Synchrotron Diffraction Study of the Crystal Structure of Ca(UO2)6(SO4)2O2(OH)6·12H2O, a Natural Phase Related to Uranopilite
Abstract
:1. Introduction
2. Materials and Methods
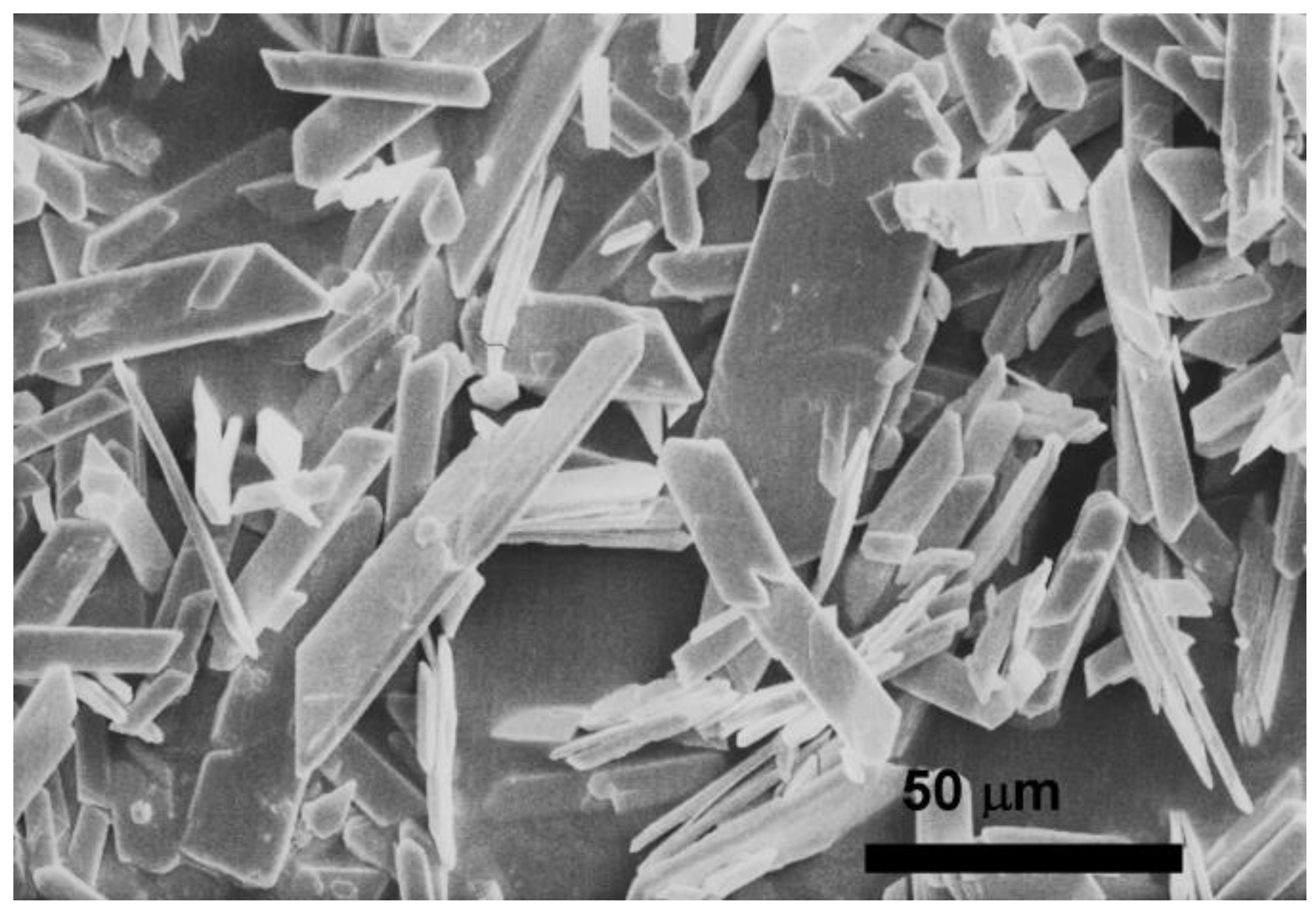
2.1. Occurrence
2.2. Chemical Composition
2.3. Synchrotron Single-Crystal X-Ray Diffraction Study
3. Results
4. Discussion
Supplementary Files
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plášil, J.; Dušek, M.; Novák, M.; Čejka, J.; Císařová, I.; Škoda, R. Sejkoraite-(Y), a new member of the zippeite group containing trivalent cations from Jáchymov (St. Joachimsthal), Czech Republic: Description and crystal structure refinement. Am. Mineral. 2011, 96, 983–991. [Google Scholar] [CrossRef]
- Plášil, J.; Hloušek, J.; Veselovský, F.; Fejfarová, K.; Dušek, M.; Škoda, R.; Novák, M.; Čejka, J.; Sejkora, J.; Ondruš, P. Adolfpateraite, K(UO2)(SO4)(OH)(H2O), a new uranyl sulphate mineral from Jáchymov, Czech Republic. Am. Mineral. 2012, 97, 447–454. [Google Scholar] [CrossRef]
- Plášil, J.; Veselovský, F.; Hloušek, J.; Šák, M.; Sejkora, J.; Cejka, J.; Škácha, P.; Kasatkin, A.V. Mathesiusite, K5(UO2)4(SO4)4(VO5)(H2O)4, a new uranyl vanadate-sulfate from Jáchymov, Czech Republic. Am. Mineral. 2014, 99, 625–632. [Google Scholar] [CrossRef]
- Plášil, J.; Hloušek, J.; Kasatkin, A.V.; Škoda, R.; Novák, M.; Čejka, J. Geschieberite, K2(UO2)(SO4)2(H2O)2, a new uranyl sulfate mineral from Jáchymov. Mineral. Mag. 2015, 79, 205–216. [Google Scholar] [CrossRef]
- Plášil, J.; Škácha, P.; Sejkora, J.; Kampf, A.R.; Škoda, R.; Čejka, J.; Hloušek, J.; Kasatkin, A.V.; Pavlíček, R.; Babka, K. Plavnoite, a new K-Mn member of the zippeite group from Jáchymov, Czech Republic. Eur. J. Mineral. 2017, 29, 117–128. [Google Scholar] [CrossRef]
- Olds, T.A.; Plášil, J.; Kampf, A.R.; Simonetti, A.; Sadergaski, L.R.; Chen, Y.S.; Burns, P.C. Ewingite: Earth’s most complex mineral. Geology 2017, 45, 1007–1010. [Google Scholar] [CrossRef]
- Plášil, J.; Kampf, A.R.; Kasatkin, A.V.; Marty, J.; Škoda, R.; Silva, S.; Čejka, K. Meisserite, Na5(UO2)(SO4)3(SO3OH)(H2O), a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2013, 77, 2975–2988. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J. Belakovskiite, Na7(UO2)(SO4)4(SO3OH)(H2O)3, a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2014, 78, 639–649. [Google Scholar] [CrossRef]
