Serpentine–Hisingerite Solid Solution in Altered Ferroan Peridotite and Olivine Gabbro
Abstract
:1. Introduction
1.1. Mechanisms of Fe3+ Substitution into Serpentine
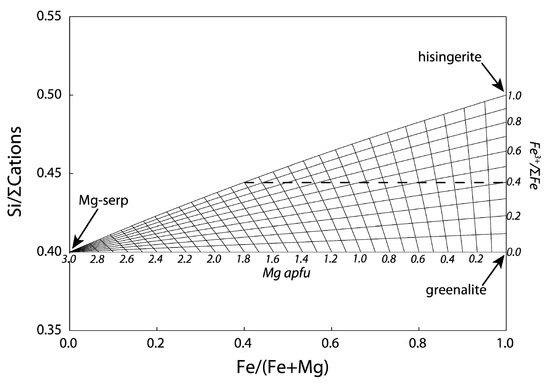
1.2. Serpentine–Hisingerite Miscibility
2. Methods
2.1. Electron Microprobe Analyses
2.2. Thermodynamic Calculations
3. Results
3.1. Layered Mafic Intrusions
3.1.1. Duluth Complex
3.1.2. Laramie Complex
3.2. Ores, Banded Iron Formation and Nakhla Meteorite
4. Discussion of Compositional and Environmental Controls
4.1. Reaction Conditions Leading to Mg Mobility
4.2. Silica Activity and Oxidation State of Hisingerite-Forming Solutions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, B.W.; Hattori, K.; Baronnet, A. Serpentinite: What, why, where? Elements 2013, 9, 99–106. [Google Scholar] [CrossRef]
- O’Hanley, D.S. Serpentinites; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Sleep, N.H.; Bird, D.K.; Pope, E.C. Serpentinite and the dawn of life. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2857–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, F.; Bach, W.; Jöns, N.; McCollom, T.; Moskowitz, B.; Berquó, T. Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15° N on the Mid-Atlantic Ridge. Geochim. Cosmochim. Acta 2009, 73, 6868–6893. [Google Scholar] [CrossRef]
- Wicks, F.J.; O’Hanley, D.S. Serpentine minerals: Structures and petrology. In Reviews in Mineralogy: Volume 19, Hydrous Phyllosilicates; BookCrafters, Inc.: Chelsea, MI, USA, 1988; pp. 91–167. [Google Scholar]
- Evans, B.W.; Dyar, M.D.; Kuehner, S.M. Implications of ferrous and ferric iron in antigorite. Am. Mineral. 2012, 97, 184–196. [Google Scholar] [CrossRef]
- Brindley, G.W.; Zussman, J. A Structural Study of the thermal transformation of serpentine mineral to forsterite. Am. Mineral. 1957, 42, 461–474. [Google Scholar]
- Faust, G.; Fahey, J. The Serpentine-Group Minerals; The U.S. Government Publishing Office: Washington, DC, USA, 1962.
- Whittaker, E.J.W.; Wicks, F.J. Chemical differences among the serpentinte “polymorphs”: A discussion. Am. Minerol. 1970, 55, 1025–1047. [Google Scholar]
- Wicks, F.J.; Plant, A.G. Electron-microprobe and xray-microbeam studies of serpentine textures. Can. Mineral. 1979, 17, 785–830. [Google Scholar]
