High-Resolution Analysis of Critical Minerals and Elements in Fe–Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean)
Abstract
:1. Introduction
2. Geological and Oceanographic Setting
3. Materials and Methods
3.1. Samples
3.2. Laboratory Methods
4. Results
4.1. Physical Properties, Textural Features and Mineralogy
4.1.1. Layering and Growth Patterns
4.1.2. Mineralogy
4.1.3. Thermic Treatment and CEE Experiments: Changes in Mineralogy
4.1.4. Scanning Electron Microscopy (SEM) and High-Resolution Transmission Electron Microscopy (HR-TEM) Analysis
4.2. Geochemistry
4.2.1. Bulk Chemistry
4.2.2. Chemistry of Mineral Phases after Leaching Experiments
4.2.3. Electron Probe Micro Analysis (EPMA) and Distribution of Elements
4.2.4. Factor Analysis and Mineral Phases
4.2.5. Growth Rate and “Cobalt Chronometer” Age
5. Discussion
5.1. Genesis of Fe–Mn Crusts Based on High-Resolution Analyses
5.2. Association of Metals with Different Mineral Phases
5.2.1. Major Elements Defining the Main Mineral Phases
5.2.2. Manganese Oxides as Critical Minerals with High Contents of Co, Ni and Cu
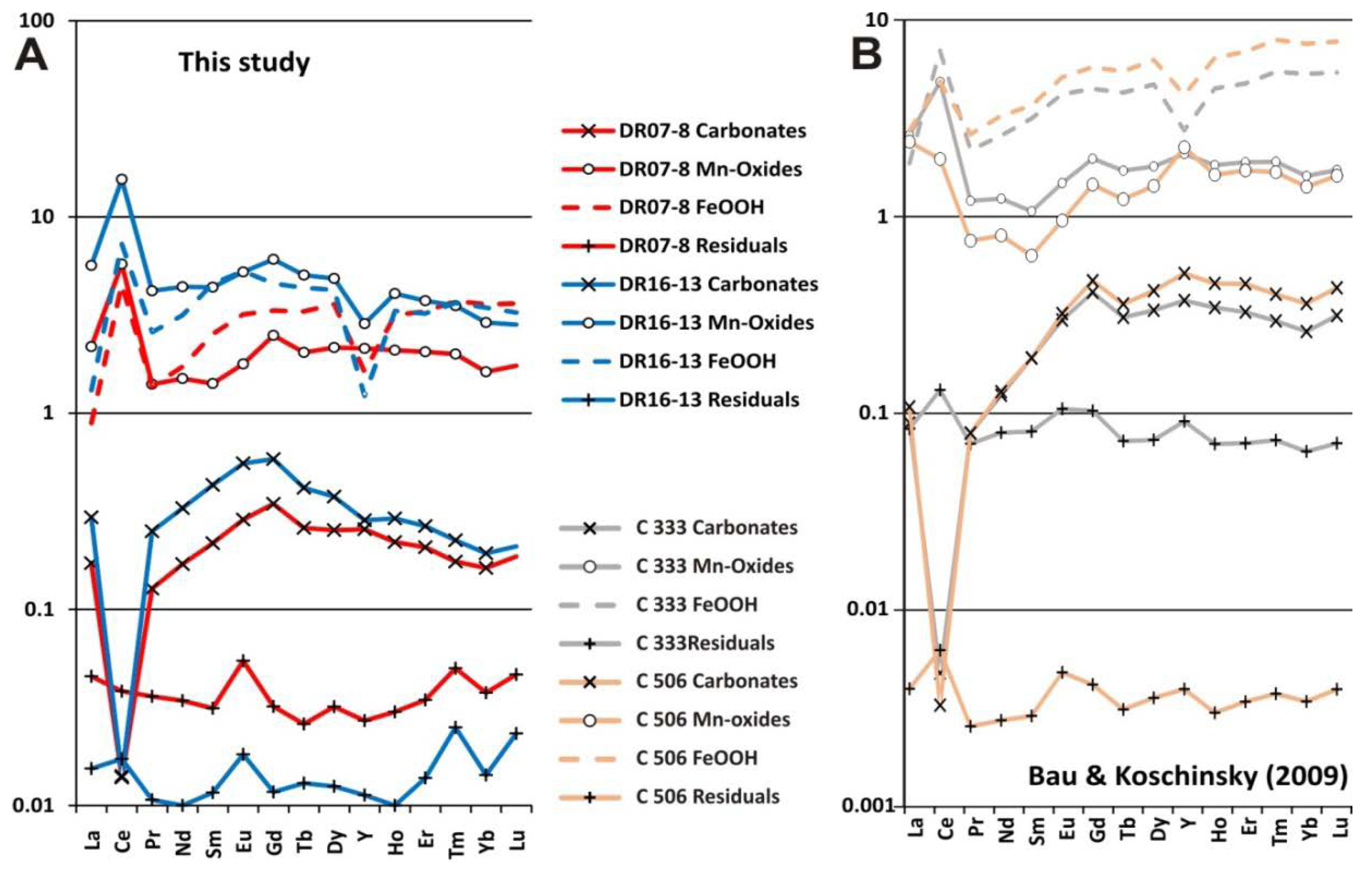
5.2.3. REYs Scavenging between Fe and Mn Phases as Record of Different Genetic Processes
5.3. Composition Changes along the Growing History of the Crusts
6. Conclusions
- (1)
- The Fe–Mn crust (DR16-13) collected on the eastern side of the Tropic Seamount has a mean thickness of 9 cm, showing no botryoids on its surface, mainly composed of dense laminations of Fe-vernadite (more than 90%) and goethite group minerals. This mineralogy reflects the predominance of the hydrogenetic process during their formation, similar to other ferromanganese crusts from the Atlantic, Pacific and Indian oceans [2,4,6,15,23,44,63,92,93]. Based on the high-resolution “cobalt chronometer” age calculation, this purely hydrogenetic crust yielded an age of 99 Ma (Upper Cretaceous). The onset of crust growth is consistent with the age of the volcanic formation of the Tropic Seamount dated at 119 Ma [39]. This means that this crust would be one of the oldest Fe–Mn crusts of the Atlantic Ocean.
- (2)
- The Fe–Mn crust (DR07-8), collected in the western side of The Paps Seamount, has a maximum thickness of up to 8 cm, showing typical botryoids on its surface reaching diameters of 1–2 cm. Based on the “cobalt chronometer” for age calculation, this crust began to grow 30 Ma ago (early Oligocene). More than 240 EPMA spot analyses of the microlayers indicate a hydrogenetic or mixed hydrogenetic/diagenetic origin. High-resolution mineralogical analyses show two main types of manganese oxides: (i) hydrogenetic Fe-vernadite, as the main Mn oxide, and (ii) a 10 Ȧ phyllomanganate interpreted as an intergrowth of buserite and asbolane. Bright laminae of diagenetic buserite/asbolane appear on overlying dendritic growths of hydrogenetic Fe-vernadite. Additionally, the occurrence of authigenic calcite, palygorskite and carbonate fluor-apatite (CFA) suggests early diagenesis and pervasive phosphatization events.
