Dissolved Silica Effects on Adsorption and Co-Precipitation of Sb(III) and Sb(V) with Ferrihydrite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Adsorption Experiments
2.3. Co-Precipitation Experiments
2.4. Analytical Methods
3. Results and Discussion
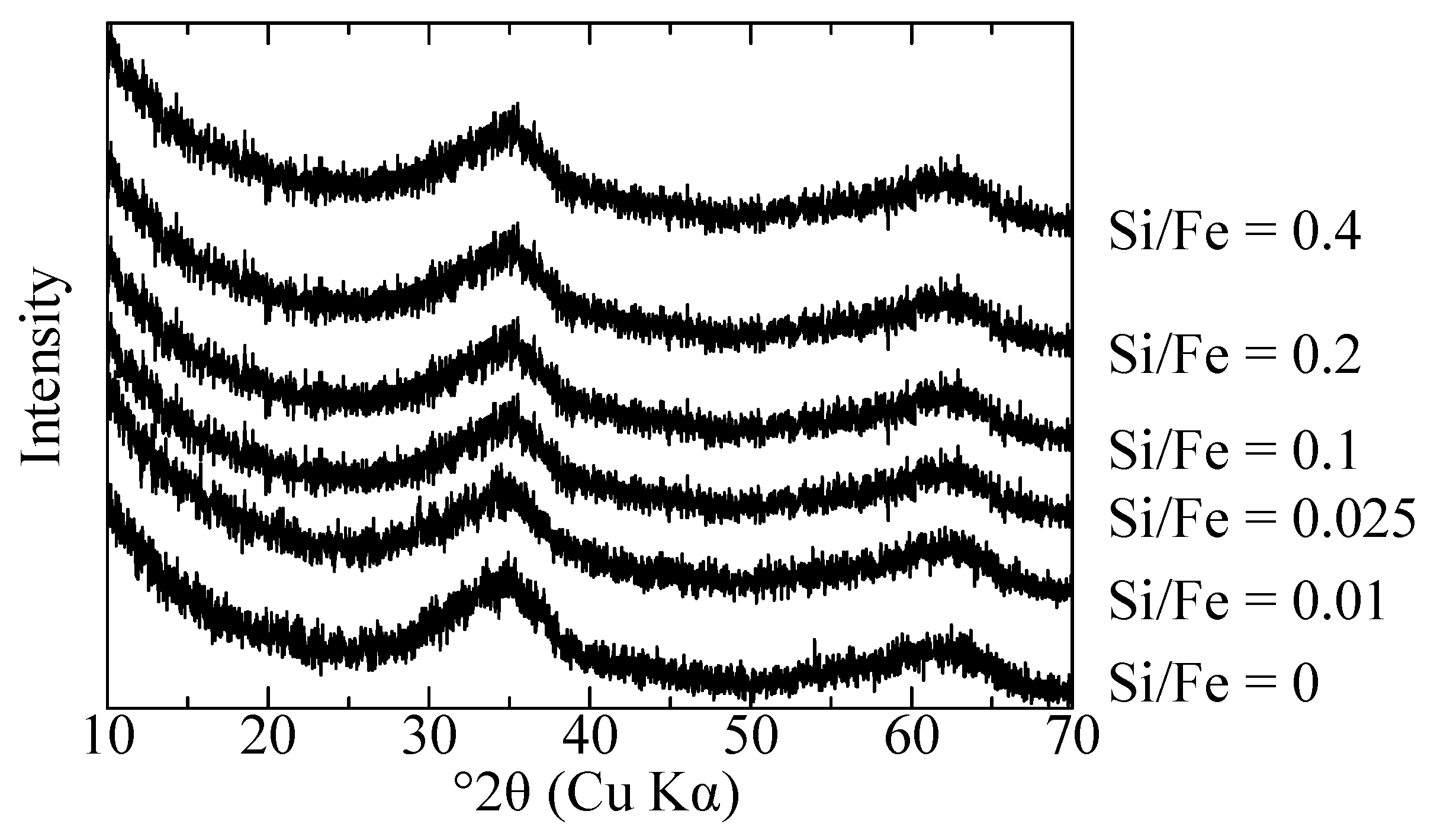
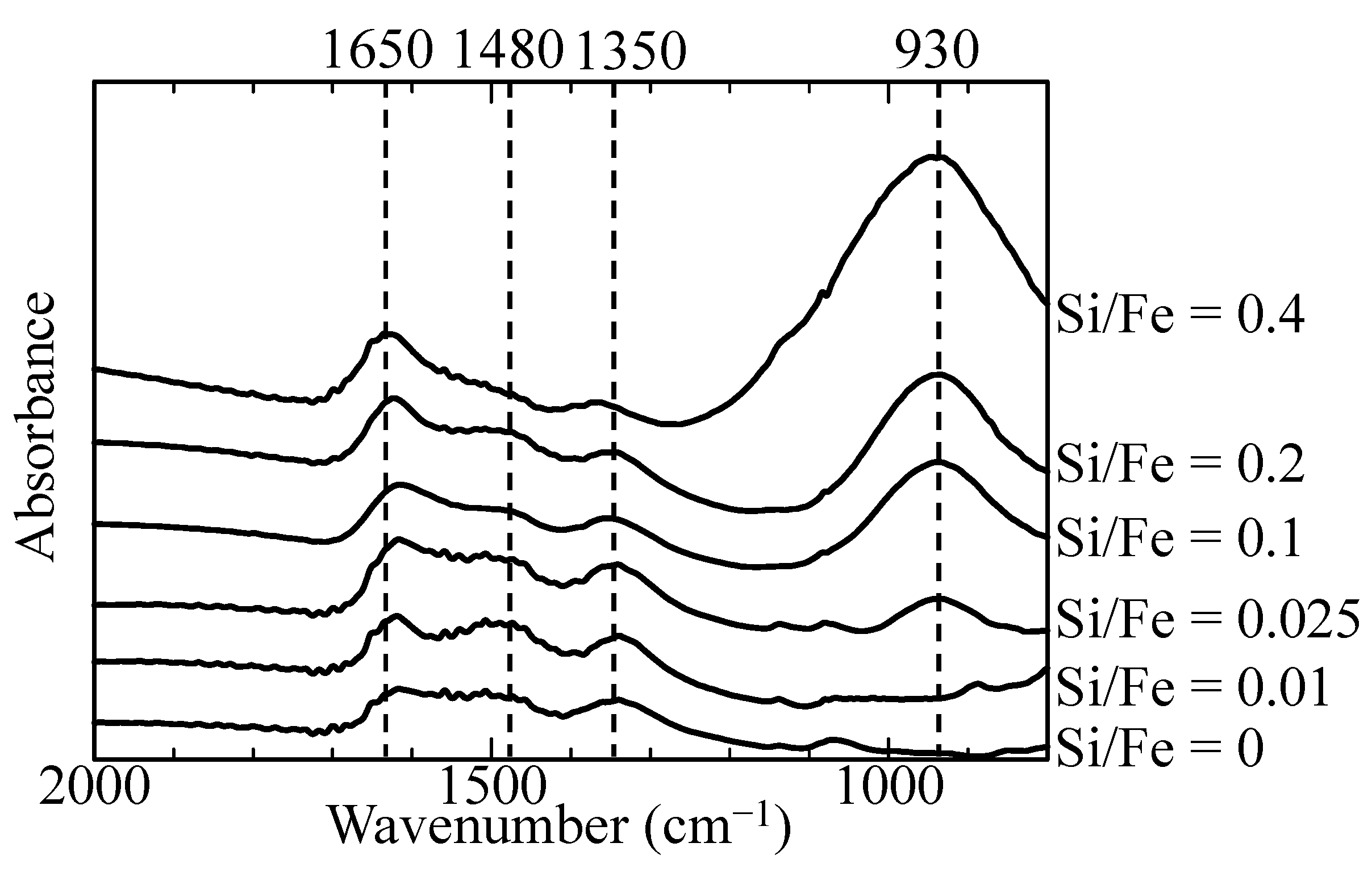
3.1. Characterization of Initial Adsorbents
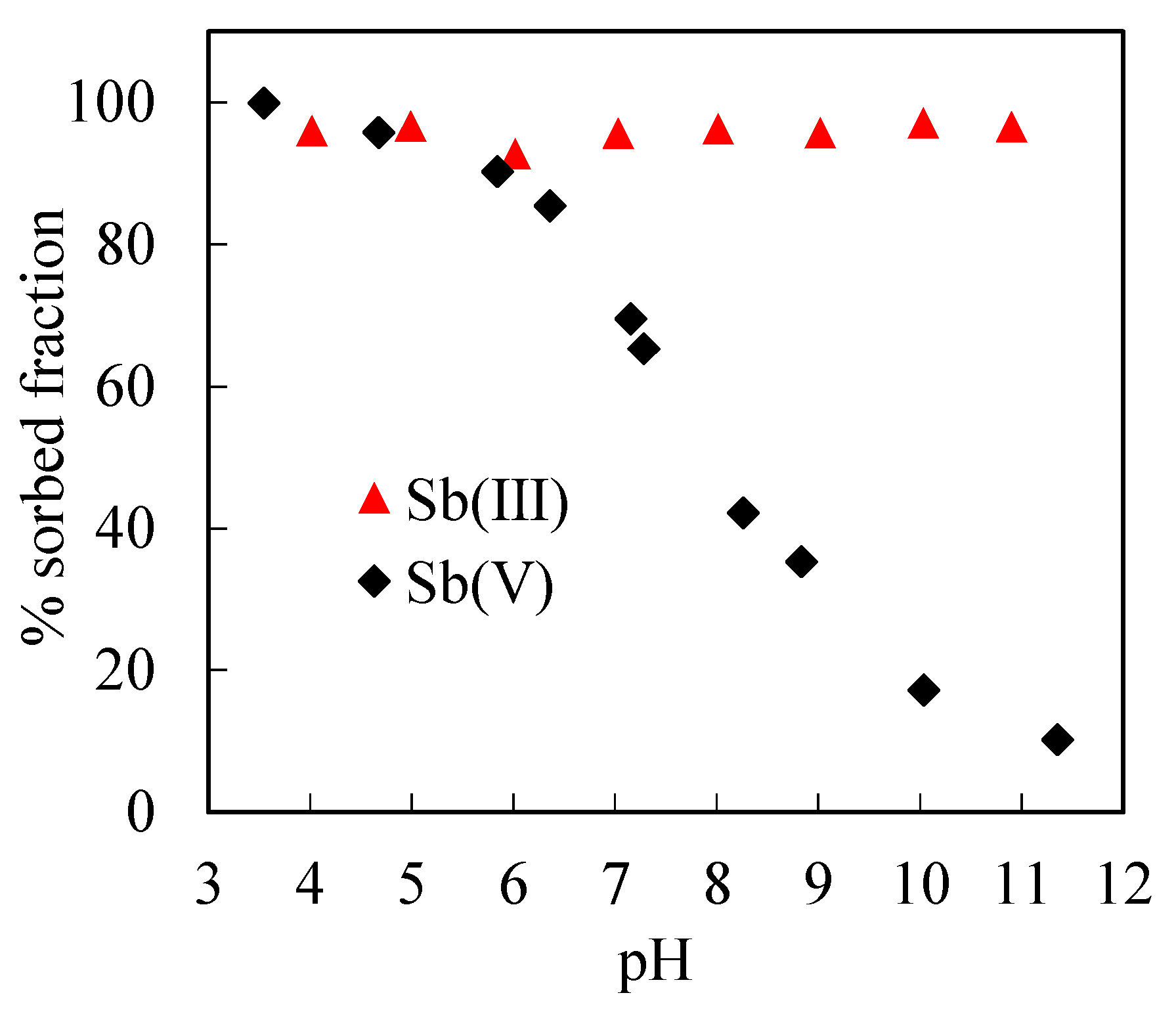
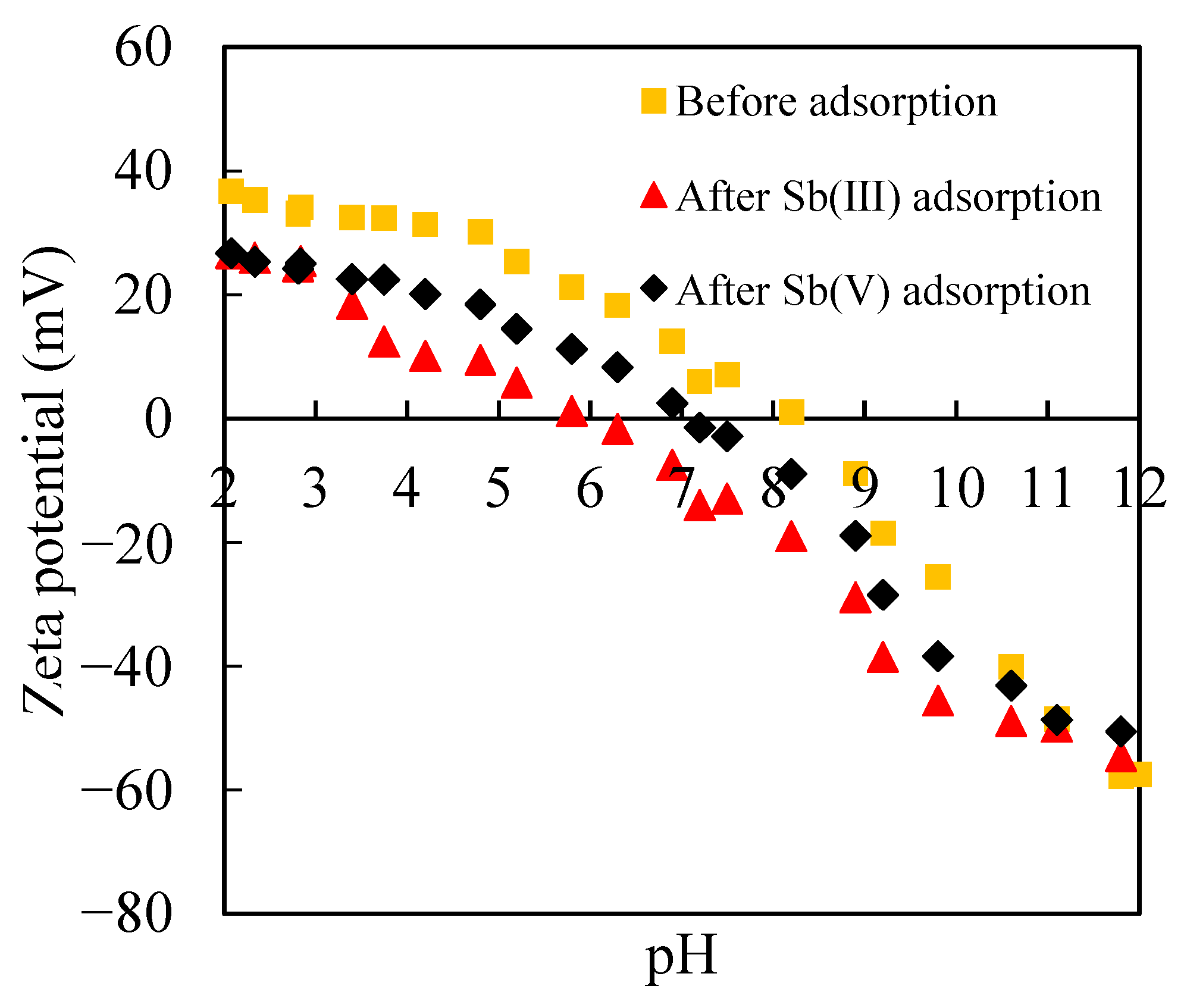
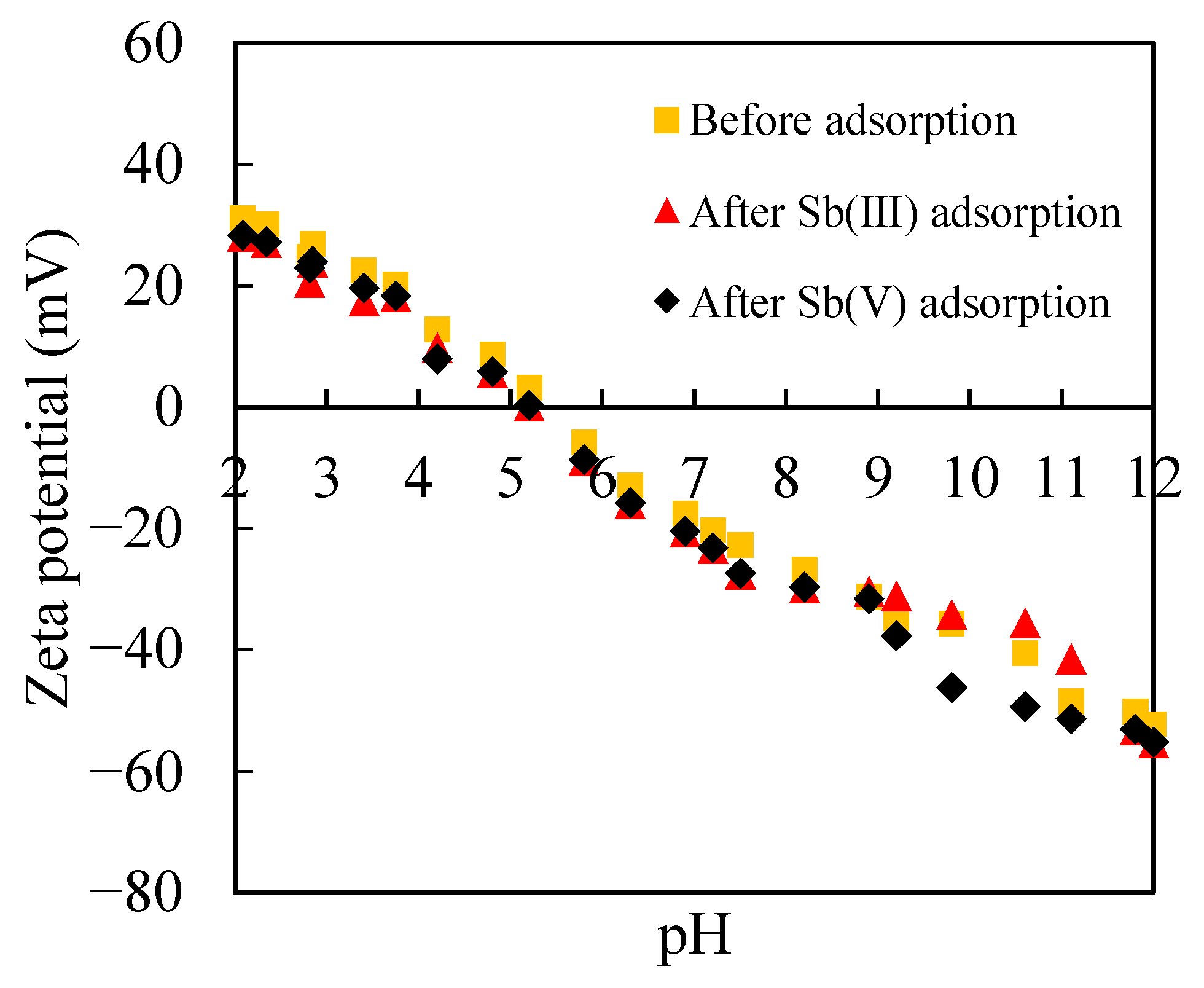
3.2. Adsorption of Sb(III) and Sb(V) on Ferrihydrite
3.2.1. Sb(III) and Sb(V) Adsorption in the Absence of Silica
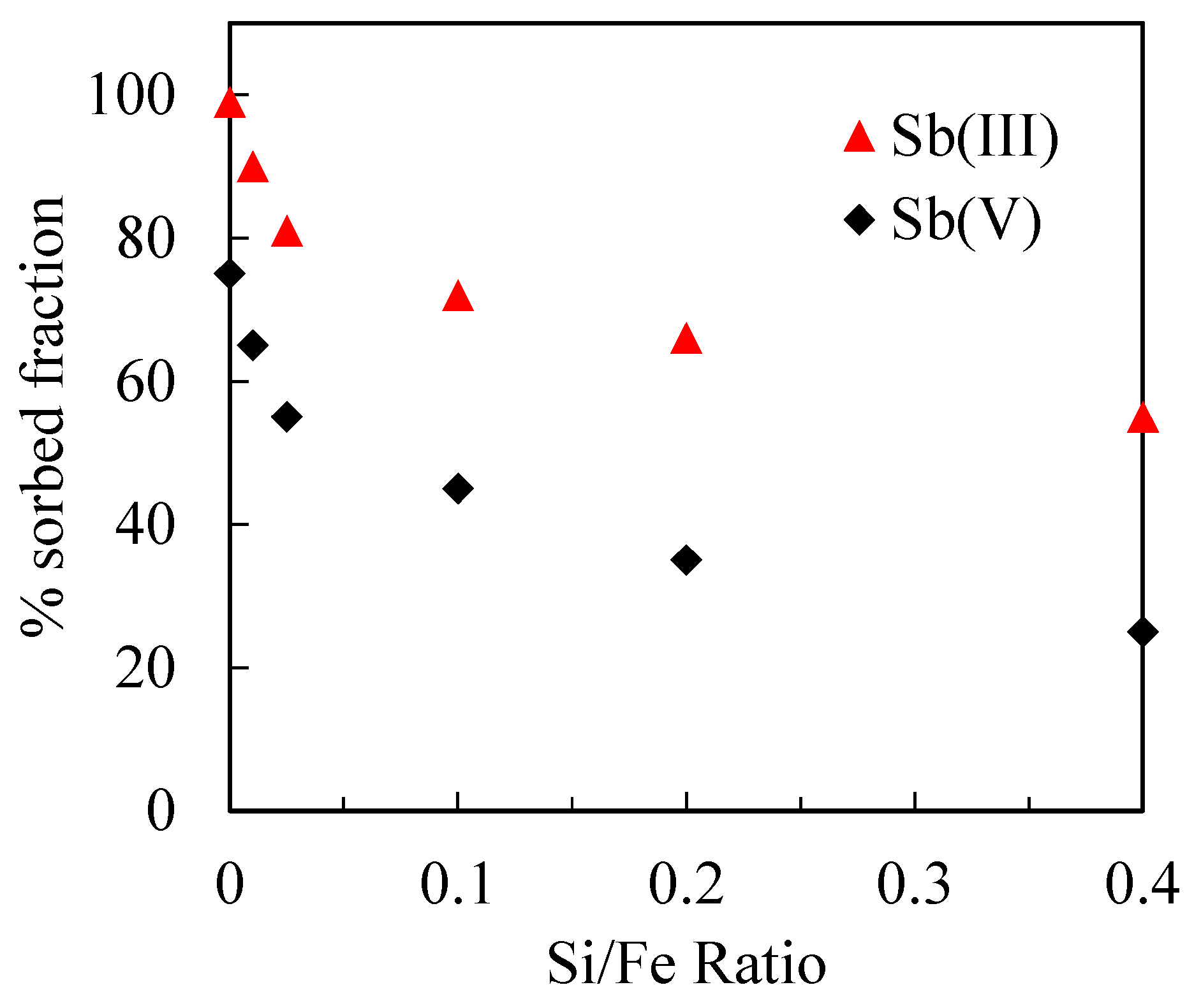
3.2.2. Sb(III) and Sb(V) Adsorption in the Presence of Silica
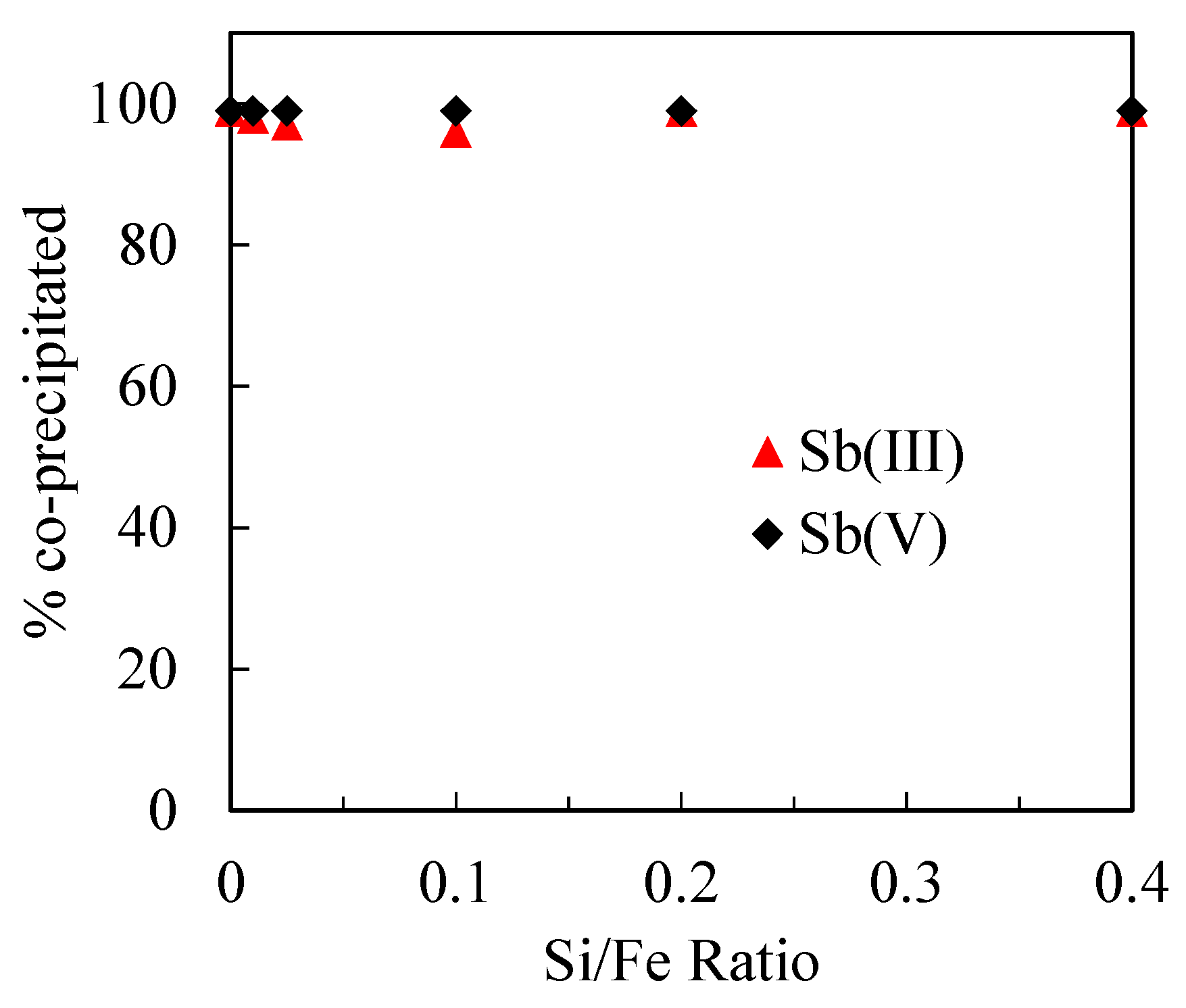
3.3. Sb(III) and Sb(V) Co-Precipitation Process with Different Si/Fe Ratios
4. Conclusions and Implications
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters I. Occurence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Carlin, J.F., Jr. Antimony. In U.S. Geological Survey Mineral Commodity Summaries; USGS: Denver, CO, USA, 2000; pp. 295–306. [Google Scholar]