- McCollom, T.M.; Shock, E.L. Geochemical constraints on chemolithoautotropic metabolism by microorganisms in seafloor hydrothermal systems. Geochim. Cosmochim. Acta 1997, 61, 4375–4391. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; Humphris, S.E.; Kahl, W.A.; Jïöns, N.; Moskowitz, B.; Berquó, T.S. Magnetite in seafloor serpentinite-Some like it hot. Geology 2014, 42, 135–138. [Google Scholar] [CrossRef]
- Evans, B.W.; Kuehner, S.M.; Chopelas, A. Magnetite-free, yellow lizardite serpentinization of olivine websterite, Canyon Mountain complex, N.E. Oregon. Am. Mineral. 2009, 94, 1731–1734. [Google Scholar] [CrossRef]
- Beard, J.S.; Frost, B.R. The stoichiometric effects of ferric iron substitutions in serpentine from microprobe data. Int. Geol. Rev. 2017, 59, 541–547. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; McCollom, T.M. Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 2013, 178, 55–69. [Google Scholar] [CrossRef]
- Evans, K.A.; Powell, R.; Frost, B.R. Using equilibrium thermodynamics in the study of metasomatic alteration, illustrated by an application to serpentinites. Lithos 2013, 168–169, 67–84. [Google Scholar] [CrossRef]
- Malvoisin, B. Mass transfer in the oceanic lithosphere: Serpentinization is not isochemical. Earth Planet. Sci. Lett. 2015, 430, 75–85. [Google Scholar] [CrossRef]
- Caruso, L.J.; Chernosky, J.V.J. The stability of lizardite. Can. Mineral. 1979, 17, 757–769. [Google Scholar]
- O’Hanley, D.S.; Dyar, M.D. The composition of lizardite 1T and the formation of magnetite in serpentinites. Am. Mineral. 1993, 78, 391–404. [Google Scholar]
- O’Hanley, D.S.; Dyar, M.D. The composition of chrysotile and its relationship with lizardite. Can. Mineral. 1998, 36, 727–739. [Google Scholar]
- Votyakov, S.L.; Chashchukhin, I.S.; Galakhova, O.L.; Gulyaeva, T.Y. Crystal Chemistry of Lizardite as an Indicator of Early Serpentinization in Ultramafic Rocks. I. Compositional and Structural Features of the Mineral According to Spectroscopic Data. Geochem. Int. 2005, 43, 947–965. [Google Scholar]
- Evans, B.W. Control of the products of serpentinization by the Fe2+ Mg-1 exchange potential of olivine and orthopyroxene. J. Petrol. 2008, 49, 1873–1887. [Google Scholar] [CrossRef]
- Fuchs, Y.; Linares, J.; Mellini, M. Mossbauer and infrared spectrometry of lizardite-1T from Monte Fico, Elba. Phys. Chem. Miner. 1998, 26, 111–115. [Google Scholar] [CrossRef]