- (3)
- Sequential leaching analysis indicated that Co, Ni, Cu, Ba and Ce are linked to Mn minerals. Therefore, Mn-oxides are enriched in Ni and Cu by diagenetic processes or in Co and Ce by hydrogenetic processes. On the other hand, Fe-oxyhydroxides concentrate V, Zn, As and Pb. The distribution of REYs depends on the type of genetic processes. Thus, mixed diagenetic/hydrogenetic crusts show enrichment in MREEs and HREEs in the Fe-oxyhydroxide phases, whereas the purely hydrogenetic crust shows a slight enrichment of all REY (and especially LREE) elements in the Mn-oxide phases.
- (4)
- Differences in the Co, Ni and Cu contents between the two types of crusts are related to the minerals associated with each genetic process. Fe-vernadite concentrates essentially Co, Ce and Ni, whereas the buserite, intergrown with asbolane, concentrates Ni and Cu, controlling the enrichment in strategic elements of the two-end member crusts. Therefore, the bright lamination of buserite/asbolane leads to enrichment in Mn, Ni and Cu, whereas hydrogenetic laminae of Fe-vernadite contribute to increasing the contents of Fe (up to 30 wt %), Co and Ce, reaching the highest enrichments in layers with very low growth rates. In this way, the mixed hydrogenetic/diagenetic crust (DR07-08) is enriched in Ni and Cu and the purely hydrogenetic crust (DR16-13) in Co and Ce.
- (5)
- The enrichment in REYs for the Mn-oxide phase is clearly evidenced by the purely hydrogenetic crust. Moreover, the enrichment in HREEs, related to Fe-hydroxides, is confirmed in the two types of crusts. An important positive anomaly of Ce is depicted higher in the Mn-oxide than the Fe-oxyhydroxide phases. A prominent negative anomaly in Y, depicted for the Fe-oxyhydroxide phases by [94], is also shown in the CISP crusts, but not in the Mn-oxide phases, corresponding to the diagenetic/hydrogenetic crusts.
- (6)
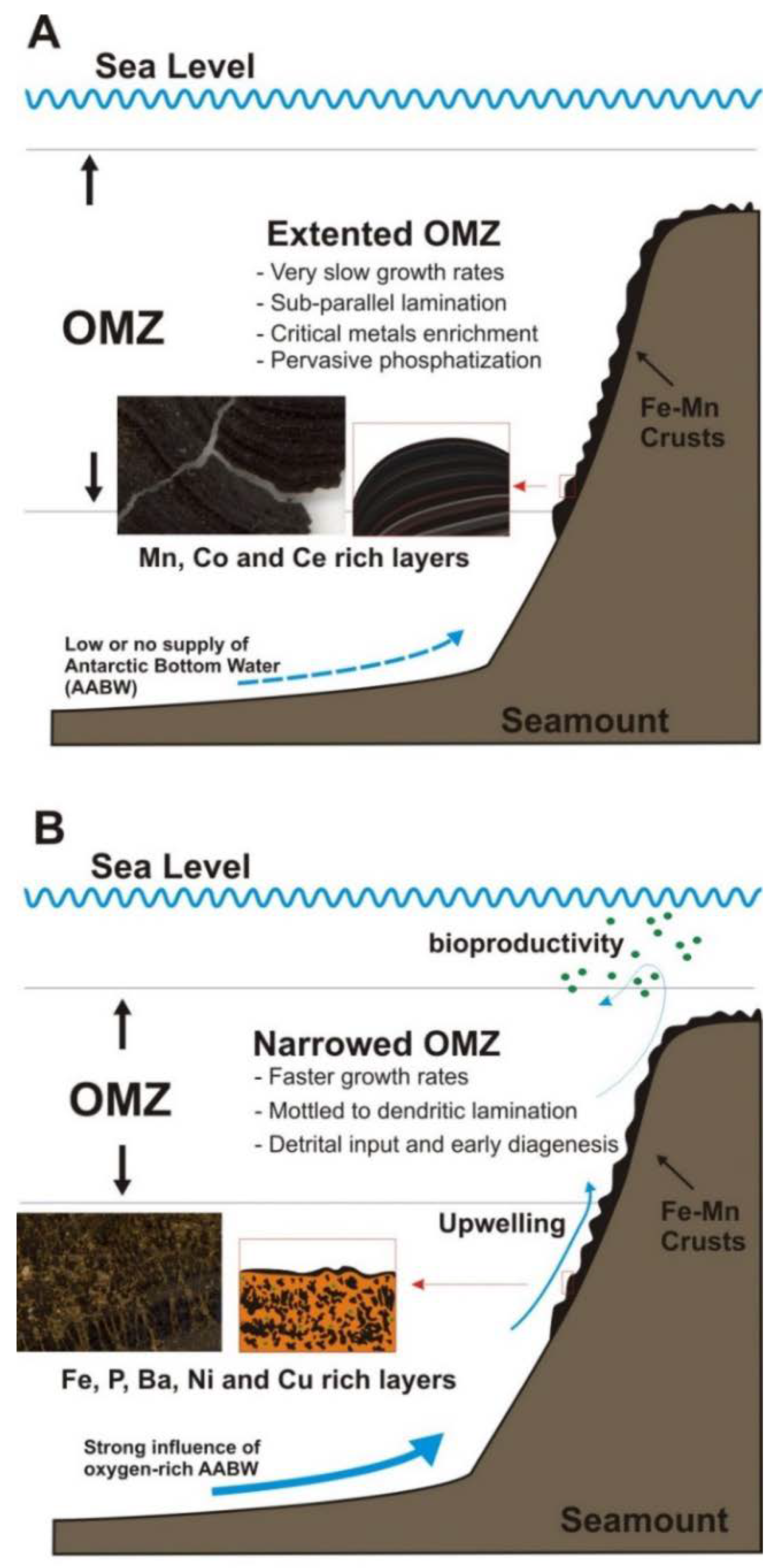
- At least one episode of enrichment in critical metals (Co, Ni) has been derived for both crusts between 29 and 22 Ma that might be extrapolated to all the CISP. Other episodes of metal-rich mineralization have taken place on the Tropic Seamount crust between 99 and 84 Ma. We suggest that these enrichment episodes in critical metals might be related to expansion events of the OMZ in the Eastern Atlantic Ocean.
- (7)
- Enrichment in critical metals might be explained by several factors controlling the type of hydrogenetic or diagenetic growth of the crusts: (i) Expansion of the OMZ in the Atlantic Ocean caused by global oceanographic/climate changes, that will promote slow growth of hydrogenetic Fe–Mn oxide minerals enriched in Co, Tl and Ce; (ii) Local factors such as micro-topography and increases in undercurrent strengths bathing the seamounts, that will produce erosion and/or sediment deposition, and thus rapid growth of diagenetic Mn-oxide laminations enriched in Cu and Ni; (iii) Early phosphatization when the OMZ was intensified and expanded, promoting the formation of CFA enriched in Y and P, in propitious layers enriched in carbonate sediments within the crusts.
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- International Seabed Authority (ISA). 2018. Available online: https://www.isa.org.jm (accessed on 11 January 2018).
