3.2. XRPD Study
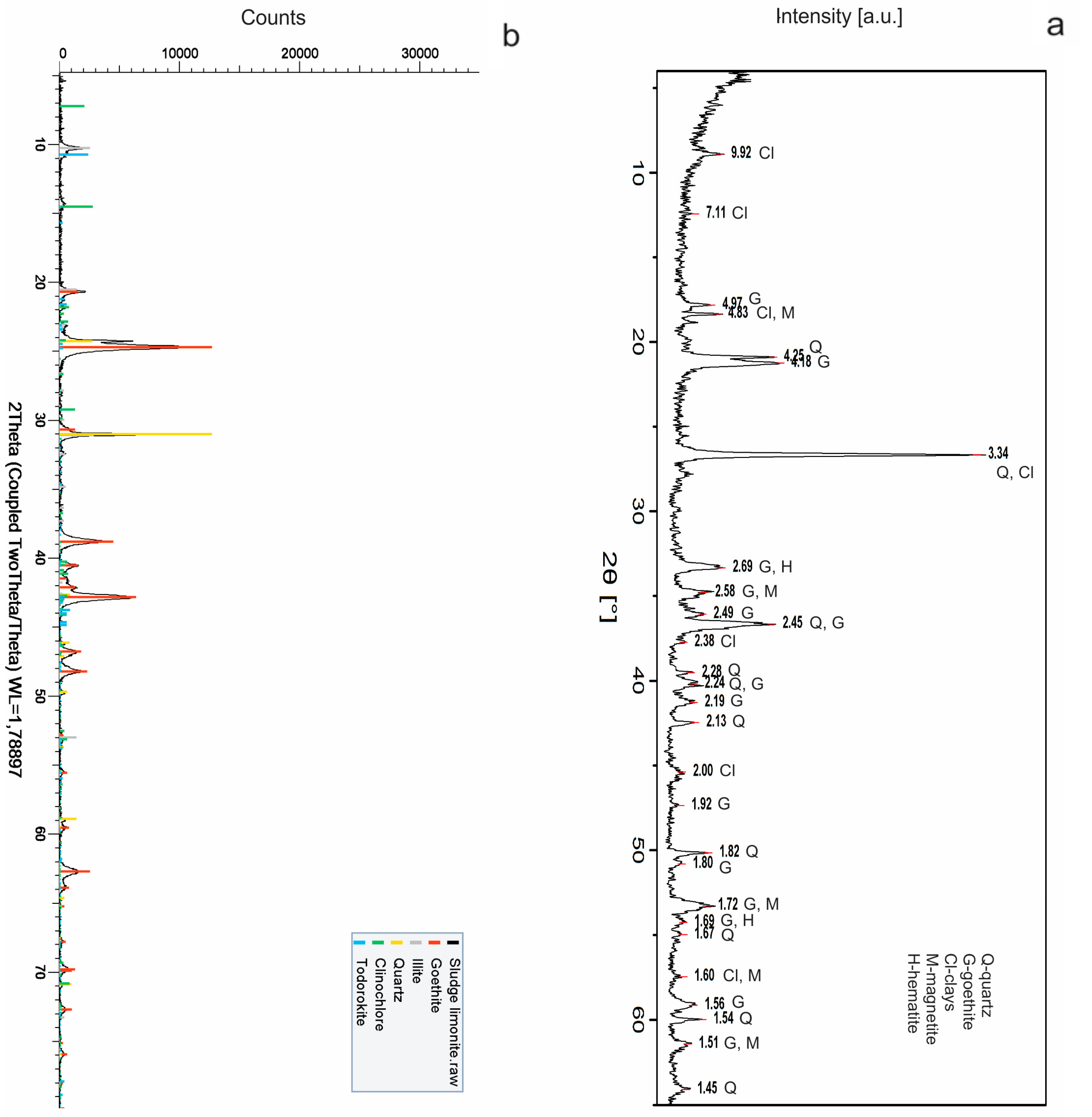
The obtained results of the XRPD studies are shown at
Figure 1 and
Table 2. By comparison of these, it can be clearly seen that there are more or less similar qualitative mineral compositions, but with their different semi-quantitative contents. It can be concluded that sludge samples consist mostly of goethite-FeO(OH) and quartz (SiO
2), which prevails over clay minerals. Also, the minor contents of magnetite, hematite, clinochlore and todorokite (with composition of: (Na,Ca,K,Ba,Sr)
1−x(Mn,Mg,Al)
6O
12·3–4H
2O), were determined.
Identification of clay minerals indicates that they are mostly of illite-sericite and kaolinite composition, and with chlorites, which appears only sporadically. Sericite is mainly of the muscovite mica type.
The results of determined densities (
Table 1), obviously confirm the XRPD results (
Table 2), and the correlation between sample mineral composition and their densities. Namely, increase of goethite (4.27–4.29 g/cm
3) content increases the sample density, whereas increase of quartz (2.65–2.66 g/cm
3) and clays (2.79–2.80 g/cm
3) contents decrease it (
https://www.mindat.org).
