Quick, Easy, and Economic Mineralogical Studies of Flooded Chalk for EOR Experiments Using Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Flooding Experiments
2.3. Field Emission Gun-Scanning Electron Microscopy with Energy-Dispersive Spectroscopy
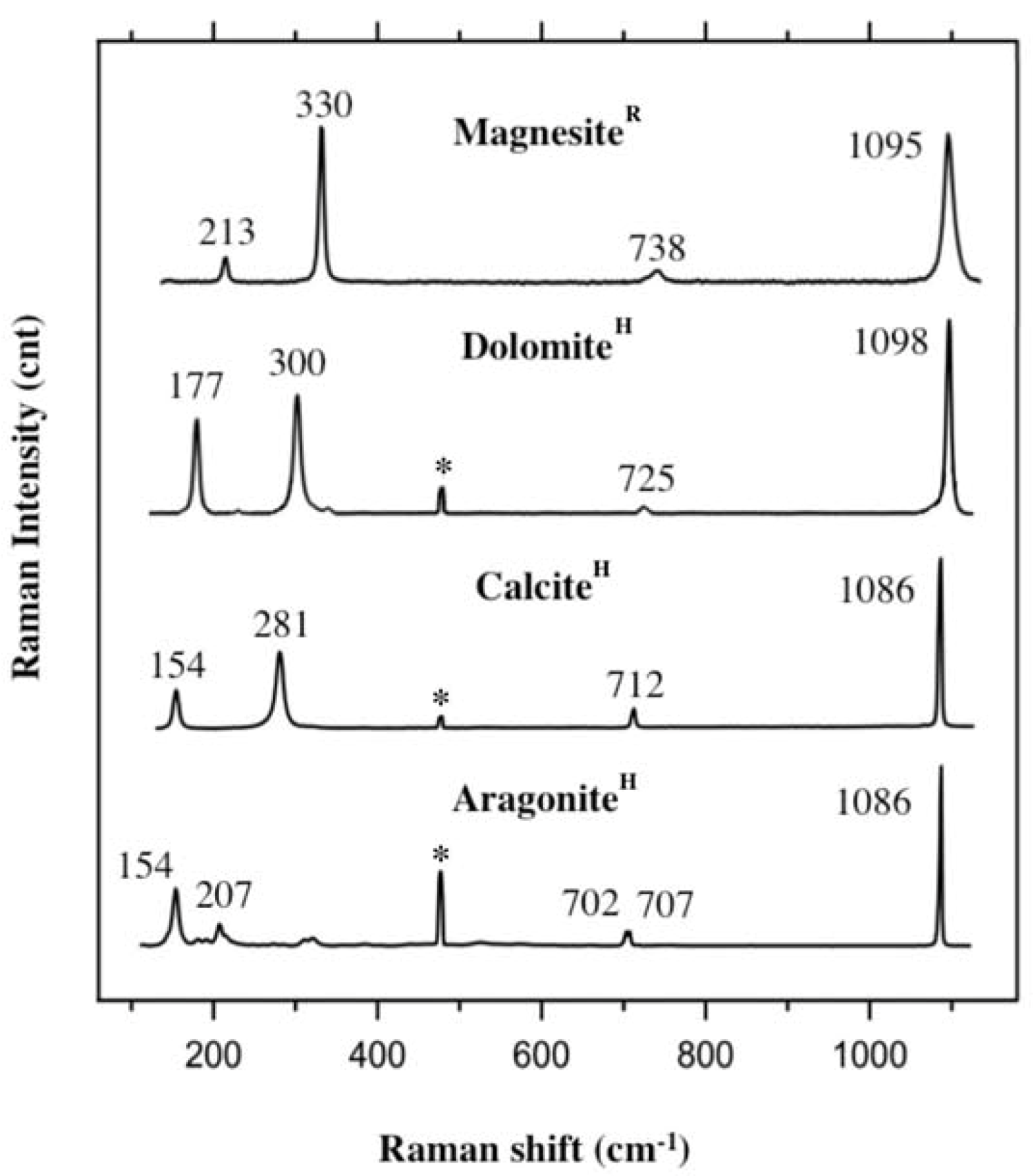
2.4. Raman Spectroscopy
2.5. Raman Spectroscopy on Carbonates
2.6. Earlier Studies and Characterisation of the Sample Material
3. Results
3.1. FEG-SEM-EDS
3.2. Raman Spectroscopy
3.2.1. Generals
3.2.2. Long Term Test
3.2.3. Ultra Long Term Test
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hermansen, H.; Landa, G.H.; Sylte, J.E.; Thomas, L.K. Experiences after 10 years of waterflooding the Ekofisk Field, Norway. J. Pet. Sci. Eng. 2000, 26, 11–18. [Google Scholar] [CrossRef]
- Nagel, N.B. Compaction and subsidence issues within the petroleum industry: From Wilmington to Ekofisk and beyond. Phys. Chem. Earth A 2001, 26, 3–14. [Google Scholar] [CrossRef]
- Risnes, R.; Madland, M.V.; Hole, M.; Kwabiah, N.K. Water weakening of chalk—Mechanical effects of water-glycol mixtures. J. Pet. Sci. Eng. 2005, 48, 21–36. [Google Scholar] [CrossRef]
- Heggheim, T.; Madland, M.V.; Risnes, R.; Austad, T. A chemical induced enhanced weakening of chalk by seawater. J. Pet. Sci. Eng. 2005, 46, 171–184. [Google Scholar] [CrossRef]
- Korsnes, R.I.; Strand, S.; Hoff, Ø.; Pedersen, T.; Madland, M.V.; Austad, T. Does the chemical interaction between seawater and chalk affect the mechanical properties of chalk? In Multiphysics Coupling and Long Term Behaviour in Rock Mechanics; Cotthem, A.V., Charlier, R., Thimus, J.F., Tshibangu, J.P., Eds.; Taylor & Francis: London, UK, 2006; pp. 427–434. [Google Scholar]
- Korsnes, R.I.; Madland, M.V.; Austad, T.; Haver, S.; Rosland, G. The effects of temperature on the water weakening of chalk by seawater. J. Pet. Sci. Eng. 2008, 60, 183–193. [Google Scholar] [CrossRef]
- Madland, M.V.; Midtgarden, K.; Manafov, R.; Korsnes, R.I.; Kristiansen, T.; Hiorth, A. The Effect of Temperature and Brine Composition on the Mechanical Strength of Kansas Chalk. In Proceedings of the International Symposium of the Society of Core Analysts, Abu Dhabi, UAE, 29 October–2 November 2008. [Google Scholar]
- Madland, M.V.; Hiorth, A.; Omdal, E.; Megawati, M.; Hildebrand-Habel, T.; Korsnes, I.R.; Evje, S.; Cathles, M.L. Chemical alterations induced by rock-fluid interactions when injecting brines in high porosity chalks. Transp. Porous Med. 2011, 87, 679–702. [Google Scholar] [CrossRef]
- Zangiabadi, B.; Korsnes, R.I.; Hildebrand-Habel, T.; Hiorth, A.; Surtarjana, I.K.; Lian, A.; Madland, M.V. Chemical water weakening of various outcrop chalks at elevated temperature. In Poromechanics IV; Ling, H.I., Smyth, A., Betti, R., Eds.; DEStech Publications, Inc.: Lancaster, UK, 2009; pp. 543–548. [Google Scholar]
- Andreassen, K.A.; Fabricius, I.L. Biot critical frequency applied to description of failure and yield of highly porous chalk with different pore fluids. Geophysics 2010, 75, E205–E213. [Google Scholar] [CrossRef]