- Halbach, P.; Hebisch, U.; Scherhag, C. Geochemical variations of ferromanganese nodules and crusts from different provinces of the Pacific Ocean and their genetic control. Chem. Geol. 1981, 34, 3–17. [Google Scholar] [CrossRef]
- Hein, J.R.; Morgenson, L.A.; Clague, D.A.; Koski, A. Cobalt-rich ferromanganese crusts from the Exclusive Economic Zone of the United States and nodules from the oceanic Pacific. In Geology and Resource Potential of the Continental Margin of Western North America and Adjacent Ocean Basins-Beaufort Sea to Baja California; Scholl, D.W., Grantz, A., Vedder, J.G., Eds.; Circum-Pacific Council for Energy and Mineral Resources: Washington, DC, USA, 1987; pp. 753–771. [Google Scholar]
- Hein, J.R.; Schwab, W.C.; Davis, A. Cobalt- and platinum-rich ferromanganese crusts and associated substrate rocks from the Marshall Islands. Mar. Geol. 1988, 78, 255–283. [Google Scholar] [CrossRef]
- Baturin, G.N. The Geochemistry of Manganese and Manganese Nodules in the Ocean; Springer: Dordrecht, The Netherlands, 1988; ISBN 978-94-009-3731-4. [Google Scholar]
- Hein, J.R.; Koschinsky, A.; Bau, M.; Manheim, F.T.; Kang, J.K.; Roberts, L. Co-rich ferromanganese crusts in the Pacific. In Handbook of Marine Mineral Deposits; CRC Marine Science Series; Cronan, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 239–279. [Google Scholar]
- Hein, J.R.; Conrad, T.A.; Staudigel, H. Seamount mineral deposits: A source of rare metals for high technology industries. Oceanography 2010, 23, 184–189. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Hein, J.R.; Spinardi, F.; Okamoto, N.; Mizell, K.; Thorburn, D.; Tawake, A. Critical metals in manganese nodules from the Cook Islands EEZ, abundance and distributions. Ore Geol. Rev. 2015, 68, 97–116. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.; Mizell, K.; Banakar, V.K.; Frey, F.A.; Sager, W.W. Controls on ferromanganese crust composition and reconnaissance resource potential, Ninetyeast Ridge, Indian Ocean. Deep-Sea Res. Part 1 2016, 110, 1–19. [Google Scholar] [CrossRef]
- Muiños, S.B.; Hein, J.R.; Frank, M.; Monteiro, J.H.; Gaspar, L.; Conrad, T.; Pereira, H.G.; Abrantes, F. Deep-sea Fe–Mn crusts fromthe northeast Atlantic Ocean: Composition and resource considerations. Mar. Georesour. Geotechnol. 2013, 31, 40–70. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A. Deep-Ocean Ferromanganese Crusts and Nodules. Treatise Geochem. 2014, 13, 273–291. [Google Scholar]
- González, F.J.; Somoza, L.; Hein, J.R.; Vázquez, J.T.; Medialdea, T.; León, R.; Martín Rubí, J.A.; Bellido, E.; Reyes, J. Deep-water seamounts and banks along the Atlantic Spanish continental margin as a potential source of raw materials. Eur. Mineral. Conf. 2012, 1, EMC2012-422. [Google Scholar]
- González, F.J.; Somoza, L.; Hein, J.R.; Medialdea, T.; León, R.; Urgorri, V.; Reyes, J.; Martín-Rubí, J.A. Phosphorites, Co-rich Mn nodules, and Fe–Mn crusts from Galicia Bank, NE Atlantic: Reflections of Cenozoic tectonics and paleoceanography. Geochem. Geophys. Geosyst. 2016, 17, 346–374. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halbach, P.; Manheim, F.T.; Bau, M.; Kang, J.K.; Lubick, N. Iron and manganese oxide mineralization in the Pacific. Geol. Soc. Lond. Spec. Publ. 1997, 119, 123–138. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T. The influence of suboxic diagenesis on the formation of manganese nodules in the Clarion Clipperton nodule belt of the Pacific Ocean. Mar. Geol. 2014, 357, 123–138. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T.; Dohrmann, R.; Wirth, R.; Grangeon, S. Mineralogical characterization of individual growth structures of Mn-nodules with different Ni + Cu content from the central Pacific Ocean. Am. Mineral. 2015, 100, 2497–2508. [Google Scholar] [CrossRef]
- Kuhn, T.; Wegorzewski, A.; Rühlemann, C.; Vink, A. Composition, formation, and occurrence of polymetallic nodules. In Deep-Sea Mining: Resource Potential, Technical and Environmental Considerations; Sharma, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 23–63. ISBN 978-3-319-52557-0. [Google Scholar]
- Hodkinson, R.A.; Stoffers, P.; Scholten, J.; Cronan, D.S.; Jeschke, G.; Rogers, T.D.S. Geochemistry of hydrothermal manganese deposits from the Pitcairn Island hotspot, southeastern Pacific. Geochim. Cosmochim. Acta 1994, 58, 5011–5029. [Google Scholar] [CrossRef]
- Nath, N.; Plüger, W.; Roelandts, I. Geochemical constraints on the hydrothermal origin of ferromanganese encrustations from the Rodrigues Triple Junction, Indian Ocean. Geol. Soc. Lond. Spec. Publ. 1997, 119, 199–212. [Google Scholar] [CrossRef]
- Kuhn, T.; Bau, M.; Blum, N.; Halbach, P. Origin of negative Ce anomalies in mixed hydrothermal–hydrogenetic Fe–Mn crusts from the Central Indian Ridge. Earth Planet. Sci. Lett. 1998, 163, 207–220. [Google Scholar] [CrossRef]
- Dekov, V.M.; Savelli, C. Hydrothermal activity in the SE Tyrrhenian Sea: An over-view of 30 years of research. Mar. Geol. 2004, 204, 161–185. [Google Scholar] [CrossRef]
- Hein, J.R. Cobalt-rich ferromanganese crusts. Global distribution, composition, origin and research activities. In Workshop on Minerals Other than Polymetallic Nodules of the International Seabed Area; International Seabed Authority: Kingston, Jamaica, 2004; pp. 188–256. [Google Scholar]
- Halbach, P.; Segl, M.; Puteanus, D.; Mangini, A. Co-fluxes and growth rates in ferromanganese deposits from central Pacific seamount areas. Nature 1983, 304, 716–719. [Google Scholar] [CrossRef]
- Rona, P.A. The changing vision of marine minerals. Ore Geol. Rev. 2008, 33, 618–666. [Google Scholar] [CrossRef]
- González, F.J.; Somoza, L.; Lunar, R.; Martínez-Frías, J.; Medialdea, T.; León, R.; Martín-Rubí, J.A.; Torres, T.; Ortiz, J.E.; Marino, E. Polymetallic ferromanganese deposits research on the Atlantic Spanish continental margin. In Proceedings of the 43rd Underwater Mining Institute Conference, Lisboa, Portugal, 21–28 September 2014; Hein, J.R., Barriga, F.J.A.S., Morgan, C.L., Eds.; [Google Scholar]
- Marino, E.; González, F.J.; Somoza, L.; Lunar, R.; Ortega, L.; Vázquez, J.T.; Reyes, J.; Bellido, E. Strategic and rare elements in Cretaceous-Cenozoic cobalt-rich ferromanganese crusts from seamounts in the Canary Island Seamount Province (northeastern tropical Atlantic). Ore Geol. Rev. 2017, 87, 41–61. [Google Scholar] [CrossRef]
- European Commision. 2018. Available online: https://ec.europa.eu/ (accessed on 6 February 2018).
