REE and Y Mineralogy of the Krudum Granite Body (Saxothuringian Zone)
Abstract
:1. Introduction
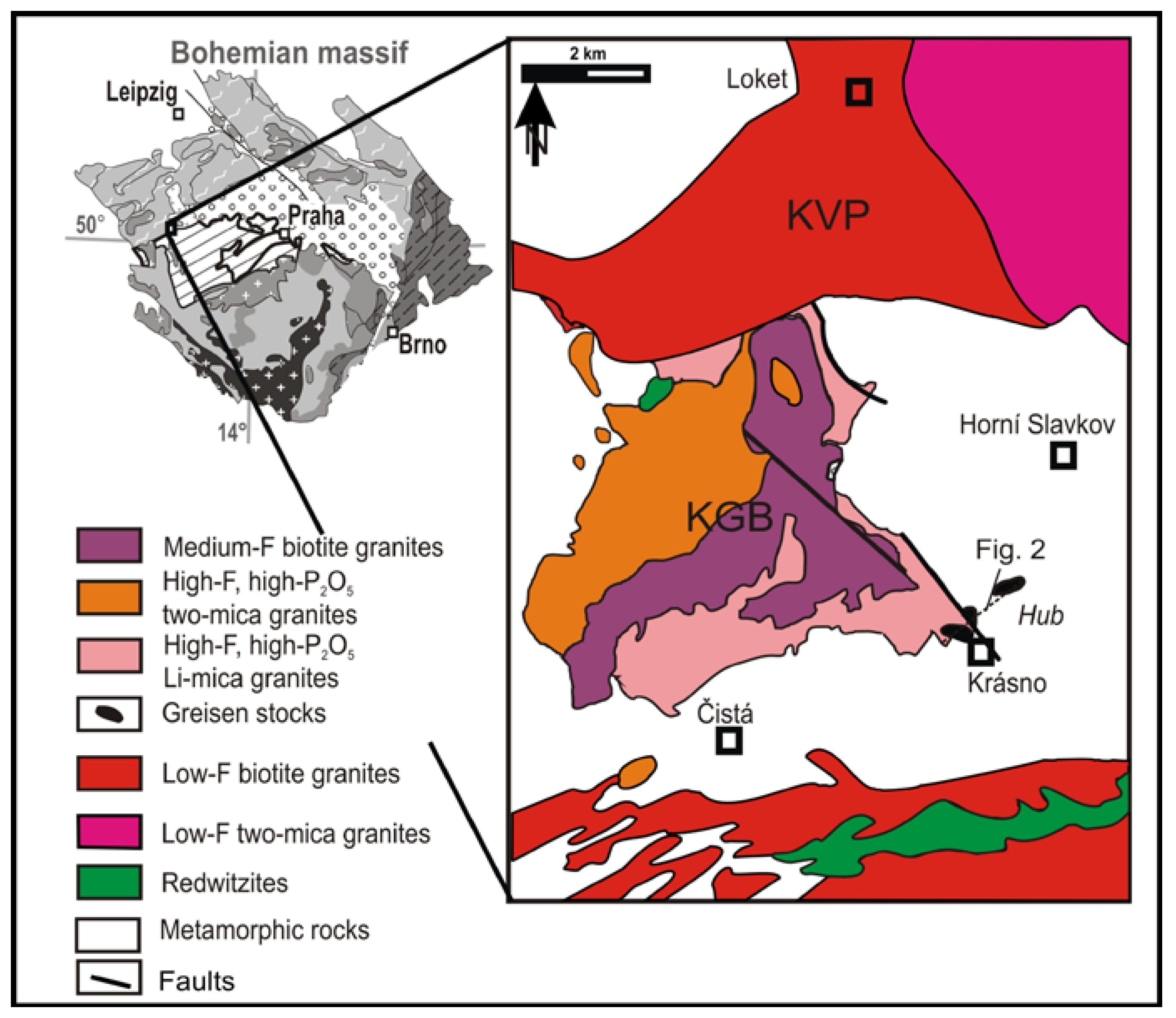
2. Geological Setting
3. Analytical Methods
4. Results
4.1. Petrography
4.2. Geochemistry
4.3. REE and Y Mineralogy
4.3.1. Accessory Minerals Textures
4.3.2. Monazite Composition
4.3.3. Xenotime Composition
4.3.4. Zircon Composition
5. Discussion
5.1. Substitution in Monazite
5.2. Substitution in Xenotime
5.3. Substitution in zircon
6. Conclusions
- (a)
- The Krudum granite body has a sequence of highly fractionated granitic rocks from the medium-F biotite granites to the high-F, high-P2O5 Li-mica granites and alkali-feldspar syenites.
- (b)
- Analyzed monazite grains from the Krudum granite body display strong preference of cheralite substitution over the huttonite substitution with up to 69.3 mol % cheralite component in the alkali-feldspar syenites. Some monazite grains from the alkali-feldspar syenites are enriched in ThO2 (up to 35.4 wt % ThO2).
- (c)
- The coupled thorite-coffinite and cheralite substitutions are dominant in the analyzed xenotime. Some xenotime analyses reveal low totals, suggesting probably their hydrothermal alteration.
- (d)
- Zircon grains from these granites are sometimes metamictized and fluorized. Zircon from the high-F, high-P2O5 Li-mica granites and alkali-feldspar syenites is usually enriched in P (up to 8.29 wt % P2O5; 0.24 apfu P). The higher concentrations of P, Y, REE and Sc returned by zircon grains from the high-F, high-P2O5 Li-mica granites could be explained by their metamictization. However, the highest concentration of P (8.29 wt % P2O5; 0.24 apfu P) that was found in zircon grains from alkali-feldspar syenite without visible metamictization could be believed as the result of a primary magmatic enrichment produced by fractionation.