- Eggleton, R.A.; Tilley, D.B. Hisingerite: A ferric kaolin mineral with curved morphology. Clays Clay Miner. 1998, 46, 400–413. [Google Scholar] [CrossRef]
- Smith, K.L.; Milnes, A.R.; Eggleton, R.A. Weathering of basalt: Formation of iddingsite. Clays Clay Miner. 1987, 35, 418–428. [Google Scholar] [CrossRef]
- Evans, B.W.; Kuehner, S.M.; Joswiak, D.J.; Cressey, G. Serpentine, iron-rich phyllosilicates and fayalite produced by hydration and Mg depletion of peridotite, Duluth Complex, Minnesota, USA. J. Petrol. 2017, 58, 495–512. [Google Scholar] [CrossRef]
- Van Aken, P.A.; Liebscher, B.; Styrsa, V.J. Quantitative determination of iron oxidation states in minerals using Fe L2,3-edge electron energy-loss near-edge structure spectroscopy. Phys. Chem. Miner. 1998, 25, 323–327. [Google Scholar] [CrossRef]
- Andreani, M.; Muñoz, M.; Marcaillou, C.; Delacour, A. μXANES study of iron redox state in serpentine during oceanic serpentinization. Lithos 2013, 178, 70–83. [Google Scholar] [CrossRef]
- Debret, B.; Andreani, M.; Muñoz, M.; Bolfan-Casanova, N.; Carlut, J.; Nicollet, C.; Schwartz, S.; Trcera, N. Evolution of Fe redox state in serpentine during subduction. Earth Planet. Sci. Lett. 2014, 400, 206–218. [Google Scholar] [CrossRef]
- Bethke, C.M.; Farrell, B.; Yeakel, S. The Geochemist’s Workbench® Release 12.0—Reaction Modeling Guide; Aqueous Solutions, LLC: Champaign, IL, USA, 2018; ISBN 9781597180948. [Google Scholar]
- Kong, X.Z.; Tutolo, B.M.; Saar, M.O. DBCreate: A SUPCRT92-based program for producing EQ3/6, TOUGHREACT, and GWB thermodynamic databases at user-defined T and P. Comput. Geosci. 2013, 51, 415–417. [Google Scholar] [CrossRef]
- Helgeson, H.C.; Delany, J.M.; Nesbitt, H.W.; Bird, D.K. Summary and critique of the thermodynamic properties of the rock-forming minerals. Am. J. Sci. 1978, 278A, 229. [Google Scholar]
- Sverjensky, D.A.; Harrison, B.; Azzolini, D. Water in the deep Earth: The dielectric constant and the solubilities of quartz and corundum to 60 kb and 1200 °C. Geochim. Cosmochim. Acta 2014, 129, 125–145. [Google Scholar] [CrossRef]
- McCollom, T.M.; Bach, W. Thermodynamic constraints on hydrogen generation during serpentinization of ultramafic rocks. Geochim. Cosmochim. Acta 2009, 73, 856–875. [Google Scholar] [CrossRef]