- Minerals for EU Project. 2018. Available online: http://www.minerals4eu.eu/ (accessed on 6 February 2018).
- Han, X.; Jin, X.; Yang, S.; Fietzke, J.; Eisenhauer, A. Rhythmic growth of Pacifc ferromanganese nodules and their Milankovitch climatic origin. Earth Planet. Sci. Lett. 2003, 211, 143–157. [Google Scholar] [CrossRef]
- Oda, H.; Usui, A.; Miyagi, I.; Joshima, M.; Weiss, B.P.; Shantz, C.; Fong, L.E.; McBride, K.K.; Harder, R.; Baudenbacher, F.J. Ultrafine-scale magnetostratigraphy of marine ferromanganese crust. Geology 2011, 39, 227–230. [Google Scholar] [CrossRef] [Green Version]
- González, F.J.; Somoza, L.; León, R.; Medialdea, T.; de Torres, T.; Ortiz, J.E.; Lunar, R.; Martínez-frías, J.; Merinero, R. Ferromanganese nodules and microhardgrounds associated with the Cadiz Contourite Channel (NE Atlantic): Palaeoenvironmental records of fluid venting and bottom currents. Chem. Geol. 2012, 310–311, 56–78. [Google Scholar] [CrossRef] [Green Version]
- Usui, A.; Nishi, K.; Sato, H.; Nakasato, Y.; Thornton, B.; Kashiwabara, T.; Tokumaru, A.; Sakaguchi, A.; Yamaoka, K.; Kato, S.; et al. Continuous growth of hydrogenetic ferromanganese crusts since 17 Myr ago on Takuyo-Daigo Seamount, NW Pacific, at water depths of 800–5500 m. Ore Geol. Rev. 2017, 87, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Konstantinova, N.; Cherkashov, G.; Hein, J.R.; Mirão, J.; Dias, L.; Madureira, P.; Kuznetsov, V.; Maksimov, F. Composition and characteristics of the ferromanganese crusts from the western Arctic Ocean. Ore Geol. Rev. 2017, 87, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Carracedo, J.C.; Day, S.J.; Guillou, H.; Rodríguez Badiola, E.; Canas, J.A.; Pérez Torrado, F.J. Hotspot volcanism close to a passive continental margin: The Canary Islands. Geol. Mag. 1998, 135, 591–604. [Google Scholar] [CrossRef]
- Geldmacher, J.; Hoernle, K.; Bogaard, P.v.d.; Duggen, S.; Werner, R. New 40Ar/39Ar age and geochemical data from seamounts in the Canary and Madeira Volcanic Provinces: A contribution to the “Great Plume Debate”. Earth Planet. Sci. Lett. 2005, 237, 85–101. [Google Scholar] [CrossRef] [Green Version]
- Geldmacher, J.; Hoernle, K.; Hanan, B.B.; Blichert-Toft, J.; Hauff, F.; Gill, J.B.; Schmincke, H.-U. Hafnium isotopic variations in East Atlantic intraplate volcanism. Contrib. Mineral. Petrol. 2011, 162, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Hoernle, K.; Schmincke, H.U. The role of partial melting in the 15-Ma geochemical evolution of Gran Canaria: A blob model for the Canary Hotspot. J. Petrol. 1993, 34, 599–626. [Google Scholar] [CrossRef]
- Van den Bogaard, P. The origin of the Canary Island Seamount Province—New ages of old Seamounts. Sci. Rep. 2013, 3, 2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carracedo, J.C.; Pérez-Torrado, F.J.; Ancochea, E.; Meco, J.; Hernán, F.; Cubas, C.R.; Casillas, R.; Rodríguez Badiola, E.; Ahijado, A. Cenozoic volcanism II: The Canary Islands. In The Geology of Spain; Gibbons, E., Moreno, M.T., Eds.; Geological Society: London, UK, 2002; pp. 439–472. [Google Scholar]
- Ancochea, E. Evolución de la actividad volcánica. In Geología de España; Vera, J.A., Ed.; SGE-IGME: Madrid, Spain, 2004; pp. 639–641. [Google Scholar]
- Schmincke, H.-U.; Krastel, S.; Hansteen, T.; Sumita, M. Preliminary Results, Leg M43/1, Rock Sampling and Description. In DECOS/OMEX II, Cruise No. 43; Schminckle, H.-U., Graf, G., Eds.; METEOR-Berichte: Hamburg, Germany, 2000; p. 99. [Google Scholar]
- Instituto Geológico y Minero de España (IGME). Presentación Parcial de Datos e Información Sobre los Límites de la Plataforma Continental de España al Oeste de las Islas Canarias, Conforme a la Parte VI y el Anexo II de la Convención de las Naciones Unidas sobre el Derecho del Mar. 2015. Unpublished work. 2015. [Google Scholar]
- Koschinsky, A.; Halbach, P. Sequential leaching of marine ferromanganese precipitates: Genetic implications. Geochim. Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- González, F.J.; Rincón-Tomás, B.; Somoza, L.; Hein, J.R.; Medialdea, T.; Madureira, P.; Reyes, J.; Hoppert, M.; Reitner, J. Fe-rich mineralized microbes from hydrothermal vents at Tagoro submarine volcano, El Hierro Island (central east Atlantic). In Proceedings of the Geological Society of America 113th Annual Meeting, Honolulu, HI, USA, 23–25 May 2017; Volume 49, p. 4. [Google Scholar] [CrossRef]
- Somoza, L.; González, F.J.; Barker, S.J.; Madureira, P.; Medialdea, T.; de Ignacio, C.; Lourenço, N.; León, R.; Vázquez, J.T.; Palomino, D. Evolution of submarine eruptive activity during the 2011–2012 El Hierro event as documented by hydroacoustic images and remotely operated vehicle observations. Geochem. Geophys. Geosyst. 2017, 18, 3109–3137. [Google Scholar] [CrossRef]
- Machín, F.; Hernández-Guerra, A.; Pelegrí, J.L. Mass fluxes in the Canary Basin. Prog. Oceanogr. 2006, 70, 416–447. [Google Scholar] [CrossRef]
- Pastor, M.V.; Vélez-Belchí, P.; Hernández-Guerra, A. Water masses in the Canary current large marine ecosystem. In Oceanographic and Biological Features in the Canary Current Large Marine Ecosystem, Proceedings of the IOCUNESCO 2015, 18–25 June, Paris, France; Valdés, L., Déniz-González, I., Eds.; IOC Technical Series 115; UNESCO: Paris, France, 2015; pp. 73–79. [Google Scholar]