Acknowledgments
Conflicts of Interest
References
- Bea, F. Residence of REE, Y, Th and U in granites and crustal protoliths; Implications for the chemistry of crustal melts. J. Petrol. 1996, 37, 521–552. [Google Scholar] [CrossRef]
- Broska, I.; Petrík, I. Genesis and stability of accessory phosphates in silicic magmatic rocks: A Western Carpathian case study. Mineralogia 2008, 39, 53–65. [Google Scholar] [CrossRef]
- Förster, H.J.; Rhede, D.; Hecht, L. Chemical composition of radioactive accessory minerals: Implications for the evolution, alteration, age, and uranium fertility of the Fichtelgebirge granites (NE Bavaria, Germany). Neues Jahrbuch für Mineralogie 2008, 185, 161–182. [Google Scholar] [CrossRef]
- Harlov, D.E.; Procházka, V.; Förster, H.J.; Matějka, D. Origin of monazite-xenotime-zircon-fluorapatite assemblages in the peraluminous Melechov granite massif, Czech Republic. Mineral. Petrol. 2008, 94, 9–26. [Google Scholar] [CrossRef]
- Hetherington, C.J.; Jercinovic, M.J.; Williams, M.L.; Mahan, K. Understanding geologic processes with xenotime: Composition, chronology, and a protocol for electron probe microanalysis. Chem. Geol. 2008, 254, 133–147. [Google Scholar] [CrossRef]
- Kelts, A.B.; Ren, M.; Anthony, E.Y. Monazite occurrence, chemistry, and chronology in the granitoid rocks of the Lachlan Fold Belt, Australia: An electron microprobe study. Am. Mineral. 2008, 93, 373–383. [Google Scholar] [CrossRef]
- Pérez-Soba, C.; Villaseca, C.; Orejana, D.; Jeffries, T. Uranium-rich accessory minerals in the peraluminous and perphosphorous Belvís de Monroy pluton (Iberian Variscan belt). Contrib. Mineral. Petrol. 2014, 167. [Google Scholar] [CrossRef]
- Uher, P.; Kohút, M.; Ondrejka, M.; Konečný, P.; Siman, P. Monazite-(Ce) in Hercynian granites and pegmatites of the Bratislava massif, Western Carpathians: Compositional variations and Th-U-Pb electron-microprobe dating. Acta Geol. Slov. 2014, 6, 215–231. [Google Scholar]
- Breiter, K. Monazite and zircon as major carriers of Th, U, and Y in peraluminous granites: Examples from the Bohemian Massif. Mineral. Petrol. 2016, 110, 767–785. [Google Scholar] [CrossRef]
- Förster, H.J.; Tischendorf, G.; Trumbull, R.B.; Gottesmann, B. Late-collisional granites in the Variscan Erzgebirge (Germany). J. Petrol. 1999, 40, 1613–1645. [Google Scholar] [CrossRef]
- Förster, H.J.; Romer, R.L. Carboniterous magmatism. In Pre-Mesozoic Geology of Saxo-Thuringia—From the Cadomian Active Margin to the Variscan Orogen; Linnemann, U., Romer, R.L., Eds.; Schweizerbart Verlag: Stuttgart, Germany, 2010; pp. 287–308. [Google Scholar]
- Blecha, V.; Štemprok, M. Petrochemical and geochemical characteristics of late Variscan granites in the Karlovy Vary Massif (Czech Republic)–implications for gravity and magnetic interpretation at shallow depths. J. Geosci. 2012, 57, 65–85. [Google Scholar] [CrossRef]
- Hofmann, Y.; Jahr, T.; Jentzch, G. Three-dimensional gravimetric modelling to detect deep structure of the region Vogtland/NW Bohemia. J. Geodyn. 2003, 35, 209–220. [Google Scholar] [CrossRef]
- Finger, F.; Gerdes, A.; René, M.; Riegler, G. The Saxo-Danubian granite belt: Magmatic response to post-collisional delamination of mantle lithosphere below the south-western sector of the Bohemian Massif (Variscan orogen). Geol. Carpath. 2009, 60, 205–212. [Google Scholar] [CrossRef]
- Tichomirova, M.; Leonhardt, D. New age determinations (Pb-Pb zircon evaporation, Rb/Sr) on the granites from Aue-Schwarzenberg and Eibenstock, Western Erzgebirge, Germany. Z. Geol. Wiss. 2010, 38, 99–123. [Google Scholar]
- Dolejš, D.; Bendl, J.; Štemprok, M. Rb-Sr isotopic composition in the Western Krušné hory/Erzgebirge pluton, Central Europe: Record of variations in source lithology, mafic magma input and postmagmatic hydrothermal events. Mineral. Petrol. 2016, 110, 601–622. [Google Scholar] [CrossRef]