- Plášil, J.; Kampf, A.R.; Kasatkin, A.V.; Marty, J. Bluelizardite, Na7(UO2)(SO4)4Cl(H2O)2, a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. J. Geosci. 2014, 59, 145–158. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J. Bobcookite, NaAl(UO2)2(SO4)4·18H2O and wetherillite, Na2Mg(UO2)2(SO4)4·18H2O, two new uranyl sulfate minerals from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2015, 79, 695–714. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J.; Čejka, J. Fermiite, Na4(UO2)(SO4)3·3H2O, and oppeneimerite, Na2(UO2)(SO4)2·3H2O, two new uranyl sulfate minerals from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2015, 79, 1123–1142. [Google Scholar] [CrossRef]
- Kampf, A.R.; Kasatkin, A.V.; Čejka, J.; Marty, J. Plášilite, Na(UO2)(SO4)(OH)·2H2O, a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. J. Geosci. 2015, 60, 1–10. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Čejka, J.; Marty, J.; Škoda, R.; Lapčák, L. Alwilkinsite-(Y), a new rare-earth uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2017, 81, 895–907. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J.; Čejka, J. Klaprothite, péligotite and ottohahnite, three new minerals with bidentate UO7-SO4 linkages from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2017, 81, 753–779. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J.; Čejka, J.; Lapčák, L. Shumwayite, [(UO2)(SO4)(H2O)2]2·H2O, a new uranyl sulfate mineral from Red Canyon, San Juan County, Utah, USA. Mineral. Mag. 2017, 81, 273–285. [Google Scholar] [CrossRef]
- Olds, T.A.; Sadergaski, L.R.; Plášil, J.; Kampf, A.R.; Burns, P.C.; Steele, I.M.; Marty, J.; Carlson, S.M.; Mills, O.P. Leószilárdite, the first Na,Mg-containing uranyl carbonate from the Markey Mine, San Juan County, Utah, USA. Mineral. Mag. 2017, 81, 1039–1050. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Olds, T.A.; Nash, B.P.; Marty, J. Ammoniozippeite, a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, and the Burro mine, San Miguel County, Colorado, USA. Can. Mineral. 2018, 56, 235–245. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Nash, B.P.; Marty, J. Greenlizardite, (NH4)Na(UO2)2(SO4)2(OH)2·4H2O, a new mineral with phosphuranylite-type uranyl sulfate sheets from Red Canyon, San Juan County, Utah, USA. Mineral. Mag. 2018, 82, 401–411. [Google Scholar] [CrossRef]
- Olds, T.A.; Plášil, J.; Kampf, A.R.; Spano, T.; Haynes, P.; Carlson, S.M.; Burns, P.C.; Simonetti, A.; Mills, O.P. Leesite, K(H2O)2[(UO2)4O2(OH)5]·3H2O, a new K-bearing schoepite-family mineral from the Jomac mine, San Juan County, Utah, U.S.A. Am. Mineral. 2018, 103, 143–150. [Google Scholar] [CrossRef]
