation reagents, surface modification of minerals by low temperature plasma has the advantages of pollution-free and completely controllable process conditions. In the process of clean reaction and short processing time modification, the surface of the material is endowed with desirable and ideal physical and chemical properties without negatively affecting the bulk structure [
20].
Low temperature plasma, an effective surface modification method, has been used for the surface pre-treatment of sulfide minerals to increase the separation efficiency between minerals. Excellent separation efficiency was achieved by the treatments of pyrite, chalcopyrite, molybdenite and chalcocite in Ar/O
2 microwave (MW) and radio frequency (RF) discharges [
21,
22,
23,
24]. The results showed a significant difference in the surface oxidation rates of various sulfides in low temperature plasma [
21,
22]. The oxidation rate of chalcopyrite is 8.4 and 19.1 times faster than that of pyrite and chalcocite, respectively [
21]. Pyrite in mixed minerals delayed the emission of sulfur dioxide (SO
2) from the oxidation of mineral Cu
2S in low temperature plasma, which resulted from the different oxidation rates of pyrite and chalcocite [
22]. The original sulfide/sulfide of sulfide mixtures was converted into oxide/sulfide system, proving that the selective surface modifications of mineral mixtures by low temperature plasma pre-treatment are feasible [
24]. However, none of the retrieved literature considered the effects of low temperature oxygen plasma modification on the floatability of arsenopyrite and the separation efficiency of pyrite from arsenopyrite.
In this study, surface modification with dielectric barrier discharge (DBD) low temperature oxygen plasma was performed to increase the separation efficiency of pyrite and arsenopyrite. The effects of plasma modification on the arsenopyrite and pyrite surface were assessed by micro-flotation tests, X-ray photoelectron spectroscopy (XPS) studies, Fourier transform infrared spectroscopy (FTIR) analysis, inductively coupled plasma mass spectrometry (ICP-MS) analysis and zeta potential measurements.
2. Materials and Methods
2.1. Experimental Setup
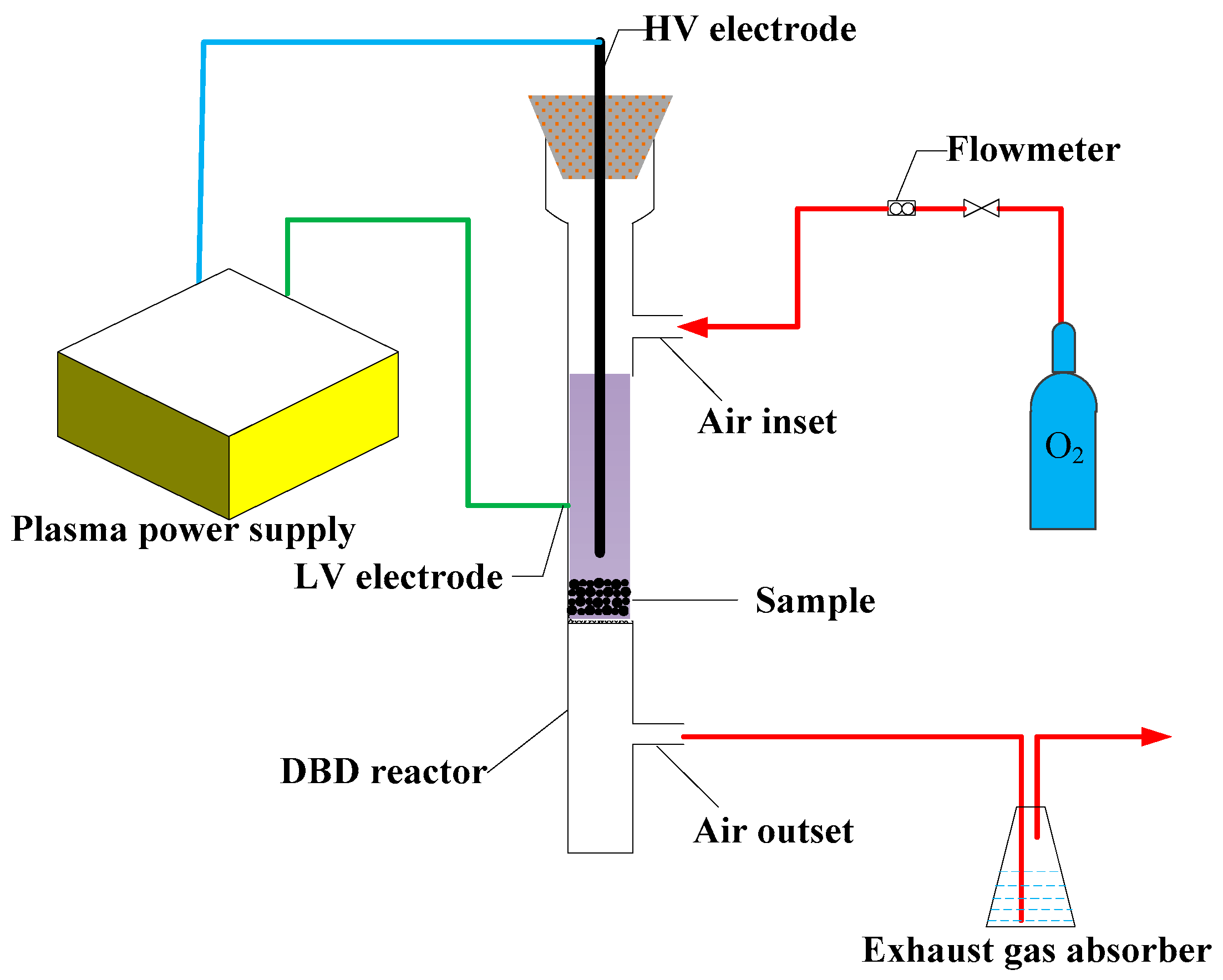
The schematic view of the setup applied in the experiments is shown in
