Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus ferrooxidans under the Induction of Surfactant Triton X-100
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Minerals
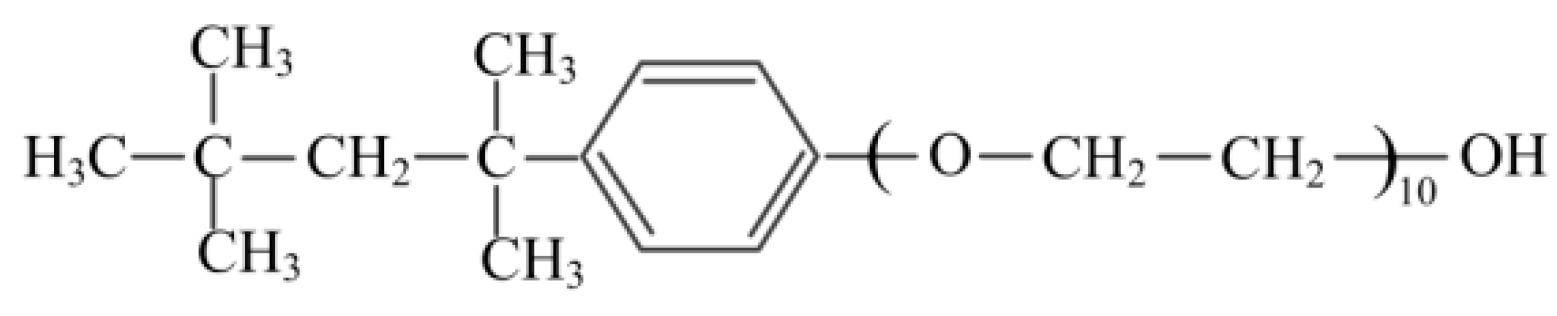
2.2. Surface Tension and Contact Angle Measurements
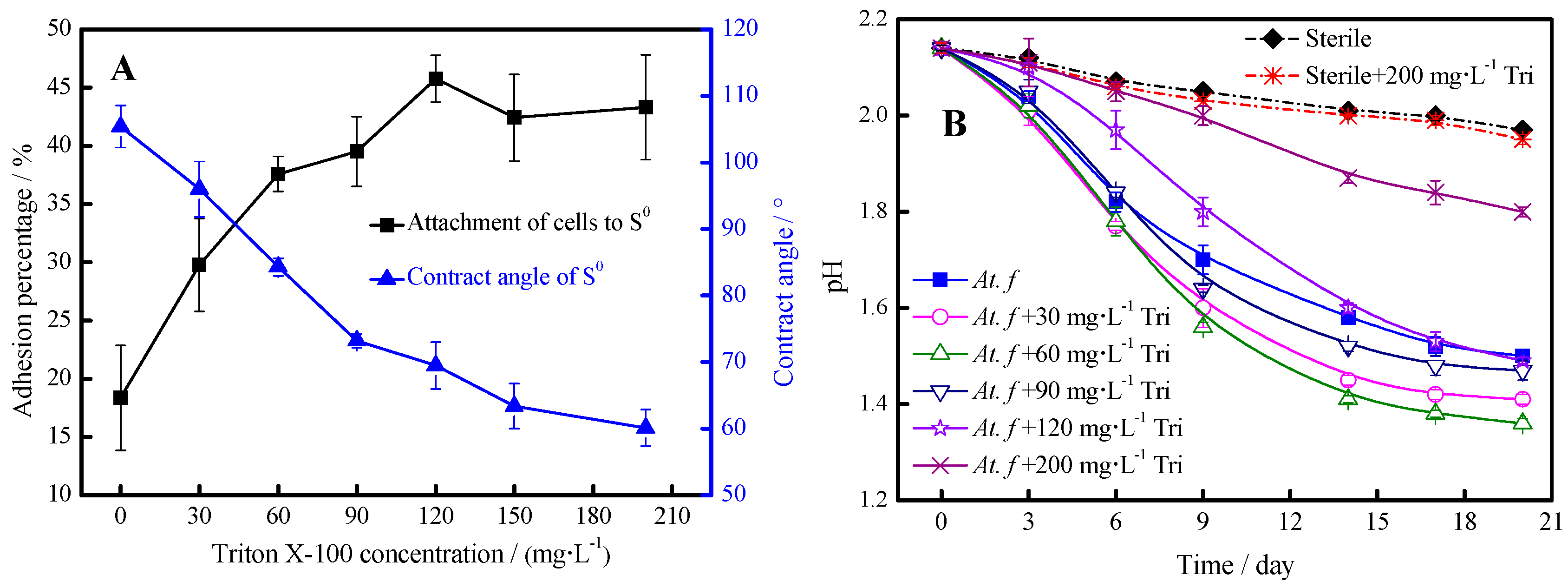
2.3. Bacterial Adhesion Experiments
2.4. Ferrous Iron and Sulfur Oxidation Measurements