- Pekov, I.V.; Krivovichev, S.V.; Yapaskurt, V.O.; Chukanov, N.V.; Belakovskiy, D.I. Beshtauite, (NH4)2(UO2)(SO4)2·2H2O, a new mineral from Mount Beshtau, Northern Caucasus, Russia. Am. Mineral. 2014, 99, 1783–1787. [Google Scholar] [CrossRef]
- Plášil, J.; Kasatkin, A.V.; Škoda, R.; Novák, M.; Kallistová, A.; Dušek, M.; Skála, R.; Fejfarová, K.; Čejka, J.; Meisser, N.; et al. Leydetite, Fe(UO2)(SO4)2(H2O)11, a new uranyl sulfate mineral from Mas d’Alary, Lodève, France. Mineral. Mag. 2013, 77, 429–441. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Actinide compounds containing hexavalent cations of the VI group elements (S, Se, Mo, Cr, W). In Structural Chemistry of Inorganic Actinide Compounds; Krivovichev, S.V., Burns, P.C., Tananaev, I.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 95–182. [Google Scholar]
- Krivovichev, S.V. Actinyl compounds with hexavalent elements (S, Cr, Se, Mo)—Structural diversity, nanoscale chemistry, and cellular automata modeling. Eur. J. Inorg. Chem. 2010, 2010, 2594–2603. [Google Scholar] [CrossRef]
- Frondel, C.; Ito, J.; Honea, R.M.; Weeks, A.M. Mineralogy of the zippeite group. Can. Mineral. 1976, 14, 429–436. [Google Scholar]
- Burns, P.C.; Deely, K.M.; Hayden, L.A. The crystal chemistry of the zippeite group. Can. Mineral. 2003, 41, 687–706. [Google Scholar] [CrossRef]
- Brugger, J.; Burns, P.C.; Meisser, N. Contribution to the mineralogy of acid drainage of uranium minerals: Marecottite and the zippeite-group. Am. Mineral. 2003, 88, 676–685. [Google Scholar] [CrossRef]
- Plášil, J.; Fejfarová, K.; Wallwork, K.S.; Dušek, M.; Škoda, R.; Sejkora, J.; Čejka, J.; Veselovský, F.; Hloušek, J.; Meisser, N.; et al. Crystal structure of pseudojohannite, with a revised formula, Cu3(OH)2[(UO2)4O4(SO4)2](H2O)12. Am. Mineral. 2012, 97, 1796–1803. [Google Scholar] [CrossRef]
- Plášil, J.; Dušek, M.; Čejka, J.; Sejkora, J. The crystal structure of rabejacite, the Ca2+-dominant member of the zippeite group. Mineral. Mag. 2014, 78, 1249–1264. [Google Scholar] [CrossRef]
- Mereiter, K. Die Kristallstruktur des Johannites, Cu(UO2)2(OH)2(SO4)2·8H2O. Tscherm. Mineral. Petrogr. Mitt. 1982, 30, 47–57. [Google Scholar] [CrossRef]
- Cejka, J.; Sejkora, J.; Mrázek, Z.; Urbanec, Z.; Jarchovský, T. Jáchymovite, (UO2)8(SO4)(OH)14·13H2O, a new uranyl mineral from Jáchymov, the Krusné hory Mts., Czech Republic, and its comparison with uranopilite. Neues Jahrb. Mineral. Abh. 1996, 170, 155–170. [Google Scholar]