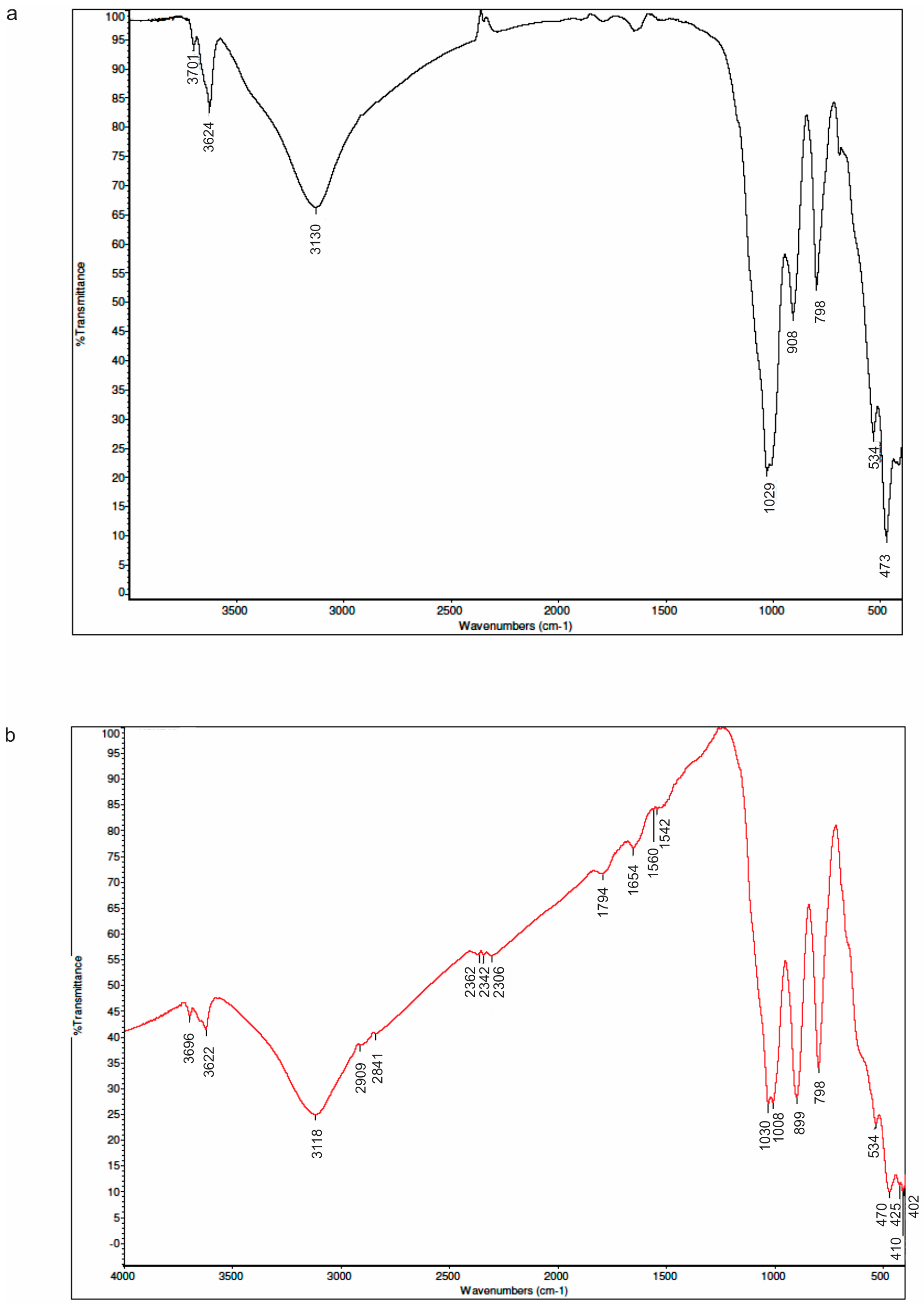
3.4. SEM-EDS
SEM-EDS was used for the morphological, structural and chemical composition study of sludge samples, and results are shown at
Figure 3 and
Figure 4, and
Table 4 and
Table 5. For defining a more precise elemental and mineral composition, analyses of selected points on the surface were done. These points were primarily selected by choosing different grains with various colors, brightness, types of crystals, shapes and textures, and with the main purpose of analyzing as many different mineral species as possible. It is obvious that majority of the visible grains are very fine or ultra-fine, with sizes less than about 10 μm; bigger grains than these are with at less quantity, and is contrary to the much bigger grains determined for natural mineral raw samples [
2].
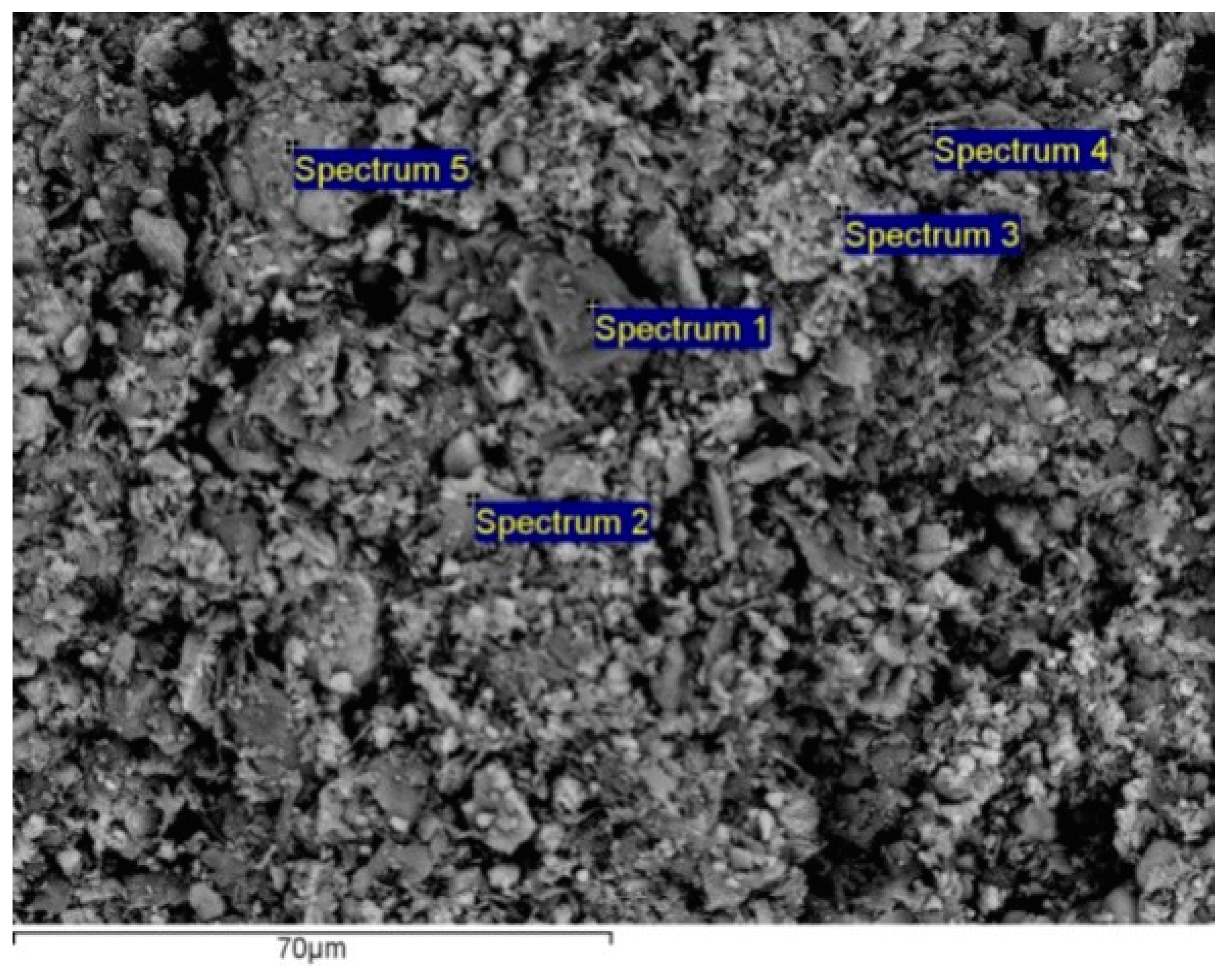
SEM images and EDS chemistry of sludge I samples (
Figure 3 and
Table 4) show heterogeneity of mineral composition with the predominant oxide (and/or hydroxide) minerals consisting mainly of iron, silicon and aluminum; and with some Mn, Na and K, as well. Also, only rarely, there are some Ti and Cu impurities. Furthermore, Si/Al ratio indicates the presence of free silica (i.e., quartz), with or without Al-silicates.