- Herbst, K.A.; Rose, G.; Hanusch, K.; Schumann, H.; Wolf, H.U. Antimony and antimony compounds. Ullmanns Encycl. Ind. Chem. 1985, 3, 55–76. [Google Scholar]
- Krachler, M.; Emons, H.; Zheng, J. Speciation of antimony for the 21st century: Promises and pitfalls. TrAC Trends Anal. Chem. 2001, 20, 79–90. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters II. Relevant solution chemistry. Earth-Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Rossberg, A.; Vantelon, D.; Xifra, I.; Kretzschmar, R.; Leuz, A.K.; Funke, H.; Johnson, C.A. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 3299–3312. [Google Scholar] [CrossRef]
- He, M. Distribution and phytoavailability of antimony at an antimony mining and smelting area, Hunan, China. Environ. Geochem. Health 2007, 29, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, M.; Xi, J.; Lu, X. Antimony distribution and mobility in rivers around the world’s largest antimony mine of Xikuangshan, Hunan Province, China. Microchem. J. 2011, 97, 4–11. [Google Scholar] [CrossRef]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Mitsunobu, S.; Harada, T.; Takahashi, Y. Comparison of antimony behavior with that of arsenic under various soil redox conditions. Environ. Sci. Technol. 2006, 40, 7270–7276. [Google Scholar] [CrossRef] [PubMed]