- Johnson, J.W.; Oelkers, E.H.; Helgeson, H.C. SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000 °C. Comput. Geosci. 1992, 18, 899–947. [Google Scholar] [CrossRef]
- Blanc, P.; Vieillard, P.; Gailhanou, H.; Gaboreau, S.; Gaucher, É.; Fialips, C.I.; Madé, B.; Giffaut, E. A generalized model for predicting the thermodynamic properties of clay minerals. Am. J. Sci. 2015, 315, 734–780. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Mildner, D.F.R.; Gagnon, C.V.L.; Saar, M.O.; Seyfried, W.E. Nanoscale constraints on porosity generation and fluid flow during serpentinization. Geology 2016, 44, 103–106. [Google Scholar] [CrossRef]
- Jolliffe, F. A study of greenalite. Am. Mineral. 1935, 20, 405–425. [Google Scholar]
- Gruner, J.W. The structure and chemical composition of greenalite. Am. Mineral. 1936, 21, 449–455. [Google Scholar]
- Floran, R.J.; Papike, J.J. Petrology of the low-grade rocks of the Gunflint Iron-Formation, Ontario-Minnesota. Bull. Geol. Soc. Am. 1975, 86, 1169–1190. [Google Scholar] [CrossRef]
- Gole, M. Low temperature retrograde minerals in metamorphosed Archean banded iron formations, western Australia. Can. Mineral. 1980, 18, 205–214. [Google Scholar]
- Gole, M. Mineralogy and petrology of very-low-metamorphic grade Archaean banded iron-formations, Weld Range, Western Australia. Am. Mineral. 1980, 65, 8–25. [Google Scholar]
- Klein, C.; Gole, M.J. Mineralogy and petrology of parts of the Marra Mamba iron formation, Hamersley Basin, Western Australia. Am. Mineral. 1981, 66, 507–525. [Google Scholar] [CrossRef]
- Guggenheim, S.; Bailey, S.W. Structural aspects of greenalite and related minerals. Can. Mineral. 1982, 20, 1–18. [Google Scholar]
- Guggenheim, S.; Eggleton, R.A. Modulated structures of greenalite and caryopilite: A system with long-range, in-plane structural disorder in the tetrahedra sheet. Can. Mineraoligist 1998, 36, 163–179. [Google Scholar]
- Rasmussen, M.G.; Evans, B.W.; Kuehner, S.M. Low-temperature fayalite, greenalite, and minnesotaite from the overlook gold deposit, Washington: Phase relations in the system FeO-SiO2-H2O. Can. Mineral. 1998, 36, 147–162. [Google Scholar]