- Frondel, C. Studies of uranium minerals (X): Uranopilite. Am. Mineral. 1952, 37, 950–959. [Google Scholar]
- Deliens, M.; Piret, P. La rabejacite, Ca(UO2)4(SO4)2(OH)6·6H2O, nouveau sulfate d’uranyle et de calcium des gîtes du Lodévois, Hérault, France. Eur. J. Mineral. 1993, 5, 873–877. [Google Scholar] [CrossRef]
- Meisser, N. La Minéralogie de L’uranium dans le Massif des Aiguilles Rouges; Matériaux pour la Géologie de la Suisse: Série Géotechnique; Office Fédéral de Topographie Swisstopo: Köniz, Switzerland, 2012; Volume 96, 183p. [Google Scholar]
- Brugger, J.; Wallwork, K.S.; Meisser, N.; Ondruš, P.; Čejka, J. Pseudojohannite from Jáchymov, Musonoï and La Creusaz: A new member of the zippeite-group. Am. Mineral. 2006, 91, 929–936. [Google Scholar] [CrossRef]
- Meisser, N.; Brugger, J.; Ansermet, S.; Thélin, P.; Bussy, F. Françoisite-(Ce), a new mineral species from La Creusaz uranium deposit (Valais, Switzerland) and from Radium Ridge (Flinders Ranges, South Australia): Description and genesis. Am. Mineral. 2010, 95, 1527–1532. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Burns, P.C.; Hawthorne, F.C.; Ewing, R.C. The crystal chemistry of hexavalent uranium: Polyhedron geometries, bond-valence parameters, and polymerization of polyhedra. Can. Mineral. 1997, 35, 1551–1570. [Google Scholar]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. 2015, B71, 562–578. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Krivovichev, S.V.; Burns, P.C. The crystal chemistry of sulfate minerals. Rev. Mineral. Geochem. 2000, 40, 1–112. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Miner Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth. Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Plášil, J. Structural complexity of natural uranyl sulfates. Acta Cryst. Sect. B 2018. accepted. [Google Scholar]
- Gurzhiy, V.V.; Krivovichev, S.V.; Tananaev, I.G. Dehydration-driven evolution of topological complexity in ethylamonium uranyl selenates. J. Solid State Chem. 2017, 247, 105–112. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Tyumentseva, O.S.; Krivovichev, S.V.; Tananaev, I.G. Cyclic polyamines as templates for novel complex topologies in uranyl sulfates and selenates. Z. Kristallogr. 2018, 233, 233–245. [Google Scholar] [CrossRef]
- Pankova, Y.A.; Gorelova, L.A.; Krivovichev, S.V.; Pekov, I.V. The crystal structure of ginorite, Ca2[B14O20(OH)6](H2O)5, and the analysis of dimensional reduction and structural complexity in the CaO-B2O3-H2O system. Eur. J. Mineral. 2018, 30, 277–287. [Google Scholar] [CrossRef]