- Lichti, K.A.; Ko, M.; Wallis, L. Galvanic corrosion study of carbon steel to arsenic and antimony couples. Geothermics 2015, 58, 15–21. [Google Scholar] [CrossRef]
- Okkenhaug, G.; Zhu, Y.G.; He, J.; Li, X.; Luo, L.; Mulder, J. Antimony (Sb) and Arsenic (As) in Sb mining impacted paddy soil from Xikuangshan, China: Differences in mechanisms controlling soil sequestration and uptake in Rice. Environ. Sci. Technol. 2012, 46, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Gebel, T. Aresnic and antimony: Comparative approach on mechanistic toxicology. Chem. Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef]
- USEPA. Water Related Fate of the 129 Priority Pollutants; DOC. 745-R-00-007; USEPA: Washington, DC, USA, 1979; Volume 1.
- CEC (Council of the European Communities). Council Directive 76/464/EEC of 4 May 1976 on pollution caused by certain dangerous substances discharged into the aquatic environment of the Community. Off. J. L 1976, 129, 23–29. [Google Scholar]
- Oorts, K.; Smolders, E.; Degryse, F.; Buekers, J.; Gascó, G.; Cornelis, G.; Mertens, J. Solubility and toxicity of antimony trioxide (Sb2O3) in soil. Environ. Sci. Technol. 2008, 42, 4378–4383. [Google Scholar] [CrossRef] [PubMed]
- Krupka, K.M.; Serne, R.J. Geochemical Factors Affecting the Behavior of Antimony, Cobalt, Europium, Technetium, and Uranium in Vadose Zone Sediments; Northwest National Lab. (PNNL): Richland, WA, USA, 2002.
- Mitsunobu, S.; Takahashi, Y.; Terada, Y.; Sakata, M. Antimony(V) incorporation into synthetic ferrihydrite, goethite, and natural iron oxyhydroxides. Environ. Sci. Technol. 2010, 44, 3712–3718. [Google Scholar] [CrossRef] [PubMed]
- Leuz, A.-K.; Mönch, H.; Johnson, C.A. Sorption of Sb(III) and Sb(V) to goethite: Influence on Sb(III) oxidation and mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, G.; Amstätter, K.; Lassen Bue, H.; Cornelissen, G.; Breedveld, G.D.; Henriksen, T.; Mulder, J. Antimony (Sb) contaminated shooting range soil: Sb mobility and immobilization by soil amendments. Environ. Sci. Technol. 2013, 47, 6431–6439. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, S.; Gieré, R.; Newville, M.; Majzlan, J. Antimony sinks in the weathering crust of bullets from Swiss shooting ranges. Sci. Total Environ. 2009, 407, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Swedlund, P.J.; Webster, J.G. Adsorption and polymerisation of silicic acid on ferrihydrite, and its effect on arsenic adsorption. Water Res. 1999, 33, 3413–3422. [Google Scholar] [CrossRef]
- Waychunas, G.A.; Rea, B.A.; Fuller, C.C.; Davis, J.A. Surface chemistry of ferrihydrite: Part 1.EXAFS studies ongeometrie of coprecipitated and adsorbed arsenate. Geochim. Cosmochim. Acta 1993, 57, 2251–2269. [Google Scholar] [CrossRef]
- Russell, J.D. Infrared spectroscopy of ferrihydrite: Evidence for the presence ofstructural hydroxyl groups. Clay Miner. 1979, 14, 109–114. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 3527302743. [Google Scholar]
- Doelsch, E.; Masion, A.; Rose, J.; Stone, W.E.E.; Bottero, J.Y.; Bertsch, P.M. Chemistry and structure of colloids obtained by hydrolysis of Fe(III) in the presence of SiO4 ligands. Colloids Surf. A Physicochem. Eng. Asp. 2003, 217, 121–128. [Google Scholar] [CrossRef]