X-ray microanalysis (EDS) data for selected points (
Figure 3 and
Table 4) confirm heterogeneity of sludge I surfaces with the following detected possible major phases, and which were calculated from compound % based on oxygen by stoichiometry: quartz (point 1); Fe minerals intricately associated with alumosilicates (point 2); Fe minerals (points 3 and 5); and alumosilicates (point 4). Small quantities of manganese minerals (or its impurities) have been also recorded at the points 2–5.
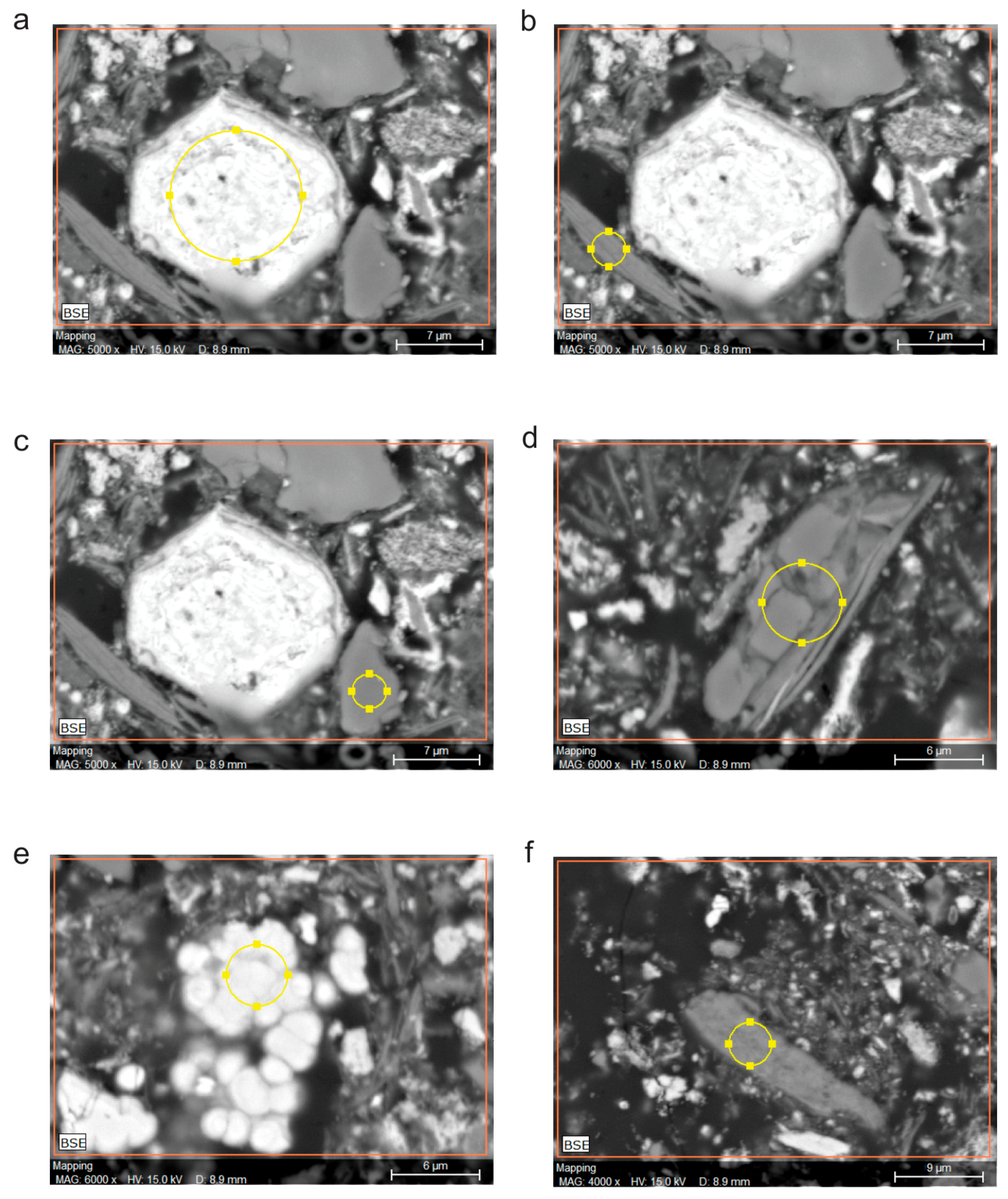
X-ray microanalysis (EDS) data for selected points of the sludge II sample (
Figure 4 and
Table 5), compared to sludge I sample, confirm the same heterogeneity of sludge surfaces. In addition, the same major possible phases were detected, i.e., Fe minerals associated with manganese (spectrums a and e); alumosilicates with K, Na and rarely Fe (spectrums b and f); and quartz (spectrums c and d). Therefore, it is obvious that the chosen points are of various types of crystals, shapes, textures, colors and brightness, and are adequate to the determined minerals. For example, Fe minerals are bright, whereas quartz and clay minerals are gray or dark.
One of the most limiting factors for determination of the mineral phases from EDS data is its inability to measure hydrogen, since goethite and clay minerals contain considerable amounts of OH
− groups and/or H
2O. According to that, we can only speculate about these possible phases. However, we believe that the obtained SEM-EDS results are also in very good accordance and that they mainly confirms XRPD results of the previously determined qualitative and semi-quantitative mineral compositions presented in this paper (
Figure 1 and
Table 2). Besides that, all of the presented results are further in very good agreement with those previously obtained for the mineral raw material [
2].