2.5. Leaching Experiments
3. Results and Discussion
3.1. The Iron-Oxidizing Activity of At. ferrooxidans
3.2. The Induction of Sulfur Oxidation by At. ferrooxidans
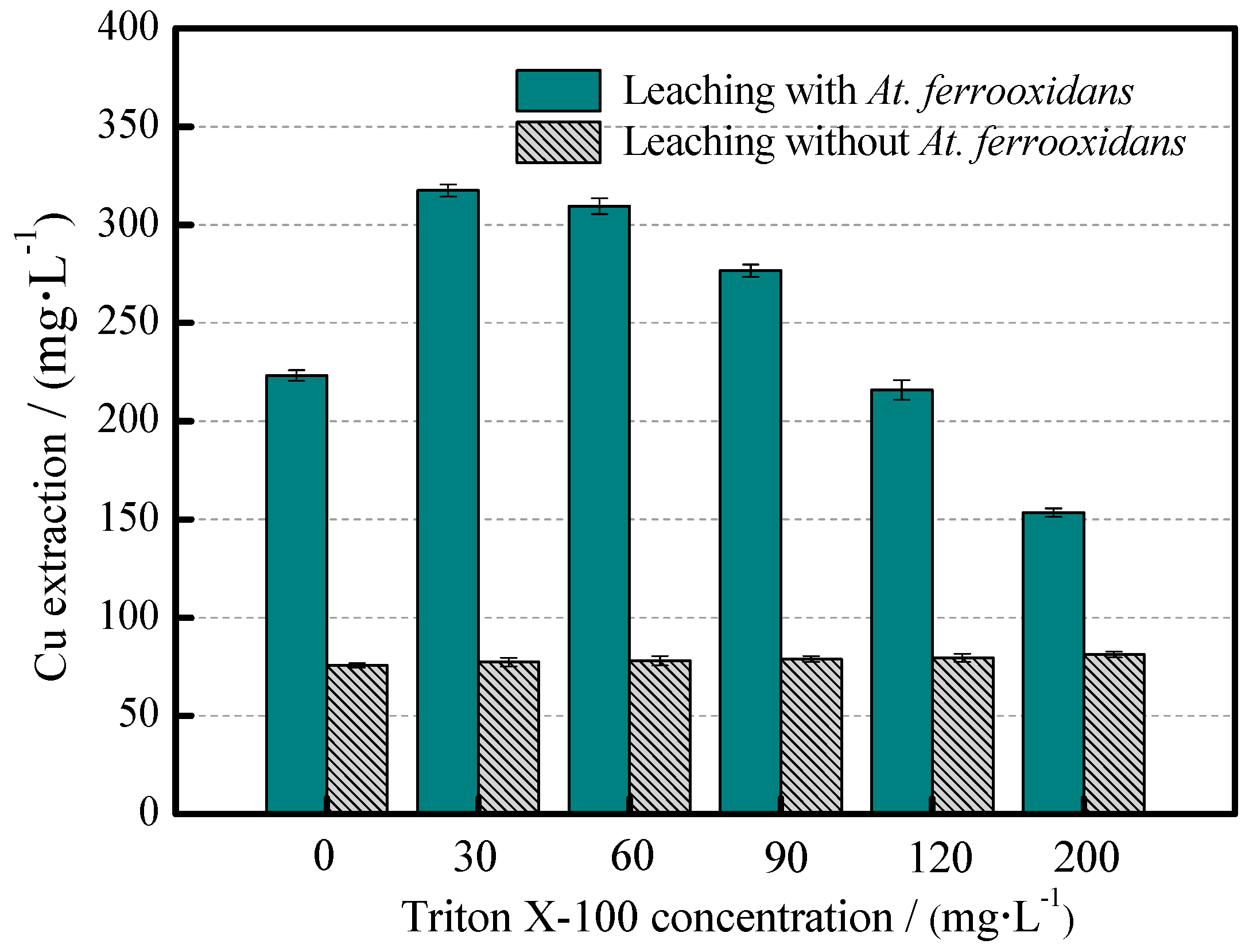
3.3. The Leaching Efficiency of Chalcopyrite
3.4. The Induction of Chalcopyrite Bioleaching
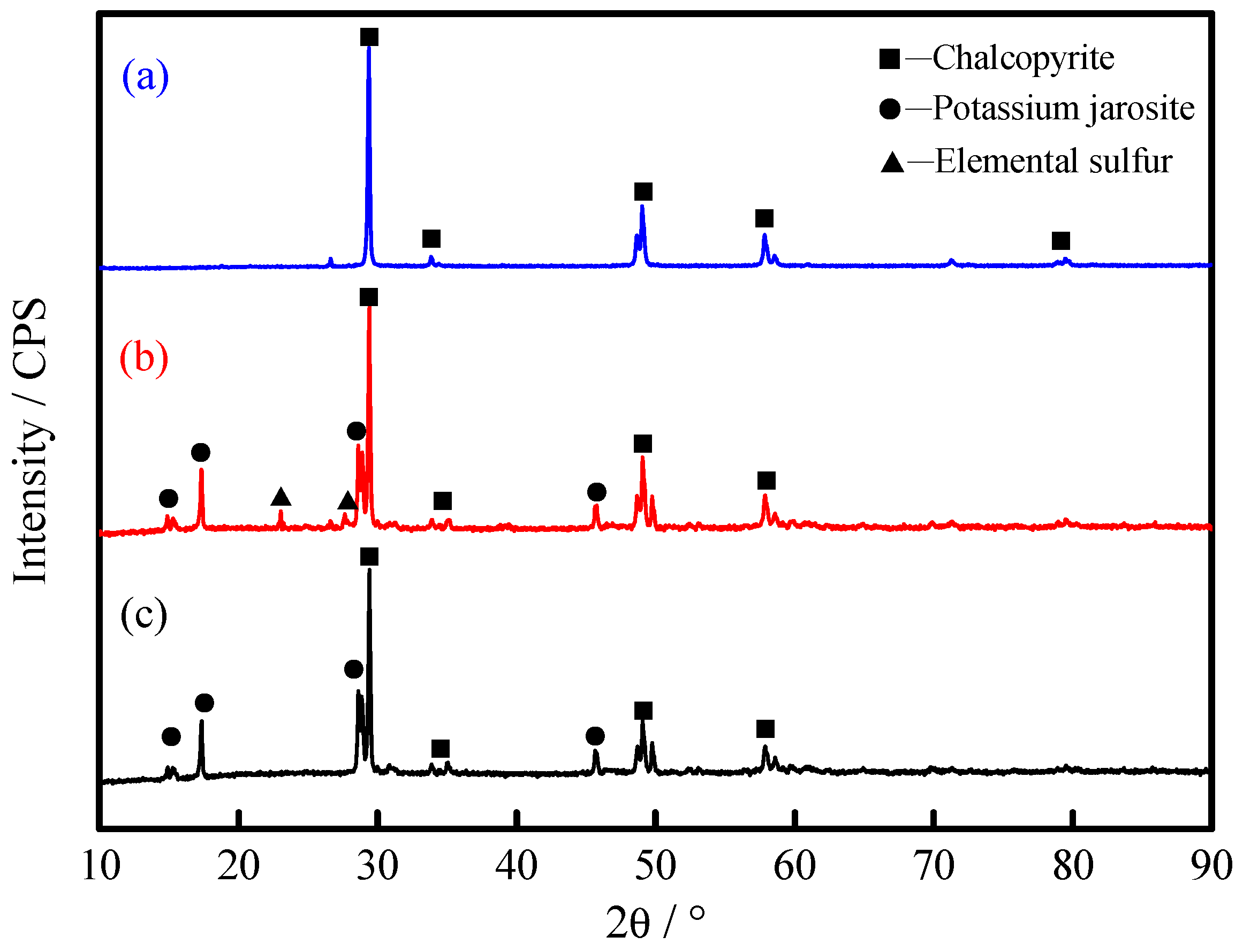
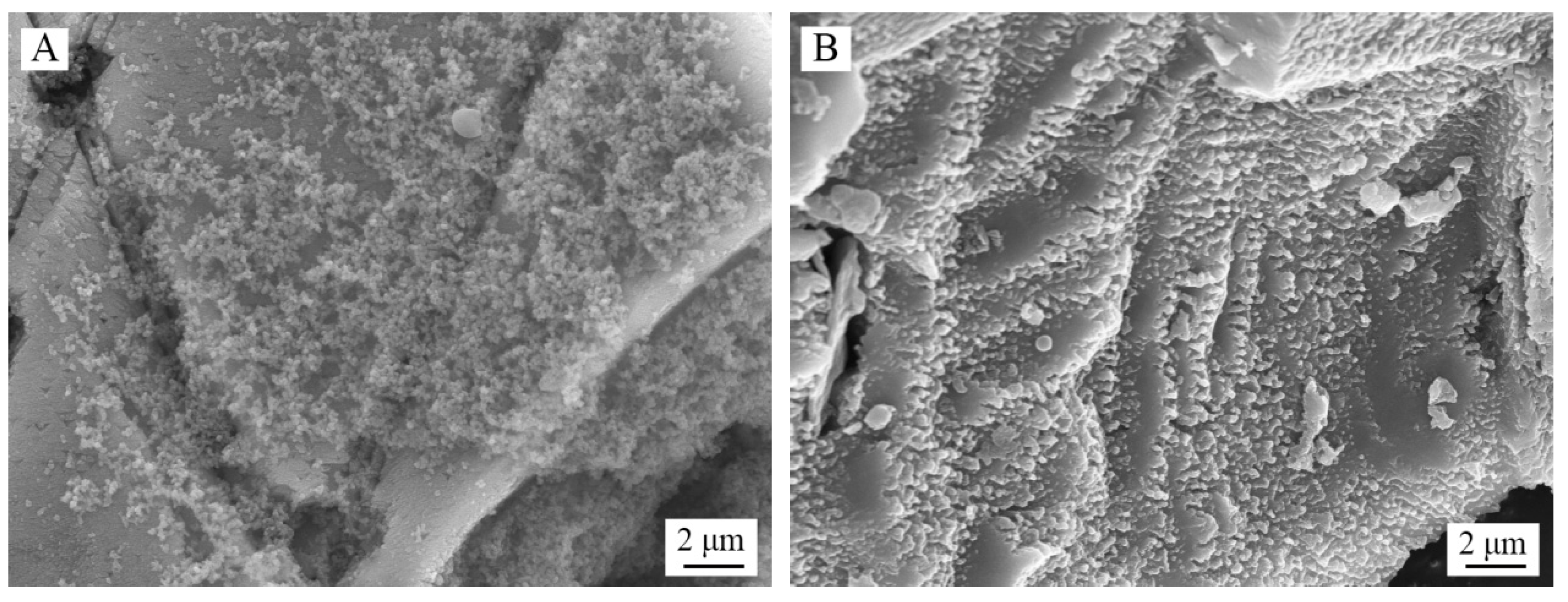
3.5. Analysis of Chalcopyrite Surface after Leaching