- Swedlund, P.J.; Miskelly, G.M.; McQuillan, A.J. Silicic acid adsorption and oligomerization at the ferrihydrite—Water interface: Interpretation of ATR-IR spectra based on a model surface structure. Langmuir 2010, 26, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- Pokrovski, G.S.; Schott, J.; Farges, F.; Hazemann, J.-L. Iron (III)-silica interactions in aqueous solution: Insights from X-ray absorption fine structure spectroscopy. Geochim. Cosmochim. Acta 2003, 67, 3559–3573. [Google Scholar] [CrossRef]
- Seehra, M.S.; Roy, P.; Raman, A.; Manivannan, A. Structural investigations of synthetic ferrihydrite nanoparticles doped with Si. Solid State Commun. 2004, 130, 597–601. [Google Scholar] [CrossRef]
- Dyer, L.; Fawell, P.D.; Newman, O.M.G.; Richmond, W.R. Synthesis and characterisation of ferrihydrite/silica co-precipitates. J. Colloid Interface Sci. 2010, 348, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Cismasu, A.C.; Michel, F.M.; Tcaciuc, A.P.; Brown, G.E. Properties of impurity-bearing ferrihydrite III. Effects of Si on the structure of 2-line ferrihydrite. Geochim. Cosmochim. Acta 2014, 133, 168–185. [Google Scholar] [CrossRef]