3.5. Particle Size Distribution Analyzes
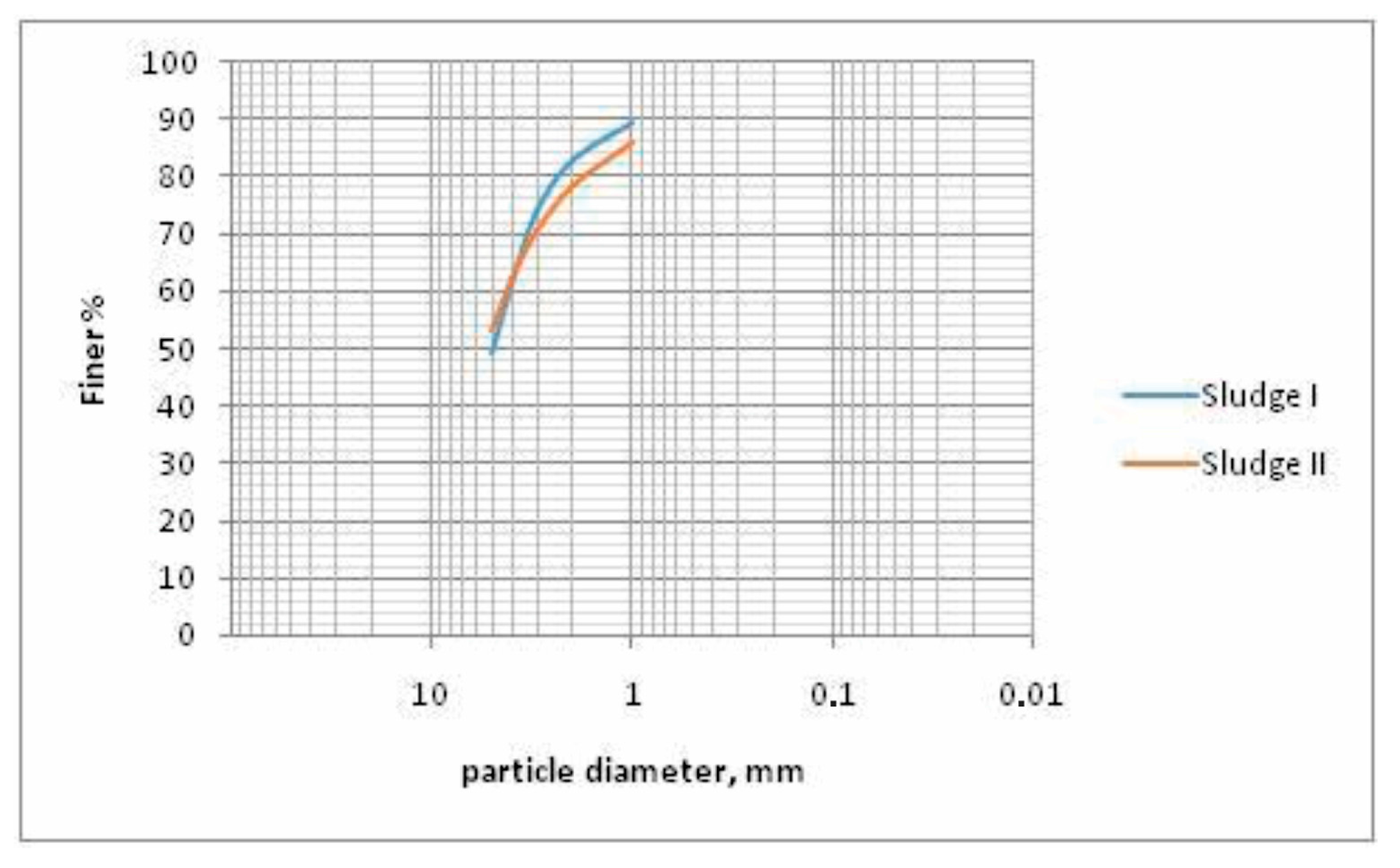
Comparative results of particle size distribution analyzes of two samples of sludge, given in
Table 6 and
Figure 5, show that there is no considerable difference in the classes of particle size distribution of these two samples. However, it is clearly noticeable that in both samples, the highest mass percentage (~50%) belongs to the finest classes, i.e., to those bellow 6 μm. Furthermore, almost 3/4 of the samples belong to the classes below 13 μm, confirming the SEM-EDS results (
Figure 3 and
Figure 4).
In both samples, iron content was also determined for every size class separately (
Table 6). The Fe content by particle size classes is quite uniform and generally is in accordance with the initial measurements. Calculated units of Fe compared to initial values show the largest iron content in the size class bellow 6 μm. Although in some classes a slightly higher iron content is observed by comparison with the initial one (such as 6–9 μm;
Table 6), these differences are not sufficient reason for further research to be done on separate classes. Particularly interesting is that there is no big difference in the classes of particle size distribution between the two samples, although the mineral phases are present in various contents (
Table 1,
Table 2 and
Table 3). This indicates that all of the identified mineral phases, both useful (such as goethite and other Fe minerals) and useless (i.e., gangue minerals, such as quartz and clays), are present in the sludge samples in very similar contents in all of the particle size classes. As these minerals mainly appear as fine and ultra-fine particles, this makes the system very complex for further separation processes.
Some other recently presented results of particle size distribution of the limonite sludge that occurs as a hydro-cyclone overflow in the Omarska iron mine [
23] are also in accordance with those listed here.
3.6. Initial Settling and Flocculation Studies
The main goal of initial settling and flocculation studies was to compare the behavior of two sludge samples with different S/L ratios; and with or without flocculant. Additionally, the possibility of selective separation of iron minerals and quartz was studied, due to the fact that quartz was determined as the main impurity by the present research (
Figure 1,
Figure 2,
Figure 3 and
Figure 4 and
Table 1,
Table 2,
Table 3,
Table 4 and
Table 5).
Based on previous experiments of limonite and clay settling [
24], it can be concluded that the distribution of limonite in settled product is significantly higher than the distribution of clay, with the use of any applied flocculants, regardless of the time of settling, and the type and concentration of applied reagents. Distributions of limonite in the settled product in laboratory experiments under test conditions were within the range of 80–100%. The distributions of clay in the overflow were within 20–50%, which, translated into industrial conditions, means that the settled product with over 51–55% of Fe can be easily obtained, with mass utilization of over 80%, compared to the initial sludge. This is possible, because industrial sludge in Omarska contains over 40%, and sometimes more than 50% of Fe. On the basis of the Fe content in settled product, we believe that it would be, after consolidation, very interesting for application in the field of metallurgy.
For initial settling investigations, under best conditions, the same procedure used for investigations of settling rate on natural limonite samples [
24] was applied. These experiments aim to demonstrate the possible impact of different input samples on the behavior of settling rate, naturally and also in the presence of the flocculant. Furthermore, the effect of solid concentration was examined by using different ratios of solid/liquid. In such manner, we tried to define the behaviors of these samples, as well as to determine if there is a difference between samples from plants for the preparation of mineral raw materials “Omarska” (which S:L ratios are usually of 1:7; 1:8; or 1:9), and possible influence of mineralogical, chemical or particle size distribution analyses.
3.6.1. Natural Settling with Different S/L Ratio
As it is well known, forms for calculating the grain fall rate cannot accurately determine the rate of precipitation, due to the defects in the correct values for correction coefficients that take into account the irregular shape of the grain, the changings of fluid density and in particular, the questions related to the flocculation or dispersion of the smallest classes resulting from the charge of the grain.
At the beginning of precipitation, in diluted pulp, and under free fall conditions, deposition rate is relatively large and constant. The smaller the angle between the straight line of the curve and the ordinates, the greater the speed of compaction. When crossing into the area of plunged fall, the rate is rapidly reduced and the graph gets a rectilinear character. As the density increases in the thickening zone, the curve is asymptotically approaching a constant value that depends on the thickness of the precipitate layer of the precipitated product. A defective point is called a critical deposition point. The prevailing opinion is that for the critical point of precipitation, the beginning of the precipitation curve should be taken. The critical rate (Vav) in this paper is calculated as: Vav = H/tcr (mm/min); where H is height of the clear zone during critical time (in mm), and tcr is deposition time required to achieve a critical deposition point (in minutes).