| Crystal System | Monoclinic |
|---|---|
| Space group | P21/c |
| a, Å | 11.931(2) |
| b, Å | 14.246(6) |
| c, Å | 20.873(4) |
| β, ° | 102.768(15) |
| V, Å3 | 3460.1(18) |
| Z | 4 |
| ρcalc, g/cm3 | 4.170 |
| μ, mm−1 | 16.244 |
| Crystal dimensions, μm | 2 × 3 × 12 |
| λ, Å | 0.800000 |
| 2Θ range, deg. | 4.92–43.22 |
| Index ranges | −12 ≤ h ≤ 12, −14 ≤ k ≤ 14, −21 ≤ l ≤ 21 |
| Reflections collected | 12545 |
| Independent reflections | 3805 [Rint = 0.144] |
| Data/restraints/parameters | 3805/12/243 |
| GOF | 1.049 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.173, wR2 = 0.380, Rfree = 0.192 |
| Final R indexes [all data] | R1 = 0.179, wR2 = 0.386, Rfree = 0.201 |
| Atom | x | y | z | Ueq | BVS |
|---|---|---|---|---|---|
| U1 | 0.7442(2) | 0.3460(2) | 0.74651(13) | 0.0448(9) | 6.36 |
| U2 | 1.0278(2) | 0.2113(2) | 0.59440(14) | 0.0471(9) | 6.29 |
| U3 | 0.4738(2) | 0.2605(2) | 0.79912(14) | 0.0472(9) | 6.23 |
| U4 | 0.0512(2) | 0.3457(2) | 0.91572(13) | 0.0447(9) | 6.28 |
| U5 | 0.7282(2) | 0.3397(2) | 0.92816(13) | 0.0450(9) | 6.26 |
| U6 | 0.2940(2) | 0.2244(2) | 1.03222(14) | 0.0476(9) | 6.02 |
| S1 | 1.0408(16) | 0.3614(15) | 0.7353(8) | 0.045(5) | 5.85 |
| S2 | 0.7350(15) | 0.2058(14) | 0.6043(9) | 0.043(5) | 6.09 |
| Ca | 0.8799(16) | 0.0915(14) | 0.8342(9) | 0.068(5) | 1.77 |
| O1 | 0.694(4) | 0.458(2) | 0.723(2) | 0.043(12) | 1.82 |
| O2 | 0.806(4) | 0.239(2) | 0.774(2) | 0.032(10) | 2.08 |
| O3 | 0.978(6) | 0.323(3) | 0.573(4) | 0.09(2) | 1.85 |
| O4 | 1.081(5) | 0.105(3) | 0.627(3) | 0.067(16) | 1.85 |
| O5 | 0.397(4) | 0.364(3) | 0.781(3) | 0.054(14) | 1.85 |
| O6 | 0.540(4) | 0.152(2) | 0.819(2) | 0.043(12) | 1.82 |
| O7 | 0.086(4) | 0.462(2) | 0.935(2) | 0.042(12) | 1.85 |
| O8 | 0.003(5) | 0.235(2) | 0.889(3) | 0.065(16) | 2.02 |
| O9 | 0.698(6) | 0.455(2) | 0.943(3) | 0.080(19) | 1.85 |
| O10 | 0.768(5) | 0.225(2) | 0.918(3) | 0.070(17) | 1.89 |
| O11 | 0.226(4) | 0.118(2) | 1.001(2) | 0.053(13) | 1.71 |
| O12 | 0.372(4) | 0.323(3) | 1.064(3) | 0.053(14) | 1.82 |
| O13 | 1.088(5) | 0.360(4) | 0.806(3) | 0.060(15) | 2.02 |
| O14 | 1.033(4) | 0.268(3) | 0.704(2) | 0.045(12) | 1.97 |
| O15 | 0.920(4) | 0.401(4) | 0.721(3) | 0.052(13) | 1.85 |
| O16 | 0.893(5) | −0.073(4) | 0.798(3) | 0.071(17) | 1.71 |
| O17 | 0.845(4) | 0.163(3) | 0.623(2) | 0.028(10) | 2.14 |
| O18 | 0.739(4) | 0.296(3) | 0.639(2) | 0.042(12) | 2.07 |