- Machek, M.; Roxerová, Z.; Janoušek, V.; Petrovský, E.; René, M. Petrophysical and geochemical constraints on alteration processes in granites. Stud. Geophys. Geodaet. 2013, 57, 710–740. [Google Scholar] [CrossRef]
- Breiter, K.; Förster, H.J.; Seltmann, R. Variscan silicic magmatism and related tin-tungsten mineralization on the Erzgebirge-Slavkovský les metallogenic province. Miner. Depos. 1999, 34, 505–521. [Google Scholar] [CrossRef]
- Jarchovský, T. The nature and genesis of greisen stocks at Krásno, Slavkovský les area–western Bohemian, Czech Republic. J. Czech Geol. Soc. 2006, 51, 201–216. [Google Scholar]
- René, M.; Škoda, R. Nb-Ta-Ti oxides fractionation in rare-metal granites: Krásno-Horní Slavkov ore district, Czech Republic. Mineral. Petrol. 2011, 103, 37–48. [Google Scholar] [CrossRef]
- Dolníček, Z.; René, M.; Prochaska, W.; Kovář, M. Fluid evolution of the Hub stock, Horní Slavkov-Krásno Sn-W ore district, Bohemian Massif, Czech Republic. Miner. Depos. 2012, 47, 821–833. [Google Scholar] [CrossRef]
- René, M. Petrology, geochemistry and mineralogy of greisens associated with tin-tungsten mineralisation: Hub stock deposit at Krásno-Horní Slavkov ore district, Czech Republic. In Contributions to Mineralisation; Al-Juboury, A.I., Ed.; InTech: Rijeka, Croatia, 2018; pp. 1–22. [Google Scholar]
- Pouchou, J.L.; Pichoir, F. “PAP” (φ-ρ-Z) procedure for improved quantitative microanalysis. In Microbeam Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Pyle, J.M.; Spear, M.S.; Rudnick, R.L.; McDonough, W.F. Monazite-xenotime-garnet equilibrium in metapelites and a new monazite-garnet thermometer. J. Petrol. 2001, 42, 2083–2107. [Google Scholar] [CrossRef]
- Chappell, B.W.; Hine, R. The Cornubian batholith: An example of magmatic fractionation on a crustal scale. Res. Geol. 2006, 56, 203–244. [Google Scholar] [CrossRef]
- Boynton, W.V. Geochemistry of the rare earth elements: Meteorite studies. In Rare Earth Elements; Henderson, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 63–114. [Google Scholar]
- Förster, H.J. The chemical composition of REE-Y-Th-U-rich accessory minerals in peraluminous granites of the Erzgebirge-Fichtelgebirge region, Germany. Part I: The monazite-(Ce)-brabantite solid solutions series. Am. Mineral. 1998, 83, 259–272. [Google Scholar] [CrossRef]
- Hoshino, M.; Watanabe, Y.; Ishihara, S. Crystal chemistry of monazite from the granitic rocks of Japan: Petrogenetic implications. Can. Mineral. 2012, 50, 1331–1346. [Google Scholar] [CrossRef]
- Gramaccioli, C.M.; Segalstad, T.V. A uranium- and thorium-rich monazite from a south-alpine pegmatite at Piona, Italy. Am. Mineral. 1978, 63, 757–761. [Google Scholar]
- Franz, G.; Andrehs, G.; Rhede, D. Crystal chemistry of monazite and xenotime from Saxothuringian-Moldanubian metapelites, NE Bavaria, Germany. Eur. J. Min. 1996, 8, 1097–1118. [Google Scholar] [CrossRef]
- Linthout, K. Tripartite division of the system 2REEPO4-CaTh(PO4)2-2ThSiO4, dicreditation of brabantite, and recognition of cheralite as a name for members dominated by CaTh(PO4)2. Can. Mineral. 2007, 45, 503–508. [Google Scholar] [CrossRef]
- Förster, H.J. The chemical composition of REE-Y-Th-U-rich accessory minerals in peraluminous granites of the Erzgebirge-Fichtelgebirge region, Germany. Part II: Xenotime. Am. Mineral. 1998, 83, 1302–1315. [Google Scholar] [CrossRef]
- Breiter, K.; Förster, H.J.; Škoda, R. Extreme P-. Bi-, Nb-, Sc-, U- and F-rich zircon from fractionated perphosphorus granites: The peraluminous Podlesí granite system, Czech Republic. Lithos 2006, 88, 15–34. [Google Scholar] [CrossRef]