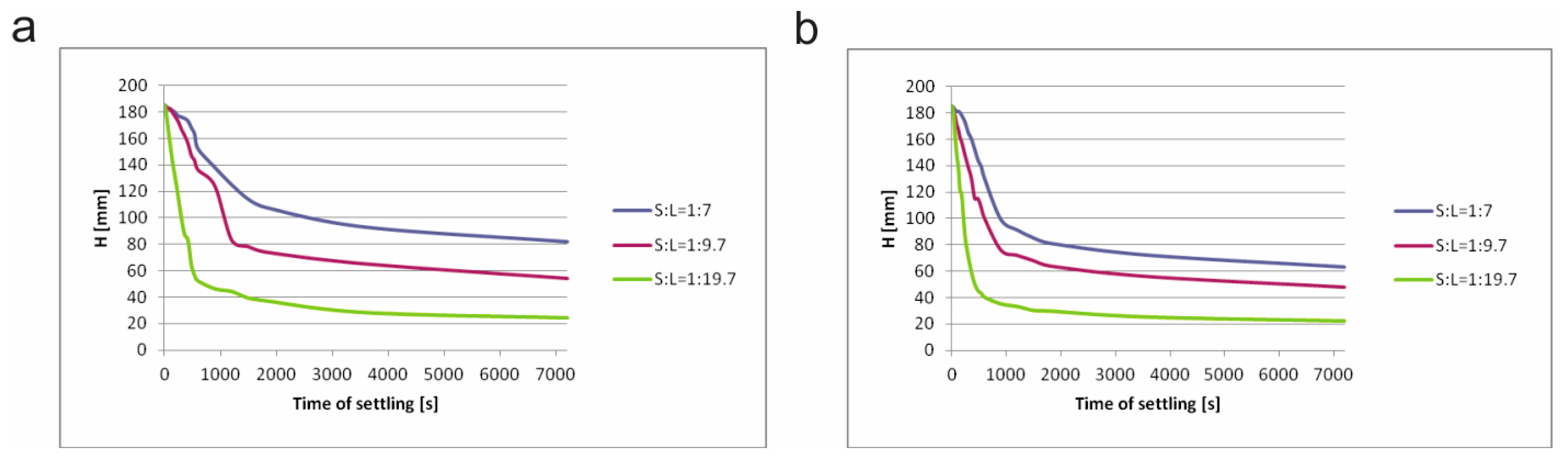
In
Figure 6, natural settling curves of sludge I and sludge II with different ratios of S:L are shown. Generally, there is no big difference in settling behavior between these samples. However, sludge II sample has a higher settling rate as expected due to its higher density and higher contents of Fe minerals in its composition (
Table 1,
Table 2 and
Table 3). If we compare the effect of solid concentration, in all three cases, sludge II has a higher deposition rate. Also, samples with smallest solid/liquid ratio have the highest settling rates in both cases, which is in accordance with some other studies [
25].
3.6.2. Settling with Different S/L Ratios with Additional Flocculant A100
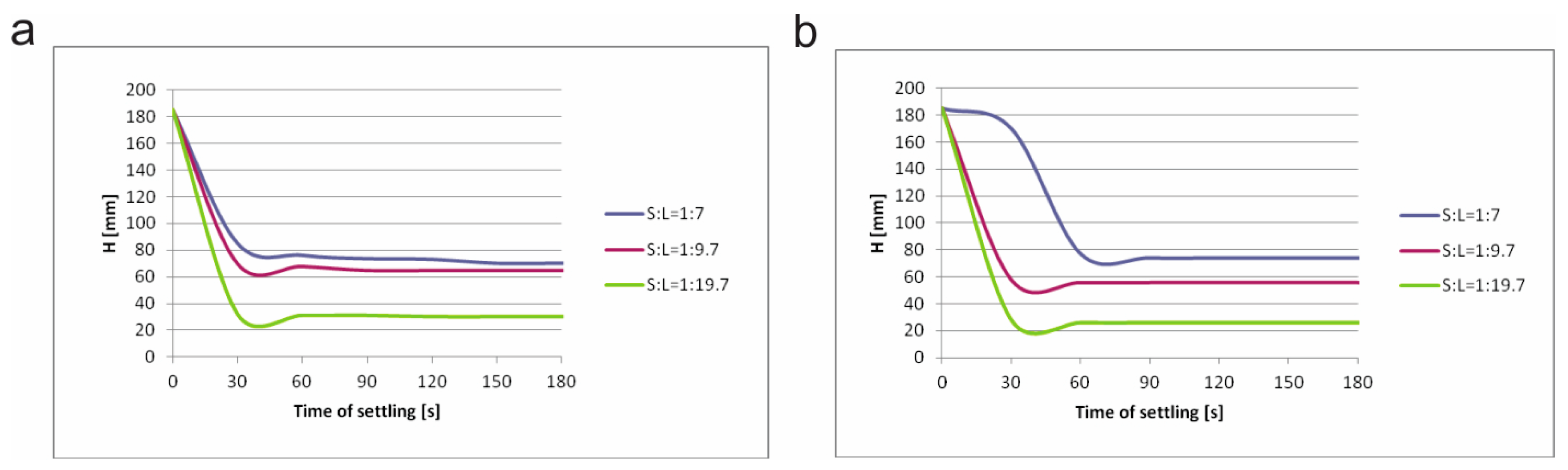
In
Figure 7, settling curves of both samples with different ratios of S/L, and with additional anionic flocculant A100, are shown.
Figure 7 shows that there is relatively small difference between sludge I and sludge II. In all cases settling starts fast, i.e., only after 30 seconds from the beginning, regardless of the S/L ratio; or the type of sample. However, by comparison with natural settling (
Figure 6 and
Figure 7), it can be clearly seen that the settling rate is obviously improved with additional flocculant.
In
Table 7 and
Figure 8, comparisons of critical settling rates are shown. From these, it can be clearly seen that in the case of natural settling, there is no significant increase in the critical settling rates, regardless of the S:L ratios. The differences between the rates in the relationship of S:L are greater with higher percentage of the liquid phase in both samples. It is important to emphasize here that such diluted pulp is unusual for plants from which the samples were taken (i.e., the overflow of the hydro cyclone), but it was used in the experiment for easier detection of the behavior of the mineral particles and the influence of the solid/liquid ratios.
However, with the addition of the flocculant, these rates increase significantly with the increase of the liquid component in both of the samples. Furthermore, the differences in critical settling rates between samples in the presence of the flocculant are also obvious. These differences obviously depend on the various mineral compositions, because sludge II contains significantly higher content of iron minerals than sludge I (
Table 1 and
Table 2). Obviously, the effect of flocculant in the heterogeneous system depends both on the mineral composition and their quantity; as well as on the S/L ratio.