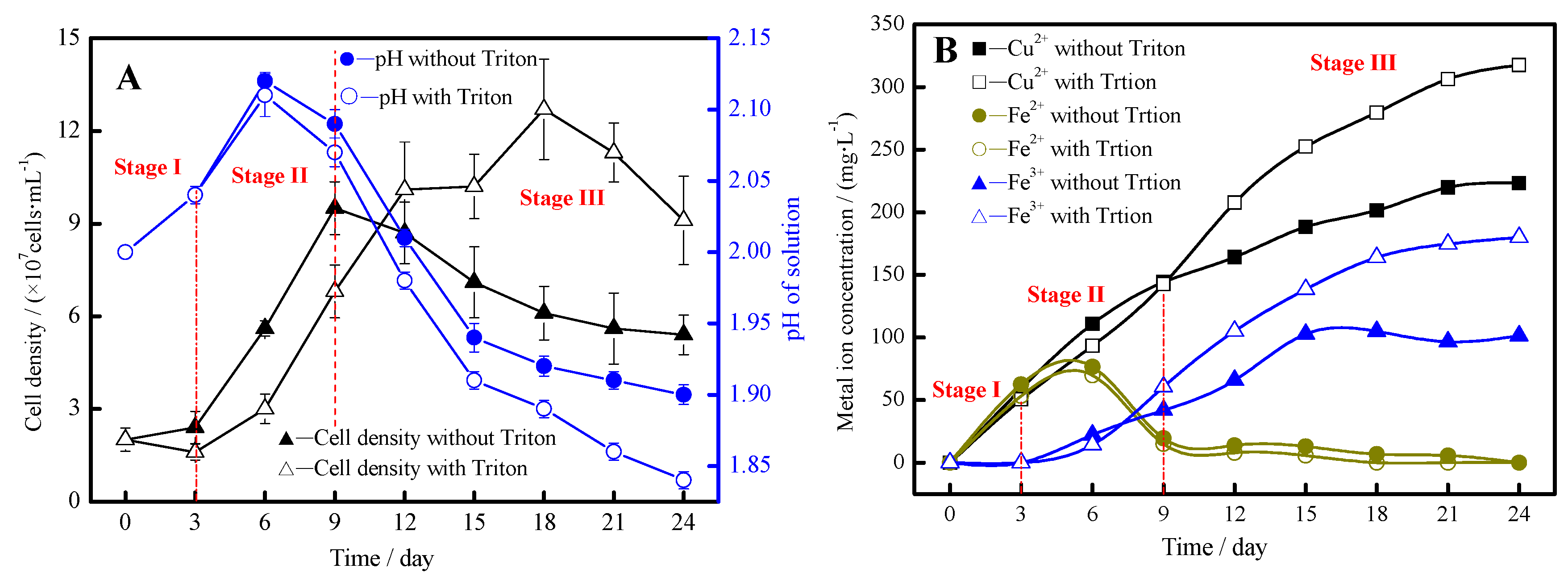
3.6. Model of the Enhancement of Bioleaching
- (i)
- In the first stage, the addition of Triton X-100 could hardly influence chalcopyrite leaching. Chalcopyrite was initially attacked by protons, simultaneously releasing ferrous iron and elemental sulfur, as Reaction (2) proceeded.
- (ii)
- In the second stage, the bacterial leaching of chalcopyrite is slightly prevented by the addition of Triton X-100. This was mainly because the surfactant addition had a negative effect on iron-oxidizing activity of the leaching bacteria, and the bacteria would first use soluble Fe2+ rather than insoluble S0 as the energy resource, as illustrated by Reactions (1) and (3).
- (iii)
- In the third stage, chalcopyrite bioleaching was promoted under the induction of Triton X-100, and the copper extraction efficiency increased remarkably. Triton X-100 induced the bioavailability of formed S0, consequently destroying the structure of the passivation layer. On the other hand, the decrease of the leaching solution pH delayed jarosite formation (Reaction (4)), and favored the “indirect” oxidation of chalcopyrite (Reaction (5)), which in turn maintained the ongoing dissolution of chalcopyrite.