- Huang, X.; Wang, R.C.; Chen, X.M.; Hu, H.; Liu, C.S. Vasrtical variations in the mineralogy of the Yichun topaz-lepidolite granit, Jiangxi province, Southern China. Can. Mineral. 2002, 40, 1047–1068. [Google Scholar] [CrossRef]
- Uher, P. Accessory zircon in orogenic to post-orogenic granites and pegmatites: Compositional variations as indicator of magmatic evolution. An example from western Carpathian, Slovakia. Geophys. Res. Abstr. 2005, 7, 09440. [Google Scholar]
- Breiter, K.; Škoda, R. Vertical zonality of fractionated granite plutons reflected in zircon chemistry: The Cínovec A-type versus the Beauvoir S-type granite suite. Geol. Carpath. 2012, 63, 383–398. [Google Scholar] [CrossRef]
- Förster, H.J. Composition and origin of intermediate solid solutions in the system thorite-xenotime-zircon-coffinite. Lithos 2006, 88, 35–55. [Google Scholar] [CrossRef]
- Hata, S. Xenotime and variety of zircon from Iisaka. Sci. Pap. Inst. Chem. Res. 1938, 34, 619–622. [Google Scholar]
- Uher, P.; Černý, P. Zircon in Hercynian granitic pegmatites of the Western Carpathians, Slovakia. Geol. Carpath. 1998, 49, 261–270. [Google Scholar]
- Hoskin, P.W.O.; Kinny, P.D.; Wyborn, D.; Chappell, B.W. Identifying accessory mineral saturation during differentiation in granitoid magmas: An integrated approach. J. Petrol. 2000, 41, 1365–1396. [Google Scholar] [CrossRef]
- Finch, R.J.; Hanchar, J.M. Structure and chemistry of zircon and zircon group minerals. Rev. Mineral. Geochem. 2003, 53, 1–26. [Google Scholar] [CrossRef]
- Bernhard, F.; Walter, F.; Ettinger, K.; Taucher, J.; Mereiter, K. Pretulite, ScPO4: A new scandium mineral from the Styrian and lower Austrian lazulite occurrences, Austria. Am. Mineral. 1998, 83, 625–630. [Google Scholar] [CrossRef]
- Geisler, T.; Rashwan, A.A.; Rahn, A.K.; Poller, U.; Zwingmann, H.; Pidgeon, R.T.; Schleicher, H.; Tolmaschek, F. Low-temperature hydrothermal alteration of natural metamict zircons from the Eastern Desert Egypt. Mineral. Mag. 2003, 67, 485–508. [Google Scholar] [CrossRef]
- Nasdala, L.; Pidgeon, R.T.; Wolf, D. Heterogeneous metamictization of zircon on a microscale. Geochem. Cosmochim. Acta 1996, 60, 1091–1097. [Google Scholar] [CrossRef]
- Nasdala, L.; Kronz, A.; Wirth, R.; Vászi, T.; Pérez-Soba, C.; Willner, A.; Kennedy, A.K. The phenomenon of different electron microprobe totals in radiation-damaged and altered zircon. Geochim. Cosmochim. Acta 2009, 73, 1637–1650. [Google Scholar] [CrossRef] [Green Version]
- René, M. Composition of coexisting zircon and xenotime in rare-metal granites from the Krušné Hory/Erzgebirge Mts. (Saxothuringian Zone, Bohemian Massif). Miner. Petrol. 2014, 108, 551–569. [Google Scholar] [CrossRef]
- Johan, Z.; Johan, V. Accessory minerals of the Cínovec (Zinnwald) granite cupola, Czech Republic. Part 1: Nb-, Ta- and Ti-bearing oxides. Mineral. Petrol. 2005, 83, 113–150. [Google Scholar] [CrossRef]
- Förster, H.J.; Rhede, D. The Be-Th-rich granite of Seiffen (eastern Erzgebirge, Germany): Accessory-mineral chemistry, composition, and age of late-Variscan Li-F granite of A-type affinity. Neues Jahrbuch für Mineralogie 2006, 182, 307–321. [Google Scholar] [CrossRef]
- Pointer, C.M.; Ashworth, J.R.; Ixer, R.A. The zircon-thorite mineral group in metasomatized granite, Ririwai, Nigeria 1. Geochemistry and metastable solid solution in thorite and coffinite. Mineral. Petrol. 1988, 38, 245–262. [Google Scholar] [CrossRef]
- Smith, D.G.W.; De St, J.L.; Reed, S.J.B.; Long, J.V.P. Zonally metamictized and other zircon from Thor Lake, Northwest Territories. Can. Mineral. 1991, 29, 301–309. [Google Scholar]
- Peréz-Soba, C.; Villaseca, C.; Gonzáles del Tánago, J. The composition of zircon in the peraluminous Hercynian granites of the Spanish Central System batholith. Can. Mineral. 2007, 45, 509–527. [Google Scholar] [CrossRef]