- Zhang, P.; Tweheyo, M.T.; Austad, T. Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: Impact of the potential determining ions Ca2+, Mg2+, and SO42+. Colloids Surf. A Physicochem. Eng. Aspects 2007, 301, 199–208. [Google Scholar] [CrossRef]
- Strand, S.; Hjuler, M.L.; Torsvik, R.; Pedersen, J.I.; Madland, M.V.; Austad, T. Wettability of chalk: Impact of silica, clay content and mechanical properties. Pet. Geosci. 2007, 13, 69–80. [Google Scholar] [CrossRef]
- Austad, T.; Strand, S.; Madland, M.V.; Puntervold, T.; Korsnes, R.I. Seawater in chalk: An EOR and compaction fluid. SPE Reserv. Eval. Eng. 2008, 11, 648–654. [Google Scholar] [CrossRef]
- Hiorth, A.; Cathles, L.; Madland, M. The Impact of Pore Water Chemistry on Carbonate Surface Charge and Oil Wettability. Transp. Porous Med. 2010, 85, 1–21. [Google Scholar] [CrossRef]
- Fathi, S.J.; Austad, T.; Strand, S. “Smart Water” as a Wettability Modifier in Chalk: The Effect of Salinity and Ionic Composition. Energy Fuels 2010, 24, 2514–2519. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.Y.; Salah Hamad, A.S.; Abdulaziz, A.K.; Mohammed Saleh, A.J. Laboratory Investigation of the Impact of Injection Water Salinity and Ionic Content on Oil Recovery from Carbonate Reservoirs. SPE Reserv. Eval. Eng. 2011. [Google Scholar] [CrossRef]
- Megawati, M.; Hiorth, A.; Madland, M.V. The impact of surface charge on the mechanical behavior of high-porosity chalk. Rock Mech. Rock Eng. 2013, 46, 1073–1090. [Google Scholar] [CrossRef]
- Zhang, P.; Tweheyo, M.T.; Austad, T. Wettability Alteration and Improved Oil Recovery in Chalk: The Effect of Calcium in the Presence of Sulfate. Energy Fuels 2006, 20, 2056–2062. [Google Scholar] [CrossRef]
- Andersen, P.Ø.; Evje, S.; Madland, M.V.; Hiorth, A. A geochemical model for interpretation of chalk core flooding experiments. Chem. Eng. Sci. 2012, 84, 218–241. [Google Scholar] [CrossRef]
- Megawati, M.; Madland, M.V.; Hiorth, A. Mechanical and physical behavior of high-porosity chalks exposed to chemical perturbation. J. Pet. Sci. Eng. 2015, 133, 313–327. [Google Scholar] [CrossRef]
- Wang, W.; Madland, M.V.; Zimmermann, U.; Nermoen, A.; Reidar, I.; Korsnes, R.; Bertolino, S.R.A.; Hildebrand-Habel, T. Evaluation of porosity change during chemo-mechanical compaction in flooding experiments on Liege outcrop chalk. In Reservoir Quality of Clastic and Carbonate Rocks: Analysis, Modelling and Prediction; Armitage, P.J., Butcher, A.R., Churchill, J.M., Csoma, A.E., Hollis, C., Lander, R.H., Omma, J.E., Worden, R.H., Eds.; Geological Society: London, UK, 2016; p. 435. [Google Scholar]
- Raman, C.V. A new radiation. Indian J. Phys. 1928, 2, 387–398. [Google Scholar]
- Krishnamurti, D. Raman spectrum of magnesite. Proc. Indian Acad. Sci. Sect. A 1956, 43, 210. [Google Scholar]
- Griffith, W.P. Raman spectroscopy of minerals. Nature 1969, 224, 264–266. [Google Scholar] [CrossRef]