- Hicks, L.J.; Bridges, J.C.; Gurman, S.J. Ferric saponite and serpentine in the nakhlite Martian meteorites. Geochim. Cosmochim. Acta 2014, 136, 194–210. [Google Scholar] [CrossRef]
- Milliken, R.; Bish, D.L. Distinguishing hisingerite from other clays and its importance for Mars. In Proceedings of the 45th Lunar and Planetary Science Conference, Woodlands, TX, USA, 17–21 March 2014; Volume 343, p. 2251. [Google Scholar]
- Baker, J.H. Greenalite, Mg-rich minnesotaite and stilpnomelane from the Oesjoeberg and Sirsjoeberg iron-ore mines, Hjulsjoe, W. Bergslagen, Sweden. Mineral. Mag. 1985, 49 Pt 4, 611–613. [Google Scholar] [CrossRef]
- Dietrich, V. Ilvait, Ferroantigorit und Greenalith als Begleiter Oxidisch-Sulfidischer Vererzungen in den Oberhalbsteiner Serpentiniten; Geologisches Institut der Eidg. Technischen Hochschule und der Universität Zürich: Zürich, Switzerland, 1972. [Google Scholar]
- Eggleton, R.A.; Pennington, J.H.; Freeman, R.S.; Threadgold, I.M. Structural aspects of the hisingerite-neotocite series. Clay Miner. 1983, 18, 21–31. [Google Scholar] [CrossRef]
- Floran, R.J.; Papike, J.J. Mineralogy and petrology of the Gunflint Iron Formation, Minnesota-Ontario: Correlation of compositional and assemblage variations at low to moderate grade. J. Petrol. 1978, 19, 215–288. [Google Scholar] [CrossRef]
- Hawkins, A.C.; Shannon, E.V. Canbyite, a new mineral. Am. Mineral. 1924, 9, 1–5. [Google Scholar]
- Klein, C. Greenalite, stilpnomelane, minnesotaite, crocidolite and carbonates in a very low-grade metamorphic Precambrian Iron-Formation. Can. Mineral. 1974, 12, 475–498. [Google Scholar]
- Kohyama, N.; Sudo, T. Hisingerite occurring as a weathering product of iron-rich saponite. Clays Clay Miner. 1975, 23, 215–218. [Google Scholar] [CrossRef]
- Lindqvist, B.; Jansson, S. On the crystal chemistry of hisingerite. Am. Mineral. 1962, 47, 1356–1362. [Google Scholar]
- Manceau, A.; Ildefonse, P.; Hazemann, J.L.; Flank, A.M.; Gallup, D. Crystal chemistry of hydrous iron silicate scale deposits at the Salton Sea geothermal field. Clays Clay Miner. 1995, 43, 304–317. [Google Scholar] [CrossRef]
- Mustoe, G.E. Hisingerite—A rare iron mineral from Walker Valley, Skagit County, Washington. Washingt. Geol. 1996, 24, 14–19. [Google Scholar]
- Schwarz, G.M. On the nature and origin of hisingerite from Parry Sound, Ontario. Am. Mineral. 1924, 9, 141–144. [Google Scholar]