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Panda, S.; Biswal, A.; Mishra, S.; Panda, P.K.; Pradhan, N.; Mohapatra, U. Reductive dissolution by waste newspaper for enhanced meso-acidophilic bioleaching of copper from low grade chalcopyrite: A new concept of biohydrometallurgy. Hydrometallurgy 2015, 153, 98–105. [Google Scholar] [CrossRef]
- Bevilaqua, D.; Leite, A.L.L.C.; Garcia, O.; Tuovinen, O.H. Oxidation of chalcopyrite by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in shake flasks. Process. Biochem. 2002, 38, 587–592. [Google Scholar] [CrossRef]
- Pathak, A.; Morrison, L.; Healy, M.G. Catalytic potential of selected metal ions for bioleaching, and potential techno-economic and environmental issues: A critical review. Bioresour. Technol. 2017, 229, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. review of acidic sulfate, sulfate-chloride and sulfate-nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Fu, K.; Lin, H.; Mo, X.; Wang, H.; Wen, H.; Wen, Z. Comparative study on the passivation layers of copper sulphide minerals during bioleaching. Int. J. Miner. Met. Mater. 2012, 19, 886–892. [Google Scholar] [CrossRef]
- Wang, J.; Gan, X.; Zhao, H.; Hu, M.; Li, K.; Qin, W.; Qiu, G. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis. Miner. Eng. 2016, 98, 264–278. [Google Scholar] [CrossRef]
- Hedrich, S.; Joulian, C.; Graupner, T.; Schippers, A.; Guézennec, A.G. Enhanced chalcopyrite dissolution in stirred tank reactors by temperature increase during bioleaching. Hydrometallurgy 2018, 179, 125–131. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z.; Ma, C.; Zheng, L.; Hong, C.; Wen, W. Bioleaching of chalcopyrite by Acidianus manzaensis under different constant pH. Miner. Eng. 2016, 98, 80–89. [Google Scholar] [CrossRef]
- Lotfalian, M.; Ranjbar, M.; Fazaelipoor, M.H.; Schaffie, M.; Manafi, Z. The effect of redox control on the continuous bioleaching of chalcopyrite concentrate. Miner. Eng. 2015, 81, 52–57. [Google Scholar] [CrossRef]
- Abdollahi, H.; Noaparast, M.; Shafaei, S.Z.; Manafi, Z.; Muñoz, J.A.; Tuovinen, O.H. Silver-catalyzed bioleaching of copper, molybdenum and rhenium from a chalcopyrite–molybdenite concentrate. Int. Biodeterior. Biodegrad. 2015, 104, 194–200. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, S. Catalytic effect of activated carbon on bioleaching of low-grade primary copper sulfide ores. Trans. Nonferr. Met. Soc. China 2007, 17, 1123–1127. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Tang, Y.; Ghorbani, Y.; Northey, S.; Yellishetty, M.; Deng, X.; McBride, D. The current state and future directions of percolation leaching in the Chinese mining industry: Challenges and opportunities. Miner. Eng. 2018, 125, 206–222. [Google Scholar] [CrossRef]
- Liu, W.; Yang, H.; Song, Y.; Tong, L. Catalytic effects of activated carbon and surfactants on bioleaching of cobalt ore. Hydrometallurgy 2015, 152, 69–75. [Google Scholar] [CrossRef]
- Deng, T.; Liao, M.; Wang, M.; Chen, Y.; Belzile, N. Investigations of accelerating parameters for the biooxidation of low-grade refractory gold ores. Miner. Eng. 2000, 13, 1543–1553. [Google Scholar] [CrossRef]
- Lan, Z.; Hu, Y.; Qin, W. Effect of surfactant OPD on the bioleaching of marmatite. Miner. Eng. 2009, 22, 10–13. [Google Scholar] [CrossRef]
- Dehghan, R.; Dianati, M. The effects of Pb-Zn flotation reagents on the bioleaching process by mesophilic bacteria. Int. J. Miner. Process. 2015, 143, 80–86. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Nakamura, T.; Tsunekawa, M.; Hirajima, T.; Ito, M. Enhancement in bacterial leaching of chalcopyrite by polyoxyethylene sorbitan monolaurate addition. Shigen-to-Sozai 1995, 111, 943–948. [Google Scholar] [CrossRef]
- Peng, A.; Liu, H.; Nie, Z.; Xia, J. Effect of surfactant Tween-80 on sulfur oxidation and expression of sulfur metabolism relevant genes of Acidithiobacillus ferrooxidans. Trans. Nonferr. Met. Soc. China 2012, 22, 3147–3155. [Google Scholar] [CrossRef]