- Kuebler, K.; Wang, A.; Abbott, K.; Haskin, L.A. Can we detect carbonate and sulfate minerals on the surface of Mars by Raman spectroscopy? In Proceedings of the 32nd Annual Lunar and Planetary Science Conference, Houston, TX, USA, 12–16 March 2001. [Google Scholar]
- Downs, R.T. The RRUFF Project: An integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals. In Proceedings of the 19th General Meeting of the International Mineralogical Association, Kobe, Japan, 23–28 July 2006. [Google Scholar]
- Andò, S.; Garzanti, E. Raman spectroscopy in heavy-mineral studies. In Sediment Provenance Studies in Hydrocarbon Exploration and Production; Scott, R.A., Smyth, H.R., Morton, A.C., Richardson, N., Eds.; Geological Society: London, UK, 2013; p. 386. [Google Scholar]
- Gillet, P.; Biellmann, C.; Reynard, B.; McMillan, P. Raman spectroscopic studies of carbonates Part I: High-pressure and high-temperature behaviour of calcite, magnesite, dolomite and aragonite. Phys. Chem. Miner. 1993, 20, 1–18. [Google Scholar] [CrossRef]
- Bendel, V.; Schmidt, B.C. Raman spectroscopic characterisation of disordered alkali feldspars along the join KAlSi3O8–NaAlSi3O8: Application to natural sanidine and anorthoclase. Eur. J. Miner. 2008, 20, 1055–1065. [Google Scholar] [CrossRef]
- Noguchi, N.; Abduriyim, A.; Shimizu, I.; Kamegata, N.; Odake, S.; Kagi, H. Imaging of internal stress around a mineral inclusion in a sapphire crystal: Application of micro-Raman and photoluminescence spectroscopy. JRS 2013, 44, 147–154. [Google Scholar] [CrossRef]
- De La Pierre, M.; Carteret, C.; Maschio, L.; André, E.; Orlando, R.; Dovesi, R. The Raman spectrum of CaCO3 polymorphs calcite and aragonite: A combined experimental and computational study. J. Chem. Phys. 2014, 140, 164509. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, B.; Paleari, C.I.; Andò, S.; Garzanti, E.; Andersson, P.S.; Petit, J.R.; Crosta, X.; Narcisi, B.; Baroni, C.; Salvatore, M.C.; et al. Causes of dust size variability in central East Antarctica (Dome B): Atmospheric transport from expanded South American sources during Marine Isotope Stage 2. Quat. Sci. Rev. 2017, 168, 55–68. [Google Scholar] [CrossRef]
- Molenaar, N.; Zijlstra, J.J.P. Differential early diagenetic low-Mg calcite cementation and rhythmic hardground development in Campanian-Maastrichtian chalk. Sediment. Geol. 1997, 109, 261–281. [Google Scholar] [CrossRef]
- Robaszynski, F.; Dhondt, A.V.; John, W.M. Cretaceous lithostratigraphic units (Belgium). Geol. Belg. 2001, 4, 121–134. [Google Scholar]
- Hjuler, M.L.; Fabricius, I.L. Engineering properties of chalk related to diagenetic variations of Upper Cretaceous onshore and offshore chalk in the North Sea area. J. Pet. Sci. Eng. 2009, 68, 151–170. [Google Scholar] [CrossRef]