Figure 1. The power supply was provided by Nanjing Surman Plasma Technology Co. Ltd. (Nanjing, China). The DBD reactor was a self-made quartz tube with a height of 260 mm, an external diameter of 21 mm and an internal diameter of 19 mm. The HV electrode was a stainless-steel bar with a diameter of 2 mm, arranged in the center of the reactor. A layer of tin foil was wrapped around the reactor as the LV electrode. Before the plasma pre-treatment experiment, gas-permeable quartz sand was arranged in the middle of the reactor and the sample was transferred onto the quartz sand.
2.2. Materials and Reagents
The representative pyrite and arsenopyrite samples employed in all the experiments were obtained from Yunnan province, China. For micro-flotation experiments and XPS analysis, the pyrite and arsenopyrite samples were overall crushed with an agate mortar. Subsequently, the fine-grained pyrite and arsenopyrite particles were sieved using Tyler screens to achieve a particle fraction of −105 + 38 μm. Besides, the fractions finer than 10 μm were screened for FTIR spectroscopic analysis and zeta potential measurements. Furthermore, the whole fractions fines than 105 μm for ICP-MS analysis. The chemical analysis suggested that the pyrite samples contained 45.87% Fe and 53.39% S and the arsenopyrite samples contained 34.30% Fe, 45.18% As and 19.69% S, which indicated that purity of pyrite and arsenopyrite samples were overall higher than 95%. To prevent the mineral surface from the oxidation, the samples employed in all experiments were ultrasonically cleaned and then vacuum dried.
Sodium butyl xanthate (SBX) served as the collector. The pH values of the solution were regulated using 0.1 mol/L sodium hydroxide (NaOH) and 0.1 mol/L hydrochloric acid (HCl) solutions. All reagents employed in this study were of analytical grade and deionized water was used throughout the experiments.
2.3. Sample Pre-treatment
For each experiment, 7 g of ultrasonically cleaned and vacuum dried ore sample was transferred to the reactor and placed uniformly on quartz sand. Subsequently, the piston with a HV electrode was plugged into the reactor and the lower end of the HV electrode was positioned 5 mm above the ore sample. Pre-inflate for half a minute before plasma ignition to remove residual air from the reactor. In the meantime, the flow rate of oxygen was controlled by the mass flow meter to 210 mL/min. Next, the voltage regulator was rotated to the required input voltage (30 V, 35 V, 40 V, 45 V) and the input current was adjusted to 1.2 ± 0.1 A. The timing started after the current was stable and the pre-treatment time was 30 s, 1 min, 2 min, 3 min, 4 min and 5 min, respectively.