| O19 | 0.709(4) | 0.224(3) | 0.533(2) | 0.042(12) | 1.91 |
| O20 | 0.654(5) | 0.140(4) | 0.626(4) | 0.09(2) | 1.43 |
| OH21 | −0.140(3) | 0.401(3) | 0.8570(19) | 0.023(9) | 1.19 |
| OH22 | 0.558(3) | 0.277(3) | 0.705(2) | 0.026(10) | 0.98 |
| OH23 | 0.247(4) | 0.304(3) | 0.920(2) | 0.037(11) | 0.88 |
| OH24 | 1.204(5) | 0.285(4) | 0.622(3) | 0.050(13) | 1.14 |
| O25 | 0.639(4) | 0.351(3) | 0.819(2) | 0.042(12) | 1.98 |
| OH26 | 0.915(4) | 0.130(3) | 0.494(2) | 0.040(11) | 1.27 |
| O27 | 1.126(6) | 0.205(5) | 0.513(3) | 0.079(19) | 2.20 |
| OH28 | 0.531(5) | 0.300(4) | 0.910(3) | 0.062(15) | 1.10 |
| OW29 | 0.460(4) | 0.171(3) | 0.995(3) | 0.049(13) | 0.49 |
| OW30 | 0.311(8) | 0.193(6) | 0.842(5) | 0.11(3) | 0.42 |
| OW31 | 0.341(5) | 0.179(4) | 0.712(3) | 0.058(15) | 0.48 |
| OW32 | 0.424(4) | 0.377(3) | 0.620(3) | 0.048(13) | 0.36 |
| OW33 | 0.730(6) | 0.025(4) | 0.884(3) | 0.074(18) | 0.27 |
| OW34 | 0.724(5) | 0.046(4) | 0.745(3) | 0.068(16) | 0.29 |
| OW35 | 1.035(8) | 0.096(7) | 0.780(5) | 0.13(3) | 0.32 |
| OW36 | 1.014(8) | 0.024(6) | 0.932(5) | 0.12(3) | 0.24 |
| OW37 | 0.396(5) | 0.461(4) | 0.938(3) | 0.064(16) | - |
| OW38 | 0.448(6) | 0.034(5) | 0.674(4) | 0.09(2) | - |
| OW39 | 0.724(6) | 0.420(5) | 0.511(4) | 0.09(2) | - |
| OW40 | 0.577(4) | 0.510(3) | 0.581(2) | 0.046(12) | - |
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|
| U1 | 0.0358(17) | 0.076(2) | 0.0297(16) | −0.0003(13) | 0.0238(14) | 0.0023(13) |
| U2 | 0.0338(17) | 0.081(2) | 0.0326(17) | −0.0028(13) | 0.0199(14) | −0.0001(14) |
| U3 | 0.0351(17) | 0.081(2) | 0.0312(16) | −0.0053(14) | 0.0190(14) | −0.0001(14) |
| U4 | 0.0339(17) | 0.078(2) | 0.0269(16) | −0.0010(13) | 0.0168(13) | 0.0020(13) |
| U5 | 0.0355(17) | 0.077(2) | 0.0281(16) | −0.0003(13) | 0.0198(13) | −0.0009(13) |
| U6 | 0.0350(18) | 0.079(2) | 0.0356(17) | 0.0000(13) | 0.0211(14) | 0.0025(14) |
| S1 | 0.026(11) | 0.067(15) | 0.036(8) | −0.006(8) | 0.014(8) | −0.005(10) |
| S2 | 0.026(10) | 0.068(13) | 0.037(11) | −0.010(9) | 0.013(9) | −0.003(8) |
| Ca | 0.061(12) | 0.082(14) | 0.065(12) | −0.007(10) | 0.025(10) | −0.027(10) |
| Bond | Bond Length | Bond | Bond Length | Bond | Bond Length |
|---|---|---|---|---|---|
| U1–O2 | 1.73(2) | U3–O5 | 1.73(3) | U5–O10 | 1.72(3) |
| U1–O1 | 1.74(3) | U3–O6 | 1.74(3) | U5–O9 | 1.73(3) |
| U1–O25 | 2.18(5) | U3–O25 | 2.31(5) | U5–O25 | 2.29(5) |
| U1–O18 | 2.34(5) | U3–OH28 | 2.33(6) | U5–OH28 | 2.37(6) |
| U1–OH22 | 2.41(4) | U3–OH22 | 2.41(4) | U5–OH26 | 2.39(5) |
