Geopolymer Synthesis Using Garnet Tailings from Molybdenum Mines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Geopolymer Synthesis
2.3. Characterization
3. Results
3.1. Transformations of Soluble Al and Si
3.2. Compressive Strength Test
3.3. X-Ray Diffraction Results
3.4. Scanning Electron Microscopy (SEM)
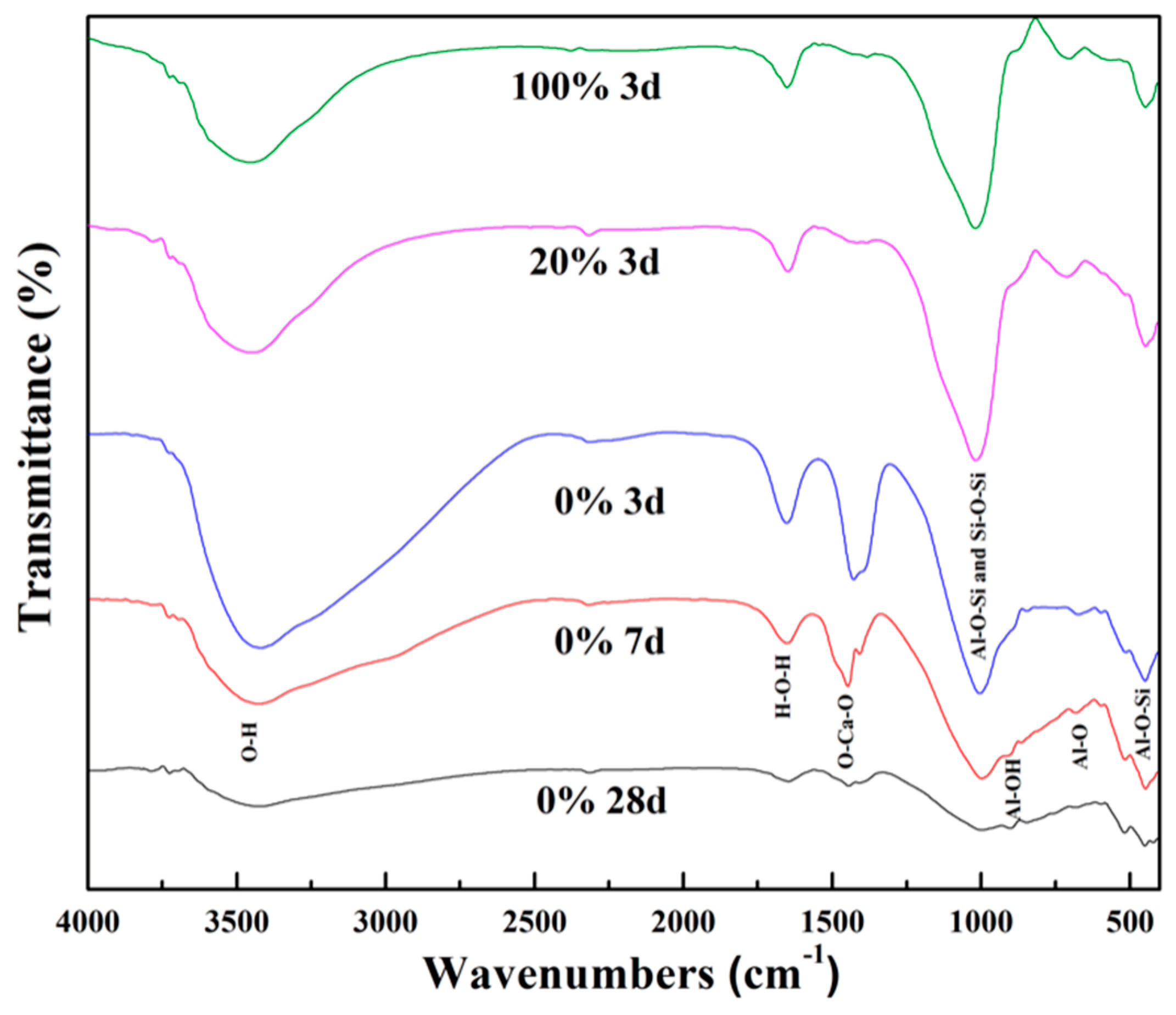
3.5. The FTIR Spectra of Sample
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gitari, W.; Thobakgale, R.; Akinyemi, S. Mobility and Attenuation Dynamics of Potentially Toxic Chemical Species at an Abandoned Copper Mine Tailings Dump. Minerals 2018, 8, 64. [Google Scholar] [CrossRef]
- Xu, D.; Yang, H.; Ouyang, J.; Zhang, Y.; Fu, L.; Chen, D. Lauric Acid Hybridizing Fly Ash Composite for Thermal Energy Storage. Minerals 2018, 8, 161. [Google Scholar] [CrossRef]
- Bian, Z.; Miao, X.; Lei, S.; Chen, S.E.; Wang, W. The challenges of reusing mining and mineral-processing wastes. Science 2012, 337, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Franks, D.M.; Boger, D.V.; Côte, C.M.; Mulligan, D.R. Sustainable development principles for the disposal of mining and mineral processing wastes. Resour. Policy 2011, 36, 114–122. [Google Scholar] [CrossRef]
- Amin, S.K.; El-Sherbiny, S.A.; El-Magd, A.A.M.A.; Belal, A.; Abadir, M.F.; Amin, S.K. Fabrication of geopolymer bricks using ceramic dust waste. Constr. Build. Mater. 2017, 157, 610–620. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Monosi, S.; Tittarelli, F.; Giosuè, C.; Ruello, M.L. Effect of two different sources and washing treatment on the properties of UFS by-products for mortar and concrete production. Constr. Build. Mater. 2013, 44, 260–266. [Google Scholar] [CrossRef]
- Kinnunen, P.; Ismailov, A.; Solismaa, S.; Sreenivasan, H.; Räisänen, M.L.; Levänen, E.; Illikainen, M. Recycling mine tailings in chemically bonded ceramics—A review. J Clean. Prod. 2018, 174, 634–649. [Google Scholar] [CrossRef]
- Muttashar, H.L.; Hussin, M.W.; Ariffin, M.A.M. Realisation of enhanced self-compacting geopolymer concrete using spent garnet as sand replacement. Mag. Concr. Res. 2017, 70, 558–569. [Google Scholar] [CrossRef]
- Dimas, D.D.; Giannopoulou, I.P.; Panias, D. Utilization of Alumina Red Mud for Synthesis of Inorganic Polymeric Materials. Min. Proc. Ext. Met. Rev. 2009, 30, 211–239. [Google Scholar] [CrossRef]
- Nikolov, A.; Rostovsky, I.; Nugteren, H. Geopolymer materials based on natural zeolite. Case Stud. Constr. Mater. 2017, 6, 198–205. [Google Scholar] [CrossRef]