- Zimmermann, U.; Madland, M.V.; Nermoen, A.; Hildebrand-Habel, T.; Bertolino, S.A.R.; Hiorth, A.; Korsnes, R.I.; Audinot, J.N.; Grysan, P. Evaluation of the compositional changes during flooding of reactive fluids using scanning electron microscopy, nano-secondary ion mass spectroscopy, X-ray diffraction, and whole-rock geochemistry. AAPG Bull. 2015, 99, 791–805. [Google Scholar] [CrossRef]
- Hjuler, M.L. Diagenesis of Upper Cretaceous Onshore and Offshore Chalk from the North Sea Area. Ph.D. Thesis, Department of Civil Engineering, Arctic Technology Centre, Technical University of Denmark, Kongens Lyngby, Denmark, 2007. [Google Scholar]
- Felder, R.M.; Spence, R.D.; Ferrell, J.K. A method for the dynamic measurement of diffusivities of gases in polymers. J. Polym. Sci. 1975, 19, 3193–3200. [Google Scholar] [CrossRef]
- Slimani, H. New species of dinoflagellate cysts from the Campanian-Danian chalks at Hallembaye and Turnhout (Belgium) and at Beutenaken (The Netherlands). J. Micropalaeontol. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Nermoen, A.; Korsnes, R.I.; Hiorth, A.; Madland, M.V. Porosity and permeability development in compacting chalks during flooding of nonequilibrium brines: Insights from long-term experiment. JGR Solid Earth 2015, 120, 2935–2960. [Google Scholar] [CrossRef]
- Rutt, H.N.; Nicola, H.J. Raman spectra of carbonates of calcite structure. Phys. C Solid State Phys. 1974, 7, 4522. [Google Scholar] [CrossRef]
- Bischoff, W.D.; Sharma, S.K.; MacKenzie, F.T. Carbonate ion disorder in synthetic and biogenic magnesian calcites: A Raman spectral study. Am. Miner. 1985, 70, 581–589. [Google Scholar]
- Herman, R.G.; Bogdan, C.E.; Sommer, A.J.; Simpson, D.R. Discrimination among carbonate minerals by Raman spectroscopy using the laser microprobe. Appl. Spectrosc. 1987, 41, 437–440. [Google Scholar] [CrossRef]
- Edwards, H.; Villar, S.; Jehlicka, J.; Munshi, T. FT–Raman spectroscopic study of calcium-rich and magnesium-rich carbonate minerals. Spectrochim. Acta A 2005, 61, 2273. [Google Scholar] [CrossRef] [PubMed]
- Korsakov, A.; De Gussem, K.; Zhukov, V.P.; Perraki, M.; Vandenabeele, P.; Golovin, A.V. Aragonite-calcite-dolomite relationships in UHPM polycrystalline carbonate inclusions from the Kokchetav Massif, northern Kazakhstan. Eur. J. Miner. 2009, 21, 1301. [Google Scholar] [CrossRef]
- Borromeo, L.; Zimmermann, U.; Andò, S.; Coletti, G.; Bersani, D.; Basso, D.; Gentile, P.; Schulz, B.; Garzanti, E. Raman spectroscopy as a tool for magnesium estimation in Mg-calcite. JRS 2017, 48, 983–992. [Google Scholar] [CrossRef]
- Dandeu, A.; Humbert, B.; Carteret, C.; Muhr, H.; Plasari, E.; Bossoutrot, J.M. Raman Spectroscopy—A Powerful Tool for the Quantitative Determination of the Composition of Polymorph Mixtures: Application to CaCO3 Polymorph Mixtures. Chem. Eng. Technol. 2006, 29, 221–225. [Google Scholar] [CrossRef]
- Sun, J.; Zeguang, W.; Hongfei, C. A Raman spectroscopic comparison of calcite and dolomite. Spectrochim. Acta A 2014, 117, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purgstaller, B.; Mavromatis, V.; Immenhauser, A.; Dietzel, M. Transformation of Mg-bearing amorphous calcium carbonate to Mg-calcite—In situ monitoring. Geochim. Cosmochim. Acta 2016, 174, 180–195. [Google Scholar] [CrossRef]