| U1–O15 | 2.41(5) | U3–OW31 | 2.42(6) | U5–O19 | 2.42(5) |
| U1–OH21 | 2.54(4) | U3–OW30 | 2.49(9) | U5–OH21 | 2.54(4) |
| U2–O3 | 1.73(3) | U4–O7 | 1.73(3) | U6–O12 | 1.74(3) |
| U2–O4 | 1.73(3) | U4–O8 | 1.73(3) | U6–O11 | 1.77(3) |
| U2–O27 | 2.26(7) | U4–O27 | 2.16(7) | U6–O27 | 2.20(7) |
| U2–OH24 | 2.30(5) | U4–OH23 | 2.39(5) | U6–OH24 | 2.37(5) |
| U2–O14 | 2.41(5) | U4–O13 | 2.43(6) | U6–OW29 | 2.40(5) |
| U2–O17 | 2.48(4) | U4–OH21 | 2.47(4) | U6–OH23 | 2.55(5) |
| U2–OH26 | 2.51(5) | U4–OH26 | 2.57(5) | U6–OW32 | 2.56(5) |
| Ca–OW35 | 2.37(10) | S1–O13 | 1.46(6) | S2–O17 | 1.42(5) |
| Ca–OW34 | 2.41(6) | S1–O14 | 1.48(5) | S2–O18 | 1.47(5) |
| Ca–OW33 | 2.44(7) | S1–O16 | 1.48(6) | S2–O19 | 1.49(5) |
| Ca–O16 | 2.49(6) | S1–O15 | 1.51(6) | S2–O20 | 1.49(2) |
| Ca–OW36 | 2.49(10) | <S1–O> | 1.483 | <S2–O> | 1.468 |
| Ca–O2 | 2.51(3) | ||||
| Ca–O8 | 2.62(4) | ||||
| <Ca–O> | 2.476 |
| υ (atoms) | IG (bits/atom) | IG, total (bits/cell) | Contribution to IG, total (%) | |
|---|---|---|---|---|
| Topological complexity of the US chain | 108 | 4.755 | 513.528 | 25.78 |
| Structural complexity of the US chain | 108 | 5.755 | 621.528 | 31.20 |
| Structural complexity of the US substructure (two chains per cell) | 216 | 5.755 | 1243.056 | 62.40 |
| Total structural complexity for CaUS | 316 | 6.304 | 1991.995 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivovichev, S.V.; Meisser, N.; Brugger, J.; Chernyshov, D.V.; Gurzhiy, V.V. Synchrotron Diffraction Study of the Crystal Structure of Ca(UO2)6(SO4)2O2(OH)6·12H2O, a Natural Phase Related to Uranopilite. Minerals 2018, 8, 569. https://doi.org/10.3390/min8120569
Krivovichev SV, Meisser N, Brugger J, Chernyshov DV, Gurzhiy VV. Synchrotron Diffraction Study of the Crystal Structure of Ca(UO2)6(SO4)2O2(OH)6·12H2O, a Natural Phase Related to Uranopilite. Minerals. 2018; 8(12):569. https://doi.org/10.3390/min8120569
Chicago/Turabian StyleKrivovichev, Sergey V., Nicolas Meisser, Joel Brugger, Dmitry V. Chernyshov, and Vladislav V. Gurzhiy. 2018. "Synchrotron Diffraction Study of the Crystal Structure of Ca(UO2)6(SO4)2O2(OH)6·12H2O, a Natural Phase Related to Uranopilite" Minerals 8, no. 12: 569. https://doi.org/10.3390/min8120569
APA StyleKrivovichev, S. V., Meisser, N., Brugger, J., Chernyshov, D. V., & Gurzhiy, V. V. (2018). Synchrotron Diffraction Study of the Crystal Structure of Ca(UO2)6(SO4)2O2(OH)6·12H2O, a Natural Phase Related to Uranopilite. Minerals, 8(12), 569. https://doi.org/10.3390/min8120569