- Sarnthein, M.; Thiede, J.; Pflaumann, U.; Erlenkeuser, H.; Fiitterer, D.; Koopmann, B.; Lange, H.; Seibold, E. Atmospheric and oceanic circulation patterns off northwest Africa during the past 25 million years. In Geology of Northwest Africa Continental Margin; von Rad, U., Hinz, K., Sarnthein, M., Seibold, E., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1982; pp. 545–604. [Google Scholar]
- Hernández-Guerra, A.; Fraile-Nuez, E.; López-Laatzen, F.; Martínez, A.; Parrilla, G.; Vélez- Belchí, P. Canary current and north equatorial current from an inverse box model. J. Geophys. Res. 2005, 110, C12019. [Google Scholar] [CrossRef]
- Knoll, M.; Hernández-Guerra, A.; Lenz, B.; López-Laatzen, F.; Machín, F.; Müller, T.; Siedler, G. The Eastern Boundary Current system between the Canary Islands and the African coast. Deep Sea Res. Part II 2002, 19, 3427–3440. [Google Scholar] [CrossRef]
- Mémery, L.; Arhan, M.; Alvarez-Salgado, X.A.; Messias, M.-J.; Mercier, H.; Castro, C.G.; Rios, A.F. The water masses along the western boundary of the south and equatorial Atlantic. Prog. Ocean. 2000, 47, 69–98. [Google Scholar] [CrossRef]
- Brandt, P.; Hormann, V.; Körtzinger, A.; Visbeck, M.; Krahmann, G.; Stramma, L.; Lumpkin, R.; Schmid, C. Changes in the ventilation of the oxygen minimum zone of the tropical North Atlantic. J. Phys. Oceanogr. 2010, 40, 1784–1801. [Google Scholar] [CrossRef]
- Brandt, P.; Greatbatch, R.J.; Claus, M.; Didwischus, S.-H.; Hormann, V.; Funk, A.; Hahn, J.; Krahmann, G.; Fischer, J.; Körtzinger, A. Ventilation of the equatorial Atlantic by the equatorial deep jets. Phys. Oceanogr. Oceans 2012, 117, C12015. [Google Scholar] [CrossRef]
- Bashmachnikov, I.; Nascimento, Â.; Neves, F.; Menezes, T.; Koldunov, N.V. Distribution of intermediate water masses in the subtropical northeast Atlantic. Ocean Sci. 2015, 11, 803–827. [Google Scholar] [CrossRef] [Green Version]
- Helmers, E.; Schrems, O. Wet deposition of metals to the tropical North and the South Atlantic ocean. Atmos. Environ. 1995, 29, 2475–2484. [Google Scholar] [CrossRef]
- Eltayeb, M.A.H.; Injuk, J.; Manhaut, W.; Van Grieken, R.E. Elemental Composition of mineral aerosol generated from Sudan Sahara Sand. J. Atmos. Chem. 2001, 40, 247–273. [Google Scholar] [CrossRef]
- Bristow, C.S.; Hudson-Edwards, K.A.; Chappell, A. Fertilizing the Amazon and equatorial Atlantic with West African dust. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Palomino, D.; Vázquez, J.-T.; Somoza, L.; León, R.; López-González, N.; Medialdea, T.; Fernández-Salas, L.-M.; González, F.-J.; Rengel, J.A. Geomorphological features in the southern Canary Island volcanic province: The importance of volcanic processes and massive slope instabilities associated with seamounts. Geomorphology 2016, 255, 125–139. [Google Scholar] [CrossRef]
- Golden, D.C.; Chen, C.C.; Dixon, J.B. Transformation of birnessite to buserite, todorokite, and manganite under mild hydrothermal treatment. Clays Clay Min. 1987, 35, 271. [Google Scholar] [CrossRef]
- Tessier, A.P.; Campbell, P.G.C.; Bisson, M.X. Sequential Extraction Procedure for the Speciation of Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Manheim, F.T.; Lane-Bostwick, C.M. Cobalt in ferromanganese crusts as amonitor of hydrothermal discharge on the Pacific sea floor. Nature 1988, 335, 59–62. [Google Scholar] [CrossRef]
- Hein, J.R.; Schulz, M.S.; Kang, J.-K. Insular and submarine ferromanganese mineralization of the Tonga-Lau region. Mar. Min. 1990, 9, 305–354. [Google Scholar]
- Kustova, G.N.; Burgina, E.B.; Sadykov, V.A.; Poryvaev, S.G. Vibrational spectroscopic investigation of the goethite thermal decomposition products. Phys. Chem. Miner. 1992, 18, 379–382. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Infrared spectroscopy of goethite dehydroxylation: III. FT-IR microscopy of in situ study of the thermal transformation of goethite to hematite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 967–981. [Google Scholar] [CrossRef]
- Saito, G.; Kunisada, Y.; Nomura, T.; Sakaguchi, N.; Akiyama, T. Twin formation in hematite during dehydration of goethite. Phys. Chem. Miner. 2016, 43, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–51. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust; Its Composition and Evolution; An Examination of the Geochemical Record Preserved in Sedimentary Rocks; Blackwell: Oxford, UK, 1985. [Google Scholar]
- Koschinsky, A.; Hein, J.R. Uptake of elements from seawater by ferromanganese crusts: Solid-phase associations and seawater speciation. Mar. Geol. 2003, 198, 331–351. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchuk, V.T. Mineralogy and chemistry of ferromanganese crusts from the Atlantic Ocean. Geochem. Int. 2011, 49, 578–593. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchuk, V.T.; Rashidov, V.A. Ferromanganese crusts from the Sea of Okhotsk. Oceanology 2012, 52, 88–100. [Google Scholar] [CrossRef]
- Bonatti, E.; Kraemer, T.; Rydell, H. Classification and genesis of submarine ironmanganese deposits. In Ferromanganese Deposits of the Ocean Floor; Horn, D.R., Ed.; Arden House: New York, NY, USA, 1972; pp. 149–165. [Google Scholar]