Effect of solid/liquid ratio on settling rate was confirmed by the results of hindered settling rate which was calculated according to the Richardson–Zaki equation [
25]. This equation describes a method for calculating the sedimentation rate in a liquid-solid system as a function of the free falling rate of a single particle and the concentration of particles. From
Table 8, it can be seen that samples with the smallest solid/liquid ratio have the highest settling rates in both cases.
3.6.3. Testing Selectivity of Flocculation with Flocculant A100 and Dispersant SHMP
As noted above, the dispersant and the flocculant were selected on the basis of results obtained earlier of the effects of certain reagents on the behavior of limonite and clay [
24]. Namely, it was then concluded that the distribution of limonite in settled product is significantly higher than the distribution of clay, with the use of applied flocculants, regardless of the time of settling. However, at that time, behavior of quartz was not studied, due to the supposition of its insignificant contents and role. Some earlier studies showed that hydrolyzed polyacrylamide may be used for selective flocculation of hematite from quartz [
25,
26,
27].
In this chapter, the influence of settling time on the selectivity was tested only at the iron richer sample (sludge II) with addition of sodium hexametaphosphate (SHMP) as dispersant. Initially, settling behavior was tested by monitoring the weight distribution and the content grade and recovery of Fe and SiO
2 in floating and sinking part. The results of the distribution of masses and grade and recovery of Fe and SiO
2 contents between the sinking and floating part in the function of settling time are presented in
Table 9.
According to the obtained results, settling time does not play a significant role in the distribution of masses between the sinking and floating parts. Partial increasing of iron ore content after flocculation by comparison to the initial sample can be seen especially in the floating part. However, taking into account the distribution of masses, recovery of iron ore is relatively low, i.e., within a few Fe%; and, therefore, it is not quite satisfactory. However, as the content of iron partially increases by flocculation, this method should be considered as appropriate.
Separation indices are not calculated, because it is obvious from
Table 9 that the selectivity has not been achieved. Theoretically, selective flocculation depends on many parameters, such as: surface chemistry of particles, particle size distribution, degree of dispersion of particles, functional groups of the polymer, etc. Specifically, heterogeneity of the mineral surface is one of the important factors in these cases. According to the cited papers, we expected that polyacrylamide is much less adsorbed on quartz. Unfortunately, in this case, this did not happen.
3.7. Zeta Potential Study
In order to better understand the influence of mineral composition on dispersion, in this chapter, results of zeta potential (ζ-potential) measurements were analyzed for the sludge itself, as well as for the individual goethite and quartz phases, which were previously identified as major minerals in the sludge samples (
Figure 2 and
Figure 3;
Table 1,
Table 2 and
Table 3).
An important step in the selective flocculation process is to achieve stable dispersion of mineral particles. Dispersion stability of mineral particles is dependent on the zeta potential of present particles. The knowledge of zeta potential plays a significant role in understanding dispersion and interfacial processes, such as flocculation. Zeta potential is a measure of the magnitude of the electrostatic or charge repulsion/attraction between particles, and it is one of the fundamental parameters known to affect stability. Its measurement brings detailed insight into the causes of dispersion, aggregation or flocculation, and it can be used to improve the formulation of dispersions, emulsions and suspensions.
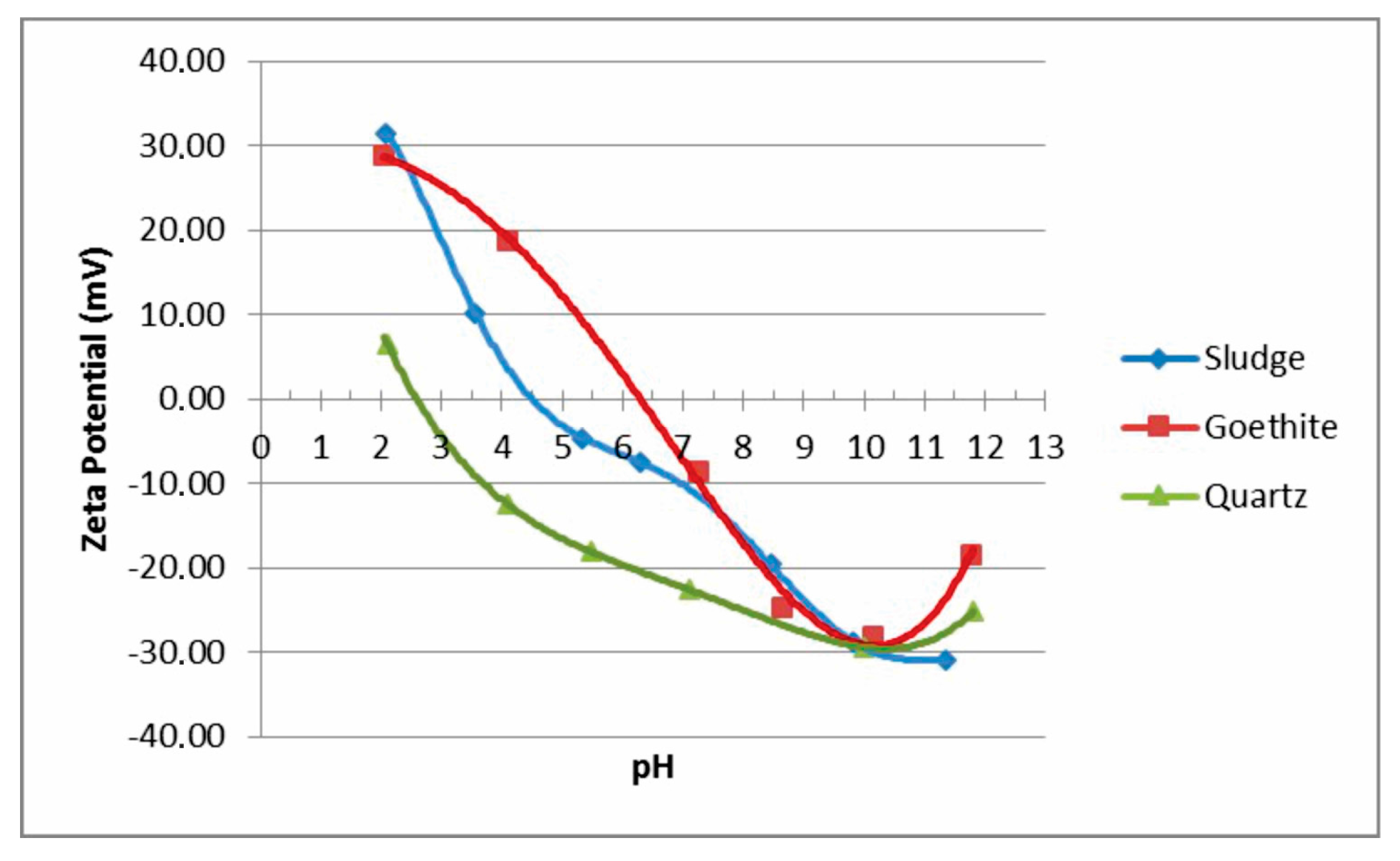
From a plot of zeta potential as a function of pH, a number of important data can be noted. Namely, isoelectric point (IEP) represents a condition when the value of zeta potential is zero. At such conditions, the dispersion is unstable, and there are no reflective forces present among the particles. A higher zeta potential produces dispersion. Also, particles with an IEP below pH = 7 have an acidic character, whereas those with an IEP above pH = 7 are basic (i.e., alkaline). This study was done by measuring of zeta potential in the various combinations of absence/presence of dispersant (SHMP), and also in the absence/presence of anionic polyacrylamide A100 (PAM) flocculant.