- Swedlund, P.J.; Miskelly, G.M.; McQuillan, A.J. An attenuated total reflectance IR study of silicic acid adsorbed onto a ferric oxyhydroxide surface. Geochim. Cosmochim. Acta 2009, 73, 4199–4214. [Google Scholar] [CrossRef]
- Hiemstra, T.; Barnett, M.O.; van Riemsdijk, W.H. Interaction of silicic acid with goethite. J. Colloid Interface Sci. 2007, 310, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Pichler, T. Sequential and simultaneous adsorption of Sb(III) and Sb(V) on ferrihydrite: Implications for oxidation and competition. Chemosphere 2016, 145, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 126. [Google Scholar]
- Wang, L.; Wan, C.; Zhang, Y.; Lee, D.-J.; Liu, X.; Chen, X.; Tay, J.-H. Mechanism of enhanced Sb(V) removal from aqueous solution using chemically modified aerobic granules. J. Hazard. Mater. 2015, 284, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; He, M. Removal of antimony(V) and antimony(III) from drinking water by coagulation-flocculation-sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.; Marmier, N.; Lomenech, C.; Giffaut, E.; Ehrhardt, J.J. Competition between selenium (IV) and silicic acid on the hematite surface. Chemosphere 2009, 75, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.M.; Ehm, L.; Antao, S.M.; Lee, P.L.; Chupas, P.J.; Liu, G.; Strongin, D.R.; Schoonen, M.A.A.; Phillips, B.L.; Parise, J.B. The Structure of Ferrihydrite, a Nanocrystalline Material. Science 2007, 316, 1726–1729. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Sato, T.; Otake, T. Dissolved Silica Effects on Adsorption and Co-Precipitation of Sb(III) and Sb(V) with Ferrihydrite. Minerals 2018, 8, 101. https://doi.org/10.3390/min8030101
Zhou S, Sato T, Otake T. Dissolved Silica Effects on Adsorption and Co-Precipitation of Sb(III) and Sb(V) with Ferrihydrite. Minerals. 2018; 8(3):101. https://doi.org/10.3390/min8030101
Chicago/Turabian StyleZhou, Shuang, Tsutomu Sato, and Tsubasa Otake. 2018. "Dissolved Silica Effects on Adsorption and Co-Precipitation of Sb(III) and Sb(V) with Ferrihydrite" Minerals 8, no. 3: 101. https://doi.org/10.3390/min8030101
APA StyleZhou, S., Sato, T., & Otake, T. (2018). Dissolved Silica Effects on Adsorption and Co-Precipitation of Sb(III) and Sb(V) with Ferrihydrite. Minerals, 8(3), 101. https://doi.org/10.3390/min8030101