- Anderson, A.J.; Wirth, R.; Thomas, R. The alteration of metamict zircon and its role in the remobilization of high-field-strength elements in the Georgeville granite, Nova Scotia. Can. Mineral. 2008, 46, 1–18. [Google Scholar] [CrossRef]
- Abd El-Naby, H.H. High and low temperature alteration of uranium and thorium minerals, Um Ara granites, south Eastern Desert, Egypt. Ore Geol. Rev. 2009, 35, 436–446. [Google Scholar] [CrossRef]
- Hoshino, M.; Kimata, M.; Nishida, N.; Shimizu, M.; Akasaka, T. Crystal chemistry of zircon from granitic rocks, Japan: Genetic implications of HREE, U and Th enrichment. Neues Jahrbuch für Mineralogie 2010, 187, 167–188. [Google Scholar] [CrossRef]










| Sample | Kru-126 | 1162 | 1250 | 1008 | 1192 | 1617 | 1542 | 1618 |
|---|---|---|---|---|---|---|---|---|
| Rock Type wt % | Medium-F Biotite Granite | High-F Two-Mica Granite | High-F Two-Mica Granite | High-F Li-Mica Granite | High-F Li-Mica Granite | Leucocratic High-F Li-Mica Granite | Alkali-Feldspar Syenite | Alkali-Feldspar Syenite |
| SiO2 | 74.59 | 72.88 | 72.45 | 72.08 | 74.55 | 76.14 | 65.18 | 63.95 |
| TiO2 | 0.16 | 0.19 | 0.18 | 0.08 | 0.06 | 0.05 | 0.04 | 0.04 |
| Al2O3 | 13.47 | 13.84 | 14.53 | 14.42 | 13.91 | 13.19 | 19.31 | 19.73 |
| Fe2O3 | 0.15 | 0.35 | 0.32 | 0.12 | 0.05 | 0.10 | 0.37 | 0.32 |
| FeO | 1.43 | 1.08 | 0.96 | 0.89 | 0.93 | 0.04 | 0.04 | 0.29 |
| MnO | 0.06 | 0.03 | 0.03 | 0.06 | 0.06 | 0.01 | 0.02 | 0.02 |
| MgO | 0.21 | 0.43 | 0.32 | 0.22 | 0.14 | 0.05 | 0.07 | 0.07 |
| CaO | 0.38 | 0.64 | 0.46 | 0.71 | 0.49 | 0.31 | 0.39 | 0.27 |
| Na2O | 3.17 | 3.06 | 3.22 | 3.60 | 3.25 | 4.21 | 4.58 | 5.81 |
| K2O | 4.96 | 5.22 | 5.38 | 4.93 | 4.67 | 3.91 | 7.82 | 6.65 |
| P2O5 | 0.16 | 0.23 | 0.23 | 0.30 | 0.27 | 0.39 | 0.69 | 0.43 |
| H2O+ | 1.00 | 0.41 | 0.51 | 1.30 | 0.05 | 0.32 | 0.55 | 0.58 |
| H2O− | 0.31 | 0.64 | 0.46 | 0.00 | 0.39 | 0.12 | 0.10 | 0.14 |
| F | 0.09 | 0.31 | 0.22 | 0.64 | 0.44 | 0.14 | 0.12 | 0.22 |
| O=F | 0.04 | 0.13 | 0.09 | 0.27 | 0.19 | 0.06 | 0.05 | 0.09 |
| Total | 100.10 | 99.18 | 99.18 | 99.08 | 99.07 | 98.92 | 99.23 | 98.43 |
| ASI | 1.19 | 1.17 | 1.22 | 1.15 | 1.36 | 1.13 | 1.16 | 1.14 |
| ppm | ||||||||
| Ba | 144.0 | 296.0 | 488.0 | 47.0 | 55.0 | 23.0 | 22.0 | 31.0 |
| Rb | 588.9 | 543.0 | 580.0 | 883.0 | 1240.0 | 927.0 | 1800.0 | 1703.0 |
| Sr | 25.4 | 91.0 | 85.0 | 22.0 | 29.0 | 12.0 | 15.0 | 21.0 |
| Y | 25.2 | 34.2 | 32.8 | 9.0 | 16.7 | 5.7 | 7.0 | 3.4 |
| Zr | 109.6 | 177.0 | 170.0 | 37.0 | 54.0 | 20.0 | 19.0 | 27.0 |
| Nb | 21.3 | 23.5 | 23.3 | 22.0 | 44.0 | 32.1 | 15.1 | 14.4 |
| Th | 17.3 | 24.7 | 23.6 | 6.7 | 13.0 | 3.7 | 5.5 | 3.7 |
| Ga | 23.1 | 18.0 | 38.0 | 16.0 | 46.0 | 33.0 | 53.0 | 37.0 |
| Zn | 56.0 | 48.0 | 68.0 | 76.0 | 77.0 | 17.0 | 22.6 | 63.0 |
| Hf | 3.7 | 5.5 | 5.2 | 1.8 | 3.4 | 1.4 | 1.9 | 1.4 |
| Cs | 51.5 | 53.0 | 48.1 | 86.8 | 113.0 | 37.6 | 41.8 | 80.9 |
| Ta | 5.4 | 6.0 | 6.3 | 9.8 | 26.1 | 21.4 | 10.6 | 21.4 |
| U | 13.6 | 10.3 | 14.9 | 9.3 | 22.2 | 4.7 | 4.4 | 4.7 |
| W | 12.2 | 7.1 | 13.8 | 11.1 | 62.7 | 4.1 | 4.9 | 4.1 |
| Sn | 39.0 | 34.0 | 33.0 | 50.0 | 50.0 | 46.0 | 16.0 | 23.0 |
| La | 20.30 | 34.00 | 32.20 | 4.99 | 4.84 | 2.20 | 1.42 | 1.42 |
| Ce | 43.20 | 71.40 | 67.40 | 11.50 | 11.30 | 3.42 | 3.78 | 1.69 |
| Pr | 5.08 | 8.20 | 7.90 | 1.38 | 1.30 | 0.41 | 0.48 | 0.21 |
| Nd | 19.40 | 31.10 | 29.60 | 5.05 | 5.43 | 2.12 | 1.82 | 1.15 |
| Sm | 3.95 | 7.29 | 6.75 | 1.41 | 1.81 | 0.67 | 0.75 | 0.38 |
| Eu | 0.22 | 0.72 | 0.68 | 0.06 | 0.10 | 0.01 | 0.02 | 0.01 |
| Gd | 3.54 | 6.72 | 6.48 | 1.24 | 1.93 | 0.61 | 0.76 | 0.34 |
| Tb | 0.71 | 1.20 | 1.13 | 0.28 | 0.46 | 0.14 | 0.21 | 0.08 |