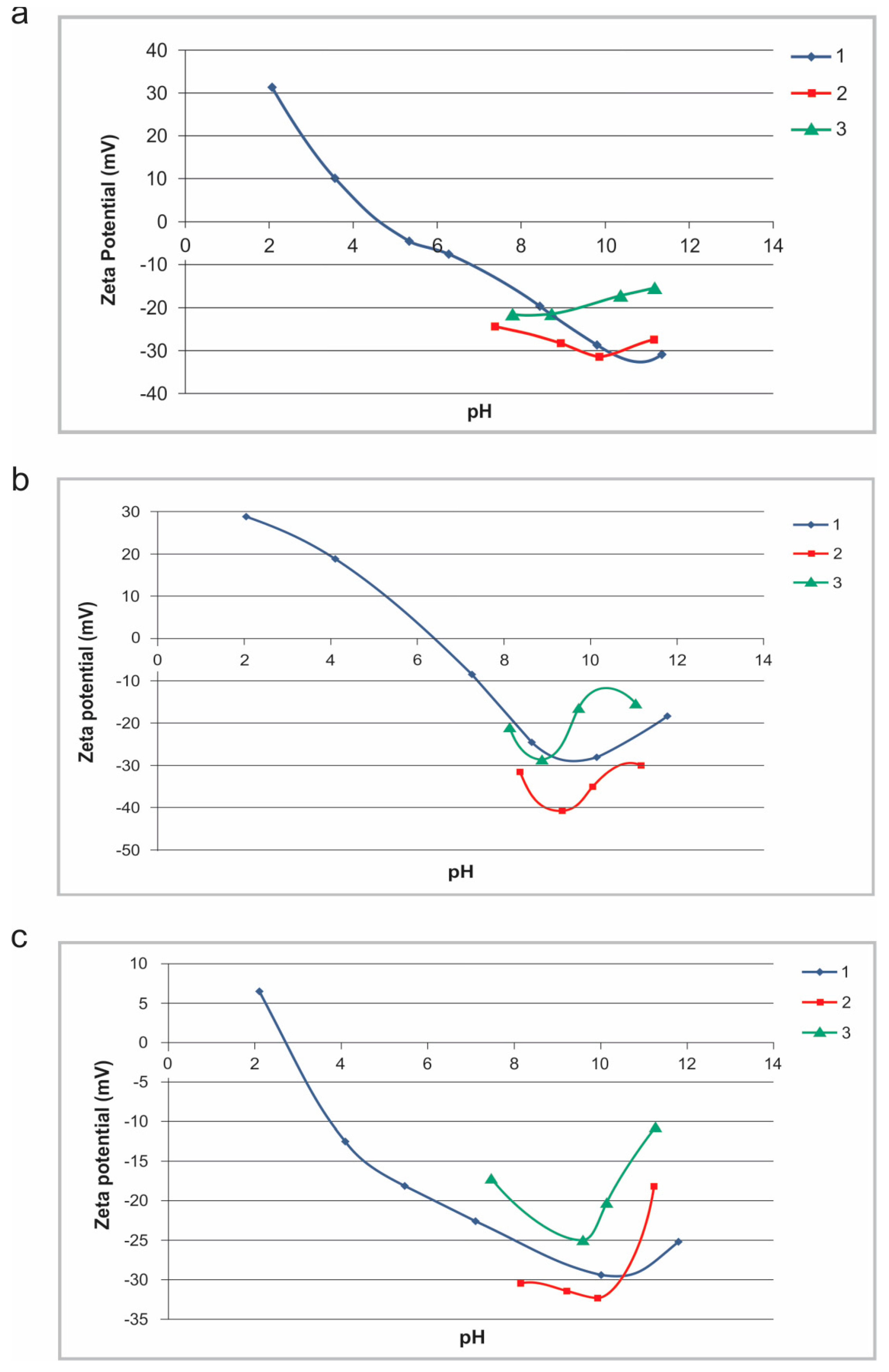
Zeta-potential curves for sludge II, and also for natural mineral raw samples of goethite II and quartz II, which were previously determined [
2], are shown at
Figure 9. The zeta potential curves for natural quartz and goethite are similar to various curves available in the literature [
28], with the IEPs at pH = 2.6 and pH = 6.6 values, respectively. The IEP for sludge II that was found at pH = 4.5 confirms mineralogical heterogeneity on surfaces of particles. Namely, from the results obtained, it is obvious that the measured zeta potential and IEP value of sludge II mostly lie between the values of individual mineral goethite and quartz phases. Therefore, this most probably indicates to the presence of both of these individual phases on the surface of sludge particles. The magnitude of the negative zeta potential of all mineral particles is maximized at pH = 10 value.
The presence of sodium-hexametaphosphate (SHMP) dispersant obviously causes the increase of negative zeta potential magnitudes in all of the investigated cases, as it can be seen from
Figure 10. Also, from these, it can be clearly seen that the surface charge remains negative throughout all of the investigated pH values, and for all of the samples. According to the well-known DLVO (Derjaguin, Landau, Verwey, Overbeek) theory, the stability of the suspension of colloidal particles is determined by the balance between the electrostatic interaction and the van der Waals interaction between particles. The addition of SHMP increases the negative charges on particle surfaces, causing better dispersion.
After addition of SHMP dispersant, the biggest difference of zeta potential magnitudes between goethite and quartz are also at pH = 10 (
Figure 10b). For example, such zeta potential values at pH = 10 increase from −30 mV (without SHMP) to −45 mV (with SHMP) for goethite, but only from −29 mV (without SHMP) to −31 mV (with SHMP) for quartz. After adding SHMP, goethite is mostly more negatively charged than quartz. Also, behavior of sludge surface charge is very similar to goethite, and the surface charge remains negative throughout all of the investigated pH region (
Figure 10a,b). The particles, regardless of their mineralogy, with negative zeta potentials of significant magnitude will remain dispersed due to the electrostatic repulsion. The energy barrier can be eliminated by neutralization of surface charge due to the adsorption of a suitable flocculant onto the particle surface and bridging between the particles. Flocculant adsorption can be measured by changing of the zeta potential after adsorption. If the polymer is adsorbed, there is a change in the surface of the particles, and thus the change in zeta potential.