- Villa, C.; Pecina, E.T.; Torres, R.; Gómez, L. Geopolymer synthesis using alkaline activation of natural zeolite. Constr. Build. Mater. 2010, 24, 2084–2090. [Google Scholar] [CrossRef]
- Song, L.; Li, Z.; Duan, P.; Huang, M.; Hao, X.; Yu, Y. Novel low cost and durable rapid-repair material derived from industrial and agricultural by-products. Ceram. Int. 2017, 43, 14511–14516. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Influence of preheating of fly ash precursors to produce geopolymers. J. Am. Ceram. Soc. 2017, 100, 3165–3174. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Behnia, A.; Alengaram, U.J.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar. Mater. Des. 2014, 59, 532–539. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Glid, M.; Sobrados, I.; Rhaiem, H.B.; Sanz, J.; Amara, A.B.H. Alkaline activation of metakaolinite-silica mixtures: Role of dissolved silica concentration on the formation of geopolymers. Ceram. Int. 2017, 43, 12641–12650. [Google Scholar] [CrossRef]
- Sabbatini, A.; Vidal, L.; Pettinari, C.; Sobrados, I.; Rossignol, S. Control of shaping and thermal resistance of metakaolin-based geopolymers. Mater. Des. 2017, 116, 374–385. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.J.; Zhou, W.; Ren, D. Fresh properties, compressive strength and microstructure of fly ash geopolymer paste blended with iron ore tailing under thermal cycle. Constr. Build. Mater. 2016, 118, 76–88. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.J.; Zhou, W.; Ren, D. Development of fly ash and iron ore tailing based porous geopolymer for removal of Cu(II) from wastewater. Ceram. Int. 2016, 42, 13507–13518. [Google Scholar] [CrossRef]
- Pooria, G.; Navid, R. Clayey soil stabilization using geopolymer and portland cement. Constr. Build. Mater. 2018, 188, 361–371. [Google Scholar]
- Djobo, J.N.Y.; Tchakouté; Kouamo, H.; Ranjbar, N.; Elimbi, A.; Tchadjié, L.N.; Njopwouo, D. Gel composition and strength properties of alkali-activated oyster shell-volcanic ash: Effect of synthesis conditions. J Am. Ceram. Soc. 2016, 188, 361–371. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kashefi, A.; Maheri, M.R. Hot-pressed geopolymer: Dual effects of heat and curing time. Cem. Concr. Comp. 2018, 86, 1–8. [Google Scholar] [CrossRef]
- Ye, N.; Chen, Y.; Yang, J.; Liang, S.; Hu, Y.; Hu, J.; Zhu, S.; Fan, W.; Xiao, B. Transformations of Na, Al, Si and Fe species in red mud during synthesis of one-part geopolymers. Cem. Concr. Res. 2017, 101, 123–130. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Rosas-Casarez, C.A.; Arredondo-Rea, S.P.; Cruz-Enríquez, A.; Corral-Higuera, R.; Gómez-Soberón, J.M.; Medina-Serna, T.D.J. Influence of Size Reduction of Fly Ash Particles by Grinding on the Chemical Properties of Geopolymers. Appl. Sci. 2018, 8, 365. [Google Scholar] [CrossRef]