| Dy | 4.18 | 6.51 | 6.04 | 1.71 | 1.67 | 0.94 | 1.18 | 0.52 |
| Ho | 0.88 | 1.15 | 1.06 | 0.29 | 0.28 | 0.16 | 0.19 | 0.09 |
| Er | 2.61 | 3.30 | 3.08 | 0.84 | 1.67 | 0.47 | 0.48 | 0.27 |
| Tm | 0.43 | 0.47 | 0.47 | 0.14 | 0.28 | 0.09 | 0.09 | 0.06 |
| Yb | 3.22 | 2.88 | 2.72 | 1.04 | 2.02 | 0.59 | 0.62 | 0.37 |
| Lu | 0.41 | 0.39 | 0.37 | 0.12 | 0.28 | 0.08 | 0.08 | 0.05 |
| ΣREE | 108.13 | 175.34 | 165.87 | 30.06 | 34.92 | 11.90 | 11.88 | 6.63 |
| LaN/YbN | 4.25 | 7.96 | 7.98 | 3.23 | 1.62 | 2.52 | 1.55 | 2.59 |
| Eu/Eu* | 0.18 | 0.31 | 0.31 | 0.14 | 0.17 | 0.04 | 0.06 | 0.09 |
| Sample | KRU-34 | KRU-35 | 1162-2 | 1162-13 | 1008-33 | 1371-3 | 1617-26 |
|---|---|---|---|---|---|---|---|
| Variety wt % | Medium-F Granite | Medium-F Granite | High-F Two-Mica Granite | High-F Two-Mica Granite | High-F Li-Mica Granite | High-F Li-Mica Granite | Alkali-Feldspar Syenite |
| P2O5 | 29.27 | 29.09 | 29.22 | 28.12 | 28.70 | 28.67 | 30.18 |
| SiO2 | 0.62 | 0.75 | 0.58 | 0.55 | 1.15 | 0.63 | 0.27 |
| ThO2 | 8.23 | 10.33 | 5.81 | 2.71 | 10.08 | 24.53 | 32.82 |
| UO2 | 0.93 | 0.88 | 1.23 | 0.19 | 0.31 | 2.13 | 4.54 |
| Y2O3 | 2.13 | 2.53 | 2.91 | 0.38 | 3.38 | 2.12 | 1.49 |
| La2O3 | 11.68 | 11.36 | 11.51 | 15.44 | 10.47 | 4.80 | 3.84 |
| Ce2O3 | 26.29 | 24.73 | 26.17 | 32.57 | 25.22 | 15.42 | 9.19 |
| Pr2O3 | 2.80 | 2.77 | 3.03 | 3.49 | 2.95 | 1.98 | 1.01 |
| Nd2O3 | 10.02 | 9.48 | 11.36 | 12.04 | 10.60 | 6.79 | 3.39 |
| Sm2O3 | 1.79 | 1.81 | 2.54 | 1.88 | 2.25 | 2.84 | 0.97 |
| Gd2O3 | 1.20 | 1.12 | 1.95 | 0.72 | 1.83 | 1.75 | 0.80 |
| Dy2O3 | 0.80 | 0.80 | 1.02 | 0.11 | 1.14 | 1.02 | 0.59 |
| Er2O3 | 0.17 | 0.17 | 0.21 | 0.03 | 0.23 | 0.14 | 0.11 |
| CaO | 1.30 | 1.75 | 1.53 | 0.32 | 1.29 | 5.18 | 8.04 |
| PbO | 0.17 | 0.17 | 0.16 | 0.04 | 0.14 | 0.42 | 0.62 |
| As2O5 | 0.03 | 0.06 | 0.05 | 0.06 | 0.04 | b.d.l. | 0.08 |
| F | b.d.l. | b.d.l. | b.d.l. | 0.01 | 0.56 | b.d.l. | 0.40 |
| O=F | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 | 0.00 | 0.10 |
| Total | 97.43 | 97.80 | 99.28 | 98.66 | 100.20 | 98.42 | 98.24 |
| apfu, O=4 | |||||||
| P | 0.986 | 0.977 | 0.973 | 0.960 | 0.955 | 0.969 | 1.000 |
| Si | 0.025 | 0.030 | 0.023 | 0.022 | 0.045 | 0.025 | 0.011 |
| Th | 0.075 | 0.093 | 0.052 | 0.025 | 0.090 | 0.223 | 0.292 |
| U | 0.008 | 0.008 | 0.011 | 0.002 | 0.003 | 0.019 | 0.040 |
| Y | 0.045 | 0.053 | 0.061 | 0.008 | 0.071 | 0.045 | 0.031 |
| La | 0.171 | 0.166 | 0.167 | 0.230 | 0.152 | 0.071 | 0.055 |
| Ce | 0.383 | 0.359 | 0.376 | 0.481 | 0.363 | 0.225 | 0.131 |
| Pr | 0.041 | 0.040 | 0.043 | 0.051 | 0.042 | 0.029 | 0.014 |
| Nd | 0.142 | 0.134 | 0.159 | 0.173 | 0.149 | 0.097 | 0.047 |
| Sm | 0.025 | 0.025 | 0.034 | 0.026 | 0.030 | 0.039 | 0.013 |
| Gd | 0.016 | 0.015 | 0.025 | 0.010 | 0.024 | 0.023 | 0.010 |
| Dy | 0.010 | 0.010 | 0.013 | 0.001 | 0.014 | 0.013 | 0.007 |
| Er | 0.002 | 0.002 | 0.003 | 0.000 | 0.003 | 0.002 | 0.001 |
| Ca | 0.055 | 0.074 | 0.064 | 0.014 | 0.054 | 0.222 | 0.337 |
| Pb | 0.002 | 0.002 | 0.002 | 0.000 | 0.001 | 0.005 | 0.007 |
| As | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.002 |
| F | 0.000 | 0.000 | 0.000 | 0.003 | 0.139 | 0.000 | 0.087 |
| LREEPO4 | 0.7758 | 0.7312 | 0.7633 | 0.9397 | 0.7308 | 0.4333 | 0.2541 |
| HREEPO4 | 0.0295 | 0.0282 | 0.0420 | 0.0111 | 0.0424 | 0.0390 | 0.0185 |
| CaTh(PO4)2 | 0.1158 | 0.1548 | 0.1311 | 0.0281 | 0.1118 | 0.4559 | 0.6934 |
| ThSiO4 | 0.0316 | 0.0303 | 0.0010 | 0.0131 | 0.0414 | 0.0257 | 0.0021 |
| YPO4 | 0.0474 | 0.0554 | 0.0625 | 0.0080 | 0.0735 | 0.0462 | 0.0319 |
| Sample | 1007-4 | 1007-11 | 1007-19 | 997-9 | 1542-18 | 1542-20 |
|---|---|---|---|---|---|---|
| Variety wt % | High-F Li-Mica Granite | High-F Li-Mica Granite | High-F Li-Mica Granite | Alkali-Feldspar Syenite | Alkali-Feldspar Syenite | Alkali-Feldspar Syenite |