- Torma, A.E.; Gabra, G.G.; Guay, R.; Silver, M. Effects of surface active agents on the oxidation of chalcopyrite by thiobacillus ferrooxidans. Hydrometallurgy 1976, 4, 301–309. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Xin, X.; Zhang, H.; Shi, X. Surface tension and dilational viscoelasticity of water in the presence of surfactants tyloxapol and Triton X-100 with cetyl trimethylammonium bromide at 25 °C. J. Chem. Eng. Data 2009, 54, 989–995. [Google Scholar] [CrossRef]
- Sałek, K.; Kaczorek, E.; Guzik, U. Bacterial properties changing under Triton X-100 presence in the diesel oil biodegradation systems: From surface and cellular changes to mono- and dioxygenases activities. Environ. Sci. Pollut. Res. 2015, 22, 4305–4315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, D.; Liu, W.; Gao, S. Effects of Triton X-100 on oxidative activity of Acidithiobacillus ferrooxidans and on chalcopyrite bioleaching. J. Northeast. Univ. Natl. Sci. 2016, 6, 861–865. (In Chinese) [Google Scholar]
- Zhang, R.; Wei, D.; Shen, Y.; Liu, W.; Lu, T.; Han, C. Catalytic effect of polyethylene glycol on sulfur oxidation in chalcopyrite bioleaching by Acidithiobacillus ferrooxidans. Miner. Eng. 2016, 95, 74–78. [Google Scholar] [CrossRef]
- Peng, T.; Zhou, D.; Liu, X.; Yu, R.; Jiang, T.; Gu, G.; Zeng, W. Enrichment of ferric iron on mineral surface during bioleaching of chalcopyrite. Trans. Nonferr. Met. Soc. China 2016, 26, 544–550. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, B.; Li, Y.; Song, Q.; Zhao, Y.; Zhang, R.; Shu, X. Effect of chemical fractionation treatment on structure and characteristics of pyrolysis products of Xinjiang long flame coal. Fuel 2018, 234, 1193–1204. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Zhu, P.; Chen, A.; Liu, H.; Yin, H.; Liang, Y. Carbon material with high specific surface area improves complex copper ores’ bioleaching efficiency by mixed moderate thermophiles. Minerals 2018, 8, 301. [Google Scholar] [CrossRef]
- Tuttle, J.H.; Dugan, P.R. Inhibition of growth, iron, and sulfur oxidation in Thiobacillus ferrooxidans by simple organic compounds. Can. J. Microbiol. 1976, 22, 719–730. [Google Scholar] [CrossRef]
- Jafari, M.; Shafaei, S.Z.A.; Abdollahi, H.; Gharabaghi, M.; Chehreh, C.S. A comparative study on the effect of flotation reagents on growth and iron oxidation activities of Leptospirillum ferrooxidans and Acidithiobacillus ferrooxidans. Minerals 2016, 7, 2. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Sun, F.; Liu, C. Catalytic effect of a combined silver and surfactant catalyst on cobalt ore bioleaching. JOM 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Pakostova, E.; Mandl, M.; Tuovinen, O.H. Cellular ATP and biomass of attached and planktonic sulfur-oxidizing Acidithiobacillus ferrooxidans. Process Biochem. 2013, 48, 1785–1788. [Google Scholar] [CrossRef]
- Tan, S.; Chen, M. Early stage adsorption behaviour of Acidithiobacillus ferrooxidans on minerals I: An experimental approach. Hydrometallurgy 2013, 119–120, 87–94. [Google Scholar] [CrossRef]
- Devasia, P.; Natarajan, K.A. Adhesion of Acidithiobacillus ferrooxidans to mineral surfaces. Int. J. Miner. Process. 2010, 94, 135–139. [Google Scholar] [CrossRef]
- Knickerbocker, C.; Nordstrom, D.K.; Southam, G. The role of “blebbing” in overcoming the hydrophobic barrier during biooxidation of elemental sulfur by Thiobacillus thiooxidans. Chem. Geol. 2000, 169, 425–433. [Google Scholar] [CrossRef]
- Porro, S.; Ramírez, S.; Reche, C.; Curutchet, G.; Alonso-Romanowski, S.; Donati, E. Bacterial attachment: Its role in bioleaching processes. Process Biochem. 1997, 32, 573–578. [Google Scholar] [CrossRef]
- Valdés, J.; Pedroso, I.; Quatrini, R.; Dodson, R.; Tettelin, H.; Blake, R. Acidithiobacillus ferrooxidans metabolism: From genome sequence to industrial applications. BMC Genom. 2008, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Bevilaqua, D.; Lahti, H.; Suegama, P.H.; Benedetti, A.V.; Puhakka, J.A. Effect of Na-chloride on the bioleaching of a chalcopyrite concentrate in shake flasks and stirred tank bioreactors. Hydrometallurgy 2013, 138, 1–13. [Google Scholar] [CrossRef]