- Josso, P.; Pelleter, E.; Pourret, O.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Bollinger, C. A new discrimination scheme for oceanic ferromanganese deposits using high field strength and rare earth elements. Ore Geol. Rev. 2017, 87, 3–15. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Puteanus, D.; Halbach, P. Correlation of Co concentration and growth rate—A method for age determination of ferromanganese crusts. Chem. Geol. 1988, 69, 73–85. [Google Scholar] [CrossRef]
- Hein, J.R.; Yeh, H.-W.; Gunn, S.H.; Sliter, W.V.; Benninger, L.M.; Wang, C.-H. Two major Cenozoic episodes of phosphogenesis recorded in equatorial Pacific seamount deposits. Paleoceanography 1993, 8, 293–311. [Google Scholar] [CrossRef]
- Koschinsky, A.; Stascheit, A.; Bau, M.; Halbach, P. Effects of phosphatization on the geochemical and mineralogical composition of marine ferromanganese crusts. Geochim. Cosmochim. Acta 1997, 61, 4079–4094. [Google Scholar] [CrossRef]
- Hyeong, K.; Kim, J.; Yoo, C.M.; Moon, J.-W.; Seo, I. Cenozoic history of phosphogenesis recorded in the ferromanganese crusts of central and western Pacific seamounts: Implications for deepwater circulation and phosphorus budgets. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 392, 293–301. [Google Scholar] [CrossRef]
- Conrad, T.; Hein, J.R.; Paytan, A.; Clague, D.A. Formation of Fe–Mn crusts within a continental margin environment. Ore Geol. Rev. 2017, 87, 25–40. [Google Scholar] [CrossRef]
- Henderiks, J. Coccolith Studies in the Canary Basin: Glacial-interglacial Paleoceanography of the Eastern Boundary Current System. Unpublished Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 2001; p. 175. [Google Scholar]
- Frierdich, A.J.; Hasenmueller, E.A.; Catalano, J.G. Composition and structure of nanocrystalline Fe and Mn oxide cave deposits: Implications for trace element mobility in karst systems. Chem. Geol. 2011, 284, 82–96. [Google Scholar] [CrossRef]
- Lysyuk, G.N. Biomineral nanostructures of manganese oxides in oceanic ferromanganese nodules. Geol. Ore Depos. 2008, 50, 647–649. [Google Scholar] [CrossRef]
- Reith, F.; Zammit, C.M.; Shar, S.S.; Etschmann, B.; Bottrill, R.; Southam, G.; Ta, C.; Kilburn, M.; Oberthür, T.; Ball, A.S.; et al. Biological role in the transformation of platinum-group mineral grains. Nat. Geosci. 2016, 9, 294–298. [Google Scholar] [CrossRef]
- Bogdanov, Y.A.; Sorochtin, O.G.; Zonenshain, L.P.; Kuptzov, V.M.; Lisitzina, N.A.; Podrajanski, A.M. Ferromanganese Crust and Nodules of Pacific Seamounts; Nauka: Moscow, Russia, 1990; p. 229. [Google Scholar]
- Bogdanov, Y.A.; Bogdanova, O.Y.; Dubinin, A.V.; Gorand, A.; Gorshkov, A.I.; Gurvich, E.G.; Isaeva, A.B.; Ivanov, G.V.; Jansa, L.F.; Monaco, A. Composition of ferromanganese crusts and nodules at northwest Pacific guyots and geologic and paleoceanographic considerations. In Proceedings of the Ocean Drilling Program: Scientific Results 144; Haggerty, J.A., Premoli Silva, I., Rack, F.R., McNutt, M.K., Eds.; Ocean Drilling Program: College Station, TX, USA, 1995; p. 1059. [Google Scholar]
- Bogdanova, O.Y.; Gorshkov, A.I.; Novikov, G.V.; Bogdanov, Y.A. Mineralogy of morphogenetic types of ferromanganese deposits in the world ocean. Geol. Ore Depos. 2008, 50, 462–469. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Feng, X.; Tan, W.; He, J.; Hu, R.; Liu, F. Synthesis of todorokite-type manganese oxide from Cu-buserite by controllingthe pH at atmospheric pressure. Microporous Mesoporous Mater. 2009, 117, 41–47. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, M.; Ginder-Vogel, M.; Ni, C.; Parikh, S.J.; Sparks, D.L. Formation of nano-crystalline todorokite from biogenic Mn oxides. Geochim. Cosmochim. Acta 2010, 74, 3232–3245. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, H.; Liu, F.; Cui, H.; Tan, W.; Li, W. Transformation from Phyllomanganates to Todorokite under Various Conditions: A Review of Implication for Formation Pathway of Natural Todorokite. In Advances in the Environmental Biogeochemistry of Manganese Oxides; ACS Symposium Series 2016; American Chemical Society: Washington, DC, USA, 2016; Volume 1197. [Google Scholar]
- Manceau, A.; Lanson, M.; Takahashi, Y. Mineralogy and crystal chemistry of Mn, Fe, Co, Ni, and Cu in a deep-sea Pacific polymetallic nodule. Am. Mineral. 2014, 99, 2068–2083. [Google Scholar] [CrossRef]
- Koschinsky, A.; van Gerven, M.; Halbach, P. First discovery and investigation ofmassive ferromanganese crusts in the NE Atlantic in comparison to hydrogenetic Pacific occurrences. Mar. Georesour. Geotechnol. 1995, 13, 375–391. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P.; Hein, J.R.; Mangini, A. Ferromanganese crusts as indicators for paleoceanographic events in the NE Atlantic. Geol. Rundschau 1996, 85, 567–576. [Google Scholar] [CrossRef]
- Bau, M.; Koschinsky, A. Oxidative scavenging of cerium on hydrous Fe oxide: Evidence from the distribution of rare earth elements and yttrium between Fe oxides and Mn oxides in hydrogenetic ferromanganese crusts. Geochem. J. 2009, 43, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Chukhrov, F.V.; Gorshkov, A.I.; Vitovskaya, I.V.; Drits, V.A.; Sivtsov, A.I.; Rudnitskaya, E.S. Crystallochemical nature of Co-Ni asbolan. Izvestia Akademia Nauk, SSSR. Seriya Geol. 1982, 6, 73–81. [Google Scholar]
- Chukhrov, F.V.; Gorshkov, A.I.; Drits, V.A.; Shterenberg, A.V.; Sakharov, B.A. Mixed-layer asbolan-buserite minerals and asbolans in oceanic iron-manganese concretions. Int. Geol. Rev. 1983, 25, 838–847. [Google Scholar]