A temperature probe (Testo108, GER) was used to measure the temperatures during the plasma modification. The highest temperatures at input voltages of 30 V, 35 V, 40 V and 45 V were 328.5 K, 333.4 K, 342.1 K and 355.4 K, respectively. The DBD reactor containing 7.0 g samples was placed in the above constant temperatures water and keep the liquid level above the sample position and inflating for 1 min at the oxygen flow rate of 210 mL/min. The same flotation procedure was used to investigate the effect of temperature on the separation efficiency of arsenopyrite and pyrite.
2.4. Flotation Experiments
The sample used in the flotation experiments was a mixed sample of arsenopyrite and pyrite with a mixed ratio of 1:1 by weight. Flotation experiments were performed in a 40-mL XFG flotation cell with an impeller speed of 1500 rpm and 5.0 g mixed samples were placed in 40 mL deionized water. 1 × 10−4 mol/L SBX solution was poured into the suspension and agitated for 3 min. The flotation was performed for 5 min. Native pH (about 6.5) and no depressants or frothers were employed for the flotation experiments. The concentrates and tailings were overall weighed to calculate the flotation recovery and separation efficiency. Each flotation experiment was measured three times.
2.5. XPS Measurements
XPS measurements were performed using a Thermo Scientific apparatus (250 Xi, Thermo fisher Scientific, Waltham, MA, USA) with an Al Kα X-ray source. To determine all elements in the measured sample, a survey scan was first performed at pass energy of 100 eV. Subsequently, multiplex (narrow) high resolution scan was performed at 30 eV to obtain the XPS spectrum of each specific element. The Thermo Avantage software was employed for data processing and fitting. The spectral intensities were converted to surface elemental concentrations by initially subtracting a Shirley-type background and then fitting peaks using an 80% to 20% Gaussian/Lorenzian line shape. The binding energies were calibrated using C 1s spectrum photoelectron peak (adventitious carbon, C-C) as a reference with a binding energy of 284.8 eV.
2.6. FTIR Spectroscopic Analysis
The infrared spectra of the untreated and plasma-modified samples were recorded with a Nicolet iS 10 Fourier Transform Infrared Spectrometer (Thermo fisher Scientific, Waltham, MA, USA) by KBr diffuse reflection method. The scanned area was from 4000 to 400 cm−1. Each spectrum was recorded with 16 scans measured at 4 cm−1 resolution.
2.7. Zeta Potential Measurements
The zeta potentials of the arsenopyrite and pyrite before and after plasma pre-treatment were performed using a Brookhaven ZetaPlus zeta potential analyzer (Brookhaven, NY, USA). By mixing 0.1 g sample with 40 mL of deionized water containing 5 × 10
−3 mol/L KNO
3 background solutions, the mineral suspension was prepared. For each experiment, the suspension pH was adjusted to a desired value using 0.1 mol/L HCl or 0.1 mol/L NaOH stock solutions. If necessary, 1 × 10
−4 mol/L freshly prepared SBX solution was added into the suspension before pH adjustment. The prepared suspension was magnetically stirred for 5 min by a magnetic stirring apparatus. The resultant suspensions were allowed to stand still for 10 min and the supernatant of the suspension was transferred to a measuring vessel using a plastic dropper for zeta potential measurements at ambient temperature. The ζ potential of each sample was measured three times [
25].
2.8. Dissolution of Arsenopyrite and Pyrite by ICP Analysis
The concentrations of Fe and S (pyrite and arsenopyrite) and As (arsenopyrite) dissolved in arsenopyrite and pyrite before and after low temperature oxygen plasma were measured by ICP-MS (ELAN-DRC II, PE, MA, USA). The suspension was prepared by adding 2 g sample into the 100 mL deionized water and subsequently the prepared suspension was stirred by a magnetic stirring apparatus for a certain time. The supernatant was aspirated by a Pasteur pipette and transferred to two 15 mL centrifugal tubes, centrifuged at a speed of 3000 r/min for 30 min by a centrifuge. Then 10 mL of supernatant was obtained as ICP-MS measurement sample. The concentration of each element was measured three times.