| P2O5 | 34.50 | 36.31 | 33.87 | 31.99 | 32.75 | 33.00 |
| SiO2 | 0.96 | b.d.l. | 0.85 | 0.23 | 0.97 | 0.93 |
| ThO2 | 0.87 | 0.04 | 0.72 | 0.53 | 1.50 | 1.29 |
| UO2 | 4.43 | 0.93 | 4.56 | 2.49 | 3.06 | 2.97 |
| Y2O3 | 38.83 | 48.50 | 38.23 | 39.59 | 40.18 | 40.19 |
| La2O3 | b.d.l. | 0.03 | 0.01 | b.d.l. | b.d.l. | 0.01 |
| Ce2O3 | b.d.l. | b.d.l. | 0.03 | 0.04 | 0.11 | 0.14 |
| Pr2O3 | b.d.l. | 0.02 | 0.07 | 0.10 | 0.08 | 0.05 |
| Nd2O3 | 0.32 | 0.08 | 0.42 | 0.26 | 0.38 | 0.44 |
| Sm2O3 | 0.87 | 0.11 | 0.79 | 0.84 | 0.67 | 0.68 |
| Gd2O3 | 2.56 | 0.61 | 2.38 | 2.72 | 2.29 | 2.21 |
| Dy2O3 | 7.59 | 3.79 | 7.35 | 6.69 | 6.16 | 6.19 |
| Ho2O3 | 0.98 | 0.69 | 1.06 | b.d.l. | b.d.l. | b.d.l. |
| Er2O3 | 3.23 | 2.48 | 3.42 | 3.15 | 3.45 | 3.43 |
| Yb2O3 | 4.23 | 3.81 | 3.96 | 4.50 | 2.31 | 2.36 |
| Lu2O3 | 0.81 | 0.56 | 0.91 | 0.02 | b.d.l. | b.d.l. |
| CaO | 0.42 | 0.89 | 0.51 | 0.49 | 0.19 | 0.16 |
| PbO | 0.18 | 0.02 | 0.10 | 0.43 | 0.51 | 0.51 |
| F | 0.09 | 1.12 | 0.10 | 0.05 | 0.10 | 0.02 |
| O=F | 0.04 | 0.47 | 0.04 | 0.02 | 0.04 | 0.01 |
| Total | 100.83 | 99.52 | 99.30 | 94.10 | 94.67 | 94.57 |
| apfu, O=4 | ||||||
| P | 0.985 | 1.001 | 0.984 | 0.973 | 0.974 | 0.978 |
| Si | 0.032 | 0.000 | 0.029 | 0.008 | 0.034 | 0.033 |
| Th | 0.007 | 0.000 | 0.006 | 0.004 | 0.012 | 0.010 |
| U | 0.033 | 0.007 | 0.035 | 0.020 | 0.024 | 0.023 |
| Y | 0.696 | 0.840 | 0.697 | 0.756 | 0.750 | 0.748 |
| La | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ce | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 |
| Pr | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 |
| Nd | 0.004 | 0.001 | 0.005 | 0.003 | 0.005 | 0.005 |
| Sm | 0.010 | 0.001 | 0.009 | 0.010 | 0.008 | 0.008 |
| Gd | 0.029 | 0.007 | 0.027 | 0.032 | 0.027 | 0.026 |
| Dy | 0.082 | 0.040 | 0.081 | .0.077 | 0.070 | 0.070 |
| Ho | 0.010 | 0.007 | 0.012 | 0.000 | 0.000 | 0.000 |
| Er | 0.034 | 0.025 | 0.037 | 0.036 | 0.038 | 0.038 |
| Yb | 0.043 | 0.038 | 0.041 | 0.049 | 0.025 | 0.025 |
| Lu | 0.008 | 0.006 | 0.009 | 0.000 | 0.000 | 0.000 |
| Ca | 0.015 | 0.031 | 0.019 | 0.019 | 0.007 | 0.006 |
| Pb | 0.002 | 0.000 | 0.001 | 0.004 | 0.005 | 0.005 |
| F | 0.019 | 0.231 | 0.022 | 0.011 | 0.022 | 0.004 |
| LREEPO4 | 0.0144 | 0.0020 | 0.0153 | 0.0148 | 0.0154 | 0.0165 |
| HREEPO4 | 0.2117 | 0.1226 | 0.2112 | 0.1917 | 0.1644 | 0.1644 |
| CaTh(PO4)2 | 0.0308 | 0.0616 | 0.0388 | 0.0375 | 0.0144 | 0.0124 |
| ThSiO4 | 0.0270 | 0.0000 | 0.0230 | 0.0090 | 0.0340 | 0.0320 |
| YPO4 | 0.7153 | 0.8375 | 0.7112 | 0.7470 | 0.7708 | 0.7735 |
| Sample | 23-44 | 23-45 | 1162-5 | 1250-27 | 1007-2 | 1283-21 | 997-1 | 1542-2 |
|---|---|---|---|---|---|---|---|---|
| Variety wt % | Medium-F Granite | Medium-F Granite | High-F Two-Mica Granite | High-F Two-Mica Granite | High-F Li-Mica Granite | High-F Li-Mica Granite | Alkali-Feldspar Syenite | Alkali-Feldspar Syenite |
| SiO2 | 31.60 | 32.16 | 31.85 | 32.30 | 31.49 | 26.49 | 32.32 | 24.22 |
| Al2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.11 | 0.01 | 0.85 |
| ZrO2 | 64.19 | 65.40 | 63.05 | 66.00 | 61.73 | 49.81 | 65.32 | 54.02 |
| HfO2 | 1.43 | 1.03 | 2.52 | 1.36 | 3.60 | 1.83 | 1.33 | 2.67 |
| CaO | 0.01 | 0.03 | 0.05 | b.d.l. | 0.03 | 0.40 | 0.04 | 1.54 |
| FeO | b.d.l. | b.d.l. | 0.05 | 0.02 | 0.16 | 0.01 | 0.02 | 1.61 |
| MnO | 0.02 | b.d.l. | 0.02 | b.d.l. | 0.04 | 0.03 | 0.02 | b.d.l. |
| MgO | b.d.l. | 0.01 | 0.01 | 0.02 | b.d.l. | b.d.l. | 0.01 | 0.04 |
| P2O5 | 0.50 | 0.05 | 0.59 | 0.02 | 0.79 | 5.46 | 0.38 | 4.58 |
| Sc2O3 | 0.11 | b.d.l. | 0.35 | b.d.l. | 0.31 | 0.42 | 0.09 | 0.50 |
| As2O5 | b.d.l. | b.d.l. | 0.01 | 0.05 | 0.10 | 0.05 | 0.04 | 0.30 |
| Bi2O3 | 0.09 | 0.08 | 0.05 | 0.10 | 0.07 | 0.12 | 0.09 | 0.10 |