- He, H.; Xia, J.; Yang, Y.; Jiang, H.; Xiao, C.; Zheng, L.; Qiu, G. Sulfur speciation on the surface of chalcopyrite leached by Acidianus manzaensis. Hydrometallurgy 2009, 99, 45–50. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z. Relatedness of Cu and Fe speciation to chalcopyrite bioleaching by Acidithiobacillus ferrooxidans. Hydrometallurgy 2015, 156, 40–46. [Google Scholar] [CrossRef]
- Li, L.; Lv, Z.; Yuan, X. Effect of L-glycine on bioleaching of collophanite by Acidithiobacillus ferrooxidans. Int. Biodeterior. Biodegrad. 2013, 85, 156–165. [Google Scholar] [CrossRef]
- Castro, C.; Donati, E. Effects of different energy sources on cell adhesion and bioleaching of a chalcopyrite concentrate by extremophilic archaeon Acidianus copahuensis. Hydrometallurgy 2016, 162, 49–56. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, R.; Lu, X.; Lu, J.; Li, C.; Li, J. Bioleaching of chalcopyrite by Acidithiobacillus ferrooxidans. Miner. Eng. 2013, 53, 184–192. [Google Scholar] [CrossRef]
- Lara, R.; García-Meza, J.; González, I.; Cruz, R. Influence of the surface speciation on biofilm attachment to chalcopyrite by Acidithiobacillus thiooxidans. Appl. Microbial. Biot. 2013, 97, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.C.; Sherman, D.M.; Purton, J.A. Surface oxidation of chalcopyrite (CuFeS2) under ambient atmospheric and aqueous (pH 2–10) conditions: Cu, Fe L- and O K-edge X-ray spectroscopy. Geochim. Cosmochim. Acta 2013, 67, 2137–2146. [Google Scholar] [CrossRef]
- Pearce, C.I.; Pattrick, R.A.D.; Vaughan, D.J.; Henderson, C.M.B.; Van der Laan, G. Copper oxidation state in chalcopyrite: Mixed Cu d9 and d10 characteristics. Geochim. Cosmochim. Acta 2006, 70, 4635–4642. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Tomashevich, Y.; Tauson, V.; Vyalikh, D.; Molodtsov, S.; Szargan, R. A comparative X-ray absorption near-edge structure study of bornite, Cu5FeS4, and chalcopyrite, CuFeS2. J. Electron. Spectrosc. 2005, 142, 83–88. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Tao, L.; Cao, P.; Yang, C.; Qin, W.; Qiu, G. Roles of oxidants and reductants in bioleaching system of chalcopyrite at normal atmospheric pressure and 45 °C. Int. J. Miner. Process. 2017, 162, 81–91. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential. Hydrometallurgy 2008, 93, 88–96. [Google Scholar] [CrossRef]
- Acres, R.G.; Harmer, S.L.; Beattie, D.A. Synchrotron XPS studies of solution exposed chalcopyrite, bornite, and heterogeneous chalcopyrite with bornite. Int. J. Miner. Process. 2010, 94, 43–51. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Wang, J.; Li, Y.; Liao, R.; Wang, X. Comparison of bioleaching and dissolution process of p-type and n-type chalcopyrite. Miner. Eng. 2017, 109, 153–161. [Google Scholar] [CrossRef]
- Acres, R.G.; Harmer, S.L.; Shui, H.; Chen, C.; Beattie, D.A. Synchrotron scanning photoemission microscopy of homogeneous and heterogeneous metal sulfide minerals. J. Synchrotron Radiat. 2011, 18, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Nathsarma, K.C.; Rao, K.S.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Sun, C.; Kou, J.; Zhao, H.; Wei, D.; Xing, Y. Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus ferrooxidans under the Induction of Surfactant Triton X-100. Minerals 2019, 9, 11. https://doi.org/10.3390/min9010011
Zhang R, Sun C, Kou J, Zhao H, Wei D, Xing Y. Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus ferrooxidans under the Induction of Surfactant Triton X-100. Minerals. 2019; 9(1):11. https://doi.org/10.3390/min9010011
Chicago/Turabian StyleZhang, Ruiyang, Chunbao Sun, Jue Kou, Hongyu Zhao, Dezhou Wei, and Yi Xing. 2019. "Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus ferrooxidans under the Induction of Surfactant Triton X-100" Minerals 9, no. 1: 11. https://doi.org/10.3390/min9010011