4. Discussion
This study showed that the low temperature oxygen plasma can achieve effective separation of minerals. The effects of plasma on the floatability of arsenopyrite are stronger than that of pyrite, which is consistent with the fact that arsenopyrite is more sensitive to oxidation than pyrite [
38]. The pre-treatment time of the oxygen plasma had a negative correlation with the flotation recovery. The reactions of excitation, dissociation and ionization of oxygen took place at the plasma ignition, through which the reactive species were produced [
39]. With the increase in plasma pre-treatment time, more reactive species have the opportunity to bombard the mineral surfaces, thereby breaking the original covalent bonds of the crystals and promoting the oxidation and increasing the hydrophilic of mineral surfaces by binding O with Fe-, As- and S- [
40].
With a constant input current (1.2 ± 0.1 A), a higher input voltage indicates higher input power and output power in the experiment system. It is generally believed that the reaction rate increased at high input power is correlated with the energy density. Accordingly, the acceleration in reaction rates can be interpreted by higher densities of reactive oxygen species as well as by rising the gas temperatures [
21]. In other words, higher input voltage has higher density of reactive species and reaction temperature. The modification temperature experiments (35 V, 1 min) indicated that the recovery of arsenopyrite and pyrite decreased to 88.35% and 97.51%, respectively. Though the modification temperature increased the separation efficiency of pyrite from arsenopyrite to a certain extent, the changes of minerals hydrophobicity should be primarily attributed to the reaction of the plasma reactive species with the mineral surfaces. It is noteworthy that excessive input voltage will increase the hydrophilicity of pyrite, thereby reducing the flotation separation efficiency.
This study indicates that the effects of plasma modification on the surface properties of arsenopyrite and pyrite are attributed to the changes of the concentrations of oxidation products. XPS analysis indicates that low temperature oxygen plasma pre-treatment of the arsenopyrite surface resulted in substantial enrichment of O and diminution of Fe, As and S. Under the modification of the plasma reaction species, the original sulfide on the surface of arsenopyrite was converted into sulfide/oxide mixed. Consistent with arsenopyrite, oxygen-enrichment region was formed on the surface of low temperature oxygen plasma-modified pyrite. However, the O atomic concentrations on the untreated (21.5%) or plasma-modified (29.8%) pyrite surfaces were much lower than that of the arsenopyrite (37.6% and 42.7% respectively), which may be one of the reasons for the difference of hydrophobicity between the arsenopyrite and pyrite.
In addition, compared with the samples of untreated, the proportions of Fe(III)-O and SO42− on the surfaces of plasma-modified arsenopyrite and pyrite increased, while the concentrations of iron sulfides, monosulfide and polysulfide favorable for flotation show a deceasing tendency. After plasma pretreated, the original sulfide states of the elements Fe and S were oxidized to a more hydrophilic oxidation state. Moreover, the changes of chemical states proportions enhanced the dissolutions of the mineral particles. The lack of iron sulfides and dissolution of iron ions on the surfaces of the plasma-modified minerals resulted in a lower reaction opportunity between the mineral surfaces and collector, which decreased the flotation recovery. It is also noteworthy that the proportions of hydrophilic sulfates and iron oxides species on the pyrite surfaces after plasma pretreated were significantly lower than those of arsenopyrite, which may be another reason for the difference in hydrophobicity between the arsenopyrite and pyrite.
In particular, the proportion of high oxidation states of arsenic on the surface of plasma-modified arsenopyrite was not significantly changed, which is probably correlated with arsenic was the most readily oxidized element in the arsenopyrite surfaces [
41]. Consistent with the slight changes in the concentrations of high-valence As(III)-O and As(V)-O (
Table 1), plasma has little effect on the dissolution of arsenic on the arsenopyrite surface. In addition, the concentrations of arsenic and sulfur dissolved from arsenopyrite surface were higher than those of iron, suggesting that the measured elemental ratios in the solution are different from those in the solid phase of arsenopyrite. A similar phenomenon was also observed in the dissolution experiments of pyrite. The non-equimolar ion concentrations indicated that the dissolution of the oxidized arsenopyrite and pyrite is proceeded non-stoichiometrically, which is primarily correlated with the degree of oxidation of mineral components [
42]. Plasma modification further increased the differences of “incongruently” or “non-stoichiometrically” dissolution of the mineral components and resulted in a significantly increase in the S/Fe ration of the mineral surfaces. Since the interaction sites on the arsenopyrite and pyrite surfaces with flotation reagents are iron, the decrease in Fe concentration on the mineral surfaces has negative effects on flotation recovery [
30].