- Krishnan, R.S. Raman spectra of the second order in crystals; part. I: Calcite. Proc. Math. Sci. 1945, 22, 182. [Google Scholar]
- White, W.B. Infrared Spectra of Minerals; Farmer, V.C., Ed.; Mineralogical Society Monograph 4; Mineralogical Society: London, UK, 1974; pp. 87–110, 227–284. [Google Scholar]
- Urmos, J.; Sharma, S.K.; Mackenzie, T. Characterization of some biogenic carbonates with Raman spectroscopy. Am. Miner. 1991, 76, 641–646. [Google Scholar]
- Minde, M.W.; Haser, S.; Korsnes, R.I.; Zimmermann, U.; Madland, M.V. Comparative Studies of Mineralogical Alterations of Three Ultra-long-term Tests of Onshore Chalk at Reservoir Conditions. In Proceedings of the IOR 2017-19th European Symposium on Improved Oil Recovery 2017, Stavanger, Norway, 24–27 April 2017. [Google Scholar]
- Minde, M.W.; Zimmermann, U.; Madland, M.V.; Korsnes, R.I. Submicron Investigations—What Can We Learn? IOR: Stavanger, Norway, 2016; Available online: http://www.uis.no/getfile.php/13300942/IOR-senter/IOR_16_MMinde%20-%20dag%202_theme%205.pdf (accessed on 18 May 2018).
- Borromeo, L.; Minde, M.; Toccafondi, C.; Zimmermann, U.; Andò, S.; Ossikovski, R. A New Frontier Technique for Nano-analysis on Flooded Chalk-TERS (Tip Enhanced Raman Spectroscopy). In Proceedings of the IOR 2017—19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017. [Google Scholar]
- Borromeo, L.; Toccafondi, C.; Minde, M.; Zimmermann, U.; Andò, S.; Madland, M.V.; Korsnes, R.I.; Ossikovski, R. Application of Tip Enhanced Raman Spectroscopy in the nanoscale characterization of flooded chalk. JRS 2018. submitted. [Google Scholar]
- Egeland, N.; Minde, M.W.; Kobayashi, K.; Ota, T.; Nakamura, E.; Zimmermann, U.; Madland, M.W.; Korsnes, R.I. Quantification of Mineralogical Changes in Flooded Carbonate under Reservoir Conditions. In Proceedings of the IOR 2017—19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017. [Google Scholar]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Wang, A.; Freeman, J.J.; Jolliff, B.L. Understanding the Raman spectral features of phyllosilicates. JRS 2015, 46, 829–845. [Google Scholar] [CrossRef]
- Egeland, N. Raman Spectroscopy Applied to Enhanced Oil Recovery. Master’s Thesis, University of Stavanger, Stavanger, Norway, 2015, unpublished. [Google Scholar]
- Gorelik, V.S.; Chervyakov, A.V.; Kol’tsova, L.V.; Veryaskin, S.S. Raman Spectra of Saturated Hydrocarbons and Gasolines. J. Russ. Laser Res. 2000, 21, 323–334. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Sant’Ana, A.C.; Corio, P.; Temperini, M.L.A. Chemical analysis of polycyclic aromatic hydrocarbons by surface-enhanced Raman spectroscopy. Talanta 2006, 70, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Sebek, J.; Pele, L.; Potma, E.O.; Gerber, R.B. Raman spectra of long chain hydrocarbons: Anharmonic calculations, experiment and implications for imaging of biomembranes. PCCP 2011, 13, 12724–12733. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.B.; Wang, D.; Marzec, K.M.; Mullins, O.C.; Crozier, K.B. Surface enhanced Raman spectroscopy of polycyclic aromatic hydrocarbons and molecular asphaltenes. Chem. Phys. Lett. 2015, 620, 139–143. [Google Scholar] [CrossRef]