- Shayan, A. Hisingerite material from a basalt quarry near Geelong, Victoria, Australia. Clays Clay Miner. 1984, 32, 272–278. [Google Scholar] [CrossRef]
- Sudo, T.; Nakamura, T. Hisingerite from Japan. Am. Mineral. 1952, 37, 618–621. [Google Scholar]
- Whelan, J.A.; Goldich, S.S. New data for hisingerite and neotocite. Am. Mineral. 1961, 46, 1412–1423. [Google Scholar]
- Wilshire, H.G. Alteration of olivine an orthopyroxene in basic lavas and shallow intrusions. Am. Mineral. 1958, 43, 120–147. [Google Scholar]
- Zajak, I.S. The Stratigraphy and Mineralogy of the Sokoman Iron Formation in the Knob Lake Area, Quebec and Newfoundland; University of Michigan: Ann Arbor, MI, USA, 1972. [Google Scholar]
- Bailey, S.W. Structures and compositions of other trioctahedral 1:1 phyllosilicates. Rev. Mineral. Geochem. 1988, 19, 169–188. [Google Scholar]
- Janecky, D.R.; Seyfried, W.E. Hydrothermal serpentinization of peridotite within the oceanic crust: Experimental investigations of mineralogy and major element chemistry. Geochim. Cosmochim. Acta 1986, 50, 1357–1378. [Google Scholar] [CrossRef]
- Seyfried, W.E.; Pester, N.J.; Ding, K.; Rough, M. Vent fluid chemistry of the Rainbow hydrothermal system (36° N, MAR): Phase equilibria and in situ pH controls on subseafloor alteration processes. Geochim. Cosmochim. Acta 2011, 75, 1574–1593. [Google Scholar] [CrossRef]
- Foster, M.E.; Hudleston, P.J. “Fracture cleavage” in the Duluth Complex, northeastern Minnesota (USA). Geol. Soc. Am. Bull. 1986, 97, 85–96. [Google Scholar] [CrossRef]
- Wones, D.R.; Eugster, H.P. Stability of biotite: Experiment, theory, and application. Am. Mineral. 1965, 50, 1228–1272. [Google Scholar] [CrossRef]
- Bish, D.L. X-ray Diffraction Results from Mars Science Laboratory: Mineralogy of Rocknest at Gale Crater. Science 2014, 341, 1–6. [Google Scholar] [CrossRef] [PubMed]

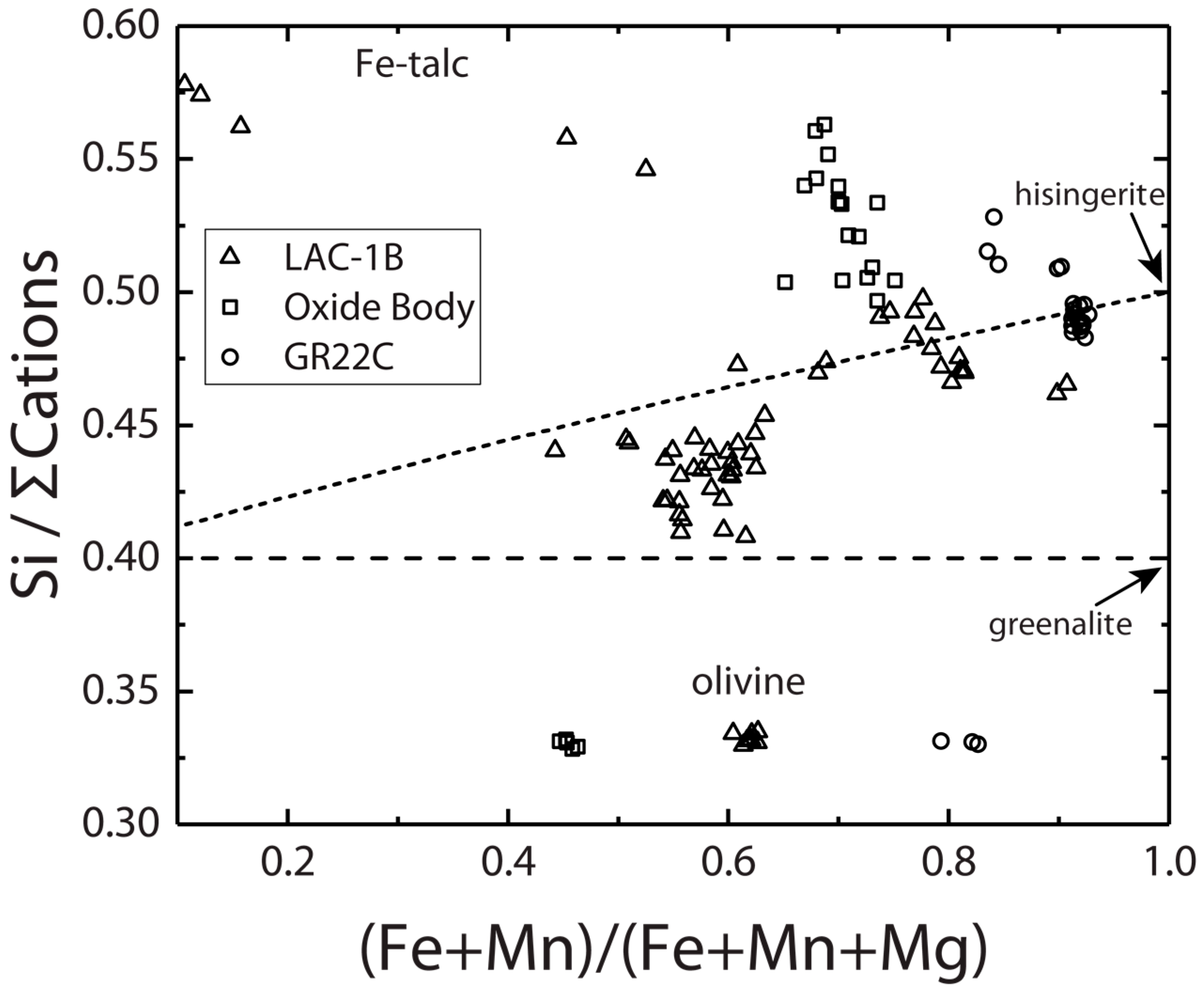
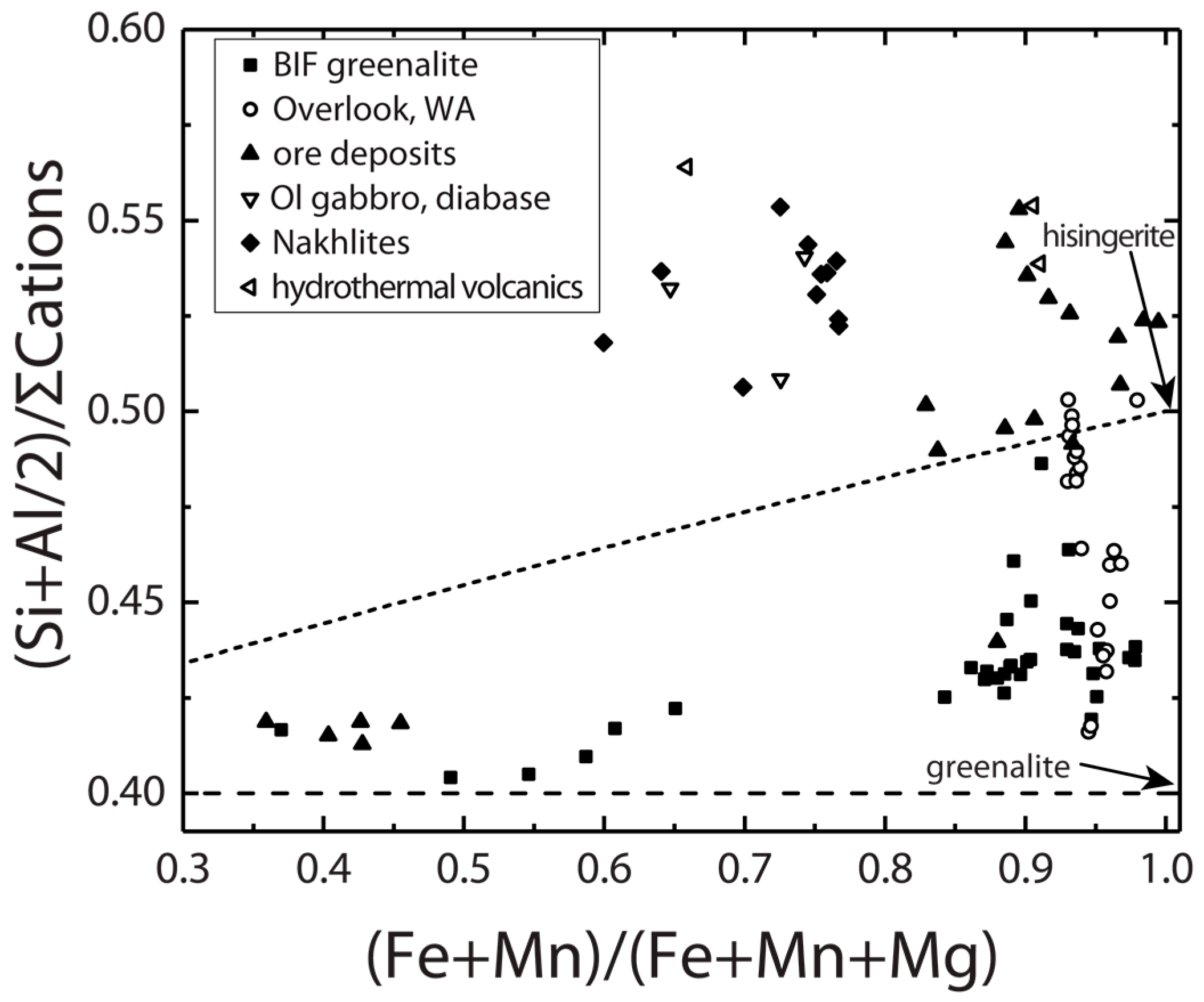

| Location | Duluth Complex | Laramie Complex | Overlook Ore Deposit | Mars | ||||
|---|---|---|---|---|---|---|---|---|
| Sample | DM561A | Bardon Peak | LAC-1B | Oxide Body | GR22C | 19-821 | 19-821 | Nakhla |
| Mineral | Mg–His | Mg–His | Gre–His | His | His | His? | Gre? | His |
| Number of Spots | 9 | 20 | 27 | 13 | 16 | 9 | 9 | 7 |