In the presence of SBX, the zeta potentials of arsenopyrite and pyrite surfaces became further negative. This indicates that the negatively charged collector adsorbed on the mineral surfaces. With the pH range tested here, most of the zeta potentials of the sample surfaces were negative, suggesting that a chemical adsorption took place through the interaction between collector and the negatively charged arsenopyrite and pyrite surfaces [
27]. Regardless of the bare or the plasma-modified arsenopyrite particles, the extent of negative zeta potential change on the mineral surfaces was increased with the increase in the pulp pH. In the pulp with pH greater than 9, a slight effect of SBX on the zeta potentials of the bare arsenopyrite surface was observed from
Figure 6a, indicating that there was low adsorption concentration of xanthate on the surface of the arsenopyrite in the alkaline pulp solution (pH > 9). Flotation of arsenopyrite in the pulp using SBX as the collector has been attributed to the oxidation reaction of the xanthate ions (X
−) to dixanthogen (X
2), which can be described as follows [
3]:
The mentioned change in the zeta potential can be primarily attributed to the pulp redox potential (
EH) values in the alkaline pulp solution below the reversible potential for the X
−/X
2 pair [
3]. A similar trend was found on the plasma-modified arsenopyrite when the pulp pH was larger than 5, suggesting that the plasma pre-treatment reduced the formation of dixanthogen on the mineral surface. Consistent results were obtained from the results of FTIR spectroscopic analysis, the disappearance of characteristic band of dixanthogen at 1261.22 cm
−1 on the plasma-modified arsenopyrite. As a result, the adsorption of xanthate on the surface of the arsenopyrite only occurred in strongly acidic pulp solution. Compared with arsenopyrite, plasma had slight effects on the adsorption of SBX on the surface of pyrite. The differences in the adsorption of xanthate on the mineral surfaces lay the conditions for flotation separation of pyrite from arsenopyrite by low temperature oxygen plasma modified.
5. Conclusions
To evaluate the underlying mechanisms of the mineral surface modification process and selective flotation, detailed surface analysis was investigated for plasma modified arsenopyrite and pyrite. On the basis of the above discussion and results, the major conclusions were summarized as follows:
Low-temperature oxygen plasma can achieve selective modification of the surface of arsenopyrite and pyrite by controlling the reasonable treating time and input voltage. The modified arsenopyrite was completely hydrophilic, while pyrite still showed an excellent flotation performance.
XPS analysis indicated that the oxidation degree of arsenopyrite was much higher than that of pyrite. Plasma modification resulted in the formation of arsenopyrite surface of substantial enrichment of O and diminution of Fe, As and S. A large number of iron and sulfur on the plasma-modification arsenopyrite surface were converted into higher-valence hydrophilic oxides.
Plasma pre-treatment increased the dissolution of arsenopyrite and pyrite and the dissolution of minerals in aqueous solution was determined according to the oxidation degree of mineral components. Zeta potential determination and FTIR spectroscopic analysis demonstrated that plasma significantly reduced the adsorption of SBX on the surface of the arsenopyrite, which had little effect on the adsorption of SBX on pyrite surface. Plasma modification reduced the floatable pH range of the arsenopyrite and resulted in the adsorption of xanthate on the surface of the arsenopyrite, which was only shown in strongly acidic pulp solution.
It has been demonstrated that low temperature plasmas is expected to achieve efficient separation of sulfide minerals by surface modification. By developing the plasma source for industrial scale material throughputs and researching the atmospheric pressure plasma process, low-temperature plasma might contribute to reduce the dosages of flotation reagents, thereby limiting the hazard of toxic reagents in the beneficiation backwater to the environment.