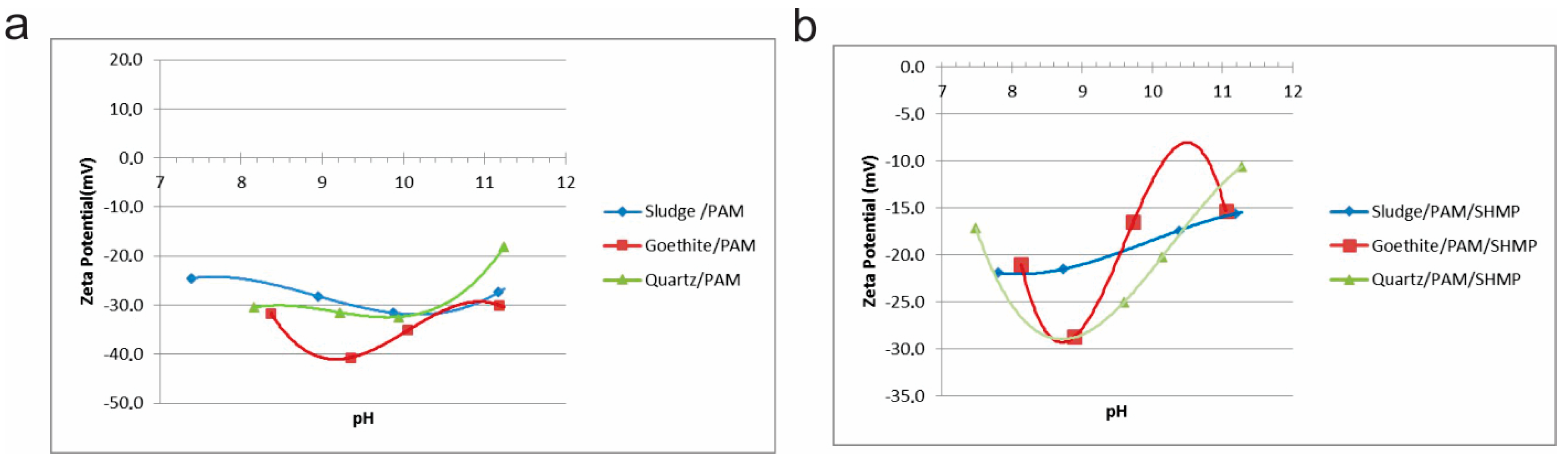
In
Figure 11 and
Figure 12, zeta potential curves as a function of pH for the same studied samples in the presence of anionic polyacrylamide (PAM, A100), and with/without SHMP dispersant, are shown. These curves are presented in two different ways: as mutually compared samples (
Figure 11); and as each individual sample with/without reagents (
Figure 12). This offers a better view of the situation.
It is obvious that the used anionic polyacrylamide (PAM) causes changes on the surface of all tested samples (
Figure 11a and
Figure 12). The relatively high zeta potential values indicate a fairly stable dispersion, regardless of the mineral composition. The further additional inclusion of a SHMP dispersant significantly reduces the zeta potential (
Figure 11b and
Figure 12), indicating favorable conditions for flocculation, particularly for goethite at pH = 10 (
Figure 12b). The difference of the zeta potentials between quartz and goethite indicates a more pronounced flocculation for goethite than for quartz. It is also evident that zeta potential values of sludge in this case are only partially between those for the individual oxides, and depends on the pH range.
The mechanism of flocculation takes place in two stages, i.e., adsorption of reagent onto surface of particles and formation of aggregates. Polymer adsorption onto mineral surface takes place due to typical chemical bonds, i.e., ionic, dispersive (van der Waals), covalent, semi-polar, hydrogen of hydrophobic bonds, as well as their combination [
29].
The nature of the mechanisms involved in flocculation of mineral suspensions is very complex, and depends on many parameters, such as: surface chemistry of particles, particle size distribution, degree of dispersion of particles, functional groups of the polymer, etc. The flocculation in the presence of polymer molecules can be attributed to charge neutralization and inter-particle bridging. Electrostatic bonding is the predominant mechanism when it comes to oppositely charged particles. Polymer molecules, by adsorption on oppositely charged oxide particles, can neutralize the charge on particle surfaces and reduce the inter-particle repulsive forces. A polymer can also flocculate similarly charged particles if the electrostatic repulsive forces are not strong enough to prevent the particles from bridging by the long chains of the polymer. The mechanism of flocculation negatively charged oxide with anionic polyacrylamide cannot be explained by electrostatic bonds. Flocculation of negatively charged oxide particles by anionic polymer is probably due to the hydrogen bonding [
10]. Electronegative MO
− groups are predominant at high pH values on oxide surfaces, and some authors believe that there is a possibility that it can bond with the weakly acidic NH
2 function, forming a weaker bond [
30].
PAM has flocculating effects on both goethite and quartz, as well as on their mixture (i.e., sludge). Our study of the possibility of selective activity was based on the fundamental differences of surface charge of quartz and goethite. According to the zeta potential measurements observed, there is a difference in the surface charge of quartz and goethite, even in presence of the PAM and SHMP. However, the behavior of the mixture is quite different, and their zeta potential values are mostly between those for the individual oxides (
Figure 9,
Figure 10,
Figure 11 and
Figure 12). This could also further indirectly indicate that goethite and quartz (and/or clays) are most probably more or less intricately associated or even included in each other, in accordance with the SEM-EDS study (points 2, 3, 5, a, e and f;
Figure 3 and
Figure 4, and
Table 4 and
Table 5).
Initial settling experiments presented in this paper indicate that despite some differences in the behavior of individual phases, these are not sufficient enough to achieve selective PAM activity under the studied conditions. In the case of such low-grade limonite ore, gangue minerals (which are usually of quartz and clay composition) are finely distributed in a porous matrix of goethite, or intricately associated and even included in each other, as zeta potential and SEM-EDS studies indicated. This makes interfacial particle surfaces heterogeneous, even when they are well dispersed, as our results have shown. In such a case, the presence of another mineral can activate the quartz surface and make it much more reactive to the polyacrylamide chain, than would be expected [
26]. Another reason may be the relatively high solid concentration. All cited positive results have been obtained with a low solid concentration of 1–5%. We selected an S/L ratio of 1:7 (i.e., of 12.5%), because it corresponds to the industrial conditions. Under such conditions, the flocculant more easily bridges the iron mineral particles simultaneously with quartz particles in this heterogeneous system. This leads to the hetero flocculation and reduces selectivity.