- Toniolo, N.; Boccaccini, A.R.; Toniolo, N.; Boccaccini, A.R. Fly ash-based geopolymers containing added silicate waste: A review. Ceram. Int. 2017, 43, 14545–14551. [Google Scholar] [CrossRef]
- Moukannaa, S.; Loutou, M.; Benzaazoua, M. Recycling of phosphate mine tailings for the production of geopolymers. J. Clean. Prod. 2018, 185, 891–903. [Google Scholar] [CrossRef]
- Dabbebi, R.; de Aguiar, J.L.B.; Camões, A.; Samet, B.; Baklouti, S. Effect of the calcinations temperatures of phosphate washing waste on the structural and mechanical properties of geopolymeric mortar. Constr. Build. Mater. 2018, 185, 489–498. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Bao, S. Preparation of geopolymers from vanadium tailings by mechanical activation. Constr. Build. Mater. 2017, 145, 236–242. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, Y.; Chen, T. Thermal stability of a silica-rich vanadium tailing based geopolymer. Constr. Build. Mater. 2013, 38, 43–47. [Google Scholar] [CrossRef]
- Muttashar, H.L.; Ariffin, M.A.M.; Hussein, M.N.; Hussin, M.W.; Ishaq, S.B. Self-compacting geopolymer concrete with spend garnet as sand replacement. J. Build. Eng. 2018, 15, 85–94. [Google Scholar] [CrossRef]
- Muttashar, H.L.; Ali, N.B.; Ariffin, M.A.M.; Hussin, M.W. Microstructures and physical properties of waste garnets as a promising construction materials. Case Stud. Constr. Mater. 2018, 8, 87–96. [Google Scholar] [CrossRef]
- Ye, N.; Yang, J.; Ke, X.; Zhu, J.; Li, Y.; Xiang, C.; Wang, H.; Li, L.; Xiao, B. Synthesis and characterization of geopolymer from Bayer red mud with thermal pretreatment. J. Am. Ceram. Soc. 2014, 97, 1652–1660. [Google Scholar] [CrossRef]
- Fernández, R.; Ruiz, AI.; Cuevas, J. Formation of CASH phases from the interaction between concrete or cement and bentonite. Clay Miner. 2016, 51, 223–235. [Google Scholar] [CrossRef]
- Mijarsh, M.J.; Johari, M.M.; Ahmad, Z.A. Effect of delay time and Na2SiO3 concentrations on compressive strength development of geopolymer mortar synthesized from TPOFA. Constr. Build Mater. 2015, 1, 64–74. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash–Portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Onisei, S.; Pontikes, Y.; Van Gerven, T.; Angelopoulos, G.N.; Velea, T.; Predica, V. Synthesis of inorganic polymers using fly ash and primary lead slag. J. Hazard. Mater. 2012, 9, 101–110. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, F.; Liu, M.; Hu, X. Novel sustainable geopolymer based syntactic foams: An eco-friendly alternative to polymer based syntactic foams. Chem. Eng. J. 2017, 313, 74–82. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Lin, J.; Zhang, P.; Fang, L.; Ma, R.; Quan, Z. Geopolymer synthetized from sludge residue pretreated by the wet alkalinizing method: Compressive strength and immobilization efficiency of heavy metal. Constr. Build. Mater. 2018, 170, 619–626. [Google Scholar] [CrossRef]
- Fardjaoui, N.E.H.; Wicklein, B.; Aranda, P. Modulation of inorganic matrices for functional nanoarchitectures fabrication: The simultaneous effect of moisture and temperature in the preparation of metakaolin based geopolymers. Bull. Chem. Soc. Jpn. 2018, 91, 1158–1167. [Google Scholar] [CrossRef]
- Provis, J.; van Deventer, J.S.J. Alkali-Activated Materials: State-of-the-Art Report; RILEM TC. 224-AAM; Springer/RILEM: Dordrecht, The Netherlands, 2014; p. 126. [Google Scholar]
- Wan, Q.; Rao, F.; Song, S.; Cholico-González, D.F.; Ortiz, N.L. Combination formation in the reinforcement of metakaolin geopolymers with quartz sand. Cem. Concr. Comp. 2017, 80, 115–122. [Google Scholar] [CrossRef]
- Król, M.; Minkiewicz, J.; Mozgawa, W. IR spectroscopy studies of zeolites in geopolymeric materials derived from kaolinite. J. Mol. Struct. 2016, 1126, 200–206. [Google Scholar] [CrossRef]






| Components (wt %) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| MK | 53.56 | 43.92 | 1.08 | 0.14 | 0.10 | 0 | 0.78 |
| GT | 44.28 | 5.57 | 13.82 | 30.26 | 0.81 | 0.23 | 0.13 |
| Samples | Garnet/g | Metakaolin/g | Sodium Silicate/g | Water/g |
|---|---|---|---|---|
| GTGs-0 | 600 | 0 | 180 | 30 |
| GTGs-1 | 540 | 60 | 40 | |
| GTGs-2 | 480 | 120 | 60 | |
| GTGs-3 | 420 | 180 | 80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Liu, H.; Hao, X.; Wang, Y.; Liu, X.; Li, Z. Geopolymer Synthesis Using Garnet Tailings from Molybdenum Mines. Minerals 2019, 9, 48. https://doi.org/10.3390/min9010048
Wang A, Liu H, Hao X, Wang Y, Liu X, Li Z. Geopolymer Synthesis Using Garnet Tailings from Molybdenum Mines. Minerals. 2019; 9(1):48. https://doi.org/10.3390/min9010048
Chicago/Turabian StyleWang, An, Hongzhao Liu, Xiaofei Hao, Yang Wang, Xueqin Liu, and Zhen Li. 2019. "Geopolymer Synthesis Using Garnet Tailings from Molybdenum Mines" Minerals 9, no. 1: 48. https://doi.org/10.3390/min9010048
APA StyleWang, A., Liu, H., Hao, X., Wang, Y., Liu, X., & Li, Z. (2019). Geopolymer Synthesis Using Garnet Tailings from Molybdenum Mines. Minerals, 9(1), 48. https://doi.org/10.3390/min9010048