| Oxide | Weight Percent | |||||||
| SiO2 | 39.49 | 39.13 | 37.74 | 42.67 | 37.82 | 40.46 | 40.46 | 43.36 |
| Al2O3 | 0.06 | 0.16 | 0.03 | 1.22 | 0.03 | 0.61 | 0.61 | 1.09 |
| Cr2O3 | 0.03 | 0 | 0.02 | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 |
| Fe2O3 t | 29.96 | 34.22 | 35.83 | 46.94 | 51.38 | 36.57 | ||
| FeO t | 33.65 | 46.23 | ||||||
| MnO | 0.32 | 0.34 | 0.25 | 0.15 | 0.49 | 0.58 | 0.58 | 0.44 |
| MgO | 12.73 | 13.01 | 13.99 | 7.46 | 2.15 | 1.86 | 1.86 | 6.09 |
| NiO | 0.13 | 0.08 | 0.05 | 0.05 | 0.01 | 0.02 | 0.02 | 0.02 |
| CaO | 0.79 | 0.78 | 0.15 | 0.81 | 0.37 | 0.09 | 0.09 | 0.41 |
| Anh. Total | 83.51 | 87.72 | 85.88 | 88.22 | 87.85 | 95.04 | 89.89 | 88.02 |
| Charge | 14 | 14 | 14 | 14 | 14 | 14 | 12 | 14 |
| Si | 2.078 | 1.989 | 2.115 | 2.131 | 1.994 | 1.975 | 1.956 | 2.17 |
| Al | 0.003 | 0.010 | 0.003 | 0.072 | 0.003 | 0.035 | 0.035 | 0.064 |
| Cr | 0.001 | 0 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.001 |
| Fe3+ | 1.186 | 1.309 | 1.346 | 1.862 | 1.887 | 1.377 | ||
| Fe2+ | 1.577 | 1.869 | ||||||
| Mn | 0.014 | 0.015 | 0.012 | 0.006 | 0.022 | 0.024 | 0.024 | 0.019 |
| Mg | 0.999 | 0.986 | 1.167 | 0.556 | 0.169 | 0.135 | 0.134 | 0.454 |
| Ca | 0.045 | 0.043 | 0.009 | 0.043 | 0.021 | 0.005 | 0.004 | 0.022 |
| Total | 4.326 | 4.352 | 4.884 | 4.155 | 4.073 | 4.063 | 4.023 | 4.107 |
| Si/ΣCations | 0.48 | 0.457 | 0.433 | 0.513 | 0.49 | 0.486 | 0.486 | 0.528 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutolo, B.M.; Evans, B.W.; Kuehner, S.M. Serpentine–Hisingerite Solid Solution in Altered Ferroan Peridotite and Olivine Gabbro. Minerals 2019, 9, 47. https://doi.org/10.3390/min9010047
Tutolo BM, Evans BW, Kuehner SM. Serpentine–Hisingerite Solid Solution in Altered Ferroan Peridotite and Olivine Gabbro. Minerals. 2019; 9(1):47. https://doi.org/10.3390/min9010047
Chicago/Turabian StyleTutolo, Benjamin M., Bernard W. Evans, and Scott M. Kuehner. 2019. "Serpentine–Hisingerite Solid Solution in Altered Ferroan Peridotite and Olivine Gabbro" Minerals 9, no. 1: 47. https://doi.org/10.3390/min9010047
APA StyleTutolo, B. M., Evans, B. W., & Kuehner, S. M. (2019). Serpentine–Hisingerite Solid Solution in Altered Ferroan Peridotite and Olivine Gabbro. Minerals, 9(1), 47. https://doi.org/10.3390/min9010047