| Calcite | Magnesite | Unknown | Other | |||||
|---|---|---|---|---|---|---|---|---|
| Spectra | % | Spectra | % | Spectra | % | Spectra | % | |
| LT1_p1A | 20 | 77% | 6 | 23% | ||||
| LT1_p1B | 3 | 13% | 20 | 87% | ||||
| LT1_p1C | 20 | 87% | 3 | 13% | ||||
| LT1_p1D | 10 | 31% | 19 | 63% | 2 | 6% | ||
| LT1_p1E | 7 | 26% | 20 | 74% | ||||
| LT1_p1F | 18 | 60% | 12 | 40% | ||||
| LT1_p1G | 0 | 0% | 25 | 100% | 1 Brookite | 4% | ||
| LT1_p1H | 0 | 0% | 25 | 100% | ||||
| LT1_p1I | 0 | 0% | 25 | 100% | ||||
| LT1_p2A | 18 | 82% | 4 | 18% | ||||
| LT1_p2B | 2 | 10% | 18 | 90% | ||||
| LT1_p2C | 13 | 39% | 20 | 61% | ||||
| LT1_p2D | 16 | 53% | 14 | 47% | ||||
| LT1_p2E | 4 | 82% | 16 | 18% | ||||
| LT2_A | 70 | 92% | 6 | 8% | ||||
| LT2_B | 49 | 86% | 7 | 12% | 1 | 2% | ||
| LT2_C | 48 | 61% | 31 | 39% | ||||
| LT2_D | 30 | 41% | 43 | 58% | 1 Rutile | 1% | ||
| LT4_A | 19 | 79% | 5 | 21% | ||||
| LT4_B | 18 | 69% | 6 | 23% | 2 | 8% | ||
| LT4_C | 20 | 100% | 0 | 0% | ||||
| LT4_D | 20 | 100% | 0 | 0% | ||||
| ULT1_1 | 1 | 9% | 10 | 91% | 1 | |||
| ULT2_4 | 1 | 9% | 10 | 91% | ||||
| ULT2_5 | 0 | 0% | 10 | 100% | ||||
| ULT2_5B | 1 | 9% | 10 | 91% | ||||
| ULT2_6 | 1 | 9% | 10 | 91% | ||||
| ULT2_7 | 2 | 17% | 10 | 83% | ||||
| ULT2_8 | 1 | 9% | 10 | 91% | ||||
| ULT1_9 | 0 | 0% | 10 | 100% | ||||
| ULT1_10 | 1 | 9% | 10 | 91% | 3 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borromeo, L.; Egeland, N.; Wetrhus Minde, M.; Zimmermann, U.; Andò, S.; Madland, M.V.; Korsnes, R.I. Quick, Easy, and Economic Mineralogical Studies of Flooded Chalk for EOR Experiments Using Raman Spectroscopy. Minerals 2018, 8, 221. https://doi.org/10.3390/min8060221
Borromeo L, Egeland N, Wetrhus Minde M, Zimmermann U, Andò S, Madland MV, Korsnes RI. Quick, Easy, and Economic Mineralogical Studies of Flooded Chalk for EOR Experiments Using Raman Spectroscopy. Minerals. 2018; 8(6):221. https://doi.org/10.3390/min8060221
Chicago/Turabian StyleBorromeo, Laura, Nina Egeland, Mona Wetrhus Minde, Udo Zimmermann, Sergio Andò, Merete Vadla Madland, and Reidar Inge Korsnes. 2018. "Quick, Easy, and Economic Mineralogical Studies of Flooded Chalk for EOR Experiments Using Raman Spectroscopy" Minerals 8, no. 6: 221. https://doi.org/10.3390/min8060221
APA StyleBorromeo, L., Egeland, N., Wetrhus Minde, M., Zimmermann, U., Andò, S., Madland, M. V., & Korsnes, R. I. (2018). Quick, Easy, and Economic Mineralogical Studies of Flooded Chalk for EOR Experiments Using Raman Spectroscopy. Minerals, 8(6), 221. https://doi.org/10.3390/min8060221