| Y2O3 | 0.48 | 0.23 | 0.26 | b.d.l. | 0.38 | 5.47 | 0.20 | 0.17 |
| La2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.02 | 0.02 | b.d.l. |
| Ce2O3 | b.d.l. | b.d.l. | 0.02 | b.d.l. | b.d.l. | 0.36 | 0.01 | 0.09 |
| Pr2O3 | 0.03 | b.d.l. | b.d.l. | 0.03 | b.d.l. | 0.02 | 0.02 | 0.06 |
| Nd2O3 | b.d.l. | 0.10 | b.d.l. | b.d.l. | 0.04 | 0.02 | 0.08 | 0.06 |
| Sm2O3 | 0.01 | 0.01 | b.d.l. | 0.01 | b.d.l. | 0.11 | b.d.l. | 0.05 |
| Gd2O3 | b.d.l. | 0.01 | 0.01 | b.d.l. | b.d.l. | 0.35 | 0.02 | 0.02 |
| Dy2O3 | 0.03 | 0.05 | 0.01 | b.d.l. | b.d.l. | 0.81 | 0.02 | b.d.l. |
| Er2O3 | 0.12 | 0.07 | 0.02 | 0.06 | 0.10 | 0.50 | 0.06 | 0.05 |
| Yb2O3 | 0.21 | 0.06 | 0.18 | 0.03 | 0.29 | 0.78 | 0.05 | 0.15 |
| UO2 | 0.50 | 0.09 | 0.65 | 0.04 | 0.52 | 3.09 | 0.11 | 2.06 |
| ThO2 | 0.01 | 0.04 | 0.02 | 0.02 | 0.01 | 0.92 | b.d.l. | 0.25 |
| PbO | 0.02 | b.d.l. | 0.03 | b.d.l. | 0.04 | 0.87 | 0.01 | 0.03 |
| F | b.d.l. | b.d.l. | 0.03 | b.d.l. | b.d.l. | 0.28 | 0.01 | 0.65 |
| O=F | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.12 | 0.00 | 0.27 |
| Total | 99.36 | 99.42 | 99.77 | 100.06 | 99.68 | 98.33 | 100.28 | 94.07 |
| apfu, O=4 | ||||||||
| Si | 0.982 | 0.995 | 0.987 | 0.994 | 0.981 | 0.859 | 0.990 | 0.810 |
| P | 0.013 | 0.001 | 0.015 | 0.001 | 0.021 | 0.150 | 0.010 | 0.130 |
| Al | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 | 0.033 |
| ΣT-site | 0.995 | 0.996 | 1.002 | 0.995 | 1.002 | 1.013 | 1.000 | 0.973 |
| Zr | 0.973 | 0.987 | 0.952 | 0.990 | 0.938 | 0.788 | 0.976 | 0.881 |
| Hf | 0.013 | 0.009 | 0.022 | 0.012 | 0.032 | 0.017 | 0.012 | 0.025 |
| Ca | 0.000 | 0.001 | 0.002 | 0.000 | 0.001 | 0.014 | 0.001 | 0.055 |
| Fe | 0.000 | 0.000 | 0.001 | 0.001 | 0.004 | 0.000 | 0.001 | 0.045 |
| Mn | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 |
| Mg | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.002 |
| Sc | 0.003 | 0.000 | 0.009 | 0.000 | 0.008 | 0.012 | 0.002 | 0.015 |
| As | 0.000 | 0.000 | 0.000 | 0.001 | 0.002 | 0.001 | 0.001 | 0.005 |
| Bi | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 |
| Y | 0.008 | 0.004 | 0.004 | 0.000 | 0.006 | 0.094 | 0.003 | 0.003 |
| La | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ce | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 | 0.001 |
| Pr | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Nd | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 |
| Sm | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 |
| Gd | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 |
| Dy | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 | 0.000 |
| Er | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.005 | 0.001 | 0.001 |
| Yb | 0.002 | 0.001 | 0.002 | 0.000 | 0.003 | 0.008 | 0.000 | 0.002 |
| U | 0.003 | 0.001 | 0.004 | 0.000 | 0.004 | 0.022 | 0.001 | 0.015 |
| Th | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 | 0.000 | 0.002 |
| Pb | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 | 0.000 |
| ΣA site | 1.004 | 1.005 | 0.997 | 1.007 | 1.000 | 0.996 | 1.000 | 1.056 |
| F | 0.000 | 0.000 | 0.006 | 0.000 | 0.000 | 0.057 | 0.002 | 0.137 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
René, M. REE and Y Mineralogy of the Krudum Granite Body (Saxothuringian Zone). Minerals 2018, 8, 287. https://doi.org/10.3390/min8070287
René M. REE and Y Mineralogy of the Krudum Granite Body (Saxothuringian Zone). Minerals. 2018; 8(7):287. https://doi.org/10.3390/min8070287
Chicago/Turabian StyleRené, Miloš. 2018. "REE and Y Mineralogy of the Krudum Granite Body (Saxothuringian Zone)" Minerals 8, no. 7: 287. https://doi.org/10.3390/min8070287
APA StyleRené, M. (2018). REE and Y Mineralogy of the Krudum Granite Body (Saxothuringian Zone). Minerals, 8(7), 287. https://doi.org/10.3390/min8070287