- Moffett, J.W. Microbially mediated cerium oxidation in sea water. Nature 1990, 345, 421–423. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Shaw, T.J.; Schneider, D.L. The geochemistry of rare earth elements in the seasonally anoxic water column and porewaters of Chesapeake Bay. Geochim. Cosmochim. Acta 1992, 56, 3389–3402. [Google Scholar] [CrossRef]
- Newman, H.R. The mineral industries of Morocco and Western Sahara U.S. In Geological Survey Minerals Yearbook V. III; U.S. Government Publishing Office (OGP): Pittsburgh, PA, USA, 2011; p. 10. [Google Scholar]
- Wang, X.; Peine, F.; Schmidt, A.; Schröder, H.C.; Wiens, M.; Schloßmacher, U.; Müller, W.E.G. Concept of Biogenic Ferromanganese Crust Formation: Coccoliths as Bio-seeds in Crusts from Central Atlantic Ocean (Senghor Seamount/Cape Verde). Nat. Prod. Commun. 2011, 6, 1–10. [Google Scholar]
- Manceau, A.; Gorshkov, A.I.; Drits, V.A. Structural chemistry of Mn, Fe, Co, and Ni in manganese hydrous oxides: Part II. Information from EXAFS spectroscopy and electron and X-ray diffraction. Am. Mineral. 1992, 77, 1144–1157. [Google Scholar]
- Varentsov, I.M.; Drits, V.A.; Gorshkov, A.I.; Sivtsov, A.V.; Sakharov, B.A. Mn-Fe oxyhydroxide crusts from Krylov Seamount (Eastern Atlantic): Mineralogy, geochemistry and genesis. Mar. Geol. 1991, 96, 53–70. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Milligan, A.J.; Saito, M.A. Marine bioinorganic chemistry: The role of trace metals in the oceanic cycles of major nutrients. Treatise Geochem. 2003, 113–143. [Google Scholar] [CrossRef]
- Vance, D.; Archer, C.; Little, S.H.; Köbberich, M.; de Souza, G.F. The oceanic cycles of the transition metals and their isotopes. Acta Geochim. 2017, 36, 359–362. [Google Scholar] [CrossRef] [Green Version]
- De Carlo, E.H.; McMurtry, G.M.; Kim, K.H. The geochemistry of ferromanganese crusts from the Hawaiian Archipelago-I. Northern survey areas. Deep-Sea Res. Part A Oceanogr. Res. Pap. 1987, 34, 441–467. [Google Scholar] [CrossRef]
- Burns, V.M.; Burns, R.G. Post-depositional metal enrichment processes inside manganese nodules from the north equatorial Pacific. Earth Planet. Sci. Lett. 1978, 39, 341–348. [Google Scholar] [CrossRef]
- Mohwinkel, D.; Kleint, C.; Koschinsky, A. Phase associations and potential selective extraction methods for selected high-tech metals from ferromanganese nodules and crusts with siderophores. Appl. Geochem. 2014, 43, 13–21. [Google Scholar] [CrossRef]
- Moorby, S.A.; Cronan, D.S. The distribution of elements between co-existing phases in some marine ferromanganese-oxide deposits. Geochim. Cosmochim. Acta 1981, 45, 1855–1877. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Takahashi, Y.; Tanimizu, M. A XAFS study on the mechanism of isotopic fractionation of molybdenum during its adsorption on ferromanganese oxides. Geochem. J. 2009, 43, 31–36. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Takahashi, Y.; Marcus, M.A.; Uruga, T.; Tanida, H.; Terada, Y.; Usui, A. Tungsten species in natural ferromanganese oxides related to its different behavior from molybdenum in oxic ocean. Geochim. Cosmochim. Acta 2013, 106, 364–378. [Google Scholar] [CrossRef]
- De Carlo, E.H.; Wen, X.-Y. The influence of redox reactions on the uptake of dissolved ce by suspended Fe and Mn oxide particles. Aquat. Geochem. 1998, 3, 357–389. [Google Scholar] [CrossRef]
- Moffett, J.W. The relationship between cerium and manganese oxidation in the marine environment. Limnol. Oceanogr. 1994, 39, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Bau, M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochim. Cosmochim. Acta 1999, 63, 67–77. [Google Scholar] [CrossRef]












| Sample | Type | T (°C) | Major Minerals | Minor Minerals |
|---|---|---|---|---|
| DR07-8 | Bulk | 40 | Goethite, δ-MnO2 (Fe-vernadite, asbolan, todorokite, birnessite) | Quartz, anorthoclase, Carbonate-fluorapatite |
| Bulk | 100 | Goethite, δ-MnO2 (Fe-vernadite, asbolan, todorokite, birnessite) | Quartz, anorthoclase, Carbonate-fluorapatite | |
| Bulk | 300 | Goethite, Hematite | Quartz, anorthite sodian, δ-MnO2 (Fe-vernadite) | |
| DR16-13 | Bulk | 40 | Goethite, δ-MnO2 (Fe-vernadite, birnessite) | Feroxyhyte |
| Bulk | 100 | Goethite, δ-MnO2 (Fe-vernadite, birnessite) | Feroxyhyte | |
| Bulk | 300 | Goethite, Hematite | δ-MnO2 (Fe-vernadite, birnessite) |
| SAMPLE | DR07-8 (Bulk) | DR07-8 (Carb.) | DR07-8 (Mn-ox) | DR07-8 (Fe-ox) | DR07-8 (Silic.) | DR16-13 (Bulk) | DR16-13 (Carb.) | DR16-13 (Mn-ox) | DR16-13 (Fe-ox) | DR16-13 (Silic.) |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe (wt %) | 14.6 | 0.01 | 1.33 | 11.71 | 0.35 | 28 | <0.1 | 1.79 | 17.13 | 0.17 |
| Mn | 15 | 0.01 | 13.82 | 0.34 | < 0.1 | 21.7 | <0.1 | 17.97 | 0.48 | <0.1 |
| Mn/Fe | 1.03 | - | - | - | - | 0.78 | - | - | - | - |
| Si | 8.6 | - | - | - | - | 1.7 | - | - | - | - |
| Al | 3.5 | 0.14 | 0.05 | 1.14 | 0.83 | 0.7 | 0.03 | 0.03 | 0.33 | 0.28 |
| Ca | 5.8 | 1.74 | 0.09 | 0.01 | 0.03 | 2.5 | 1.71 | 0.17 | 0.01 | <0.1 |
| Ti | 0.6 | <0.1 | <0.1 | 0.44 | 0.04 | 0.7 | <0.1 | <0.1 | 0.32 | 0.02 |
| K | 0.6 | 0.17 | 0.04 | 0.02 | 0.23 | 0.3 | 0.17 | 0.04 | <0.1 | 0.10 |
| Mg | 2.7 | 0.91 | 0.27 | 0.13 | 0.09 | 1.1 | 0.75 | 0.07 | 0.10 | 0.04 |
| P | 1.6 | 0.01 | <0.1 | 0.40 | 0.03 | 0.5 | <0.1 | <0.1 | 0.19 | 0.05 |
| Na | 1.2 | 1.26 | 0.01 | 0.02 | 0.13 | 1.3 | 0.66 | 0.01 | <0.1 | 0.01 |
| LOI | 22.87 | - | - | - | - | 27.15 | - | - | - | - |
| Co (μg/g) | 3218 | 0.77 | 2993.01 | 99.71 | 0.65 | 5530 | 1.13 | 5114.42 | 110.98 | 0.47 |
| Ni | 4796 | 224.95 | 4188.57 | 313.69 | 3.53 | 3285 | 151.09 | 2505.50 | 333.66 | <D.L. |
| V | 777 | 0.46 | 412.18 | 310.31 | 5.53 | 1170 | 0.31 | 607.12 | 427.70 | 3.22 |
| Cu | 1239 | 72.45 | 676.86 | 413.24 | 7.30 | 696 | 22.90 | 205.48 | 376.95 | 2.40 |
| Be | 8.2 | 1.35 | 0.33 | 4.74 | 0.14 | 12.24 | 1.50 | 1.14 | 6.58 | <D.L. |
| S | 3135 | 2164 | 580 | 239 | < D.L | 3693 | 2463 | 578 | 83 | 13 |
| Ba | 1067 | 22.93 | 779.21 | 116.73 | 56.75 | 2034 | 16.15 | 1575.73 | 196.95 | 22.61 |
| Zn | 626 | 58.93 | 258.15 | 197.44 | 6.93 | 789 | 44.34 | 273.86 | 313.49 | 6.64 |
| As | 322 | 0.71 | 2.71 | 273.65 | 2.47 | 522 | 0.56 | 7.11 | 398.94 | 2.43 |
| Mo | 270 | <D.L. | 5.51 | 240.96 | 1.80 | 670 | <D.L. | 14.58 | 572.58 | 1.89 |
| Nb | 67 | 0.11 | <D.L. | 63.3 | 4.6 | 23 | 0.1 | <D.L. | 13.3 | 1.4 |
| Se | 22.7 | 1.48 | 9.96 | 13.06 | 0.15 | 40 | 1.24 | 23.47 | 16.61 | 0.04 |
| Ag | 0.16 | <D.L. | <D.L. | <D.L. | 0.11 | 0.2 | <D.L. | <D.L. | <D.L. | 0.18 |
| Cd | 5.3 | 0.28 | 4.23 | <D.L. | <D.L. | 1.8 | <D.L. | 1.39 | <D.L. | <D.L. |
| Sb | 47.7 | <D.L. | 0.22 | 22.73 | 1.26 | 81 | <D.L. | 0.13 | 58.80 | 1.16 |
| Tl | 112 | 22.61 | 81.79 | 12.00 | 0.39 | 142 | 16.68 | 106.08 | 12.84 | 0.30 |
| Pb | 976 | <D.L. | 77.53 | 989.54 | 3.56 | 1586 | <D.L. | 160.54 | 1450.74 | 2.23 |
| Th | 25.4 | <D.L. | <D.L. | 28.98 | 0.82 | 64 | <D.L. | <D.L. | 69.49 | 0.71 |
| U | 11 | 4.03 | 0.14 | 7.09 | 0.12 | 11.3 | 4.37 | 0.14 | 6.45 | <D.L. |
| Y | 225 | 6.91 | 57.7 | 43.9 | 0.73 | 173 | 3.84 | 77.05 | 33.05 | 0.305 |
| La | 142.8 | 6.52 | 82.95 | 33.8 | 1.73 | 304 | 5.585 | 215 | 49.8 | 0.585 |
| Ce | 938 | 1.11 | 459.5 | 363 | 3.055 | 2079 | 0.565 | 1243.5 | 583.5 | 1.38 |
| Pr | 30.6 | 1.135 | 12.5 | 12.2 | 0.32 | 73 | 1.11 | 37.35 | 23 | 0.095 |
| Nd | 126 | 5.435 | 47.95 | 55.2 | 1.095 | 286 | 5.245 | 141 | 101 | 0.32 |
| Sm | 27.3 | 1.215 | 7.935 | 14.2 | 0.175 | 59.2 | 1.205 | 24.55 | 25.65 | 0.065 |
| Eu | 6.9 | 0.315 | 1.96 | 3.51 | 0.06 | 13.8 | 0.305 | 5.76 | 5.815 | 0.02 |
| Gd | 33.2 | 1.62 | 11.7 | 15.65 | 0.15 | 59.4 | 1.37 | 28.6 | 21.5 | 0.055 |
| Tb | 4.88 | 0.2 | 1.57 | 2.54 | 0.02 | 8.4 | 0.16 | 3.885 | 3.35 | 0.01 |
| Dy | 30.3 | 1.115 | 9.505 | 15.8 | 0.14 | 46.6 | 0.825 | 21.35 | 18.7 | 0.055 |
| Ho | 6.3 | 0.22 | 2.09 | 3.16 | 0.03 | 8.7 | 0.145 | 4.065 | 3.315 | 0.01 |
| Er | 18.4 | 0.6 | 5.95 | 9.57 | 0.1 | 23.5 | 0.385 | 10.85 | 9.33 | 0.04 |
| Tm | 2.6 | 0.07 | 0.8 | 1.49 | 0.02 | 3.3 | 0.045 | 1.41 | 1.46 | 0.01 |
| Yb | 16.9 | 0.455 | 4.54 | 10.04 | 0.105 | 20.4 | 0.27 | 8.095 | 9.65 | 0.04 |
| Lu | 2.7 | 0.08 | 0.75 | 1.555 | 0.02 | 3 | 0.045 | 1.215 | 1.4 | 0.01 |
| DR07-8 | DR16-13 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fact. 1 | Fact. 2 | Fact. 3 | Fact. 4 | Fact. 5 | Fact. 1 | Fact. 2 | Fact. 3 | Fact. 4 | |
| Mn | 0.87 | 0.11 | 0.29 | 0.14 | 0.95 | 0.18 | |||
| Fe | 0.61 | −0.34 | −0.61 | 0.19 | −0.29 | 0.88 | 0.16 | ||
| Co | −0.19 | 0.55 | −0.28 | 0.15 | −0.61 | 0.82 | −0.17 | ||
| Ni | −0.47 | 0.62 | 0.33 | 0.17 | 0.17 | 0.88 | −0.24 | 0.17 | |
| Cu | −0.60 | −0.16 | 0.21 | 0.20 | −0.21 | 0.67 | 0.14 | ||
| Ce | 0.67 | 0.37 | −0.23 | 0.29 | 0.18 | 0.59 | 0.58 | −0.30 | 0.21 |
| Mo | 0.79 | 0.27 | 0.38 | 0.67 | 0.12 | −0.16 | 0.14 | ||
| V | 0.75 | −0.18 | −0.47 | 0.16 | 0.14 | 0.92 | 0.15 | ||
| Si | −0.71 | 0.16 | 0.56 | −0.15 | −0.55 | 0.76 | |||
| Al | −0.32 | −0.22 | 0.22 | 0.67 | −0.37 | −0.46 | 0.31 | ||
| K | 0.38 | −0.37 | 0.64 | 0.31 | −0.20 | −0.30 | −0.50 | 0.72 | |
| Ca | 0.84 | 0.27 | 0.35 | 0.73 | 0.21 | 0.17 | |||
| Na | −0.44 | −0.32 | 0.31 | 0.59 | 0.29 | −0.46 | 0.31 | −0.12 | |
| Mg | −0.43 | 0.42 | 0.35 | 0.40 | 0.69 | 0.18 | |||
| P | 0.88 | 0.15 | 0.32 | −0.16 | 0.71 | 0.53 | |||
| Ba | 0.28 | 0.12 | −0.45 | 0.28 | 0.32 | 0.24 | 0.66 | −0.53 | 0.17 |
| W | 0.35 | 0.20 | −0.17 | 0.15 | −0.21 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, E.; González, F.J.; Lunar, R.; Reyes, J.; Medialdea, T.; Castillo-Carrión, M.; Bellido, E.; Somoza, L. High-Resolution Analysis of Critical Minerals and Elements in Fe–Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals 2018, 8, 285. https://doi.org/10.3390/min8070285
Marino E, González FJ, Lunar R, Reyes J, Medialdea T, Castillo-Carrión M, Bellido E, Somoza L. High-Resolution Analysis of Critical Minerals and Elements in Fe–Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals. 2018; 8(7):285. https://doi.org/10.3390/min8070285
Chicago/Turabian StyleMarino, Egidio, Francisco Javier González, Rosario Lunar, Jesús Reyes, Teresa Medialdea, Mercedes Castillo-Carrión, Eva Bellido, and Luis Somoza. 2018. "High-Resolution Analysis of Critical Minerals and Elements in Fe–Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean)" Minerals 8, no. 7: 285. https://doi.org/10.3390/min8070285
APA StyleMarino, E., González, F. J., Lunar, R., Reyes, J., Medialdea, T., Castillo-Carrión, M., Bellido, E., & Somoza, L. (2018). High-Resolution Analysis of Critical Minerals and Elements in Fe–Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals, 8(7), 285. https://doi.org/10.3390/min8070285







